Abstract
To prevent biomaterial-associated infections, antibiotic agents are recommended for various medical conditions requiring biomaterial implants, but resistance often appears after the introduction of antibiotics into clinical use. Therefore, new strategies for the prevention or treatment for biomaterial-associated infections are required. The purpose of this study was to evaluate the effects of antimicrobial peptides on growth and biofilm formation of Staphylococcus aureus isolated from implant-associated infections. A total of 20 patients with culture-proven staphylococcal infection associated with stable orthopedic implants were selected as the experimental group. S. aureus were isolated from tissue biopsies for identification, the isolated strains were mixed with Tet213 incubated at 37°C and viable bactrial number of S. aureus was counted. For the biofilm formation, the broad spectrum AMP Tet213 was selected and loaded onto the Ti coating first. At the same time Ti coated with Tet213 were mixed with S. aureus in vitro to form biofilm. After 30 min, 2 h, 4 h, 6 h, 8 h, the population of S. aureus in the biofilm was counted. Tet213 showed significant antibacterial effect on 16 strains (P < 0.05, Table 1). The inhibition rate reached above 80% among 12 strains of the clinically isolated strain. In biofilm experiments, counts of the NO. 1, 2, 3, 4 strains in biofilms decreased significantly after 2 h (P < 0.05), while there was no obvious difference in counts of NO. 5 strain (P > 0.05). The broad spectrum AMP Tet213 could strongly reduce the growth and biofilm formation of S. aureus in vitro, and the use of this might be an important new approach to target implant-associated infections.
Keywords: Implant-associated infection, antimicrobial peptide, titanium, biofilm
Introduction
Bacterial infections associated with implanted devices pose a significant threat to patients and a serious challenge to clinicians [1]. Inadequate treatment may result in failure of the medical device before the lifetime of the device. Staphylococcus aureus is a major cause of potentially life-threatening infections acquired in health care settings and is a leading cause of biomaterial-associated infections [2]. To prevent biomaterial-associated infections, antibiotic agents are recommended for various medical conditions requiring biomaterial implants [3,4]. However, resistance often appears after the introduction of antibiotics into clinical use [5]. Therefore, new strategies for the prevention or treatment of biomaterial-associated infections are required.
Cationic antimicrobial peptides (AMPs) make up a promising class of antimicrobial agents. AMPs are predominantly expressed in epithelial cells lining the respiratory, digestive, and genitourinary tracts, and also in the granules of circulating phagocytes., These peptides exert their activity mainly by disrupting the cell membranes of cellular pathogens [6], and this non-specific mode of action may be responsible for the broad-spectrum activity of many peptide antibiotics [7].
The antibacterial properties of AMPs have recently become a hot area of study in biomaterial- associated infections [8,9]. One peptide in particular, a membrane-active peptide known as Tet213, has shown significant antibacterial effects [10,11]. However, as is the case with most studies on antibacterial compounds, the S. aureus used in the studies on Tet213 were standard strains [12], and studies on the efficacy of antibacterial compounds on clinically isolated strains of S. aureus are limited. In this study, we isolated S. aureus from patients with implant-associated infections to study the antibacterial activity of AMPs against clinically isolated strains and the effect of AMPs on their biofilm formation.
Materials and methods
Peptides
Tet-213 (amino acid sequence: KRWWKWWRRC) was synthesized by Shanghai Apeptide Co. Ltd. (Shanghai, China). Tet-213 was purified by high-performance liquid chromatography, and its purity (> 95%) and mass were confirmed by ion-spray mass spectrometry.
Bacterial isolation and culture
Twenty patients with stable orthopedia implant-associated, culture-proven, short infection symptom duration (exclusion limit < 1 year; actual experience 0-21 days) [13] staphylococcal strains were isolated from tissue biopsies and were incubated on Columbia sheep blood agar (BioMérieux, France) at 37°C under microaerophilic conditions (5% O2, 10% CO2 and 85% N2) for 24 h. The S. aureus were verified by Gram-staining and the species was verified using Vitek (BioMérieux, France) [14]. After identification, S. aureus strains were purified on Columbia sheep blood agar once more and were kept frozen at -80°C until needed.
Interference test
Tet213 was diluted to a 1 mg/mL solution with PBS. S. aureus clinical isolates were grown on Columbia sheep blood agar under microaerophilic conditions at 37°C for 24 h. After incubation, the S. aureus culture concentration was adjusted to 0.1 McF (3 × 107 CFU/ml) with PBS using a Densicheck analyzer (BioMérieux, France) [15]. Finally, 500 μl of Tet213 and 100 μl of the S. aureus cultures were combined with 2 ml BHI broth and incubated under microaerophilic conditions at 37°C for 24 h. Fifty microliters PBS were used as blank control.
