Abstract
Ageing impairs the muscle anabolic effect of food intake, which may explain muscle loss and an increased risk of sarcopenia. Ageing is also associated with low grade inflammation (LGI), which has been negatively correlated with muscle mass and strength. In rodents, the muscle anabolic resistance observed during ageing and sarcopenia has been ascribed to the development of the LGI. We aimed to investigate this relationship in humans. We studied protein metabolism and physical fitness in healthy elderly volunteers with slight chronic C-reactive protein. Two groups of healthy elderly volunteers were selected on the presence (or not) of a chronic, slight, elevation of CRP (Control: <1; CRP+: >2 mg l−1 and <10 mg l−1, for 2 months). Body composition, short performance battery test, aerobic fitness and muscle strength were assessed. Whole body and muscle protein metabolism and the splanchnic extraction of amino acids were assessed using [13C]leucine and [2H]leucine infusion. The anabolic effect of food intake was measured by studying the volunteers both at the post-absorptive and post-prandial states. Slight chronic CRP elevation resulted in neither an alteration of whole body, nor skeletal muscle protein metabolism at both the post-absorptive and the post-prandial states. However, CRP+ presented a reduction of physical fitness, increased abdominal fat mass and post-prandial insulin resistance. Plasma cytokines (interleukin-1, interleukin-6, tumour necrosis factor α) and markers of endothelial inflammation (intercellular adhesion molecule, vascular cell adhesion molecule, selectins) were similar between groups. An isolated elevated CRP in healthy older population does not indicate an impaired skeletal muscle anabolism after food intake, nor an increased risk of skeletal muscle wasting. We propose that a broader picture of LGI (notably with elevated pro-inflammatory cytokines) is required to impact muscle metabolism and mass. However, an isolated chronic CRP elevation could predict a decrease in aerobic fitness and insulin resistance installation in elderly individuals.
Key points
Development of low grade inflammation has been correlated with sarcopenia in humans and shown to induce an anabolic resistance of muscle protein metabolism to dietary amino acid intake in an animal model.
Low grade inflammation is clinically and routinely detected in humans by measurement of the plasma C-reactive protein level.
In healthy elderly individuals presenting a slight but chronic elevation of C-reactive protein, we show that neither muscle, nor whole body protein metabolism was affected.
By contrast, a decrease in aerobic fitness, an increase in abdominal fat mass and a post-prandial insulin resistance was detected.
Our results show that the unique detection of chronic C-reactive protein elevation could predict a decrease in aerobic fitness and insulin resistance installation in elderly individuals but not muscle anabolic resistance to food intake.
Introduction
Normal ageing is associated with a progressive loss of muscle mass and strength, a condition known as sarcopenia (Rosenberg, 1989; Dardevet et al. 2012a). This phenomenon is inevitable and has also been reported among healthy and physically active elderly subjects (Hughes et al. 2001). Skeletal muscle is the major reservoir of body proteins and amino acids that can be used to cope with nutritional, infectious or traumatic stresses. Sarcopenia is then a highly predictive factor of frailty, limited mobility, increased susceptibility to injury and impaired recovery (Evans, 1997; Harris, 1997; Tavassoli et al. 2014). Muscle mass and function depend directly on muscle protein content and integrity. Proteins in skeletal muscle undergo a continuous process of synthesis and degradation. Thus, sarcopenia results from an imbalance between those fluxes of protein turnover. Skeletal muscle protein synthesis decreases in the post-absorptive (PA) state and increases in the post-prandial (PP) state, whereas protein breakdown follows the opposite pattern. In adults, net positive protein balance in the PP state and net negative protein balance in the PA state cancel each other and allow the maintenance of muscle mass. There is now strong evidence that the stimulatory effect of food intake on protein synthesis and its inhibitory effect on proteolysis is blunted in older muscles from both animals (Mosoni et al. 1995; Dardevet et al. 2002; Combaret et al. 2005) and humans (Guillet et al. 2004; Cuthberston et al. 2005) and this has been related to the ‘muscle anabolic threshold concept’ (Dardevet et al. 2012b). It has been hypothesized that this impairment may be responsible for muscle wasting during ageing. However, the cause of this impairment is still under question.
Levels of inflammatory markers, such as interleukin (IL)-6 and C-reactive protein (CRP), increase slightly with ageing, and these higher levels are correlated with disability and mortality in humans (Harris et al. 1999; Bautmans et al. 2005). Even if the increase is moderate, higher levels of cytokines and CRP increase the risk of muscle strength loss (Schaap et al. 2006) and are correlated with lower muscle mass in older individuals (Visser et al. 2002). We have recently shown that older rats also exhibit a moderate increase in plasma inflammatory markers such as α2-macroglobulin, fibrinogen or IL-6 concentrations (Balage et al. 2010). Furthermore, the presence of this low grade inflammation was shown to be correlated with a decrease in muscle protein synthesis in humans (Toth et al. 2005) and an impaired PP stimulation of muscle protein synthesis in older rats (Balage et al. 2010). Interestingly, when this low grade inflammation was prevented pharmacologically with non-steroidal anti-inflammatory drugs, muscle protein synthesis response to food intake was restored and muscle mass was improved in older rats (Rieu et al. 2009). Cytokines, and particularly tumour necrosis factor (TNF)α, impair the skeletal muscle protein synthesis by decreasing the stimulation of the mechanistic target of rapamycin signalling pathways (Lang et al. 2002). Furthermore, this signalling pathway has been shown to be responsible for muscle protein synthesis stimulation by food intake and amino acids and also to be altered during ageing (Dardevet et al. 2000; Kimball & Jefferson, 2002).
The presence of low grade inflammation may have also indirect effect on muscle anabolism without affecting the mechanistic target of rapamycin signalling pathways directly. Indeed, Boirie et al. (1997) and Volpi et al. (1999) have shown, in human volunteers, that the first-pass splanchnic uptake of amino acids increases with age and may limit the availability of amino acids to the peripheral tissues such as skeletal muscle. In older rats, the increase in amino acid splanchnic extraction has been correlated with the presence of low grade inflammation and an increased synthesis of plasma proteins by the liver (albumin) and also increased protein synthesis in the spleen (Papet et al. 2003).
