Abstract
Numerous studies have investigated association of methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism with lung cancer (LC) susceptibility in Chinese; however, the findings are inconsistent. Therefore, we performed a meta-analysis. PubMed, ISI Web of Knowledge, Chinese National Knowledge Infrastructure, and Wanfang were searched. Pooled ORs and 95% CIs were used to assess the strength of the associations. Overall, 10 studies with 2487 cases and 3228 controls investigating the MTHFR C677T polymorphism and LC risk were included. We did not find a significant association between MTHFR C677T polymorphism and LC risk. However, significantly increased LC risk was found in the population from North China, which was not found in the population from South China. In conclusion, our meta-analysis suggested that MTHFR C677T polymorphism might influence the risk of LC.
Keywords: Lung cancer, MTHFR, meta-analysis, polymorphism
Introduction
Lung cancer (LC) is a major cause of cancer death worldwide with 1 million deaths each year [1]. While the disease is largely preventable as most cases are due to tobacco smoking, global statistics estimate that 15% of lung cancer cases in men and 50% in women are not attributable to smoking. Thus, if considered a separate category, lung cancer in never-smokers would rank as the seventh most common cause of cancer death worldwide, with a higher incidence than cancers of the cervix, pancreas and prostate.
Methylenetetrahydrofolate reductase (MTHFR), whose gene maps to chromosome 1p36.3 and encodes a 77-kDa protein, plays a key role in folate metabolism by irreversibly catalyzing the reduction of 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, the predominant circulatory form of folate, which serves as both a cofactor and substrate for the regeneration of methionine. The latter leads to production of S-adenosylmethionine (SAM); the universal methyl donor in humans for DNA methylation [2]. Reduced enzyme activity may result in lower levels of SAM and an increased risk of cancer, including LC, as a consequence of gene hypomethylation [3]. One common single nucleotide polymorphism of MTHFR has been indicated: C677T (rs1801133), which results in the amino acid product changing from alanine to valine [4]. Studies have confirmed that the variant genotypes are associated with a significant reduction of enzyme activity [5], suggesting that the polymorphisms of C677T may be related to the risk of LC. A series of studies have investigated the association between the MTHFR C677T polymorphism and LC susceptibility, but provided controversial or inconclusive results [6-15]. We performed this meta-analysis to assess the relationship of MTHFR C677T polymorphism with risk of LC.
Materials and methods
Search for publications
Two researchers independently performed a computerized search in four databases-PubMed, ISI Web of Knowledge, Chinese National Knowledge Infrastructure, and Wanfang-up to June 2014. The search terms were “methylenetetrahydrofolate reductase” or MTHFR, “lung cancer or lung carcinoma or lung neoplasm” in various combinations, with the language limited to English and Chinese. The reference list of each relevant publication was also reviewed to ensure that all appropriate studies were included in the meta-analysis.
Inclusion and exclusion criteria
Studies included in this meta-analysis have to meet the following criteria: (1) case-control study or cohort study on the associations between MTHFRC677T polymorphism and LC susceptibility; (2) sufficient published data about sample size, odds ratio (OR), and their 95% confidence interval (CI); (3) the distribution of the genotypes in control groups was in the Hardy-Weinberg equilibrium (HWE). Studies were excluded when they were: (1) duplicate publication; (2) meta-analyses, letters, reviews, or editorial articles.
Data extraction
Two investigators independently extracted the following information from each study: the first author, year of publication, number of genotyped cases and controls, numbers of genotypes for MTHFR C677T polymorphism in cases and controls, and main findings.
Qualitative assessment
Two authors completed the quality assessment independently. The Newcastle-Ottawa Scale (NOS) was used to evaluate the methodological quality, which scored studies by the selection of the study groups, the comparability of the groups, and the ascertainment of the outcome of interest. We considered a study awarded 0-3, 4-6, or 7-9 as a low-, moderate-, or high-quality study, respectively. Discrepancies were resolved by consensus and discussion.
Statistical analysis
Statistical analysis was conducted by using STATA statistical package (version 10, STATA, College Station, TX). The distributions of genotypes in controls were tested by HWE using the Chi-square test. The association of polymorphisms of MTHFRC677T polymorphism and LC risk was estimated by odds ratio (ORs) with 95% confidence intervals (CIs). The random effect model (DerSimonian and Laird) was selected to summarize the combined OR and their 95% CI. The significance of the pooled OR was determined by the Z test. Publication bias was investigated with the funnel plot, in which the Standard Error (SE) of log OR of each study was plotted against its OR. Funnel-plot asymmetry was further assessed by the method of Egger’s linear regression test. All the P values were two sided. P value less than 0.05 was considered statistically significant.
