Abstract
Taste buds release ATP to activate ionotropic purinoceptors composed of P2X2 and P2X3 subunits, present on the taste nerves. Mice with genetic deletion of P2X2 and P2X3 receptors (double knockout mice) lack responses to all taste stimuli presumably due to the absence of ATP-gated receptors on the afferent nerves. Recent experiments on the double knockout mice showed, however, that their taste buds fail to release ATP, suggesting the possibility of pleiotropic deficits in these global knockouts. To test further the role of postsynaptic P2X receptors in afferent signalling, we used AF-353, a selective antagonist of P2X3-containing receptors to inhibit the receptors acutely during taste nerve recording and behaviour. The specificity of AF-353 for P2X3-containing receptors was tested by recording Ca2+ transients to exogenously applied ATP in fura-2 loaded isolated geniculate ganglion neurons from wild-type and P2X3 knockout mice. ATP responses were completely inhibited by 10 μm or 100 μm AF-353, but neither concentration blocked responses in P2X3 single knockout mice wherein the ganglion cells express only P2X2-containing receptors. Furthermore, AF-353 had no effect on taste-evoked ATP release from taste buds. In wild-type mice, i.p. injection of AF-353 or simple application of the drug directly to the tongue, inhibited taste nerve responses to all taste qualities in a dose-dependent fashion. A brief access behavioural assay confirmed the electrophysiological results and showed that preference for a synthetic sweetener, SC-45647, was abolished following i.p. injection of AF-353. These data indicate that activation of P2X3-containing receptors is required for transmission of all taste qualities.
Key points
Acute inhibition of purinergic receptors with a selective P2X3 antagonist prevents transmission of information from taste buds to sensory nerves.
The P2X3 antagonist has no effect on taste-evoked release of ATP, confirming the effect is postsynaptic.
The results confirm previous results with P2X2/3 double knockout mice that ATP is required for transmission of all taste qualities, including sour and salty. Previously, ATP was confirmed to be required for bitter, sweet and umami tastes, but was questioned for salty and sour tastes due to pleomorphic deficits in the double knockout mice.
The geniculate ganglion in mouse contains two populations of ganglion cells with different subunit composition of P2X2 and P2X3 receptors making them differently susceptible to pharmacological block and, presumably, desensitization.
Introduction
Taste buds are unique among the special sensory end organs in utilizing ATP as the primary transmitter that links activation of receptor cells to excitation of afferent nerve fibres. Taste stimuli evoke release of ATP from taste receptor cells (Huang et al. 2007; Romanov et al. 2007; Murata et al. 2010; Taruno et al. 2013), which then activates gustatory afferent fibres expressing the ionotropic purinoceptors composed of P2X2 and/or P2X3 subunits (Bo et al. 1999). The released ATP is then degraded by a specific ectoATPase, NTPDase2, expressed on the membranes of glial-like support cells (type I cells) in the taste bud (Bartel et al. 2006; Vandenbeuch et al. 2013a).
Evidence for the essential role of ATP in taste function is based largely on recordings from mice lacking both P2X2 and P2X3 subunits: P2X2/P2X3 double knockout (DKO) mice (Cockayne et al. 2005; Finger et al. 2005). These mice lack gustatory nerve responses to all taste stimuli (Finger et al. 2005), which suggests that all taste qualities require functional homotrimeric P2X2, P2X3 and/or heterotrimeric P2X2/3 receptors to communicate with nerve fibres. However, to date, ATP release has been detected only from type II taste cells, those that possess the receptors and transduction machinery for sweet, bitter and umami taste stimuli (Huang et al. 2007; Romanov et al. 2008; Murata et al. 2010). Several investigators have failed to detect ATP release from type III cells (Huang et al. 2007, 2008b; Murata et al. 2010), which are required for sour (Huang et al. 2006, 2008b) and possibly salty (Oka et al. 2013) transduction although these cells do release both serotonin and GABA (Huang et al. 2009). Hence, it is controversial whether sour and salty stimuli utilize ATP as a taste transmitter, despite the lack of responses to these stimuli in the P2X2/P2X3 DKO mice.
Further confounding interpretation of the role of ATP as a taste transmitter are recent experiments on P2X2/P2X3 DKO mice, which show that taste buds in these KOs fail to release ATP normally in response to taste stimuli (Huang et al. 2011b). Thus, the absence of taste responses may be due to reduced ATP release in addition to the lack of postsynaptic P2X receptors. Such a failure to release ATP could result in pleiotropic developmental defects unrelated to the role of P2X receptors on the afferent fibres. As conditional KOs of P2X2 and P2X3 subunits are not available, we have adopted a pharmacological approach to resolve whether postsynaptic P2X receptors are required for transmission of all taste qualities to the primary afferent fibres innervating the taste buds. We utilize the selective, membrane permeant P2X3 antagonist, AF-353, to block the afferent purinergic receptor responses to ATP chemically rather than genomically. When tested in vitro, AF-353 potently and non-competitively blocks recombinant human P2X purinoceptors that contain P2X3 subunits, i.e. P2X3 homotrimers and P2X2/3 heterotrimers, with respective IC50 values of approximately 3–10 nm and 20–100 nm (Gever et al. 2010). In the taste system, while 50–60% of the geniculate ganglion neurons express the P2X2 subunit, nearly all of the ganglion cells express the P2X3 subunit (Ishida et al. 2009). Further, gustatory afferent fibres innervating fungiform papillae from rats and mice apparently co-express both P2X2 and P2X3 subunits (Ishida et al. 2009) leaving them potentially sensitive to AF-353. Our Ca2+ imaging results on isolated geniculate ganglion neurons demonstrate that ATP responses are mediated exclusively by receptors containing P2X2 and P2X3 subunits and confirm the selectivity of AF-353 for P2X3 over P2X2 receptors. Furthermore, we demonstrate that AF-353 has no effect on the taste-evoked release of ATP. Finally, chorda tympani nerve recordings and brief access lickometer assays confirm our earlier studies with P2X2/P2X3 DKO mice and show that all taste qualities are affected in a dose-dependent manner by pharmacological inhibition of the P2X3 and P2X2/3 receptors.
Methods
Ethical approval
All experimental procedures were conducted under the approval of the Animal Care and Use Committee at the University of Colorado Medical School.
Animals
Ca2+ imaging experiments were conducted on P2X2/P2X3 DKO mice (P2rx2tm1Ckn/P2rx2tm1Ckn × P2rx3tm1Ckn/P2rx3tm1Ckn) on a mixed background (C57BL/6 and 129P2/OlaHsd) as in previous studies (Finger et al. 2005; Eddy et al. 2009; Huang et al. 2005b). P2X3 single KO mice (P2rx3tm1Ckn/P2rx3tm1Ckn) and P2X2 single KO mice (P2rx2tm1Ckn/P2rx2tm1Ckn) were obtained from breeding the P2X2/P2X3 DKO mice to wild-type (WT) mice from the same mixed background then selecting for the single KO animals in the subsequent generations. To test for possible effects of AF-353 on serotonergic ionotropic 5-HT3 receptors (Carter et al. 2009), we obtained 5-HT3A-GFP [Tg(Htr3a-EGFP)DH30Gsat/Mmnc] mice from the Mutant Mouse Regional Resource Centers and bred them on a FVB/N-Swiss Webster background. Nerve recordings and behavioural experiments were conducted on male C57BL/6 J mice obtained from The Jackson Laboratory (Bar Harbor, ME). All animals were housed at the University of Colorado in ventilated cages on a 12/12 h light cycle and fed standard chow ad libitum.