Taken together, we can hypothesize that the development of low-grade, chronic inflammation in elderly individuals could explain the decrease in the PP anabolic effect of food intake and also explain the development of sarcopenia. However, this has not been demonstrated in elderly humans considered to be healthy and free of any established disease. Furthermore, the inflammatory status is usually assessed by the measurement of CRP in humans. CRP is a homopentameric acute-phase protein produced by the liver. Its levels rise dramatically during inflammation. This increase in plasma CRP is the result of a rise in the plasma concentration of IL-6, produced predominantly by macrophages and adipocytes. CRP is a general non-specific marker for inflammation and infection. A large number of studies have shown that the development of chronic, silent, low-level inflammation plays a pivotal role in the increase in the risk of many age-related diseases (Ansar & Ghosh, 2013). The present study aimed to show whether a moderate, chronic increase of CRP may be related to an impaired muscle anabolic effect of food intake and, ultimately, be predictive of sarcopenia in healthy, aged volunteers.
Methods
Experimental design
Subject recruitment
Sixteen healthy elderly male subjects (aged 65–78 years) participated in the present study. All subjects recruited had normal blood biochemical profiles, appeared normal and healthy during physical examination, did not show any chronic diseases and were not receiving statins, β-blockers, non-steroidal anti-inflammatory drugs, corticosteroids or anti-coagulant medication. The experimental protocol was approved by the local ethical committee (Comité Consultatif pour la Protection des Personnes en Recherche Biomédicale, registered to Clinical Trials.gov Identifier: NCT00862615) and was conducted in accordance with the Declaration of Helsinki. The nature and potential risks of the study were fully explained to each volunteer and written informed consent was obtained from each participant. Plasma CRP levels were assessed for 2 months (at weeks 0, 6 and 8) (Fig.1). When CRP levels were repeatedly detected under 1 mg l−1, the volunteer was considered as control (C); when CRP levels were >2 mg l−1 and <10 mg l−1 during the entire 2 month experimental period, the volunteers were assigned to the elevated CRP group (CRP+).
Figure 1.
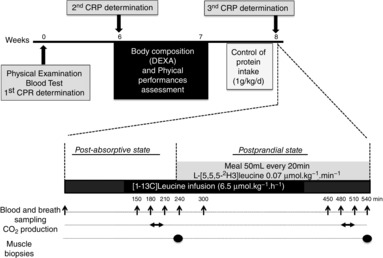
Study design
Schedule for the CRP concentration and physical fitness measurements in volunteers and metabolic study design. DEXA, dual-energy X-ray absorptiometry
Body composition and physical fitness
In addition to anthropometric measures (waist, hip, mid-upper arm and calf circumferences), body fat, fat-free mass and trunk fat mass were determined using dual-energy X-ray absorptiometry (HOLOGIC, QDR-4500 Hologic, Inc., Marlborough, MA, USA).
Performance-based measures of lower extremities functioning were assessed using the Short Performance Battery tests (tests of standing balance, time to walk 4 m, time to rise from a chair five times) (Guralnik et al. 1994) and the timed get-up-and-go (Mathias et al. 1986).
Aerobic fitness (i.e. maximal O2 uptake or 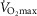 ) was determined during maximal exercise test on a cycle ergometer. All the subjects performed a progressive cycling test until volitional exhaustion to evaluate the main determinants of aerobic fitness: maximal values of ventilation (
) was determined during maximal exercise test on a cycle ergometer. All the subjects performed a progressive cycling test until volitional exhaustion to evaluate the main determinants of aerobic fitness: maximal values of ventilation (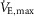 ), oxygen uptake (
), oxygen uptake (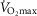 ), carbon dioxide output and respiratory exchange ratio (RERmax) by direct method (Oxycon Pro; Erich Jaeger GmbH, Hoechberg, Germany). Electrocardiogram and heart rate (HR) were measured continuously. The first stage of the test lasted 3 min, and the initial power output was 35 W. Power output was then increased by 35 W every 2 min 30 s. Pedalling rate was maintained at 60 r.p.m. Tests were considered maximal when the participants’ maximal HR (HRmax) was closed to their age-predicted maximum HR and when RERmax was greater than 1.10.
), carbon dioxide output and respiratory exchange ratio (RERmax) by direct method (Oxycon Pro; Erich Jaeger GmbH, Hoechberg, Germany). Electrocardiogram and heart rate (HR) were measured continuously. The first stage of the test lasted 3 min, and the initial power output was 35 W. Power output was then increased by 35 W every 2 min 30 s. Pedalling rate was maintained at 60 r.p.m. Tests were considered maximal when the participants’ maximal HR (HRmax) was closed to their age-predicted maximum HR and when RERmax was greater than 1.10.
Peripheral muscle strength (maximal voluntary contraction force) was measured using Myotest (Acceltec, Sion, Switzerland) for knee extensors (lower extremity functions). Strength measurement was taken twice, and the higher value divided by fat-free mass (N kg−1 FFM) was analysed. Leisure time and habitual physical activity (in MET h–1 week−1) of each volunteer were assessed using the International Physical Activity Questionnaire assisted by the same investigator (Craig et al. 2003).
Metabolic study
Materials
l-[1-13C]Leucine (99 atom%, as determined by Eurisotop), sodium [13C] bicarbonate (99 atom%, as determined by Eurisotop) and l-[5,5,5-2H3]leucine (98 atom%, as determined by Eurisotop) were obtained from Eurisotop (Gyf-sur-Yvette, France). Intravenous tracer solutions were tested for sterility and pyrogenicity before use, prepared in sterile non-pyrogenic saline and filtered through 0.22 μm filters before use.
Experimental protocol
To reduce inter-individual differences in protein intake and protein metabolism, the volunteers were asked to follow a controlled protein intake adjusted to their body weight providing 1.0 (g protein)−1 kg−1 day−1 for 4 days before the metabolic study at week 8 (Fig.1).
All subjects were studied in a PA state after an overnight fast for 12 h (T0 to T240) and then after a feeding period (T240 to T540) (Fig.1). A catheter was inserted retrogradely into a dorsal vein of a hand and placed in a box heated at 60°C for sampling arterialized blood. A second catheter was introduced into a vein of the contralateral arm for tracer infusion. Whole body leucine fluxes before and after the meal were measured with the use of l-[1-13C]leucine as an i.v. tracer. A second tracer (l-[5,5,5-2H3]leucine) was added to the meal to assess first-pass splanchnic leucine extraction.
