Abstract
We reported a 9-year-old boy with mycoplasma pneumonia who developed pulmonary infarction. The child first had fever and cough, and then had difficult breathing. But, the signs of his lung were not obvious. Mycoplasma antibody IgM was positive. The child was given intravenous azithromycin for anti-infection, and intravenous low molecular weight heparin and oral warfarin for anti-coagulation. Although difficult breathing was relieved, sudden cardiac arrest occurred. His parents requested to give up treatment.
Keywords: Pulmonary infarction, mycoplasma, pneumonia, child
Introduction
Mycoplasma pneumonia is the most common respiratory disease among children. Generally, its symptoms are mild and its prognosis is good. However, in rare patients, it can cause vascular infarction in different sites of the body, especially pulmonary infarction whose clinical manifestations are similar to that of mycoplasma pneumonia, easily leading to misdiagnosis and high mortality. We present a patient whose pulmonary infarction proved to be caused by mycoplasma pneumonia.
Case report
All study methods were approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The boy’s mother gave written formal consent.
A 9-year-old boy was admitted to our hospital due to fever for more than 10 days, cough for 6 days and difficult breathing for 4 days. The child had fever without apparent cause 10 days before admission with a fever peak of 38.5°C. The child took cephalosporin, but his condition was not improved. Later, the child was referred to his local county hospital when he had paroxysmal cough without sputum and difficult breathing with cyanosis. In the county hospital, laboratory data were 20.4 × 109/L of WBC count, 88.3% of neutrophil, 102 g/L of hemoglobin and 95 × 109/L of platelet, the child received intravenous cefepime and oxygen inhalation for 2 days, but his symptoms such as difficult breathing and cyanosis were not improved, so he was referred to our hospital. The child was admitted to hospital, color echocardiography showed mild tricuspid regurgitation and tachycardia, and chest radiograph showed increased heart shadow in our outpatient service.
The child was sound and had normal development in the past. He had no related histories of congenital metabolic disease, congenital heart disease and family thrombus. He denied hematological and renal complaints, and exposure to tuberculosis. His parents are non-consanguineous marriage. The boy had a healthy sister.
Physical examination: Temperature 37.7°C, pulse: 136 per minute, respiratory rate 24 per minute, BP 100/65 mmHg. He was conscious, but his spirit was poor. He had tachypnea, nasal breathing and three-concave sign. Lungs were rough to auscultation bilaterally, without dry or moist rales. There was no murmur to auscultation of the heart valves. Abdomen was soft without tenderness and rebound tenderness. Liver and spleen were not impalpable. Extremities had no edema with normal activities. Neurological examination was negative.
Laboratory data included (1) blood Rt: WBC count of 12.3 × 109/L, hemoglobin of 107 g/L, neutrophil of 80.2%, C-reactive protein of 31.4 mg/L, procalcitonin of 0.18 ng/ml and erythrocyte sedimentation rate of 78 mm/h; (2) arterial blood gas analysis: pH of 7.49, PCO2 of 30 mmHg and PO2 of 45 mmHg; (3) liver and renal function, myocardial enzymes, and humoral and cellular immunity were normal; (4) tuberculosis specific secretory antigen antibody, tuberculosis specific envelope antigen antibody and mycobacterium tuberculosis IgG antibody all were negative; (5) pathogenic examination: mycoplasma pneumonia IgM antibody was positive (1:160); (6) coagulation function: plasma prothrombin time of 12.70 s (normal range: 8.8-13.6 s), prothrombin international normalized ratio (INR) of 1.15 (normal range: 0.8-1.6), activated partial thromboplastic time (APTT) of 30.30 s (normal range: 26-40 s), D-dimer of 6.81 mg/L (normal range: 0-0.55 mg/L), fibrinogen of 47.5 μg/ml (normal range: 0-5 μg/ml); (7) Chest CT showed bilateral lung inflammation (Figures 1 and 2); (8) Echocardiogram indicated sinus tachycardia, non-specific ST segment elevation and abnormal T wave.
Figure 1.
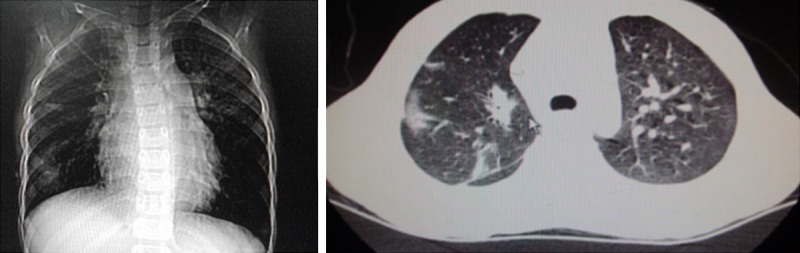
CT shows multiple high density patchy shadows in bilateral lungs, no pleural effusion and pleural thickening.