Drug
Stock solutions of AF-353 (Afferent Pharmaceuticals, San Mateo, CA, USA) were made by dissolving the compound in 10% propylene glycol (PG) in saline for intraperitoneal (i.p.) injections or in 100% dimethyl sulfoxide for Ca2+ imaging experiments. There was no effect of the vehicles on behavioural, taste nerve, or ganglion cell responses.
Geniculate ganglion isolation
Adult (2–6 month) mice were killed by CO2 followed by cervical dislocation before rapid excision of the geniculate ganglia. Isolated geniculate ganglion neurons were obtained following a protocol adapted from King and Bradley (2000). Ganglia were placed in enzyme solution [1.25 mg ml−1 trypsin (Sigma, St Louis, MO, USA), 2.5 mg ml−1 collagenase A (Roche Diagnostics, Indianapolis, IN, USA)] in minimum essential medium with Earle's balanced salts (Hyclone, Logan, UT, USA) for 30 min. Enzyme-treated ganglia were washed three times with minimum essential medium with Earle's balanced salts before gentle trituration with fire-polished glass pipettes of decreasing tip diameter. Cells were plated on poly-d-lysine (0.02 mg ml−1; BD Bioscience, San Jose, CA, USA) and laminin-coated coverslips (0.02 mg ml−1; Sigma).
Calcium imaging
Ganglion cells mounted on the coverslips were treated for 20 min with Fura-2-am (Molecular Probes, Eugene, OR, USA) at a final concentration of 2 μm. After loading, cells were perfused with Hepes buffer (in mm): 136 NaCl, 5.6 KCl, 1 MgCl2, 2.2 CaCl2, 11 glucose, 10 Hepes; pH adjusted to 7.4 with NaOH. All test compounds (ATP, Sigma; AF-353, Afferent Pharmaceuticals, San Mateo, CA, USA; 55 mm KCl, with KCl replacing equimolar NaCl) were added to Hepes buffer. Fura-2-am loaded cells were imaged through a ×40 oil immersion objective lens of an inverted microscope and images were acquired with a Sensicam QE CCD camera (PCO-Tech, Romulus, MI, USA). Emission at ∼510 nm was collected from sequential excitations at 350 and 380 nm. Images were collected every 3 s using Imaging Workbench software (Indec BioSystems, Santa Clara, CA, USA). Raw data are represented as the change in fluorescence ratio normalized to the baseline fluorescence ratio. Statistical significance among multiple groups was determined using a two-way repeated measures ANOVA using Statistica (StatSoft, Tulsa, OK, USA). Tukey post-hoc comparisons were made on statistically significant main effects. Dose–response curves were fit with a four-parameter logistic equation using Sigmaplot (Systat Software, San Jose, CA, USA) and IC50 values were compared using the Student's t test (Statistica software).
ATP release
To isolate the taste epithelium from the circumvallate papilla, we removed the tongue from the animal and made a subepithelial injection of an enzyme mixture of Dispase II (3 mg ml−1; Roche) and elastase (2.5 mg ml−1; Worthington, Lakewood, NJ, USA). After 17 min incubation in Tyrode's, the epithelium of the circumvallate papilla was peeled from the underlying mesenchyme. The piece of tissue was then placed on a plastic sheet with a hole allowing the basolateral part of the papilla to dip into the bath solution (42 μl) containing Tyrode's with or without 100 μm AF-353. The experimental protocol involved testing the same preparation, first in the absence of AF-353 and second in the presence of AF-353. For each experiment, the apical side of the epithelium was stimulated for 3 min with 5 μl artificial saliva (to control for mechanical stimulation) and then for 3 min with the same volume of artificial saliva containing a bitter mix (10 mm denatonium + 100 μm cycloheximide). Following each stimulation the bath solution was collected and transferred to a 96-well plate for luminescence reading using a luciferin-luciferase (ATP Bioluminescence Assay kit HS II; Roche) assay in an automated plate reader (Synergy HT; Biotek, Winooski, VT, USA). The relative light units obtained were then converted into ATP concentration using a standard curve made each day using known ATP standards. The ratio of ATP values obtained by stimulation with artificial saliva or the taste mixture to the resting (unstimulated) ATP values for the same 3 min duration were calculated and represented in Fig. 1F. A paired t test (P < 0.05) (GraphPad Prism5, La Jolla, CA, USA) was used to compare the amount of ATP released with and without drug. The Tyrode's solution contained (in mm): 140 NaCl, 5 KCl, 4 CaCl2, 1 MgCl2, 10 glucose, 1 sodium pyruvate and 10 Hepes, adjusted to pH 7.4 with NaOH. The artificial saliva contained (in mm): 2 NaCl, 5 KCl, 3 NaHCO3, 3 KHCO3, 1.8 HCl, 0.25 CaCl2, 0.25 MgCl2, 0.12 K2HPO4 and 0.12 KH2PO4.
Figure 1.
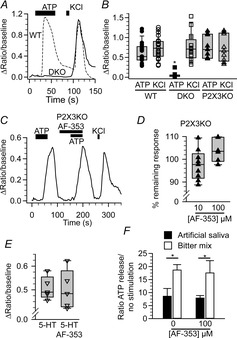
AF-353 is specific for P2X3 in isolated geniculate ganglion cells
A, change in fluorescence ratio of a P2X2/P2X3 DKO (solid line) and WT (dashed line) ganglion cell in response to 10 μm ATP and 55 mm KCl. B, summary of WT (circles; n = 23), P2X2/P2X3 DKO (DKO; squares; n = 16), and P2X3KO (triangles, n = 9) in response to ATP and KCl. C, change in fluorescence ratio of a P2X3KO ganglion cell in response to 10 μm ATP, 10 μm ATP with 100 μm AF-353, and 55 mm KCl. D, effect of 10 μm AF-353 (n = 9) or 100 μm AF-353 (n = 7) on ATP response in all P2X3KO ganglion cells. E, effect of 10 μm 5-HT or 10 μm 5-HT plus 100 μm AF-353 on intracellular Ca2+ levels in 5-HT3A-GFP expressing neurons. F, effect of 100 μm AF-353 on ATP released from isolated lingual epithelium containing circumvallate taste buds. B, D and E, individual cells are indicated by symbols while the population is represented as box plots. Asterisks indicate significance (P < 0.05 two-way repeated measures ANOVA for (B); P < 0.05 paired Student's t test for (F). 5-HT, serotonin; DKO, double knockout; KO, knockout; WT, wild-type.