After basal samples were withdrawn, a priming dose of labelled bicarbonates (NaH13CO3 0.07 mg kg−1) and l-[1-13C]leucine (6.5 μmol kg−1) were injected and then l-[1-13C]leucine was infused (6.5 μmol kg−1 h−1) for 9 h. During the first 4 h, the volunteers remained at the fasting state and then, during the next 5 h, they drank a nutritive solution representative of a complete meal divided into 15 boluses of 50 ml ingested every 20 min [meal composition: casein 0.4 g kg−1, maltodextrin 1.3 g kg−1, lipids (Isio 4 oil; Lesieur, Dunkerque, France) 0.36 g kg−1, glycerol monostearate as emulsifier, sugar free raspberry flavor, l-[5,5,5-2H3]leucine]. Arterialized blood and breath was sampled before starting the tracer infusion and every 30 min for 1.5 h at the end of the fasting and the fed periods (Fig.1). Blood samples (∼10 ml) were collected in tubes containing either lithium heparin or EDTA and immediately centrifuged at 1500 g for 10 min at 4°C. Supernatant was subsampled and stored at −80°C. Expired breath samples were collected in 10 ml evacuated tubes (Becton-Dickinson, Grenoble, France) and stored at room temperature until analysis. Production rates of carbon dioxide were measured during 20 min at the isotopic plateau at the end of the PA and the PP periods by open-circuit indirect calorimetry (Deltatrac; Datex, Geneva, Switzerland). Muscle biopsies (50–100 mg) were performed at the end of the PA period (t = 240 min) and at the end of the PP period (t = 540 min), under sterile conditions and local anesthesia using a percutaneous needle, they were immediately frozen into liquid nitrogen and then stored at –80°C.
Sample analysis
The plasma 13C and 2H enrichments of leucine and 13C enrichment of α-ketoisocaproate (KIC) were measured by gas chromatography–mass spectrometry (GC-MS, model HP5975C/7890A; Agilent, Santa Clara, USA) with the use of tertiary-buth-yldimethylsilyl derivatives and by monitoring the ions with m/z 302–303, 200–203, and 301–302 respectively. For leucine measurements, 400 μl of plasma were homogenized in eight volumes of ice-cold 10% trichloroacetic acid (TCA) and then centrifuged at 5000 g for 15 min at 4°C. The resultant pellets (TCA insoluble materials) were washed twice in four volumes of 10% TCA. The combined supernatants, which contain free amino acids, were purified by cation exchange chromatography (AG 50 X 8, 100–200 mesh, H+ form; Bio-Rad, Hercules, CA, USA) in minidisposal columns. Leucine and other amino acids were eluted with 4 m NH4OH. After evaporation of NH4OH under vacuum, free amino acids were resuspended in 0.01 m HCl for subsequent derivatization. KIC enrichment was measured as described previously by Rémond et al. (2007).
The 13C enrichments of carbon dioxide were measured on a gas chromatography combustion isotope ratio mass spectrometer (GC-C-IRMS; Fisons Instruments, VG Isotech, Middlewich, UK).
13C leucine enrichment in the muscle-free amino acid pool and total mixed muscle proteins of the two biopsies was determined. Muscle biopsies (50–100 mg) were homogenized in 10% TCA and then centrifuged at 5000 g for 15 min at 4°C. The pellets were washed twice with 10% TCA and, after solubilization in 0.6 m NaOH, proteins were hydrolyzed using 6 m HCl. Next, HCl was removed by evaporation and amino acids purified by cation exchange chromatography as describe above. Amino acids were derivatized as their N-acetyl-propyl residues and 13C leucine enrichment was assessed using GC-C-IRMS. Muscle-free amino acids extracted in the supernatant were also purified using cation exchange chromatography and then derivatized as their ter-buthylmethylsilyl residues.
For determination of albumin synthesis, plasma proteins were precipited with TCA 10% and albumin was extracted using the TCA/ethanol method as described by Dardevet et al. (2008). Albumin was then hydrolyzed into amino acids using 6 m HCl that were derivatized as their N-acetyl-propyl residues. 13C leucine enrichment was assessed as described previously using GC-C-IRMS.
Plasma glucose was assayed with an enzymatic method using an autoanalyser (Pentra 400; Horiba, Montpellier, France) and the insulin concentration was assayed by ELISA (Invitrogen, Villebon-sur-Yvette, France). The homeostatic model assessment-insulin resistance (HOMA-IR) index was calculated as: insulin (mUI l–1) × glucose (mmol l–1)/22.5 at both PA and PP states.
CRP was measured by nephelemetry using Behring method at the University Hospital. It is an ultrasensitive method for CRP detection under 1 mg ml−1. The repeatability of the assay is 2.65% and its reproducibility is 7.28%. Plasma concentrations of IL-1β, IL-6, Il-8, IL-10, IL-12 heterodimer (IL-12p70), granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein (MCP)-1, TNFα, vascular cell adhesion molecule-1, intercellular adhesion molecule-1, E-selectin and P-selectin were determined using two custom mixed assay kits with antibody-coated beads (Bio-Rad Human cytokines standard Plex; Bio-Rad, Hercules, CA, USA; Human Adhesion Molecule 4-plex; R&D Systems, Minneapolis, MN, USA) using the Luminex xMAP technology platform for multiplexing of immunochemical bioassays (BioPlex 200 with HTF system; Bio-Rad, Hercules, CA, USA).
Calculations
Whole body leucine fluxes were calculated using samples taken during the last 1.5 h of the basal period and of the feeding period. After checking the isotopic steady-state, mean plateau enrichments values were used to calculate leucine kinetics. Total whole body leucine flux (Q; μmol kg−1 min−1) was determined using:
| 1 |
where F is the [1-13C]leucine infusion rate (μmol kg−1 min−1), IEinf is the isotopic enrichment of the infusate (i.e. 99 mol% excess) and IEa (mol% excess) is the plasma [1-13C]leucine enrichment.
Whole body leucine oxidation flux (Ox, μmol kg−1 min−1) was calculated using KIC as precursor:
| 2 |
where IECO2 (mol% excess) is the expired 13CO2 enrichment, 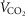 is the expired CO2 volume (μmol kg−1 min−1), IEp is the 13C enrichment of KIC and R is a factor correcting for incomplete recovery of carbon dioxide in the breath (R = 0.76 during the basal period and 0.91 during the feeding period) (Sánchez et al. 1995).
is the expired CO2 volume (μmol kg−1 min−1), IEp is the 13C enrichment of KIC and R is a factor correcting for incomplete recovery of carbon dioxide in the breath (R = 0.76 during the basal period and 0.91 during the feeding period) (Sánchez et al. 1995).
