Abstract
We reported a rare case of pure red cell aplasia in a 44-year-old man with multiple myeloma with biclonal gammophathy (IgG lambda and IgA lambda type) with severe anemia. After treatment with bortezomib, adriamycin, and dexamethasone, the patient achieved very good partial response with disappearance of monoclonal gammopathy. However, his anemia was not significantly improved. Bone marrow analysis revealed selective erythroid hypoplasia. Thus, cyclosporine A was administered, which resulted in a complete recovery from anemia. The present case may provide some insight into the pathogenesis of PRAC and malignant plasma cell disorder.
Keywords: Multiple myeloma, pure red cell aplasia, cyclosporine A, bortezomib
Introduction
Pure red cell aplasia (PRCA) is an acquired anemia that may be idiopathic or secondary to a variety of neoplastic, autoimmune, or infectious diseases or to exposure to drugs; most cases of PRCA are considered to be autoimmune-mediated [1,2]. Multiple myeloma (MM) is a bone marrow malignancy of clonal plasma cells and is characterized by osteolytic bone destruction, renal failure, an increased risk of infections, and anemia. Although anemia is a common symptom in patients with MM, PRCA has rarely been reported in MM [3,4]. Here we describe a case of PRCA associated with MM. This case of MM and PRCA were treated with bortezomib, which induced long lasting remission of myeloma, but did not result in the reversal of PRCA. However, the patient eventually responded to the treatment with cyclosporine A (CsA).
Case report
The patient, a 44-year-old man, was hospitalized in June 2009 due to symptomatic anemia. He had no history of gastrointestinal disorders or bleeding, previous malignancies or autoimmune diseases. On admission, the patient had marked facial pallor but no lymphadenopathy and hepatosplenomegaly. Laboratory findings showed hemoglobin (Hb) 34 g/L, white blood cell (WBC) of 4.6×109/L, platelets 313×109/L and reticulocyte count of 0.05%, and erythrocyte sedimentation rate (ESR) of 140 mm/h. Other investigations showed normal renal function, liver function and the levels of serum calcium and beta-2 microglobulin. Conventional cytogenetic testing showed a normal karyotype 46, XY. Lactate dehydrogenase (LDH) was elevated at 664 U/L. The serum levels of IgM was slightly decreased (0.42 g/L; [normal 0.5-2.5 g/L]) while IgA (594 g/L [normal; 0.85-3.0 g/L]) and IgG (21.5 g/L; [normal 8.0-15 g/L]) were significantly elevated. Levels of kappa and lambda light chains in blood were 1.76 g/L (normal 1.72-3.83 g/L) and 5.03 g/L (normal 0.81-1.02 g/L), respectively. The SFLC ratio was 0.35. Immunofixation electrophoresis revealed the presence of biclonal gammopathy (IgG lambda and IgA lambda) (Figure 1A). Bone marrow (BM) aspirate revealed evidence of myeloma and BM aspiration showed plasmacytosis of 14% and a virtually complete lack of erythropoiesis (Figure 1B). The plasma cell population showed the following immunophenotype: CD38 (88.8%), CD138 (89.4%), CD56 (88.8%), intracytoplasmic kappa light-chain (5.7%) and lamda (90.8%). According to the above data, the diagnosis of MM with biclonal gammopathy was made. The patient was treated bortezomib, adriamycin, and dexamethasone (PAD regimen) chemotherapy every 4 weeks (bortezomib 1.3 mg/m2/day D1, D4, D8, D11; adriamycin 9 mg/m2/day D1-4; dexamethasone 40 mg/day D1-4). After four cycles of PAD, the patient achieved very good partial response. Then he was commenced on thalidomide as maintenance therapy. However, he remained blood transfusion dependent with persisting severe anemia (Hb=68.0 g/L) although recombinant erythropoietin (rhEPO) therapy was given. At that time, a BM aspiration was performed again, which showed that normal hematopoiesis was hardly detected and erythroid precursors were lacking (Figure 1C). Reticulocyte count was consistently low (0.3%). However, there was no evidence of thymoma and infection of parvovirus B19 (viral DNA was examined). Therefore, he was diagnosed with acquired PRCA on the basis of these findings. Since dexamethasone had failed to improve his anemia in the past, we decided to use CsA. CsA was administered at an initial dose of 5 mg/kg/day (300 mg/day). After four weeks, his Hb level and reticulocyte count showed dramatic improvement (120 g/L and 1.0%, respectively).