Geniculate ganglion immunocytochemistry
Mice were anaesthetized with Fatal-Plus (50 mg kg−1 sodium pentobarbital; Vortech Pharmaceuticals, Ltd., Dearborn, MI, USA) and then perfused transcardially with 4% paraformaldehyde in phosphate buffer (mmol l−1: 29 NaH2PO4, 75 Na2HPO4, pH 7.2–7.4) before removal of geniculate ganglia. Harvested tissue was post-fixed in 4% buffered paraformaldehyde for 3 h before cryoprotection in 20% sucrose in 0.1 m phosphate buffer. Sections (16 μm) were cut on a cryostat, dried on to charged slides and, after blocking non-specific binding with 1% normal serum, immunoreacted overnight at 4°C with primary antibodies against P2X2 (cat. no. APR-003, RRID:AB_2040054; Alomone, Jerusalem, Israel) and P2X3 (cat. no. GP10108-100, RRID:AB_2283325; Neuromics, Edina, MN, USA). Fluorescent secondary antibodies [donkey antirabbit 568 (cat. no. A10042; RRID: AB_11180183; Invitrogen, Grand Island, NY, USA) and donkey anti-guinea pig 647 (cat. no. 706-605-148; RRID:AB_2340476; Jackson Immuno Research, West Grove, PA, USA)] were reacted with tissue sections for 2 h at room temperature at a concentration of 1:800 before mounting with Fluoromount G (Southern Biotech, Birmingham, AL, USA). Z-stack images were collected with sequential acquisition of the two-colour channels on an Olympus Fluoview FV300 laser scanning confocal microscope (Center Valley, PA, USA).
Quantification of P2X2 and P2X3 immunoreactivity
We used ImageJ (RRID: nif-0000-30467) to draw regions of interest in non-nuclear compartments of individual ganglion cells that were identified by P2X3 immunoreactivity. Images used in this study were confocal maximum z-stack images with a total thickness of 7–10 μm. Fluorescence intensities were measured in both channels (P2X2, P2X3). Background fluorescence was measured in a region of the image lacking tissue and then subtracted from the measured value for each cell. These background subtracted values were normalized to the maximum background subtracted value of its respective channel for each image. Linear adjustments to the brightness levels were made in Photoshop to enhance visibility in the micrographs but were not then used for quantification.
Nerve recordings
Mice were anaesthetized with sodium pentobarbital (i.p. injection; 50 mg kg−1) and maintained in a head holder. A cannula was introduced in the trachea to ease breathing. The chorda tympani nerve was exposed via a ventral approach, cut near the tympanic bulla and placed on a platinum-iridium wire electrode. A reference electrode was placed in the nearby tissue. Responses were amplified (P511; Grass Instruments, W. Warwick, NJ, USA), integrated (time constant 0.5 s), and collected using Acknowledge software (Biopac, Goleta, CA, USA).
The anterior taste field was stimulated with different tastants applied with a constant flow pump (Mini-pump, variable flow; Fisher Scientific, Pittsburgh, PA, USA): NH4Cl 100 mm, sucrose 500 mm, MSG 300 mm + amiloride (100 μm, to block the sodium component of MSG), quinine 10 mm, NaCl 100 mm, citric acid 10 mm and HCl 10 mm. The stimuli were applied for 30 s and rinsed with water for 50 s between successive stimulations.
Topical application of AF-353
Stimuli representing all taste qualities (sweet, bitter, sour, salty and umami) were applied successively on the tongue and responses were recorded. A solution of AF-353 diluted in water was then applied to the tongue for 10 min. The same taste stimuli containing AF-353 were then reapplied to the tongue. Different animals received different concentrations of AF-353: from 1 μm to 2 mm.
Systemic injection of AF-353
Mice were prepared for electrophysiological recording and after stimulation with each of the seven taste stimuli, were injected i.p. with various doses (0.125–6 mg kg−1) of AF-353 diluted in 10% PG (in saline). Each mouse received only one dose of the antagonist and 10 min after the injection, taste stimuli were again applied orally. To determine the plasma concentration of AF-353 a blood sample was collected at the end of the recording session, so that the response magnitude could be plotted as a function of plasma concentration of AF-353.
Data analysis
Amplitudes of the taste responses were compared before and after AF-353 treatment (topical application or i.p. injection). The amplitude of the integrated response for each stimulus was averaged using Acknowledge software (Biopac). Data were plotted and dose–response curves were fit with a four-parameter log dose–response curve using Prism6 (GraphPad Software; rid_000081).
Lickometer
A Davis Rig Lickometer (DiLog Instruments, Tallahassee, FL, USA), which records licking behaviour in mice, was used for training and brief-access testing of mice. Initially, mice were placed on a 23 h water deprivation schedule for 3 days before training in the Davis Rig. On training and testing days, mice were water-deprived for 16–20 h. The 3 day training regimen consisted of: day 1, a 15 min trial in which mice had access to distilled water through an open shutter; day 2, a 15 min trial in which mice had 5 s access after the first lick to different tubes of distilled water with the shutter opening and closing at 7.5 s intervals; day 3, repeating day 2 schedule but mice were given different concentrations of the sweetener SC-45647 (CID 5289342; at 0; 3; 10, 30; 100; 300 μm) presented randomly. Each concentration was presented multiple times as the 15 min experiment time allowed. This sweetener was chosen due to its pure sweet taste and the lack of post-ingestive reward mechanisms, which can occur with nutritive sugars within 5 min of ingestion (Sclafani & Glendinning, 2003, 2005). Testing occurred on 2 successive days, days 4 and 5 of the testing sequence. On these test days, 15 min before testing, mice were injected i.p. either with the vehicle, PG 10% in saline (day 4), or with AF-353 in PG 10% in saline (day 5). Thus, each mouse served as its own control. The same concentrations of SC-45647 and the same presentation sequence were used as in training. To analyse the data, the number of total licks for each concentration was compared in the presence or absence of AF-353. In the 15 min testing period, the mice were exposed several times to the same concentrations but, to diminish the impact of thirst on the consumption of liquids, only the last presentation for each concentration was used for analysis (Hallock et al. 2009). On day 5, mice were killed with CO2 and cervical dislocation immediately after testing, and a blood sample was collected to measure the concentration of AF-353 in the plasma. Lick data were analysed by a two-way repeated measures ANOVA using Statistica (StatSoft). Tukey post-hoc comparisons were made on statistically significant main effects.
Blood collection and dosage of AF-353
At the end of experiments involving i.p. injection of AF-353 (both behaviour and electrophysiology), a blood sample was collected by cardiac puncture to measure the AF-353 concentration in plasma. Animals were killed with CO2 and cervical dislocation then blood was collected by cardiac puncture. The blood was centrifuged and the plasma was frozen at −20°C for future analysis. The concentration of AF-353 in plasma was measured by mixing 50 μl of plasma with an equal volume of acetonitrile that contained a close structural analogue of AF-353 as internal standard. Samples were mixed and centrifuged, and an aliquot of the supernatant was assayed by liquid chromatography with a YMC ODS-A, 100 × 2 mm, 3.5 μm column (YMC America, Allentown, PA, USA) and detected with an API3000 mass spectrometer (MDS SCIEX, Concord, Ontario, Canada) operating in positive ion mode monitoring the m/z transitions of 401→111 and 357→111 for AF-353 and the internal standard, respectively. Concentrations of AF-353 were determined by comparing the peak height ratios (AF-353/internal standard) to those of a standard curve generated by adding a known amount of AF-353 to untreated plasma.