According to the basic compartment model of plasma leucine, the following equation applies:
| 3 |
where I is the rate of ingested unlabeled leucine (I = 0 in the basal period), Ra is the endogenous leucine appearance rate (index of protein breakdown) and Rd is the non-oxidative leucine disposal rate (NOLD index of protein synthesis) from plasma (all in μmol kg−1 min−1). Knowing I, Q, and Ox:
The net leucine balance, index of protein deposition, is calculated as Rd – Ra.
Leucine splanchnic extraction was calculated as:
where 13CQ and 2HQ are total leucine fluxes calculated according to eqn 1 with either the i.v. 13C or oral 2H3 tracer. Splanchnic extraction represents the fraction of ingested leucine taken up by the gut and/or liver during its first pass.
Fractional synthesis rate of total mixed muscle proteins was calculated at the end of feeding period by measuring the incorporation rate of [1-13C]leucine according to the equation:
where ΔIEpb is the increment of protein bound leucine enrichment between the two biopsies, t is the time (day) interval between the two biopsies, IEf is the leucine enrichment in the free muscle pool during the feeding period and IEbasal is the muscle free [1-13C]leucine enrichment before tracer infusion (taken as equal to that in the plasma).
Statistical analysis
All data are expressed as the mean ± SEM. Statistical analyses were performed using XLStat, version 2013.5.09 (Addinsoft, Paris, France). For insulin and glycaemia data, and after verifying the normality of the distribution (Shapiro–Wilk test), a repeated-measures mixed model was run to test group and time effects and time × group interaction. When differences were observed, Tukey–Kramer tests were used for multiple comparisons. For all other data, statistical tests were performed by non-parametric procedures. When a significant overall effect was detected, differences among individual means were assessed with the Mann–Whitney test. P < 0.05 was considered statistically significant.
Results
Recruitment of subjects
Three hundred male volunteers (> 65 years) were contacted for the present study. From the first medical exam, even if considered as healthy, 82% were not included in the study, mostly because of their medication, which was incompatible with the metabolic study or biopsies performed in the study. Six more volunteers were then not included because they had an abnormal maximal exercise test and 18 volunteers were not included because their CRP level was intermediate (between 1 and 2 mg l−1). Among the 29 remaining volunteers, most presented a very low CRP level and 10 of them were included in the control group (C). Only six volunteers were considered as CRP+ (because their CRP levels remained between 2 and 10 mg l−1 for 2 months) (i.e. 2% of the 300 volunteers contacted).
Characteristics of the subjects
The two groups of volunteers did not differ with respect to age, height, body weight, body mass index, total fat-free mass, total fat mass and waist, calf, arm or hip circumference (Table1). Only the abdominal fat was significantly higher (by 37%) in the CRP+ group (P < 0.05). Fasting plasma glucose, leucocytes, platelets or basal and PP energy expenditure also did not differ between our groups.
Table 1.
Volunteers characteristics
| Control | CRP+ | |
|---|---|---|
| Age (years) | 67.8 ± 1.2 | 70.0 ± 1.9 |
| Height (cm) | 172 ± 2 | 174 ± 5 |
| Weight (kg) | 74.8 ± 2.2 | 82.2 ± 5.9 |
| Body mass index (kg m−2) | 25.2 ± 0.6 | 27.0 ± 0.9 |
| Fat-free mass (FFM kg) | 57.5 ± 1.3 | 60.6 ± 3.7 |
| Appendicular fat-free mass (kg) | 25.8 ± 0.8 | 27.2 ± 1.5 |
| Fat mass (kg) | 17.0 ± 1.3 | 21.1 ± 2.2 |
| Abdominal fat mass (kg) | 8.8 ± 0.8 | 12.1 ± 1.4* |
| Waist circumference (cm) | 93.1 ± 2.6 | 98.2 ± 3.5 |
| Hip circumference (cm) | 94.9 ± 1.8 | 101.3 ± 4.3 |
| Arm circumference (cm) | 30.4 ± 1.2 | 30.8 ± 0.3 |
| Calf circumference (cm) | 37.3 ± 0.7 | 37.2 ± 1.3 |
| Fasting glycaemia (mmol l−1) | 5.58 ± 0.17 | 5.65 ± 0.27 |
| Leucocytes (Giga l−1) | 6.21 ± 0.32 | 6.25 ± 0.40 |
| Plateletes (Giga l−1) | 215 ± 10 | 231 ± 23 |
| Basal energy expenditure (kcal 24 h–1) | 1737 ± 71 | 1803 ± 168 |
| Basal energy expenditure (kcal 24 h–1 kg–1 FFM) | 30.2 ± 1.0 | 29.6 ± 1.6 |
| PP energy expenditure (kcal 24 h–1) | 1950 ± 71 | 2103 ± 218 |
| PP energy expenditure (kcal 24 h–1 kg−1 FFM) | 33.9 ± 1.1 | 34.4 ± 2.3 |
Data are the mean ± SEM; n = 10 for control group and n = 6 for CRP+ group. Statistical evaluation of data was performed by a non-parametric test. When significant, the Mann-Whitney test was used for post hoc analysis.*Significantly different between Control and CRP+ (P < 0.05).
The CRP+ group was characterized by a chronic mild elevation of plasma CRP level (Table2). CRP was 2.8 ± 0.3 mg l−1 during the 2 month experimental period, which was 4-fold higher than the average on the control group. Pro-inflammatory chemokines (IL-1β, IL-6, IL-8, IL-12, TNFα, colony-stimulating factor and MCP1) were very similar between the two groups. Similarly, markers of endothelial inflammatory activation (cellular adhesion molecules and selectins) were very similar between groups.
Table 2.