After incubation, the suspensions were vortexed for 1 min and diluted 100-fold. Then, 100 μl of the diluted suspension was incubated on Columbia sheep blood agar at 37°C for 24 h under microaerophilic conditions and the colonies were counted. Inhibition rate was calculated according to the following formula [16]: Inhibition rate = (b-a)/b × 100%. Where a and b represent the colony number in the experimental group and blank control group, respectively.
AMP loading on Ti
A previously published method was used to load Tet213 onto Ti coating [17]. Briefly, a 1 mg/mL peptide solution was prepared by dissolving 1 mg Tet213 in 1 mL PBS. Ti specimens were immersed in Tet213 solution separately for 1 h while slowly shaking at room temperature. To remove residual peptides, Ti specimens were washed three times for 1 min with PBS. Ti specimens were then gently air-dried and stored in dry containers.
Biofilm formation
For this assay, five of the 20 clinically isolated strains of S. aureus were randomly selected as test strains. They were grown on Columbia sheep blood agar under microaerophilic conditions at 37°C for 24 h and were then placed into PBS at a concentration of 0.5 MCF (1.5 × 108 CFU/ml). The bacterial solutions were transferred to sterile 9 cm petri dishes with 10 ml BHI broth, and either a Tet213-coated or an untreated Ti slide was added to the petri dishes. The petri dishes were incubated under microaerophilic conditions at 37°C for 24 h. Biofilm formation was examined after 0.5 h, 2 h, 4 h, 6 h and 8 h incubation. After incubation, Ti slides were placed in 1 ml PBS and vortexed for 90 s. The culture was then diluted 100 fold in PBS. Finally, 100 ul of the diluted culture was inoculated on Columbia sheep blood agar at 37°C for 24 h and the number of colonies was counted. The biofilm inhibition rate after 8 h was calculated using the formula above.
Statistical analysis
All experiments were performed at least three times and data are expressed as the mean ± SD. SPSS 14.0 software for Windows was used for data analysis. Data were analyzed for statistical significance using the paired t-test. A p value < 0.05 was considered statistically significant.
Results
Interference test
Among the 20 clinically isolated strains of S. aureus, Tet213 showed significant antibacterial effect on 16 strains (P < 0.05, Table 1). The inhibition rate exceeded 80% in 12 of the clinically isolated strains. Tet213 showed no significant antibacterial effect on strain 6, 10, 15, or 16 (P > 0.05), and the inhibition rate of Tet213 on these strains were 67.2%, 68.2%, 33.2%, and 52.2%, respectively.
Table 1.
Inhibitory effects of Tet213 on S. aureus after 24 h (×106 CFU/ml, means ± SD)
| Strains | CFU (control) | CFU | Inhibition rate (%) |
|---|---|---|---|
| 1 | 15.76 ± 3.91 | 3.81 ± 0.41* | 75.6 |
| 2 | 14.25 ± 2.82 | 1.38 ± 0.69* | 90.3 |
| 3 | 16.91 ± 2.11 | 1.44 ± 0.67** | 91.5 |
| 4 | 13.53 ± 1.02 | 1.45 ± 0.96** | 89.3 |
| 5 | 11.83 ± 1.04 | 2.38 ± 0.21* | 79.9 |
| 6 | 15.81 ± 1.49 | 5.18 ± 0.85 | 67.2 |
| 7 | 15.67 ± 1.71 | 1.82 ± 0.86* | 88.4 |
| 8 | 15.13 ± 0.78 | 1.76 ± 0.64** | 84.9 |
| 9 | 14.06 ± 0.71 | 2.14 ± 0.22* | 84.8 |
| 10 | 18.61 ± 4.05 | 5.91 ± 1.46 | 68.2 |
| 11 | 15.19 ± 0.63 | 2.44 ± 0.22* | 83.9 |
| 12 | 15.21 ± 2.07 | 2.47 ± 0.76* | 83.4 |
| 13 | 16.27 ± 0.42 | 0.94 ± 0.23* | 94.2 |
| 14 | 19.37 ± 0.71 | 2.73 ± 0.68* | 85.9 |
| 15 | 11.79 ± 7.99 | 7.87 ± 2.23 | 33.2 |
| 16 | 10.83 ± 0.82 | 5.17 ± 0.52 | 52.2 |
| 17 | 12.83 ± 0.24 | 2.98 ± 0.33* | 76.7 |
| 18 | 11.47 ± 0.58 | 1.54 ± 0.34* | 86.6 |
| 19 | 12.98 ± 0.78 | 1.88 ± 0.21* | 85.5 |
| 20 | 14.34 ± 1.11 | 3.01 ± 0.47* | 79.1 |
P < 0.05 compared with PBS controls (paired-t test);
P < 0.01 compared with PBS controls (paired-t test).