Results
Calcium imaging of geniculate ganglion neurons
To test whether purinergic receptors other than those containing P2X2 and P2X3 subunits contribute to the ATP-induced depolarization of geniculate ganglion neurons, we isolated geniculate ganglion neurons from both WT and P2X2/P2X3 DKO mice and exposed them to ATP. All ganglion cells from WT mice gave robust responses to both 10 μm ATP and 55 mm KCl. In contrast, cells from P2X2/P2X3 DKO mice failed to respond to 10 μm ATP (Fig. 1A and C; F2,44 = 45.89, P < 0.05), despite having a response for KCl (55 mm) similar to cells from WT mice. These results indicate that channels containing P2X2 and/or P2X3 subunits are probably the only purinergic receptors responsible for excitatory ATP signalling in gustatory afferents of the geniculate ganglion.
We tested the specificity of AF-353 using geniculate ganglion neurons isolated from P2X3 single KO mice (P2X3KO). In heterologous systems, AF-353 is a selective antagonist of the P2X3 subunit containing receptors (Gever et al. 2010) and therefore would be expected to have no effect in mice lacking the P2X3 subunits. Responses to 10 μm ATP and 55 mm KCl in ganglion cells from P2X3KO mice were not significantly different from responses to these compounds in WT neurons (Fig. 1C; all P = 0.60–1). However, unlike the situation in WT neurons, in P2X3KO neurons, neither 10 nor 100 μm AF-353 attenuated the ATP response presumably because, in the absence of the P2X3 subunit, the P2X3KO cells contain only P2X2 homotrimers. The lack of effectiveness of the AF-353 in these cells confirms the high specificity of AF-353 for P2X3-containing channels over P2X2 homotrimers (Fig. 1B and D).
As 5-HT3 receptors are also expressed in the geniculate ganglion (Kaya et al. 2004) and 5-HT is released from type III taste cells (Huang et al. 2005), we also tested whether AF-353 affected responses to 5-HT in neurons expressing GFP driven by the 5-HT3A promoter. Exogenously applied 10 μm 5-HT elicited an increase in intracellular Ca2+ in geniculate ganglion neurons expressing 5-HT3A-driven GFP (Fig. 1E) but not in those lacking GFP expression (data not shown). Pre-application of 100 μm AF-353 had no effect on the response to 5-HT (Fig. 1E) supporting the specificity of AF-353 for P2X3 receptors.
Geniculate ganglion neurons of WT mice fell into two classes when tested for responsiveness to AF-353: those more sensitive to the drug and those less sensitive to the drug. Application of 100 nm AF-353 did not affect responses in any ganglion cells. Subsequent testing with ascending concentrations (1 μm, 10 μm, 100 μm) of the antagonist revealed heterogeneous responses in the ganglionic cell population. At 1 μm, AF-353 partially blocked the ATP response in about half (nine of 19 cells) of the neurons while the remaining neurons were unaffected. Those neurons that were partially blocked at 1 μm, were completely blocked by 10 μm (and 100 μm). For those neurons unaffected by 1 μm AF-353, the higher dose, 10 μm AF-353, partially blocked the ATP response while 100 μm completely blocked the response (Fig. 2B). The IC50 value for the cells more sensitive to AF-353 (0.7 ± 0.3 μm, n = 9) was 10-fold lower than that of the cells less sensitive to the compound (9.6 ± 0.9 μm, n = 10; t17 = 92.62, P < 0.05). All drug effects were fully reversible after prolonged washout. These findings suggest that geniculate ganglion neurons of WT mice fall into at least two functional subsets both of which possess P2X3-containing receptors. The differential sensitivity to AF-353 probably reflects differences in P2X subunit composition where cells with more P2X3 homotrimers being more sensitive. To determine the sensitivity of P2X3 homotrimers to AF-353, we generated mice lacking P2X2 by selective breeding of WT mice to the P2X2/P2X3 DKO mice. Geniculate ganglion neurons isolated from these mice were about 10-fold more sensitive to AF-353 than any neurons of the WT mice (IC50 for P2X2 KO mice was 0.08 ± 0.01, n = 7; t14 = 5.96, P < 0.0001 vs. ‘sensitive’ WT population). These data suggest that nearly all geniculate ganglion neurons in mice contain at least some P2X2 subunits that underlie the residual responses to intermediate levels of AF-353.
Figure 2.
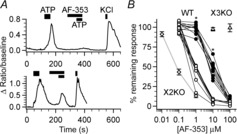
Multiple populations of geniculate ganglion cells respond differently to AF-353
A, change in fluorescence ratio of two ganglion cells in response to 10 μm ATP, 10 μm ATP with 10 μm AF-353 and 55 mm KCl. In the cell shown in the upper trace 10 μm AF-353 completely blocks the ATP response whereas it only blocks about 50% of the response in the cell shown in the lower trace. Drug application order was the same between top and bottom traces. B, effect of various concentrations of AF-353 on ganglion cells of WT (circles; n = 19 cells), X2KO (diamonds; n = 7 cells) and X3KO; triangles; n = 7–9). WT cells were separated into two categories according to their response to ATP at 1 μm AF-353. Cells above the mean response were classified as ‘less sensitive’ (closed circles) while cells below the mean response were classified as ‘more sensitive’ (open circles). For WT, individual cells are represented as circles with straight lines connecting individual cells. For X2KO and X3KO symbols indicate means ± SEM. Asterisks indicate significance (P < 0.001 Mann–Whitney test between ‘more sensitive’ and ‘less sensitive’ cells). X2KO, P2X2KO; X3KO, P2X3KO; WT, wild-type.
ATP release
Because AF-353 could theoretically inhibit the release of ATP from taste cells, as occurs in the P2X2/P2X3 DKO mice (Huang et al. 2005b), we examined the effect of 100 μm AF-353 on taste-evoked ATP release from lingual epithelium containing circumvallate taste buds. As shown in Fig. 1F, AF-353 had no effect on ATP release in response to a mix of bitter stimuli applied selectively to the apical membrane (paired t test; P < 0.05; n = 5). These data indicate that AF-353 does not inhibit ATP release from taste epithelia and suggests that its effect is due to blockade of postsynaptic P2X receptors.
Geniculate ganglion immunocytochemistry
To test whether geniculate ganglion neurons are heterogeneous with respect to expression levels of P2X3 and P2X2 proteins, we used immunohistochemistry with antibodies directed against P2X3 and P2X2 (Fig. 3A–C). Semiquantitative analysis revealed that P2X3 immunoreactivity in all ganglion cells and that the level of this immunoreactivity was distributed normally about a mean value; in contrast, P2X2 immunoreactivity was heterogeneous with cells having either high or low levels of immunoreactivity (Fig. 3D, top). Comparison of the relative intensities of P2X2 and P2X3 immunoreactivity revealed one group of cells (about one-third of total; 249 of 682 cells) with high P2X3 immunoreactivity and little P2X2 immunoreactivity over background levels (Fig. 3D, bottom), while the majority of cells had higher levels of P2X2 immunoreactivity. However, all cells, including those with high levels of P2X2 immunoreactivity had P2X3 immunoreactivity. These findings suggest that most cells probably mediate responses to ATP via the P2X2/3 heterotrimer, while those with low levels of P2X2 probably have a higher percentage of subunits dominated by P2X3 and therefore would tend to be more sensitive to AF-353.