Results
Characteristics of studies
Figure 1 shows the process of study identification. A total of 10 case-control studies with 2487 cases and 3228 controls investigating the MTHFR C677T polymorphism and LC risk met the inclusion criteria and were included in the meta-analysis [6-15]. All studies were assessed by NOS. The quality scores ranged from 6 to 9, suggesting that the methodological quality was acceptable. The characteristics of the included studies are summarized in Table 1.
Figure 1.
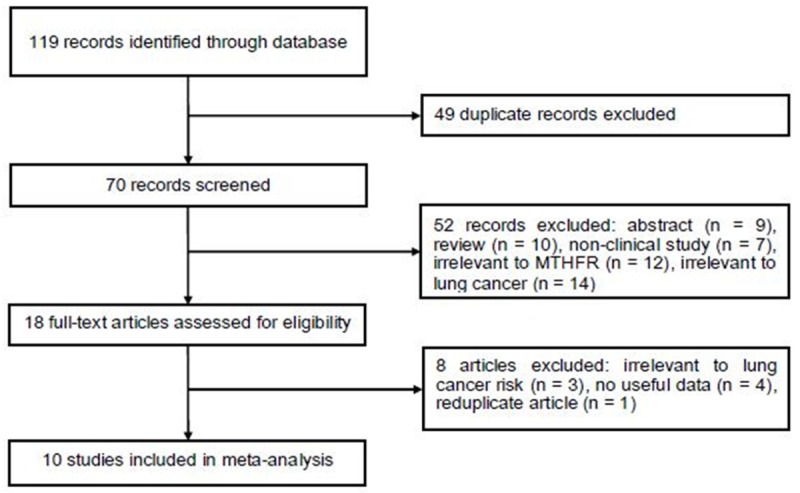
Flow diagram of study identification.
Table 1.
Characteristics of studies included in the meta-analysis
| Study | Year | Gender | Hardy Weinberg Equilibrium | Quality score | Cases | Controls | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| CC | CT | TT | CC | CT | TT | |||||
| Jeng | 2003 | Both | Yes | 6 | 36 | 22 | 1 | 123 | 95 | 14 |
| Zhang | 2005 | Both | Yes | 6 | 120 | 230 | 155 | 160 | 231 | 109 |
| Shen | 2005 | Both | Yes | 6 | 33 | 65 | 18 | 53 | 42 | 16 |
| Liu | 2008 | Both | Yes | 7 | 157 | 245 | 98 | 149 | 265 | 103 |
| Liu | 2009 | Both | Yes | 6 | 205 | 124 | 29 | 362 | 291 | 63 |
| Yao | 2010 | Both | Yes | 8 | 27 | 46 | 20 | 36 | 51 | 19 |
| Yang | 2010 | Both | Yes | 9 | 49 | 52 | 19 | 62 | 75 | 28 |
| Cui | 2011 | Both | Yes | 7 | 58 | 240 | 140 | 121 | 325 | 195 |
| Cheng | 2011 | Both | Yes | 6 | 49 | 58 | 71 | 47 | 88 | 45 |
| Ma | 2012 | Both | Yes | 8 | 20 | 54 | 46 | 22 | 28 | 10 |
Results of this meta-analysis
We did not find a significant association between MTHFR C677T polymorphism and overall LC risk. However, significantly increased LC risk was found in the population from North China (T vs. C: OR = 1.28, 95% CI: 1.14-1.44; TT vs. CC: OR = 1.67, 95% CI: 1.33-2.10; TT + CT vs. CC, OR = 1.39, 95% CI = 1.15-1.69; TT vs. CC + CT: OR = 1.46, 95% CI: 1.03-2.06), which was not found in the population from South China. In the subgroup analysis by ethnicity, significantly increased risk was not found in Han population. The main results of this meta-analysis and the heterogeneity test were shown in Table 2.
Table 2.