Inhibition of biofilm formation
After 30 min treatment with Tet213, the bacterial count in the S. aureus biofilm began to decrease, but not significant when compared with the control group (P > 0.05; Figure 1). After 2 h, the number of bacteria in the biofilm formed by strains 1, 2, 3, and 4 decreased significantly (P < 0.05), while there was no statistical difference in in the strain 5 (P > 0.05). From the fourth hour on, biofilm cell counts in strains 1, 2, 3, and 4 began to stabilize. After 4 h, biofilm cell counts in strain 5 still showed no significant changes compared to the control group (P > 0.05). After 8 h, the inhibition rate of Tet213 on biofilm formation were 77.2%, 88.2%, 83.2%, 78.2%, 41.3% in clinically isolated strains 1, 2, 3, 4, and 5, respectively (Figure 2).
Figure 1.
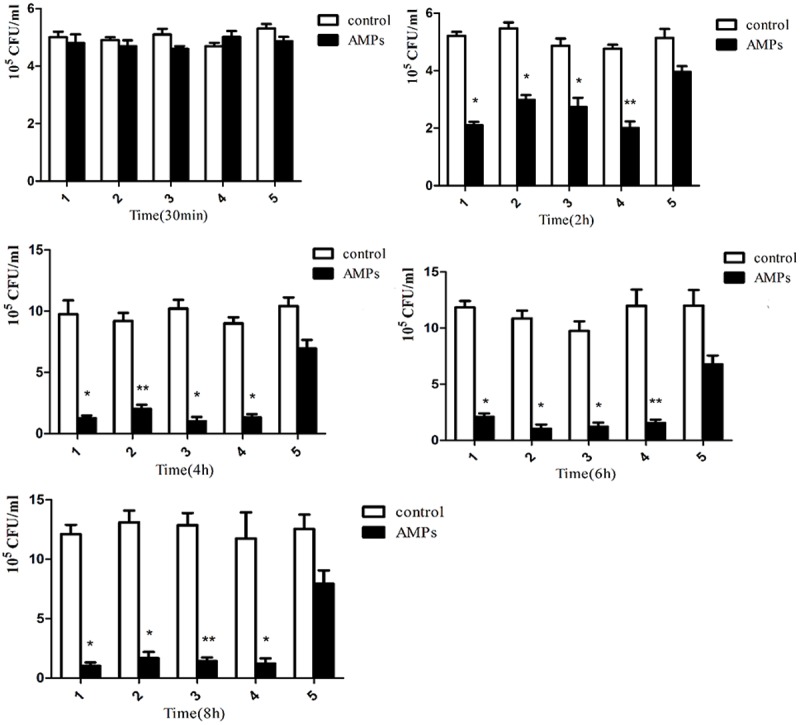
Viable S. aureus cells in the biofilms after 30 min, 2 h, 4 h, 6 h, 8 h in the presence of Tet213 or PBS. *P < 0.05, **P < 0.01 compared with PBS controls (paired-t test).
Figure 2.
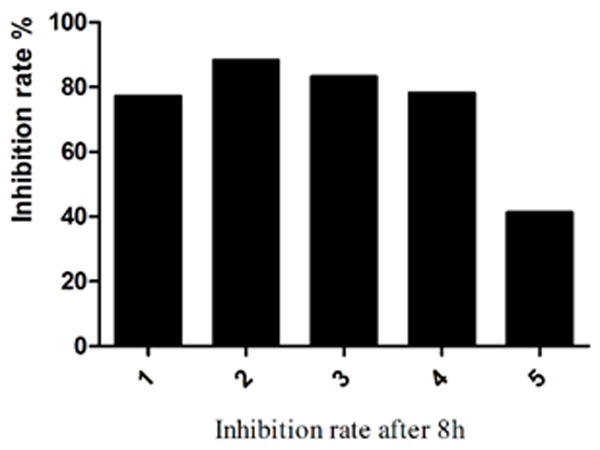
The inhibition rate of Tet213 on biofilm formation of five selected S. aureus clinically isolates after 8 h.