Plasma concentrations of CRP, pro-inflammatory chemokines and markers of endothelial inflammation
| Control | CRP + | |||||
|---|---|---|---|---|---|---|
| Week 0 | Week 8 | Week 0 | Week 6 | Week 8 | ||
| CRP | (mg ml−1) | 0.6 ± 0.1 | 0.8 ± 0.3 | 3.5 ± 0.7* | 2.5 ± 0.3* | 2.5 ± 0.5* |
| IL-1β | (ng ml−1) | 7.6 ± 1.1 | 7.6 ± 0.7 | 6.8 ± 0.7 | 6.4 ± 0.4 | 6.2 ± 0.3 |
| IL-6 | (ng ml−1) | 8.1 ± 1.1 | 8.5 ± 1.0 | 8.3 ± 0.8 | 8.1 ± 0.3 | 9.5 ± 0.5 |
| IL-8 | (ng ml−1) | 21.9 ± 7.3 | 21.6 ± 3.7 | 17.5 ± 3.0 | 16.7 ± 1.3 | 18.2 ± 0.9 |
| IL-10 | (ng ml−1) | 10.1 ± 2.0 | 9.4 ± 1.0 | 13.4 ± 3.9 | 8.1 ± 0.4 | 9.5 ± 2.4 |
| IL-12 | (ng ml−1) | 22.5 ± 4.6 | 18.4 ± 2.4 | 23.4 ± 9.9 | 17.1 ± 3.1 | 22.5 ± 5.5 |
| GM-CSF | (ng ml−1) | 16.9 ± 5.9 | 15.4 ± 3.2 | 12.1 ± 4.1 | 11.4 ± 1.3 | 13.1 ± 2.1 |
| MCP-1 | (ng ml−1) | 55.9 ± 9.4 | 54.8 ± 5.8 | 56.2 ± 3.3 | 60.9 ± 6.6 | 58.4 ± 5.7 |
| TNFα | (ng ml−1) | 27.5 ± 4.1 | 28.7 ± 3.0 | 23.3 ± 3.4 | 25.4 ± 3.9 | 24.0 ± 1.0 |
| ICAM-1 | (ng ml−1) | 150 ± 23 | 137 ± 22 | 141 ± 15 | 144 ± 16 | 145 ± 10 |
| E-selectin | (ng ml−1) | 43.8 ± 3.7 | 41.3 ± 3.5 | 43.0 ± 7.0 | 40.7 ± 5.3 | 39.3 ± 4.4 |
| P-selectin | (ng ml−1) | 43.6 ± 3.2 | 42.8 ± 2.4 | 45.0 ± 2.3 | 47.1 ± 3.4 | 47.2 ± 4.4 |
| VCAM-1 | (ng ml−1) | 722 ± 44 | 750 ± 69 | 692 ± 60 | 670 ± 87 | 620 ± 59 |
Data are the mean ± SEM; n = 10 for control group and n = 6 for CRP+ group. Statistical evaluation of data was performed by non-parametric tests. When significant, the Mann–Whitney test was used for post hoc analysis.*P < 0.05 vs. control group.GM-CSF, granulocyte-macrophage colony-stimulating factor; ICAM-1, intercellular adhesion molecule ; VCAM-1, vascular cell adhesion molecule-1.
On the day of the metabolic study, plasma glucose and insulin were measured before and after food intake. During the PA period, glucose and insulin levels did not differ significantly between the C and CRP+ groups. During the PP period, if glucose levels increased similarly between the two groups, insulin levels were over 2-fold higher in the CRP+ group than in the C group (Fig.2). Accordingly, the HOMA index did not differ at the PA state but did significantly at the PP state (P < 0.02) (Fig.2).
Figure 2.
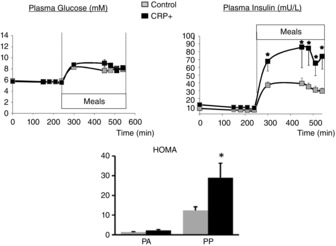
Glycaemia, insulinaemia and HOMA-IR index during the metabolic study
Data are the mean ± SEM; n = 10 for control group and n = 6 for CRP+ group. For statistical evaluation of data, a repeated-measures mixed model was run followed by Tukey–Kramer tests for glucose and insulin and a non-parametric procedure followed by the Mann–Whitney test for HOMA-IR *P < 0.05 vs. control group.
Physical fitness
Volunteers of our two groups did not differ with respect to their usual total physical activity, regardless of whether leisure, domestic or transport activities were considered. Sedentary (sitting) time was also similar in the C and the CRP+ groups. Specific performance-based measures of lower extremities functioning tests convenient for elderly individuals (Short Performance Battery test and the timed get-up-and-go) were equivalent in both groups (Table3).
Table 3.
Usual physical activity and fitness tests
| Physical activity (MET-h/week) | Sitting time (h day–1) | ||||
|---|---|---|---|---|---|
| Total | Leisure | Domestic | Transport | ||
| C | 10.5 ± 1.1 | 4.1 ± 0.7 | 5.2 ± 1.1 | 1.2 ± 0.5 | 3.8 ± 0.5 |
| CRP+ | 9.1 ± 0.8 | 2.4 ± 0.9 | 6.5 ± 1.0 | 2.0 ± 1.1 | 4.0 ± 0.7 |
| Physical fitness tests (s) | ||||
|---|---|---|---|---|
| Chair stand | Time get-up-and-go | 4 m walking (go-and-back) | ||
| C | 8.7 ± 1.0 | 6.3 ± 0.4 | 4.0 ± 0.5 | 3.8 ± 0.4 |
| CRP+ | 10.0 ± 1.3 | 6.8 ± 1.0 | 3.7 ± 0.4 | 3.7 ± 0.4 |
By contrast, when compared to the C group, the presence of higher CRP levels was associated with a decrease in aerobic fitness and peripheral maximal muscle strength. Indeed, during the maximal exercise test, although basal and maximal respiratory quotient, heart rate and blood pressure did not differ between the two groups, the maximal oxygen consumption (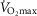 ) and the maximal developed power were 24% and 20% lower, respectively, in the CRP+ volunteers (Fig.3). The maximal voluntary contraction force developed by knee extensors assessed by Myotest tended to be lower in the CRP+ group by 24% (P < 0.1) (Fig.3).
) and the maximal developed power were 24% and 20% lower, respectively, in the CRP+ volunteers (Fig.3). The maximal voluntary contraction force developed by knee extensors assessed by Myotest tended to be lower in the CRP+ group by 24% (P < 0.1) (Fig.3).
Figure 3.