Figure 1.
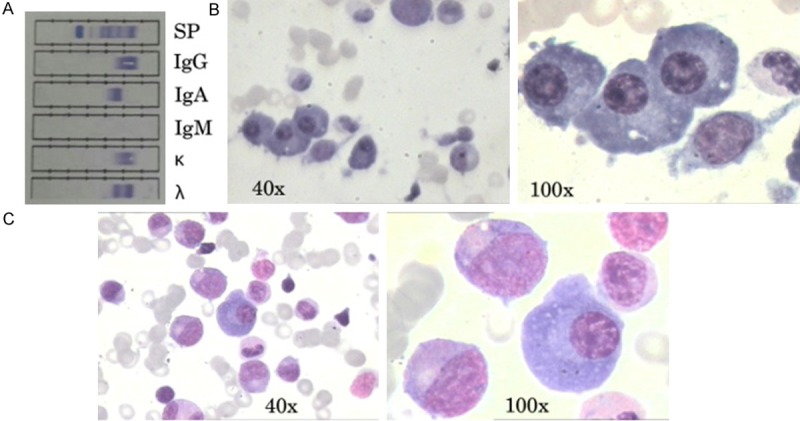
A. Serum immunofixation electrophoresis. It shows biclonal gammophthy of IgG lambda and IgA lambda type. B. Photomicrographs of bone marrow aspirate at diagnosis showing infiltration by malignant plasma cells, and absent erythropoiesis (H&E, 40× and 100×). C. Photomicrographs of bone marrow aspirate after treatment with bortezomib (H&E, 40× and 100×). There is an almost complete absence of red-cell precursors.
Discussion
We reported a rare case of MM with biclonal gammopathy (IgG lambda and IgA lambda) in a 44-years-old man diagnosed with PRCA. MM with biclonal gammopathy, a disorder characterized by the production of two distinct monoclonal proteins, accounts for 2% of MM [5]. The presence of 2 monoclonal proteins may result from the proliferation of 2 clones of plasma cells, each producing an unrelated monoclonal protein, or it may be because of the production of 2 distinct monoclonal immunoglobulins by a single clone of plasma cells [6]. The acquired PRCA can be secondary to various hematological malignancies, such as T-cell large granular lymphocyte leukemia, chronic lymphocytic leukemia, diffuse large B-cell lymphoma, and Waldenstrom’s macroglobulinaemia [7,8]. However, there are a few reports of PRCA associated with MM [3,4]. To our knowledge, this is the first report of simultaneous PRCA and MM with biclonal gammopathy.
The pathophysiology of PRCA is heterogeneous, but most cases of acquired PRCA are mediated by diverse autoimmune mechanisms, including antibody-mediated, NK cell- or T cell-mediated [7]. However, the association of MM and PRCA is still unclear. A previous report emphasizes the important role of paraproteinemia in PRCA [4]. Actually, some studies suggested the inhibition of erythropoiesis by abnormal immunoglobulins produced by MM cells [4,9]. Sarathy KS, et al [4] reported a case of acquired PRCA associated with MM. Despite immunosuppression therapies, the disease of the patient and PRCA remained refractory until complete disease control was achieved with bortezomib. Bone marrow culture studies showed a reduced BFU-E colony formation when the patient relapsed. Whereas, the recovery of erythropoiesis was observed when patient was in remission again. These data suggest that a possible mechanism of PRCA in MM may be via the suppression of BFU-E by the abnormal monoclonal immunoglobulins. Our patient presented with persistent severe anemia with selective erythroblastopenia in the bone marrow, although complete remission of the disease was achieved after treatment with bortezomib, suggesting that his PRCA is not associated with paraproteinemia. Importantly, CsA led to a dramatic recovery from anemia, which supports the notion of immunologically-mediated PRCA.