Figure 3.
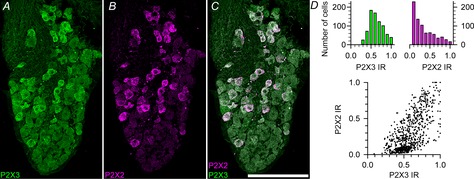
Expression of P2X2 and P2X3 in geniculate ganglion neurons
A–C, geniculate ganglion showing P2X2 (magenta) and P2X3 (green) immunoreactivity. Image is a maximum Z-projection of 12 optical sections through a ∼16 μm tissue section. Scale bar = 100 μm. Brightness and contrast were adjusted linearly to preserve relative expression level information. D, quantification of P2X2 and P2X3 immunofluorescence. D, top, histograms showing the distribution of P2X2 (magenta) and P2X3 (green) immunofluorescence of individual ganglion cells. D, bottom, scatterplot showing the relative intensities of P2X2 and P2X3 immunoreactivity for all ganglion cells. Intensity values were normalized to maximum values for each image analysed. n = 769 cells from five mice. IR, immunoreactivity.
Nerve recordings
To determine whether AF-353 modulates taste nerve responses in vivo, we compared the amplitude of taste responses before and after its application. We tested two modes of application, (1) directly applying AF-353 to the tongue, or (2) i.p. injections during the recording session.
Topical application of AF-353
When AF-353 was applied directly on to the tongue for 10 min, the taste responses to all stimuli decreased in a concentration-dependent manner. Figure 4A shows a chorda tympani recording where taste responses to all stimuli are abolished after topical application of a high concentration of AF-353 (1.1 mm). AF-353 affected all taste qualities similarly in a dose-dependent manner, so integrated responses to all stimuli were averaged at each concentration of drug and the results plotted as the means ± SD (Fig. 4B). Some responses recovered within 30 min after cessation of the antagonist but did not return to pre-exposure levels.
Figure 4.
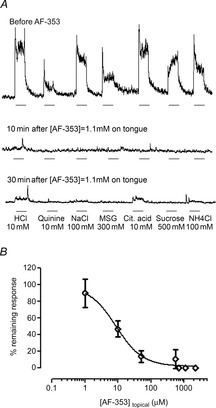
Effect of topical application of AF-353 on chorda tympani nerve responses
A, representative integrated chorda tympani nerve response to different tastants before, and 10 and 30 min after the application of AF-353 (1.1 mm) on the tongue. Responses to all tastants were totally abolished after a 10 min treatment with AF-353. Responses start recovering 30 min after a rinse with water, denoting a reversible effect of the antagonist. Taste stimuli were applied for 30 s (bar beneath recording) and rinsed for 50 s with water. B, percentage of neural response remaining after application of AF-353 at various concentrations on the tongue for 10 min. As all qualities were similarly affected, responses to all qualities were averaged (means ± SD) for each concentration of AF-353 applied to the tongue. Increasing the concentration of AF-353 proportionally decreased taste responses to all qualities.
Intraperitoneal injection of AF-353
We injected different doses of AF-353 in different animals to compare response magnitudes to each taste stimulus before and after drug injection. The percentage inhibition of chorda tympani taste responses was concentration-dependent as determined by measurements of plasma levels at the cessation of the recording session. A high dose of AF-353 (6 mg kg−1; plasma concentration: 43.7 μm) abolished the responses to all tastants while a lower dose (0.25 mg kg−1; plasma concentration: 1.3 μm) decreased the responses by about 50% (Fig. 5A). By plotting the amplitude of the remaining response relative to the plasma concentration of AF-353, the IC50 is estimated to be between 1 and 3 μm (Fig. 5B). The level of inhibition for the different taste stimuli was similar at any given dose of AF-353. Interestingly, no significant correlation exists between the plasma concentration of AF-353 and the amplitude of the electrophysiological baseline, a measure of spontaneous nerve activity. Given that the duration of the nerve activity recording session was shorter than the half-life of AF-353 in rodents (∼1.5 h; Gever et al. 2010), it was not possible to observe full recovery in these experiments.
Figure 5.
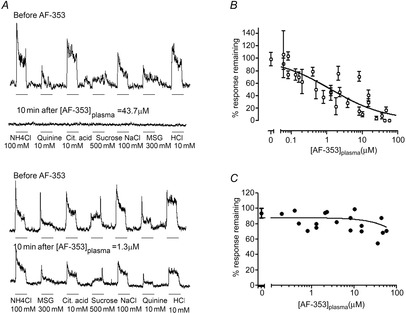
Effect of i.p. injection of AF-353 on chorda tympani nerve responses
A, representative integrated chorda tympani nerve responses to different tastants before and 10 min after the i.p. injection of AF-353. A high concentration of AF-353 (43.7 μm) measured in the plasma totally abolished responses to all taste qualities (top traces). A lower concentration of AF-353 (1.3 μm) decreased taste responses by about 50% (bottom traces). Taste stimuli were applied for 30 s (bar beneath recording) and rinsed for 50 s with water. B, percentage of neural response remaining 10 min after injection of AF-353 at various concentrations. As there was no tastant-specific effect of AF-353, all taste responses at each plasma concentration were averaged, with means ± SD plotted. Taste responses decrease with increasing concentration of AF-353 in the plasma. C, percentage of change in baseline amplitude following injection of AF-353. The concentration of AF-353 in the plasma does not significantly influence the basal activity of the nerve.
Behaviour
To determine if awake behaving mice are affected similarly by application of AF-353, and because behaviour reflects responses across all taste fields, mice were trained to the lickometer at different concentrations of the artificial sweetener SC-45647. Mice served as their own control. After 3 days of training, mice were tested on day 4 with SC-45647 following injection of the vehicle (10% PG) and on day 5 after injection with AF-353 (in vehicle). Although the mice were injected with the same dose of AF-353 at the same time before sampling, the mice showed variable plasma concentrations, possibly due to variable pharmacokinetics following i.p. injections. Figure 6A shows average lick responses to each concentration of SC-45647 in mice where the AF-353 plasma concentration was 100 μm or higher, a concentration that abolishes all chorda tympani responses in both nerve recordings and geniculate ganglion neurons. As expected, vehicle-injected mice show concentration-dependent increases in licking to SC-45647 (F1,5 = 10.19, P < 0.05). In contrast, the same mice, with plasma concentrations of AF-353 at or greater than 100 μm showed no preference for any concentration of SC-45647 (all P = 0.96–1.0). Figure 6B shows the ratio of licks to 300 μm SC-45647 compared to total licks vs. plasma concentration of AF-353 for all mice tested. In general, the concentration dependence of inhibition of preference for SC-45647 produced by AF-353 was similar to that of the electrophysiological studies, although somewhat rightward shifted.
Figure 6.