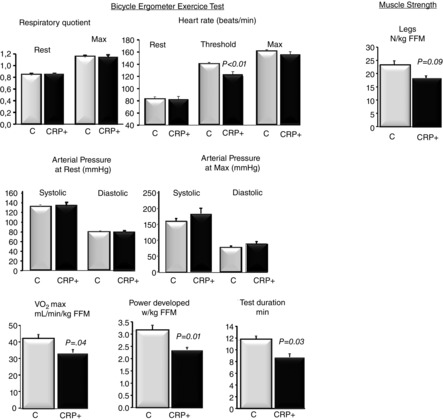
Estimation of physical fitness
Data are the mean ± SEM; n = 10 for control group and n = 6 for CRP+ group. Muscle strength was evaluated using Myotest. Statistical evaluations were performed by non-parametric procedures. When a significant overall effect was detected, differences among individual means were assessed with the Mann–Whitney test. P < 0.05 was considered statistically significant. FFM, fat-free mass.
Metabolic study: protein metabolism
Whole body leucine kinetics
A steady-state of plasma 13C-leucine, 13C-KIC and 2h-2h leucine enrichments was achieved during the final hours of the basal and the fed periods in each group of volunteers (Fig.4).
Figure 4.
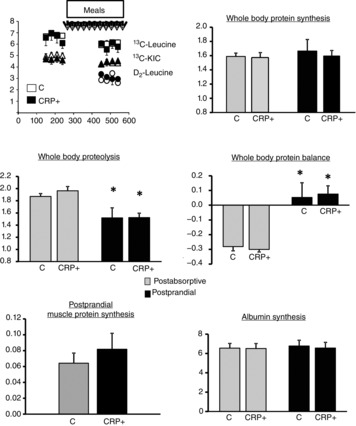
Protein metabolism
Data are the mean ± SEM; n = 10 for control group and n = 6 for CRP+ group. Statistical evaluations were performed by non-parametric procedures. When a significant overall effect was detected, differences among individual means were assessed with the Mann-Whitney test. *P < 0.05 vs. PA state. APE, atom percent excess. FSR, fractional synthesis rate.
The rate of PA whole body protein synthesis was similar in C and CRP+ groups and feeding had no significant effect. Whole body protein breakdown was decreased similarly during feeding in both groups and whole body leucine balance was improved during the PP period and became positive to the same extent in C and CRP+ volunteers (Fig.4).
Muscle and liver protein synthesis
As shown in Fig.4, PP stimulation of muscle protein synthesis was similar between the C and CRP+ groups. Dietary leucine splanchnic extraction did not differ between our groups. Liver albumin synthesis also did not differ between PA and PP states nor between groups (Fig.4).
Discussion
Ageing is characterized by a decrease in muscle mass and function named sarcopenia. Several studies have shown that impairment in the regulation of muscle protein synthesis and proteolysis after food intake, rather than their alteration at the PA state, may in part explain this age-related muscle mass loss (Mosoni et al. 1995; Guillet et al. 2004; Arnal et al. 2002). Levels of inflammatory markers, such as CRP, increase slightly with ageing, and these higher levels have been associated with disability and mortality in humans (Harris, 1999; Bautmans et al. 2005). Even a moderate elevation in plasma CRP increases the risk of muscle strength loss (Schaap et al. 2006) and has been associated with lower muscle mass in healthy older individuals (Visser et al. 2002). Our results show that an elevated CRP in healthy older volunteers is not associated with an impairment of muscle protein metabolism, nor its stimulation by food intake. However, the volunteers presenting higher levels of CRP showed an impairment of both aerobic capacity and a tendency (P < 0.09) in decreased muscle strength.
We have previously reported an association between the development of a low grade inflammation and an impaired PP stimulation of muscle protein synthesis in a model of older rats (Balage et al. 2010). Furthermore, when the low grade inflammation was prevented by chronic administration of ibuprofen, the anabolic effect of food intake on the regulation of muscle protein metabolism was maintained with ageing, and the decrease in muscle mass was alleviated (Rieu et al. 2009). Besides the specific difference between our previous studies and this clinical trial, it is important to note that the CRP levels were only slightly elevated and that other plasma pro-inflammatory biomarkers, such as IL-6, IL-1, TNFα or endothelial inflammatory markers activation, were normal (i.e. identical between our groups of volunteers).
The fed state that we studied in our experiment was induced by providing a small liquid meal every 20 min to ensure a steady-state for the different amino acid pool size and tracer enrichment during the whole PP period tested (i.e. 5 h). This steady-state was important for the tracer equation validity, although we are conscious that it did not represent a physiological situation. Indeed, food intake is defined as a bolus of nutrients intake and a non-steady-state situation. It may be then postulated that PP amino acids elevation did not occur in our experimental protocol and prevented muscle protein synthesis from being stimulated. This appears to be unlikely because, in a previous study (Rieu et al. 2006), a fed state was obtained following the same small meal composition and pattern in healthy elderly humans and we were able to show an increase in all plasma essential amino acids compared to the PA state (excepted histidine). However, recent findings suggested that the stimulation of muscle protein synthesis could be very short (90–120 min) after food intake or amino acid infusion (Bohé, 2001; Norton, 2009; Atherton, 2010; Wilson, 2011) in both humans and rodents. In the present study, a stimulation of protein synthesis may have been ‘missed’ because protein synthesis was measured for a longer period of time (i.e. 300 min). Taken together, the absence of difference between our groups should be only interpreted within our experimental conditions.