Usually, patients with primary or secondary PRCA not responding to treatment of the underlying disease should be treated with immunosuppressive drugs, such as corticosteroids (CS), cyclophosphamide (CY), CsA, and antithymocyte globulin [1]. Among them, CsA exhibits a favorable effect for PRCA. Mamiya et al. [10] analyzed the clinical features of 150 patients with acquired PRCA in Japan, which showed that CsA was the most effective form of treatment and the response rate was 82%. Whereas, the patients receiving CS or CY showed a 49% and 29% response rate, respectively. Thus, the authors recommended CsA as the first-line therapy for acquired chronic PRCA.
In conclusion, we had reported an infrequent occurrence of PRCA in a patient with MM with biclonal gammopathy, whose anemia responds to CsA therapy, but not to the therapy for the underlying disease. This case may provide some insight into the pathogenesis of PRAC and malignant plasma cell disorder.
Disclosure of conflict of interest
None.
References
- 1.Sawada K, Fujishima N, Hirokawa M. Acquired pure red cell aplasia: updated review of treatment. Br J Haematol. 2008;142:505–14. doi: 10.1111/j.1365-2141.2008.07216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charles RJ, Sabo KM, Kidd PG, Abkowitz JL. The pathophysiology of pure red cell aplasia: implications for therapy. Blood. 1996;87:4831–8. [PubMed] [Google Scholar]
- 3.Orchard J, Myint H, Hamblin TJ. A patient with myeloma who still has pure red cell aplasia despite the most intensive immune modulation. Leuk Res. 1997;21:353–4. doi: 10.1016/s0145-2126(97)00028-3. [DOI] [PubMed] [Google Scholar]
- 4.Sarathy KS, Ramakrishna R, Baig WW, Manoharan A. Acquired pure red cell aplasia in patients with plasma cell neoplasm and long term remission with bortezomib therapy. J Hematol Malig. 2013;3:37–43. [Google Scholar]
- 5.Bergsagel PL. Where we were, where we are, where we are going: progress in multiple myeloma. Am Soc Clin Oncol Educ Book. 2014;34:199–203. doi: 10.14694/EdBook_AM.2014.34.199. [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Robinson RA, Katzmann JA. The clinical aspects of biclonal gammopathies. Review of 57 cases. Am J Med. 1981;71:999–1008. doi: 10.1016/0002-9343(81)90326-0. [DOI] [PubMed] [Google Scholar]
- 7.Fisch P, Handgretinger R, Schaefer HE. Pure red cell aplasia. Br J Haematol. 2000;111:1010–22. doi: 10.1046/j.1365-2141.2000.02429.x. [DOI] [PubMed] [Google Scholar]
- 8.Li YY, Fan L, Wang L, Xu J, Li JY, Xu W. Waldenström’s macroglobulinaemia complicated by pure red cell aplasia: a case report. Blood Transfus. 2013;11:630–3. doi: 10.2450/2013.0235-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resegotti L, Dolci C, Palestro G, Peschle C. Paraproteinemic variety of pure red cell aplasia: immunological studies in 1 patient. Acta Haematol. 1978;60:227–32. doi: 10.1159/000207718. [DOI] [PubMed] [Google Scholar]
- 10.Mamiya S, Itoh T, Miura AB. Acquired pure red cell aplasia in Japan. Eur J Haematol. 1997;59:199–205. doi: 10.1111/j.1600-0609.1997.tb00978.x. [DOI] [PubMed] [Google Scholar]