Figure 2.
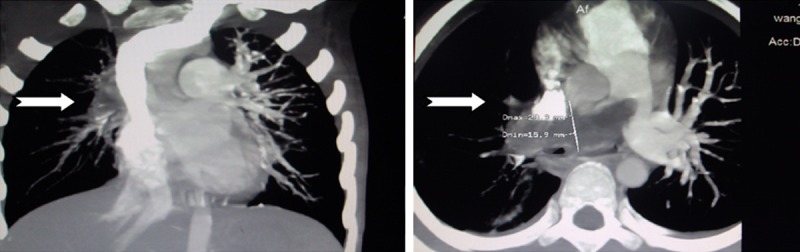
CT angiography shows lung inflammation, multiple embolisms in the pulmonary artery and its branches, mainly in the right.
Diagnosis: Mycoplasma pneumonia combined with type I respiratory failure.
Hospital course: The child was given oxygen inhalation and intravenous azithromycin, and then the body temperature was gradually decreased and difficult breathing was relieved. On the fourth day after admission, the circumference of the left lower extremity on the gastrocnemius was larger compared with the contralateral that, and was accompanied by tenderness. The child immediately underwent necessary examinations. Color echocardiography showed the enlargement of the right heart, mild tricuspid regurgitation, mild increased pulmonary arterial pressure and embolization in the right pulmonary artery. The ultrasound of the left lower extremity indicated thrombosis in the anterior and posterior tibial veins. Chest X-ray showed that the lucency was higher in the right lung than in the left lung. Laboratory data were D-dimer of 49.52 mg/L and fibrinogen of 115.4 μg/ml. CT angiography of pulmonary arteries displayed bilateral lung inflammation and multiple embolisms, especially in the right lung (Figure 2). The child was diagnosed with pulmonary infarction by the examinations above. The child was given intravenous low molecular weight heparin (4800 iv, H, QD) on the fifth day after admission and additional oral warfarin tablets (3.6 mg, QD, PO) on the seventh day after admission for anticoagulation therapy, and then his difficult breathing was improved. At 6:43 a.m on the eighth day after admission, the child unexpectedly had irritability-restlessness. Transcutaneous oxygen saturation and heart rate continuously deceased. Emergency cardiopulmonary resuscitation was immediately given. Forty minutes later, the heart rate increased to 100 per minute, but his pupillary light reflex disappeared and his parents requested to give up treatment.
Discussion
Based on these items including a 9-year-old boy, the first symptoms of fever and cough, sever cough without sputum, no sever toxic symptom, no obvious signs of the lungs, multiple patchy shadows in imaging, mild elevated EBC count, markedly increased C-reactive protein, normal procalcitonin, positive mycoplasma pneumoniae antibodies, negative blood culture, no evidence of other bacterial and viral infections, effective treatment of azithromycin, we could see that the only confirmatory pathogen was mycoplasma. According to the symptoms of chest pain and difficult breathing, laboratory data of increased levels of D-dimer and fibrinogen, and the results of pulmonary angiography, pulmonary infarction caused by mycoplasma pneumoniae was determined.
Mycoplasma pneumoniae (MP) was one of the most common pathogens in children with community-acquired pneumonia. It is reported that about 20-100 thousand individuals are hospitalized due to pneumonia in the United States every year, and mycoplasma pneumonia accounts for about 20% of acute pneumonia in middle and high school students, and about 50% in college students [1]. Mycoplasma pneumonia usually has a benign course, but sometimes it can cause severe pulmonary complications such as pleural effusion, pneumothorax, lung abscess, diffuse pneumonia, respiratory failure, and even acute respiratory distress syndrome [2]. However, mycoplasma pneumoniae accompanied by pulmonary infarction is very rare. Through literature retrieval between 1999 and 2012, only 3 children with mycoplasma pneumonia accompanied by pulmonary infarction have been reported (Table 1) [2-4]. Based on the treatment for the child in this study and review of the 3 related papers, we conclude that when un-explained dyspnea, chest pain, palpitations, syncope, hemoptysis or disease progression occur after anti-infective therapy, or un-explained enlargement of right heart, tricuspid regurgitation or dilatation of the pulmonary artery, especially deep venous thrombosis of lower extremity, we should pay attention to the possibility of pulmonary infarction.