The absence of differences between our groups in term of muscle mass and muscle protein synthesis at the PP state may also be explained by the healthy status of our volunteers. The difference in CRP levels, although predictive for disease risk, can be considered as small from a clinical viewpoint. Alemán et al. (2011), who reported an association between the loss of total appendicular skeletal muscle in free living older men and women, have shown that the odds ratio was much higher when CRP levels were >3.74 mg l−1. In the present study, the mean CRP levels in the CRP+ group was 2.83 mg l−1 only, during the 2 month experimental period. We may also hypothesize that the time of exposure to increased levels of CRP is limiting. Although the elevated CRP was confirmed over 2 months of measurements, the elevation may still be recent for some subjects. In most studies showing a positive correlation between the inflammatory markers and sarcopenia, the length of the study was over a number of years (Beyer et al. 2012). Obviously, a significant modification of muscle mass could only be measurable with a longer exposure to high CRP level. By contrast, we expected that an increase of CRP, even if recent, may impact the PP stimulation of protein metabolism. Such a modification precedes and leads to muscle mass loss during ageing (Dardevet et al. 2012b). The low magnitude in the elevation of CRP could again be an explanation, although a more probable explanation could be related to the fact that, in the present study, this elevation of CRP was isolated (i.e. without any modification or increase in systemic cytokines such as TNFα, IL-1 or IL-6). As reviewed by Beyer et al. (2012), observational studies aiming at describing the associations between the inflammatory status and sarcopenia, the populations studied also presented an increase in circulating cytokines. Even if an association has been made with CRP levels in these studies, it was also systematically concomitantly associated with a negative association with plasma IL-6 levels (Visser et al. 2002; Alemán et al. 2011; Haren et al. 2010). It may be postulated that, if an elevation of CRP is not associated with an elevation of IL6, no modification of muscle protein metabolism and, consequently, skeletal muscle mass could occur. To confirm this hypothesis, Marzetti et al. (2014) have shown very recently that distinct profiles of circulating inflammatory biomarkers characterize older subjects with different levels of physical performance. Accordingly, the difference in pro- and inflammatory markers profiles may provide novel insights into the role played by inflammation in the disabling cascade during ageing. This hypothesis is also supported by our previous observation in older rats in which a decrease in muscle mass and decrease PP anabolic response of skeletal muscle was shown in animals presenting a simultaneous increase in α2-macroglobulin (acute phase protein equivalent to CRP in rodents) and IL-6 levels (Balage et al. 2010).
Finally, the absence of alterations of protein metabolism in our volunteers could also be explained by a lack of modification of the first-pass splanchnic uptake of amino acids, which has been suspected to limit the availability of amino acids to the peripheral tissues such as skeletal muscle and then decrease the anabolic potential of food intake in elderly individuals (Boirie et al. 1997; Volpi et al. 1999). We have shown that hepatic protein synthesis (albumin), which is increased in older rats during inflammation (Papet et al. 2003) remained unchanged between our groups in the present study and may explain the unchanged splanchnic extraction of amino acids.
Sarcopenia not only is related to muscle mass loss during ageing, but also includes in its definition the loss of muscle functionality, such as strength and power (Muscaritoli et al. 2010). In the present study, we observed, in the CRP positive group, an alteration of the global fitness capacities associated with a trend in the loss in the lower limbs strength. Several studies investigating the association between inflammatory markers and physical performances have shown their negative association in term of skeletal muscle strength (Bautmans et al. 2011; Tiainen et al. 2010), performance (Bautmans et al. 2011; Santos et al. 2011), aerobic fitness (Levinger et al. 2010) and physical function (Haren et al. 2010; Bautmans et al. 2011; Tiainen et al. 2010). Interestingly, it is now well established that the loss of muscle strength in ageing is not entirely explained by muscle loss alone and, indeed, could precede a clear decrease in muscle mass. Indeed, although muscle mass changes influences the magnitude of the changes in strength over time, strength may decline, whereas muscle mass stays steady (Hughes et al. 2001). Because this decrease in physical capacities is only visible in our CRP+ group, we may assume that even a slight chronic elevation of CRP alone may be sufficient to generate these functional alterations without modification of skeletal muscle mass. The mechanisms by which the CRP would affect muscle functional capacities are still not fully determined. An explanation might be provided by Levinger et al. (2010) who showed a lower activation of protein kinase B (Akt) and its 160 kDa substrate (AS160) in older subjects with increased skeletal muscle inflammatory markers. Akt, and its phosphorylation status, comprises a downstream kinase of the insulin signalling pathways, which activates the translocation of the glucose transporter glucose transporter 4 to the membrane in skeletal muscle and increases glucose metabolism to produce either energy in the mitochondria or energy storage within the cells. The consequences of the decreased Akt activity in elderly volunteers presenting an elevation of CRP could explain the results of Safdar et al. (2010), who showed a decrease in mitochondrial oxidative capacity (energy production), which negatively correlates with i.m. CRP level. This decrease in mitochondrial activity may explain the impaired skeletal muscle performances and reduced fitness capabilities of our older subjects presenting a chronic elevation of CRP. An alteration of Akt signalling pathways is also associated with the development of insulin resistance. Interestingly, in our CRP+ volunteers, insulin secretion following the same food intake was significantly higher than in our control group, with a similar plasma glucose, indicating the presence of insulin resistance. A lower insulin sensitivity and higher abdominal fat would represent clear indications for the initiation of the metabolic syndrome in the CRP+ volunteers. We are unaware of any study demonstrating the presence of a CRP receptors in skeletal muscle that could explain directly the physical dysfunction observed. A recent study by Thiele et al. (2014) may suggest some hypothesis regarding the effect of CRP on skeletal muscle. Thiele et al. (2014) identified CRP in inflamed human striated muscle, human atherosclerotic plaque and infarcted myocardium (rat and human), as well as its colocalization with inflammatory cells, which suggests a general causal role for CRP in localized inflammation. It aggravated the pre-existing inflammatory response by inducing pathological leukocyte-endothelium interaction and the generation of reactive oxygen species. CRP deposits in tissue promoted monocyte chemotaxis and recruited circulating leukocytes. These mechanisms occur when CRP is not under a polymeric form and require phospholipase A2 activation. Whether or not CRP elevation in the elderly potentiates a pre-existing local inflammation and initiates insulin resistance in the skeletal muscle, without any detectable systemic inflammation, remains to be verified.
In conclusion, an isolated increase of CRP in a healthy older population cannot predict the alteration of PP skeletal muscle protein synthesis after food intake and then predict the risk of skeletal muscle wasting. We postulated that a simultaneous increase of CRP with cytokines (TNFα, IL-1, IL-6) is necessary for translation into anabolic resistance and sarcopenia. However, an isolated chronic CRP in elderly individuals could predict a decrease in aerobic fitness and the installation of insulin resistance. Even if our clinical study was not designed to answer this question, we may hypothesize the chronological events that would lead to sarcopenia: (i) an increase in abdominal fat mass; (ii) an increase of CRP and the development of insulin resistance; (iii) a decrease in aerobic fitness and muscle strength; (iv) reduced physical activity, decreased physical capacities, increased cytokines and altered PP skeletal muscle anabolism; and (v) increased skeletal muscle wasting.
Translational perspectives.