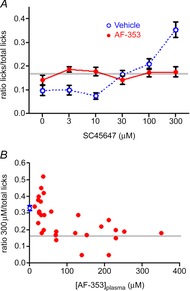
Effect of i.p. injection of AF-353 on behavioural taste responses to SC-45647
Following 3 days of training, mice were tested in the lickometer after i.p. injection of the solvent propylene glycol on day 4, and propylene glycol + AF-353 on day 5. Only the last complete presentation of each concentration was used for analysis to diminish the effect of thirst on intake. The grey horizontal line represents the expected value from random licking from all concentrations. A, average number of licks to different concentrations of SC-45647 compared to total licks for mice with plasma concentrations of AF-353 greater than 100 μm (means ± SEM, n = 12). B, ratio of number of licks for 300 μm SC-45647 to the total number of licks relative to the concentration of AF-353 in the plasma for all mice tested. Each point represents an individual mouse injected with AF-353; for injections with the vehicle propylene glycol, the lick ratios are represented by the means ± SEM for all mice tested (n = 30). Blue symbols represent behavioural responses to the vehicle, while red represents responses in the presence of AF-353.
Discussion
The principal finding of this study adds substantial confirmation to findings from P2X2/P2X3 DKO mice that ATP serves as a crucial neurotransmitter in the taste system, linking taste cell stimulation to activation of gustatory nerve fibres for all taste qualities. ATP release from type II cells occurs upon taste stimulation via a non-vesicular mechanism (Huang et al. 2007; Romanov et al. 2007). Several channels have been proposed to be involved in ATP release in taste buds, including pannexin 1 (Huang et al. 2007; Dando & Roper, 2009; Murata et al. 2010), connexins 30 and 43 (Romanov et al. 2008, 2012) and CALHM1 (Taruno et al. 2013). After release, ATP acts on purinoceptors localized on neighbouring taste cells as well as on afferent nerve fibres, which express P2X2 and P2X3 subunits (Bo et al. 1999; Ishida et al. 2009; Yang et al. 2012). Taste cells express a variety of P2 receptors, including P2X2, P2X4, P2X7 and P2Y1, P2Y2, P2Y4 (Kataoka et al. 2004; Bystrova et al. 2006; Hayato et al. 2007; Huang et al. 2009, 2005b). Recent functional studies from intact taste buds suggest that co-expression of P2X2 and P2Y1 on type II taste cells enhances ATP release from taste cells as a positive feedback mechanism (Huang et al. 2009, 2005b), while expression of P2Y4 on type III cells inhibits ATP release by a paracrine mechanism involving release of 5-HT and GABA from the type III taste cells (Huang et al. 2009). Once released, ATP is degraded to ADP via NTPDase2 (Bartel et al. 2006), which prevents the accumulation of ATP in the extracellular milieu and desensitization of afferent P2X receptors (Vandenbeuch et al. 2013a).
Our present results suggest that the receptors containing P2X3 subunits, either P2X3 homotrimers, P2X2/3 heterotrimers, or both, are essential in mice for normal taste responses. These receptors are inhibited by the selective P2X3 antagonist AF-353, a novel, non-competitive agent that shows modest affinity preference for P2X3 homotrimers over P2X2/3 heterotrimers (Gever et al. 2010). Our results using Ca2+ imaging on isolated geniculate ganglion cells show that AF-353 inhibited ATP-induced responses, and revealed a bimodal inhibition profile with many cells requiring higher concentrations of the antagonist to produce complete inhibition. As AF-353 only inhibits receptors containing the P2X3 subunit (demonstrated by the absence of inhibition in P2X3KO mice), this observation may indicate that cells in the geniculate ganglion respond to ATP via P2X3 homotrimeric and P2X2/3 heterotrimeric channels. However, it must be noted that the concentrations of AF-353 that were associated with these bimodal effects (1–100 μm) were in excess of concentrations previously reported as IC50 values at P2X3 homomeric (5–10 nm) and P2X2/3 heteromeric (30–100 nm) receptors (Gever et al. 2010). These differences could reflect a species difference, as the previous expression data were obtained using recombinant rat and human P2X receptors. Alternatively, the different measured IC50 values may reflect differences in stimulus delivery between the assay systems, where our slower delivery method may have permitted partial desensitization of the receptors. We do not expect that these differences reflect expression of other (non-P2X2 and non-P2X3) P2X receptors in the geniculate ganglion, as geniculate ganglion neurons isolated from the P2X2/P2X3 DKO mice failed to respond to ATP. Our results using taste nerve recording and behaviour showed that plasma concentrations of 100 μm AF-353 were required to block responses fully to all taste qualities in vivo. It is possible that the further right-shifted concentration dependence in vivo may be due in part to a recently discovered permeability barrier between taste buds and the surrounding lingual epithelium (Dando et al. 2014). This barrier would tend to limit access of AF-353 to the basal compartment of the taste bud containing the nerve fibres penetrating the taste bud.
Our immunocytochemical studies confirm observations from earlier studies (Ishida et al. 2009) that nearly all geniculate ganglion neurons express P2X3, while expression of P2X2 is more variable. In agreement with these data, we found two populations of geniculate ganglion neurons, with approximately 50% showing a higher sensitivity to AF-353 with the others requiring about a log dose higher concentration to block ATP responses. We speculate that the population with the higher sensitivity has a combination of P2X3 homotrimers and P2X3/P2X2 heterotrimers, while the less sensitive population predominantly expresses P2X3/P2X2 heterotrimers. In our experiments on P2X2 KO ganglia, as well as in heterologous systems and rodent DRG neurons (Gever et al. 2006) examining P2X3 homotrimers, 100 nm AF-353 inhibits responses to applied ATP. As this concentration of AF-353 fails to block responses in any ganglion cells in WT mice, we conclude that all ganglion cells contain some admixture of P2X2 subunits, which diminish the overall sensitivity to AF-353. When cells are transfected to express both P2X2 and P2X3 subunits, even with significant stoichiometric advantage for the P2X3 subunit, the active form of channel mediating ATP responses is the P2X2/3 heterotrimer, which takes the form P2X233 (Liu et al. 2001). The AF-353 doses/exposures used in our experiments are relatively selective for P2X3 and P2X2/3 receptors up to the low (∼10) micromolar range (Gever et al. 2010), and are highly unlikely to inhibit P2X2 homomeric or any other P2X purinoceptors, as confirmed by our Ca2+-imaging data on P2X3KO ganglia. Although AF-353 at supramicromolar concentrations could theoretically interact with 5-HT3 receptors (Carter et al. 2009; AF-353 was previously termed RO-4), our Ca2+ imaging data using 5-HT3a-GFP mice show no inhibition to 5-HT in 5-HT3-expressing ganglion cells by AF-353.
The differential distribution of P2X2 and P2X3 subunits may partially explain the taste selective desensitization observed in our recent study of NTPDase2 KO mice (Vandenbeuch et al. 2013a). Although responses to all stimuli were diminished in circumvallate taste buds of NTPDase2 KO mice, some stimuli were more affected than others, with sweet, sour and umami being affected more than bitter and salt. It is tempting to speculate that the ganglion cells innervating the sensitive cell types contain a higher percentage of P2X3-containing subunits, as P2X3 is rapidly desensitized by low concentrations of ATP (Grote et al. 2008).
Our previous study (Finger et al. 2005) showed that P2X2KO or P2X3KO mice have only modest differences in behavioural response to tastes compared to WT animals. This suggests that genetic deletion of a single subunit fails to eliminate taste responses as the remaining subunits still can assemble to form functional ATP receptors fully capable of responding to ATP released by the taste cells. The ageusic (tasteless) phenotype of the P2X2/P2X3 DKO mice adds further weight to the importance of the P2X2/3 heterotrimer in generating taste nerve responses.