Results of the meta-analysis
| Subgroup | OR | 95% CI | Pheterogeneity | |
|---|---|---|---|---|
| T vs. C | Overall | 1.15 | 0.97-1.37 | 0.000 |
| Han | 1.20 | 0.65-2.22 | 0.000 | |
| Not stated | 1.18 | 0.95-1.47 | 0.002 | |
| South | 1.09 | 0.86-1.39 | 0.000 | |
| North | 1.28 | 1.14-1.44 | 0.293 | |
| TT vs. CC | Overall | 1.35 | 0.99-1.83 | 0.002 |
| Han | 1.43 | 0.77-2.67 | 0.004 | |
| Not stated | 1.42 | 1.02-1.97 | 0.076 | |
| South | 1.20 | 0.77-1.89 | 0.007 | |
| North | 1.67 | 1.33-2.10 | 0.615 | |
| TT + CT vs. CC | Overall | 1.18 | 0.91-1.53 | 0.000 |
| Han | 1.10 | 073-1.65 | 0.021 | |
| Not stated | 1.35 | 0.92-1.98 | 0.000 | |
| South | 1.13 | 0.81-1.57 | 0.000 | |
| North | 1.39 | 1.15-1.69 | 0.178 | |
| TT vs. CC + CT | Overall | 1.25 | 0.99-1.57 | 0.014 |
| Han | 1.47 | 0.87-2.47 | 0.005 | |
| Not stated | 1.22 | 1.03-1.44 | 0.212 | |
| South | 1.06 | 0.87-1.30 | 0.115 | |
| North | 1.46 | 1.03-2.06 | 0.031 |
The Egger’s test indicated that there was no obvious publication bias (P = 0.568).
Discussion
Although many studies analyzing the research results about the MTHFR C677T polymorphism and LC risk in Chinese, definite conclusions cannot be drawn. Therefore, we did this meta-analysis to estimate the relationships between MTHFR C677T polymorphism and LC risk. The meta-analysis involved 10 studies with 2487 cases and 3228 controls. The results from this meta-analysis showed that the MTHFR C677T polymorphism was not significantly associated with LC risk. When we performed the subgroup analyses by geographical locations, significant associations with susceptibility for the development of LC was found in North China populations. On the one hand, it was possible that these differences might be affected by exposure to various environmental factors. However, no reported article was performed to assess the effect of MTHFR-environment interactions in different populations. In the future, more studies should be designed to analyze these associations. On the other hand, it was possible that considerable heterogeneity may have distorted the result.
Folate, a water soluble vitamin B9, has been considered as an ‘essential vitamin’ because it modulates potential DNA damage and the risk of developing cancer, although not consistently [16]. In vivo and in vitro evidences suggest that folate deficiency results in DNA damage and instability, and altered DNA methylation, and eventually result in cell death via apoptosis, all of which may promote tumor initiation [17]. The MTHFR protein, a central enzyme in folate metabolism, has been implicated with lung cancer risk [18], because it is involved in methyl group synthesis process by catalyzing the irreversible conversion of 5, 10-methylenetetrahydrofolate (THF) to 5-methyl THF, which serves as methyl donor for the remethylation of homocystein to methionine and the precursor of S-adenosylmethionine (SAM). The polymorphism in the MTHFR gene: C677T is known to have functional relevance and thus variation of MTHFR in the folate metabolic pathway may be associated with variable folate levels and is suspected of influencing the risk of lung cancer [19].
There are several limitations in this meta-analysis must also be considered. First, lack of the original data of lung cancer histological types limited our further evaluation of histological types and genotypes interactions. Second, lack of the original data limited our further evaluation of potential gene-gene and gene-environment interactions. Third, lack of information on disease status, genotypes, and well documented smoking status may also influence the results.