Discussion
In our interference test, Tet213 showed significant antibacterial activity on 80% (16 of 20 strains) of the S. aureus clinical isolates. For other four strains, Tet213 did not show any obvious antibacterial effects. This may be because some S. aureus clinical isolates have acquired the ability to sense and initiate an adaptive response to AMPs and resist their bactericidal activity [18]. In our biofilm experiments, strain 5 seemed to be resistant to Tet213, and it was likely that strain 5 became resistant to AMPs just as the four strains were not sensitive to Tet213 in the interference test.
AMPs have been isolated and characterized from tissues and organisms. They are relatively small in size (< 10 kDa), but their amino acid composition, amphipathicity, cationic charge, and size allow them to attach to and insert into membrane bilayers to form pores via the ‘barrel-stave’, ‘carpet’ or ‘toroidal-pore’ mechanisms [19]. Studies have shown that these peptides exhibit broad-spectrum antibacterial activity against a wide range of pathogenic microbes including Bacillus subtilis and S. epidermidis [20-22]. AMPs are increasingly being studied for their clinical potential in various animal models, and also in clinical trials. Our study demonstrated that the Tet213 AMP can inhibit the growth and biofilm formation of S. aureus in vitro. These results are consistent with previous experiments [23-25].
Biofilm formation on implant surfaces is the main reason why implant-associated infections are difficult to eradicate [26]. Biofilms are complex structures formed by adhered bacteria on various surfaces, including implant surfaces. Increased tolerance to antimicrobial agents is thought to be largely a consequence of biofilm formation [27]. Therefore, finding a simple and effective way to control biofilms is especially important. AMPs are attracting increasing interest as potential new antimicrobial agents to assist the prevention of biofilm formation [28,29]. Their effects on biofilm appear to be based on their ability to permeabilize and/or to form pores within the bacterial cellular membranes [30].
While it is interesting to see that antimicrobial peptides do exhibit antibacterial effects on S. aureus growth and biofilm formation, the environment inside the body is far more complex than the in vitro assay, and the distribution and metabolism of AMPs in the body will affect the antibacterial effects of therapeutic AMPs [31]. In addition, any additional potential mechanisms of action of AMPs have not yet been thoroughly studied. Therefore, further studies on the antibacterial effect of AMPs using in vivo animal models are still necessary to fully understanding the role that AMPs may play in the prevention of implant-associated infections. In the meantime, our experiment serves to confirm the short-term effects of AMPs on clinically relevant strains, but further studies are required to determine their long-term effects.
IIn conclusion, the broad spectrum AMP Tet213 strongly reduced growth and biofilm formation of S. aureus in vitro, and the use of this class of antimicrobial agents may be an important new approach in targeting implant-associated infections.
Acknowledgements
This experiment was conducted in the bacterium laboratory of Second Affiliated Hospital, Zhejiang University, School of Medicine. We acknowledge Dr. Zhendong Lin and colleagues for their assistance.
Disclosure of conflict of interest
None.
References
- 1.Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19:349–356. doi: 10.1097/01.qco.0000235161.85925.e8. [DOI] [PubMed] [Google Scholar]
- 2.Neut D, van Horn JR, van Kooten TG, van der Mei HC, Busscher HJ. Detection of biomaterial-associated infections in orthopaedic joint implants. Clin Orthop Relat Res. 2003;413:261–268. doi: 10.1097/01.blo.0000073345.50837.84. [DOI] [PubMed] [Google Scholar]
- 3.Carson CC 3rd. Efficacy of antibiotic impregnation of inflatable penile prostheses in decreasing infection in original implants. J Urol. 2004;171:1611–1614. doi: 10.1097/01.ju.0000118245.66976.e1. [DOI] [PubMed] [Google Scholar]
- 4.Mombelli A, Lang N. Antimicrobial treatment of peri-implant infections. Clin Oral Implants Res. 1992;3:162–168. doi: 10.1034/j.1600-0501.1992.030402.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith AW. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv Drug Deliv Rev. 2005;57:1539–1550. doi: 10.1016/j.addr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 7.Krause A, Neitz S, Mägert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 8.Kazemzadeh-Narbat M, Kindrachuk J, Duan K, Jenssen H, Hancock RE, Wang R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials. 2010;31:9519–9526. doi: 10.1016/j.biomaterials.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Costa F, Carvalho IF, Montelaro RC, Gomes P, Martins MC. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011;7:1431–1440. doi: 10.1016/j.actbio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Gao G, Lange D, Hilpert K, Kindrachuk J, Zou Y, Cheng JT, Kazemzadeh-Narbat M, Yu K, Wang R, Straus SK, Brooks DE, Chew BH, Hancock RE, Kizhakkedathu JN. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials. 2011;32:3899–3909. doi: 10.1016/j.biomaterials.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Lu S. Immobilization of antimicrobial peptides onto titanium surfaces. University of British Columbia; 2009. [Google Scholar]