Acute pharmacological blockade of receptors offers several advantages over reliance on global KO models (as in Finger et al. 2005). First, KO animals for P2X2/P2X3 may have abnormal development that affects their taste system. Indeed, the chorda tympani nerves of these animals are smaller than WT (unpublished observation). Second, the type II taste cells from P2X2/P2X3 DKO mice apparently do not release ATP (Huang et al. 2005b) upon taste stimulation. Consequently, it was proposed that in the DKO mice, ATP does not activate neighbouring taste cells expressing other purinergic receptors, and therefore does not generate a positive feedback for further ATP release. Nevertheless, despite these potential confounds from use of P2X2/P2X3 DKO mice, our present results show no inhibition of ATP release by AF-353, confirming that AF-353 acts postsynaptically to block taste responses and that ATP, acting on P2X3-containing receptors, is necessary for the transmission of taste signals. Whether ATP is the only transmitter involved is, however unclear, particularly for type III (sour responsive) cells for which taste-evoked ATP release has not been observed. It is possible that ATP is released by a vesicular mechanism from type III cells as these cells show presynaptic specializations (Royer & Kinnamon, 1988) and depolarization-evoked exocytosis (Vandenbeuch et al. 2010). However, the vesicular ATP transporter VNUT, normally required for transport of ATP into vesicles, is not expressed in type III cells (Iwatsuki et al. 2009). Another possibility is that tonically released ATP, from either type II cells or non-gustatory epithelial cells, is sufficient to maintain the gustatory afferent fibres in an active state, so they can respond to other transmitters released by the type III taste cells. Type III cells release a variety of transmitter candidate substances, including GABA (Cao et al. 2002; Dvoryanchikov et al. 2011; Huang et al. 2011a), serotonin (Kaya et al. 2004; Huang et al. 2005) and noradrenaline (Huang et al. 2008a; Zhang et al. 2010) but whether any of these transmitters contribute to the taste nerve response to acids and salts remains to be determined.
Taste cells are not the only sensory receptors to utilize ATP as a primary transmitter to activate afferent fibres. Similarly, the chemoreceptor cells in the carotid body use ATP as one of their principal transmitters to convey signals to the carotid sinus nerve (Piskuric & Nurse, 2013). In addition, ATP and its receptors are involved in peripheral responses to pain and irritation. P2X3 in particular is upregulated in animal models and human conditions of chronic pain (Gilchrist et al. 2005; Nagamine et al. 2006; Giniatullin et al. 2008), and thus antagonists of P2X3 have been used as pharmaceutical targets to address these conditions (Kaan et al. 2010). The relative degree of expression of P2X3 and P2X2 subunits in the different systems may determine a therapeutic window on doses of P2X3 antagonists for the treatment of specific disorders such as chronic pain.
A portion of this work has been published in abstract form (Vandenbeuch et al. 2013b; Larson et al. 2014a,b).
Acknowledgments
We thank Debra Cockayne and Roche Biosciences for the P2X2/P2X3 DKO mice, Jason Parnes and Nicole Schultz for technical assistance, Jennifer Stratford for advice with statistical analyses and Matthew Steritz for genotyping and maintaining the mouse colony.
Glossary
Abbreviations
- 5-HT
serotonin
- DKO
double knockout
- DRG
dorsal root ganglion
- KO
knockout
- PG
propylene glycol
- SC-45647
N-carboxymethyl-N′-(4-cyanophenyl)-N′′-(S)-α-phenethyl-guanidine
- WT
wild-type
Additional information
Competing interests
A.P.F. and S.A.S. are employed by Afferent Pharmaceuticals and provided the compound AF-353 for use in these studies.
Author contributions
S.C.K., T.E.F., A.V., A.P.F. and S.A.S. conceived the project and designed the research; A.V., E.L., C.B.A. and S.A.S. performed the research and analysed data. All authors contributed to writing the paper and gave approval for the final submitted version. All studies were performed at the University of Colorado Medical School except for the blood sample analyses, which were performed by Afferent Pharmaceuticals.
Funding
These studies were supported by NIH grants R01DC012555 (to S.C.K.), R01DC012931 (to T.E.F.), P30DC004657 (to D.R.) and a gift from Afferent Pharmaceuticals.
References
- Bartel DL, Sullivan SL, Lavoie EG, Sevigny J. Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo X, Alavi A, Xiang Z, Oglesby I, Ford A. Burnstock G. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport. 1999;10:1107–1111. doi: 10.1097/00001756-199904060-00037. [DOI] [PubMed] [Google Scholar]
- Bystrova MF, Yatzenko YE, Fedorov IV, Rogachevskaja OA. Kolesnikov SS. P2Y isoforms operative in mouse taste cells. Cell Tissue Res. 2006;323:377–382. doi: 10.1007/s00441-005-0098-8. [DOI] [PubMed] [Google Scholar]
- Cao Y, Shreffler C. Herness S. Localization and functional investigation of the transcription factor CREB in taste receptor cells. Neuroreport. 2002;13:1321–1325. doi: 10.1097/00001756-200207190-00022. [DOI] [PubMed] [Google Scholar]
- Carter DS, Alam M, Cai H, Dillon MP, Ford AP, Gever JR, Jahangir A, Lin C, Moore AG, Wagner PJ. Zhai Y. Identification and SAR of novel diaminopyrimidines. Part1: The discovery of RO-4, a dual P2X(3)/P2X(2/3) antagonist for the treatment of pain. Bioorganic Med Chem Lett. 2009;19:1628–1631. doi: 10.1016/j.bmcl.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G. Ford AP. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R, Pereira E, Kurian M, Barro-Soria R, Chaudhari N. Roper SD. A permeability barrier surrounds taste buds in lingual epithelia. Am J Physiol Cell Physiol. 2014 doi: 10.1152/ajpcell.00157.2014. pii: ajpcell.00157.2014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R. Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–5906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Huang YA, Barro-Soria R, Chaudhari N. Roper SD. GABA, its receptors, and GABAergic inhibition in mouse taste buds. J Neurosci. 2011;31:5782–5791. doi: 10.1523/JNEUROSCI.5559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy MC, Eschle BK, Barrows J, Hallock RM, Finger TE. Delay ER. Double P2X2/P2X3 purinergic receptor knockout mice do not taste NaCl or the artificial sweetener SC45647. Chem Senses. 2009;34:789–797. doi: 10.1093/chemse/bjp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G. Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflugers Arch. 2006;452:513–37. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- Gever JR, Soto R, Henningsen RA, Martin RS, Hackos DH, Panicker S, Rubas W, Oglesby IB, Dillon MP, Milla ME, Burnstock G. Ford AP. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol. 2010;160:1387–1398. doi: 10.1111/j.1476-5381.2010.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist LS, Cain DM, Harding-Rose C, Kov AN, Wendelschafer-Crabb G, Kennedy WR. Simone DA. Re-organization of P2X3 receptor localization on epidermal nerve fibers in a murine model of cancer pain. Brain Res. 2005;1044:197–205. doi: 10.1016/j.brainres.2005.02.081. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A. Fabbretti E. Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF. Mol Neurobiol. 2008;37:83–90. doi: 10.1007/s12035-008-8020-5. [DOI] [PubMed] [Google Scholar]