Table 1.
Children with mycoplasma pneumonia combined with pulmonary infarction
| Age (sex) | Interval (day) | Infarction | Antibody of mycoplasma pneumoniae | Clinical features | Agglutination test | References |
|---|---|---|---|---|---|---|
| 6 years (male) | 16 | Femoral vein embolism and pulmonary embolism | Complement binding (1:640) | Fever, cough, hyoxemia, tachycardia and short breath | Positive anticardiolipin antibody and acquired activated protein C resistance | [3] |
| 13 years (male) | 5 | Left popliteal vein embolism and pulmonary embolism | ELISA IgM (5260 U/L) | Fever, cough, respiratory embarrassment and hyoxemia | Increased D-dimer, protein S deficiency and positive antiphospholipid antibody | [2] |
| 6 years (male) | 17 | Pulmonary embolism in the left lower lobe | ELISA; (1:128) | Fever, cough, difficult breathing and chest pain | Positive antiphospholipid antibody, increased D-dimer and decreased activity of plasma protein C | [4] |
Notes: The precedence ordering of cases is based on the date of publication; the normal range is indicated in the parenthesis; interval refers to the duration from fever to pulmonary infarction; ELISA: enzyme linked immunosorbent assay.
At present, the pathogenesis of vascular embolisms caused by mycoplasma infections has not been completely clear. There is direct and indirect pathogenesis. The direct pathogenesis is that mycoplasma pneumoniae blood-borne metastasis may induce cytokines such as tumor necrosis factor-α, chemotactic factor and interleukin-8 which affect vessel wall and lead to local angiitis or vascular occlusion [5]. The indirect pathogenesis is that mycoplasma pneumoniae causes immunologic derangement which produces phospholipids and IgM anticardiolipin antibodies to form a temporary hypercoagulable state, leading to deep vein thrombosis [6,7]. An in vitro study indicated that lipopolysaccharide derived from mycoplasma species (including Mycoplasma pneumoniae) could induce the procoagulant activity of human monocytes, and increase the levels of anti-cardiolipin antibodies, antiphospholipid IgM antibodies, D-dimer and fibrinogen [8]. These findings reflect a systemic infection-related prothrombotic states.
Since mycoplasma pneumonia may lead to pulmonary infarction, when a patient with mycoplasma pneumonia has possible pulmonary infarction, a series of tests including blood Rt, arterial blood gas analysis, pulmonary function, ECG, echocardiography are required. However, pulmonary infarction can not be determined only based on the tests above. The level of D-miter, an important screening test, has a diagnostic rate as high as 92-100% for acute pulmonary infarction, but its specificity is poor with a false negative rate of 13-40% in children with pulmonary infarction [9,10]. Further imaging such as chest X-ray, nuclide lung ventilation perfusion imaging, CT, magnetic resonance angiography (pulmonary artery angiography) is necessary for definite diagnosis of pulmonary infarction.
For the treatment of mycoplasma pneumonia, active anti-infection should be first performed with macrolides. According to the Guidelines on the Diagnosis and Management of Acute Pulmonary Embolism of European Society of Cardiology of 2008 [11], the treatment for pulmonary infarction mainly includes anticoagulant therapy and thrombolytic therapy. The drugs for anticoagulant therapy commonly include un-fractionated heparin, low-molecular-weight heparin and vitamin K. At present, low-molecular-weight heparin which have been approved for the treatment of acute pulmonary embolism includes Enoxaparin (1.0 mg/kg, every 12 h or 1.5 mg/kg once daily), Tinzaparin (175 U/kg once daily) and Fondaparinux (5 mg for body weight < 50 kg, 7.5 mg for body weight 50-100 kg, 10 mg for body weight > 100 kg, once daily). The vitamin K antagonist which is most commonly used in clinical practice is warfarin. The initial dose of warfarin is 5-10 mg, and is given 12-48 h after heparin therapy. The combined application of warfarin and heparin should last for 5 days at least. For extensive pulmonary infarction, heparin should be given for 7-10 days. When the international normalized ratio (INR) continuously ranges between 2.0 and 3.0 for more than 2 days, administration of non-oral anticoagulants should be stopped followed by long-term anticoagulant therapy for 3 months at least. The drugs for thrombolytic therapy commonly include urokinase, streptokinase and tissue plasminogen activator. The optimal time of administration is within 48 h after symptoms appear, but thrombolytic therapy is still effective in the patients who have had symptoms for 6-14 days. Thrombolytic therapy is seldom used in children with pulmonary infarction because there has been a lack of evaluation in safety and effectiveness. Hemorrhage is a common complication of anticoagulant therapy and thrombolytic therapy. Therefore, it is necessary to weigh the advantages and disadvantages before anticoagulant therapy or thrombolytic therapy.