- 12.Basu A, Mishra B, Leong SS. Immobilization of polybia-MPI by allyl glycidyl ether based brush chemistry to generate a novel antimicrobial surface. J Materials Chem B. 2013;1:4746–4755. doi: 10.1039/c3tb20805b. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: A randomized controlled trial. JAMA. 1998;279:1537–1541. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]
- 14.Deck MK, Anderson ES, Buckner RJ, Colasante G, Coull JM, Crystal B, Della Latta P, Fuchs M, Fuller D, Harris W. Multicenter evaluation of the Staphylococcus QuickFISH method for simultaneous identification of Staphylococcus aureus and coagulase-negative staphylococci directly from blood culture bottles in less than 30 minutes. J Clin Microbiol. 2012;50:1994–1998. doi: 10.1128/JCM.00225-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Singh R, Kosaraju K, Bairy I, Ramaswamy B. A study of antibacterial and antifungal properties of human cerumen. Indian J Otology. 2012:18. [Google Scholar]
- 16.Biancalana FS, Lyra L, Moretti ML, Kamei K, Schreiber AZ. Standardization of hyphal growth inhibition rate as a means of evaluating Microsporum spp. in vitro susceptibility to terbinafine, griseofulvin, and ciclopiroxolamine. Mycopathologia. 2011;172:279–285. doi: 10.1007/s11046-011-9433-7. [DOI] [PubMed] [Google Scholar]
- 17.Kazemzadeh-Narbat M, Noordin S, Masri BA, Garbuz DS, Duncan CP, Hancock RE, Wang R. Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J Biomed Mater Res B Appl Biomater. 2012;100:1344–1352. doi: 10.1002/jbm.b.32701. [DOI] [PubMed] [Google Scholar]
- 18.Gruenheid S, Moual H. Resistance to antimicrobial peptides in Gram-negative bacteria. FEMS Microbiol Lett. 2012;330:81–89. doi: 10.1111/j.1574-6968.2012.02528.x. [DOI] [PubMed] [Google Scholar]
- 19.Park Y, Hahm KS. Antimicrobial peptides (AMPs): peptide structure and mode of action. J Biochem Mol Biol. 2005;38:507–516. doi: 10.5483/bmbrep.2005.38.5.507. [DOI] [PubMed] [Google Scholar]
- 20.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Götz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 21.Ellermeier CD, Losick R. Evidence for a novel protease governing regulated intramembrane proteolysis and resistance to antimicrobial peptides in Bacillus subtilis. Genes Dev. 2006;20:1911–1922. doi: 10.1101/gad.1440606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zasloff M. Antimicrobial peptides in health and disease. New Eng J Med. 2002;347:1199–1199. doi: 10.1056/NEJMe020106. [DOI] [PubMed] [Google Scholar]
- 23.Sobczak M, Dębek C, Olędzka E, Kozłowski R. Polymeric systems of antimicrobial peptides-strategies and potential applications. Molecules. 2013;18:14122–14137. doi: 10.3390/molecules181114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glinel K, Thebault P, Humblot V, Pradier CM, Jouenne T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012;8:1670–1684. doi: 10.1016/j.actbio.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 2004;6:269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 26.Costerton J, Montanaro L, Arciola C. Biofilm in implant infections: its production and regulation. Int J Artif Organs. 2005;28:1062–1068. doi: 10.1177/039139880502801103. [DOI] [PubMed] [Google Scholar]
- 27.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 28.Park SC, Park Y, Hahm KS. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int J Mol Sci. 2011;12:5971–5992. doi: 10.3390/ijms12095971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chennupati SK, Chiu AG, Tamashiro E, Banks CA, Cohen MB, Bleier BS, Kofonow JM, Tam E, Cohen NA. Effects of an LL-37-derived antimicrobial peptide in an animal model of biofilm Pseudomonas sinusitis. Am J Rhinol Allergy. 2009;23:46–51. doi: 10.2500/ajra.2009.23.3261. [DOI] [PubMed] [Google Scholar]
- 30.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 31.Deslouches B, Islam K, Craigo JK, Paranjape SM, Montelaro RC, Mietzner TA. Activity of the de novo engineered antimicrobial peptide WLBU2 against Pseudomonas aeruginosa in human serum and whole blood: implications for systemic applications. Antimicrob Agents Chemother. 2005;49:3208–3216. doi: 10.1128/AAC.49.8.3208-3216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