- Grote A, Hans M, Boldogkoi Z, Zimmer A, Steinhäuser C. Jabs R. Nanomolar ATP decelerates P2X3 receptor kinetics. Neuropharmacology. 2008;55:1212–1218. doi: 10.1016/j.neuropharm.2008.07.051. [DOI] [PubMed] [Google Scholar]
- Hallock RM, Tatangelo M, Barrows J. Finger TE. Residual chemosensory capabilities in double P2X2/P2X3 purinergic receptor null mice: intraoral or postingestive detection? Chem Senses. 2009;34:799–808. doi: 10.1093/chemse/bjp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayato R, Ohtubo Y. Yoshii K. Functional expression of ionotropic purinergic receptors on mouse taste bud cells. J Physiol. 2007;584:473–488. doi: 10.1113/jphysiol.2007.138370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ. Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Dando R. Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 2009;29:13909–13918. doi: 10.1523/JNEUROSCI.2351-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N. Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D. Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y. Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci. 2008;28:13088–13093. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R. Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Pereira E. Roper SD. Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PloS One. 2011;6:e25471. doi: 10.1371/journal.pone.0025471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Stone LMP E, Yang R, Kinnamon JC, Dvoryanchikov G, Chaudhari N, Finger TE, Kinnamon SC. Roper SD. Knocking out P2X receptors reduces transmitter secretion in taste buds. J Neurosci. 2011;31:13654–13661. doi: 10.1523/JNEUROSCI.3356-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Ugawa S, Ueda T, Yamada T, Shibata Y, Hondoh A, Inoue K, Yu Y. Shimada S. P2X2- and P2X3-positive fibers in fungiform papillae originate from the chorda tympani but not the trigeminal nerve in rats and mice. J Comp Neurol. 2009;514:131–144. doi: 10.1002/cne.22000. [DOI] [PubMed] [Google Scholar]
- Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, Torii K. Uneyama H. Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophys Res Commun. 2009;388:1–5. doi: 10.1016/j.bbrc.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Kaan TK, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, Nunn PA, Dickenson AH, Ford AP, Zhong Y, Malcangio M. McMahon SB. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain. 2010;133:2549–2564. doi: 10.1093/brain/awq194. [DOI] [PubMed] [Google Scholar]
- Kataoka S, Toyono T, Seta Y, Ogura T. Toyoshima K. Expression of P2Y1 receptors in rat taste buds. Histochem Cell Biol. 2004;121:419–426. doi: 10.1007/s00418-004-0647-3. [DOI] [PubMed] [Google Scholar]
- Kaya N, Shen T, Lu SG, Zhao FL. Herness S. A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am J Physiol Regul Integr Comp Physiol. 2004;286:R649–R658. doi: 10.1152/ajpregu.00572.2003. [DOI] [PubMed] [Google Scholar]
- King MS. Bradley RM. Biophysical properties and responses to glutamate receptor agonists of identified subpopulations of rat geniculate ganglion neurons. Brain Res. 2000;866:237–246. doi: 10.1016/s0006-8993(00)02292-7. [DOI] [PubMed] [Google Scholar]
- Larson ED, Finger TE. Kinnamon SC. Heterogeneity of P2X2, P2X3, and 5-HT3 receptors on gustatory geniculate ganglion neurons. J Gen Physiol. 2014;144:204. [Google Scholar]
- Larson E, Vandenbeuch A, Kinnamon SC. Finger TE. Serotonin signaling to 5-HT3A on taste sensory nerve fibers is not required for sour taste. Chem Senses. 2014 in press. Abstr. 206. [Google Scholar]
- Liu M, King BF, Dunn PM, Rong W, Townsend-Nicholson A. Burnstock G. Coexpression of P2X3 and P2X2 receptor subunits in varying amounts generates heterogeneous populations of P2X receptors that evoke a spectrum of agonist responses comparable to that seen in sensory neurons. J Pharmacol Exp Ther. 2001;296:1043–1050. [PubMed] [Google Scholar]
- Murata Y, Yasuo T, Yoshida R, Obata K, Yanagawa Y, Margolskee RF. Ninomiya Y. Action potential-enhanced ATP release from taste cells through hemichannels. J Neurophysiol. 2010;104:896–901. doi: 10.1152/jn.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine K, Ozaki N, Shinoda M, Asai H, Nishiguchi H, Mitsudo K, Tohnai I, Ueda M. Sugiura Y. Mechanical allodynia and thermal hyperalgesia induced by experimental squamous cell carcinoma of the lower gingiva in rats. J Pain. 2006;7:659–670. doi: 10.1016/j.jpain.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Oka Y, Butnaru M, Buchholtz L, von, Ryba NJ. Zuker CS. High salt recruits aversive taste pathways. Nature. 2013;494:472–475. doi: 10.1038/nature11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskuric NA. Nurse CA. Expanding role of ATP as a versatile messenger at carotid and aortic body chemoreceptors. J Physiol. 2013;591:415–422. doi: 10.1113/jphysiol.2012.234377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Bystrova MF, Rogachevskaya OA, Sadovnikov VB, Shestopalov VI. Kolesnikov SS. The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable. J Cell Sci. 2012;125:5514–5523. doi: 10.1242/jcs.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF. Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Khokhlov AA. Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol. 2008;132:731–744. doi: 10.1085/jgp.200810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer SM. Kinnamon JC. Ultrastructure of mouse foliate taste buds: synaptic and nonsynaptic interactions between taste cells and nerve fibers. J Comp Neurol. 1988;270:11–24. doi: 10.1002/cne.902700103. 58–59. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav. 2003;79:783–788. doi: 10.1016/s0031-9384(03)00174-4. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6 J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol. 2005;289:R712–R720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P. Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Anderson CB, Ford AP, Smith S, Finger TE. Kinnamon SC. A selective P2X3, P2X2/3 receptor antagonist abolishes responses to all taste qualities. Chem Senses. 2013:86. Abstr. 165, doi:10.1093/bjt036. [Google Scholar]
- Vandenbeuch A, Anderson CB, Parnes J, Enjyoji K, Robson SC, Finger TE. Kinnamon SC. Role of the ectonucleotidase NTPDase2 in taste bud function. Proc Natl Acad Sci U S A. 2013;110:14789–14794. doi: 10.1073/pnas.1309468110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Zorec R. Kinnamon SC. Capacitance measurements of regulated exocytosis in mouse taste cells. J Neurosci. 2010;30:14695–14701. doi: 10.1523/JNEUROSCI.1570-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Montoya A, Bond A, Walton J. Kinnamon JC. Immunocytochemical analysis of P2X2 in rat circumvallate taste buds. BMC Neurosci. 2012;13:51. doi: 10.1186/1471-2202-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kolli T, Hivley R, Jaber L, Zhao FI, Yan J. Herness S. Characterization of the expression pattern of adrenergic receptors in rat taste buds. Neuroscience. 2010;169:1421–37. doi: 10.1016/j.neuroscience.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