In conclusion, this meta-analysis suggested that MTHFR C677T polymorphism increased the susceptibility to LC in Chinese.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay F, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kim YI. Methylenetetrahydrofolate reductase polymorphisms, folate, and cancer risk: a paradigm of gene-nutrient interactions in carcinogenesis. Nutr Rev. 2000;58:205–9. doi: 10.1111/j.1753-4887.2000.tb01863.x. [DOI] [PubMed] [Google Scholar]
- 3.Stern LL, Mason JB, Selhub J, Choi SW. Genomic DNA hypomethylation, a characteristic of most cancers, is present in peripheral leukocytes of individuals who are homozygous for the C677T polymorphism in the methylenetetrahydrofolate reductase gene. Cancer Epidemiol Biomarkers Prev. 2000;9:849–53. [PubMed] [Google Scholar]
- 4.van der Put NM, Gabreëls F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, van den Heuvel LP, Blom HJ. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. 1998;62:1044–51. doi: 10.1086/301825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 6.Jeng YL, Wu MH, Huang HB, Lin WY, You SL, Chu TY, Chen CJ, Sun CA. The methylenetetrahydrofolate reductase 677C--&T polymorphism and lung cancer risk in a Chinese population. Anticancer Res. 2003;23:5149–52. [PubMed] [Google Scholar]
- 7.Zhang XM, Miao XP, Tan W, Qu S, Sun T, Zhou YF, Lin DX. Association between genetic polymorphisms in methylenetetrahydrofolate reductase and risk of lung cancer. Acta Acad Med Sin. 2005;27:700–3. [PubMed] [Google Scholar]
- 8.Shen M, Rothman N, Berndt SI, He X, Yeager M, Welch R, Chanock S, Caporaso N, Lan Q. Polymorphisms in folate metabolic genes and lung cancer risk in Xuan Wei, China. Lung Cancer. 2005;49:299–309. doi: 10.1016/j.lungcan.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Jin G, Wang H, Wu W, Liu Y, Qian J, Fan W, Ma H, Miao R, Hu Z, Sun W, Wang Y, Jin L, Wei Q, Shen H, Huang W, Lu D. Association of polymorphisms in one-carbon metabolizing genes and lung cancer risk: a case-control study in Chinese population. Lung Cancer. 2008;61:21–9. doi: 10.1016/j.lungcan.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Liu CS, Tsai CW, Hsia TE, Wang RF, Liu CJ, Hang LW, Chiang SY, Wang CH, Tsai RY, Lin CC, Bau DT. Interaction of methylenetetrahydrofolate reductase genotype and smoking habit in Taiwanese lung cancer patients. Cancer Genomics Proteomics. 2009;6:325–9. [PubMed] [Google Scholar]
- 11.Yao QF, Chen X, Xue JR, Luo M, Xiao L, Yang C. Relationship of polymorphisms of MTHFR gene and hypermethylation of tumor suppressor gene in lung cancers. Cancer Prev Treat. 2010;37:531–4. [Google Scholar]
- 12.Yang XX, Li FX, Yi JP, Li X, Sun JZ, Hu NY. Association between C677T genetic polymorphisms of methylenetetrahydrofolate reductase and risk of gastric cancer, colorectal cancer and lung cancer. Guangdong Med J. 2010;31:2375–8. [Google Scholar]
- 13.Cui LH, Yu Z, Zhang TT, Shin MH, Kim HN, Choi JS. Influence of polymorphisms in MTHFR 677 C→T, TYMS 3R→2R and MTR 2756 A→G on NSCLC risk and response to platinum-based chemotherapy in advanced NSCLC. Pharmacogenomics. 2011;12:797–808. doi: 10.2217/pgs.11.27. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Z, Wang W, Song YN, Xia J, Kang Y, Dai L, et al. Association between C677T genetic polymorphisms of methylenetetrahydrofolate reductase and risk of lung cancer. Chin J Tuberc Respir Dis. 2011;34:57–8. [Google Scholar]
- 15.Ma QL, Li YF, Ji M, Yang KY, Wang JY, Li S, et al. Study of the association between C677T gene polymorphisms of methylenetetrahydrofolate reductase and susceptibility to lung cancer. Chin J Clinicians. 2012;6:213–5. [Google Scholar]
- 16.Kim YI. Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem. 1999;10:66–88. doi: 10.1016/s0955-2863(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 17.Knock E, Deng L, Wu Q, Leclerc D, Wang XL, Rozen R. Low dietary folate initiates intestinal tumors in mice, with altered expression of G2-M checkpoint regulators polo-like kinase 1 and cell division cycle 25c. Cancer Res. 2006;66:10349–56. doi: 10.1158/0008-5472.CAN-06-2477. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Matsuo K, Hiraki A, Saito T, Sato S, Yatabe Y, Mitsudomi T, Hida T, Ueda R, Tajima K. Impact of one-carbon metabolism-related gene polymorphisms on risk of lung cancer in Japan: a case control study. Carcinogenesis. 2007;28:1718–25. doi: 10.1093/carcin/bgm104. [DOI] [PubMed] [Google Scholar]
- 19.Parle-McDermott A, Mills JL, Molloy AM, Carroll N, Kirke PN, Cox C, Conley MR, Pangilinan FJ, Brody LC, Scott JM. The MTHFR 1298CC and 677TT genotypes have opposite associations with red cell folate levels. Mol Genet Metab. 2006;88:290–4. doi: 10.1016/j.ymgme.2006.02.011. [DOI] [PubMed] [Google Scholar]