Most of patients with acute pulmonary infarction receiving adequate anticoagulation therapy are survival. At present, there have not been precise epidemiological data about the prognosis of children with pulmonary infarction. A long-term follow-up of 405 children with pulmonary embolism and deep vein thrombosis indicated that the mortality was 16% in Canada [12]. In addition, the recurrence rate of pulmonary infarction and the long-term effects of pulmonary infarction on lung function remain yet to be studied.
Disclosure of conflict of interest
None.
References
- 1.Garcia AV, Fingeret AL, Thirumoorthi AS, Kadenhe-Chiweshe A, Kandel JJ. Severe Mycoplasma pneumoniae infection requiring extracorporeal membrane oxygenation with concomitant ischemic stroke in a child. Pediatr Pulmonol. 2013;48:98–101. doi: 10.1002/ppul.22552. [DOI] [PubMed] [Google Scholar]
- 2.Graw-Panzer KD, Verma S, Rao S, Miller ST, Lee H. Venous thrombosis and pulmonary embolism in a child with pneumonia due to Mycoplasma pneumoniae. J Natl Med Assoc. 2009;101:956–958. doi: 10.1016/s0027-9684(15)31045-2. [DOI] [PubMed] [Google Scholar]
- 3.Brown S, Padley S, Bush A, Cummins D, Davidson S, Buchdahl R. Mycoplasma pneumonia and pulmonary embolism in a child due to acquired prothrombotic factors. Pediatr Pulmonol. 2008;43:200–202. doi: 10.1002/ppul.20739. [DOI] [PubMed] [Google Scholar]
- 4.Su HY, Jin WJ, Zhang HL, Li CC. Clinical analysis of pulmonary embolism in a child with Mycoplasma pneumoniae pneumonia. Zhonghua Er Ke Za Zhi. 2012;50:151–154. [PubMed] [Google Scholar]
- 5.Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother. 2010;16:162–169. doi: 10.1007/s10156-010-0044-x. [DOI] [PubMed] [Google Scholar]
- 6.Leonardi S, Pavone P, Rotolo N, La Rosa M. Stroke in two children with Mycoplasma pneumoniae infection A causal or causal relationship? Pediatr Infect Dis J. 2005;24:843–845. doi: 10.1097/01.inf.0000177284.88356.56. [DOI] [PubMed] [Google Scholar]
- 7.Senda J, Ito M, Atsuta N, Watanabe H, Hattori N, Kawai H, Sobue G. Paradoxical brain embolism induced by Mycoplasma pneumoniae infection with deep venous thrombus. Intern Med. 2010;49:2003–2005. doi: 10.2169/internalmedicine.49.3570. [DOI] [PubMed] [Google Scholar]
- 8.Joo CU, Kim JS, Han YM. Mycoplasma pneumoniae induced popliteal artery thrombosis treated with urokinase. Postgrad Med J. 2001;77:723–724. doi: 10.1136/pmj.77.913.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biss TT, Branda LR, Kahr WH, Chan AK, Williams S. Clinical features and outcome of Pulmonary embolism in children. Br J Haematol. 2008;142:808–818. doi: 10.1111/j.1365-2141.2008.07243.x. [DOI] [PubMed] [Google Scholar]
- 10.Biss TT, Brandao LR, Kahr WH, Chan AK, Williams S. Clinical probability score and D-dimer estimation lack utility in the diagnosis of childhood pulmonary embolism. Thromb Haemost. 2009;7:1633–1638. doi: 10.1111/j.1538-7836.2009.03572.x. [DOI] [PubMed] [Google Scholar]
- 11.Torbicki A, Perrier A, Konstenfinides S, Agnelli G, Galle N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D, Janssens U, Klepetko W, Remy-jardin M, Bassand JP ESC Committee for Practice Guidelines (CPG) Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29:2276–2315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 12.Monagle P, Adams M, Mahoney M, Ali K, Barnard D, Bernstein M, Brisson L, David M, Desai S, Scully MF, Halton J, Israels S, Jardine L, Leaker M, McCusker P, Sila M, Wu J, Anderson R, Andrew M, Massicotte MP. Outcome of pediatric thromboembolic disease: a report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000;47:763–766. doi: 10.1203/00006450-200006000-00013. [DOI] [PubMed] [Google Scholar]