Normal ageing is associated with a progressive loss of muscle mass and strength, a condition known as sarcopenia. This phenomenon is inevitable and has been reported not only in subjects from the frail elderly population, but also among healthy elderly subjects. The resulting weakness increases the incidence of falls and the length of recovery and, when advanced, muscle wasting is correlated with morbidity and increased mortality. A decrease of food-induced anabolism has been suspected to be partly responsible for the development of sarcopenia, even if the official daily protein requirement is respected. The origin of this muscle anabolic resistance has been positively correlated with the inflammatory status in elderly animal models. Because C-reactive protein (CRP) levels are routinely assessed to detect an inflammation, the present study aimed to show whether a moderate, chronic increase of CRP may be related to an impaired muscle anabolic effect of food intake and, ultimately, be predictive of sarcopenia in healthy aged volunteers. Our results show that a chronic, moderate and isolated CRP elevation in healthy elderly individuals does not correlate with a muscle anabolic resistance to food intake but could already predict a decrease of aerobic fitness and the installation of insulin resistance at the post-prandial state, which was not visible at the fast state. Accordingly, such patients, even if considered healthy, should be followed for complementary inflammation biomarkers, such as interleukin-6, which, in combination with CRP, could represent the first signs of muscle anabolic resistance and the development of sarcopenia.
Acknowledgments
The authors thank Mrs Véronique Mathé (INRA) for her expert technical assistance in the plasma immunochemical assays, as well as the staff of UEN (Unité d'Etudes Nutritionnelles, Mrs Noëlle Mathieu, Mrs Suzanne Faure).
Glossary
Abbreviations
- Akt
protein kinase B
- C
control
- CRP
C-reactive protein
- GC-C-IRMS
gas chromatography combustion isotope ratio mass spectrometer
- GC-MS
gas chromatography–mass spectrometry
- HOMA-IR
homeostatic model assessment-insulin resistance
- HR
heart rate
- IL
interleukin
- KIC
α-ketoisocaproate
- MCP
monocyte chemoattractant protein
- PA
post-absorptive
- PP
post-prandial
- TCA
trichloroacetic acid
- TNF
tumour necrosis factor
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
C.B., D.D., D.R., I.S.A., M.C., S.H., and N.M., designed the research (project conception, development of overall research plan and study oversight). C.B., D.D., F.M., C.M., and M.D., conducted the research (hands-on conduct of the experiments and data collection). C.B., D.D., F.M., and C.M., analysed the data and performed the statistical analysis. CB, DD, FM and MD wrote the paper.
Funding
The study was supported by a National French Grant: Agence Nationale de la Recherche ‘Compalimage’ Project.
References
- Alemán H, Esparza J, Ramirez FA, Astiazaran H. Payette H. Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Ageing. 2011;240:469–475. doi: 10.1093/ageing/afr040. [DOI] [PubMed] [Google Scholar]
- Ansar W. Ghosh S. C-reactive protein and the biology of disease. Immunol Res. 2013;56:131–142. doi: 10.1007/s12026-013-8384-0. [DOI] [PubMed] [Google Scholar]
- Arnal MA, Mosoni L, Dardevet D, Ribeyre MC, Bayle G, Prugnaud J. Patureau Mirand P. Pulse protein feeding pattern restores stimulation of muscle protein synthesis during the feeding period in old rats. J Nutr. 2002;132:1002–1008. doi: 10.1093/jn/132.5.1002. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K. Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010;92:1080–1088. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- Balage M, Averous J, Remond D, Bos C, Pujos E, Mosoni L, Papet I, Combaret L. Dardevet D. Development of low grade inflammation during aging impaired postprandial stimulation of muscle protein synthesis in rat skeletal muscle. J Nutr Biochem. 2010;21:325–331. doi: 10.1016/j.jnutbio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Bautmans I, Njemini R, Lambert M, Demanet C. Mets T. Circulating acute phase mediators and skeletal muscle performance in hospitalized geriatric patients. J Gerontol A Biol Sci Med Sci. 2005;60:361–367. doi: 10.1093/gerona/60.3.361. [DOI] [PubMed] [Google Scholar]
- Bautmans I, Onyema O, Van Puyvelde K, Pleck S. Mets T. Grip work estimation during sustained maximal contraction: validity and relationship with dependency and inflammation in elderly persons. J Nutr Health Agin. 2011;15:731–736. doi: 10.1007/s12603-010-0317-1. [DOI] [PubMed] [Google Scholar]
- Beyer I, Mets T. Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15:12–22. doi: 10.1097/MCO.0b013e32834dd297. [DOI] [PubMed] [Google Scholar]
- Bohé J, Low JF, Wolfe RR. Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirie Y, Gachon P. Beaufrère B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr. 1997;65:489–495. doi: 10.1093/ajcn/65.2.489. [DOI] [PubMed] [Google Scholar]
- Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D, Taillandier D, Grizard J. Attaix D. A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J Physiol. 2005;569:489–499. doi: 10.1113/jphysiol.2005.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF. Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM. Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Balage M. Grizard J. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130:2630–2635. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C. Grizard J. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-suppleme-nted meal. J Nutr. 2002;132:95–100. doi: 10.1093/jn/132.1.95. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Kimball SR, Jefferson LS, Cherrington AD, Rémond D, DiCostanzo CA. Moore MC. Portal infusion of amino acids is more efficient than peripheral infusion in stimulating liver protein synthesis at the same hepatic amino acid load in dogs. Am J Clin Nutr. 2008;88:986–996. doi: 10.1093/ajcn/88.4.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardevet D, Marzetti E, Dionne IJ, Buford TW, Buehring B, Manini TM, Fonseca HM, Delbono O, Aubertin-Leheudre M, Thornell LE, Savary-Auzeloux I, Remond D, Mosoni L, Buford TW, Bernabei R, Kirchner E, Calabrese L, Clark BC, Taylor JR, Barbat-Artigas S, Pion CH, Gustafsson T, Cederholm T. Ulfhake B. Commentaries on Viewpoint: muscle atrophy is not always sarcopenia. J Appl Physiol. 2012;113:680–684. doi: 10.1152/japplphysiol.00667.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardevet D, Rémond D, Peyron MA, Papet I, Savary-Auzeloux I. Mosoni L. Muscle wasting and resistance of muscle anabolism: the ‘anabolic threshold concept’ for adapted nutritional strategies during sarcopenia. Scientific World Journal. 2012. Special Issue ‘Skeletal Muscle Physiology’., Article ID 269531. [DOI] [PMC free article] [PubMed]
- Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J. Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18:1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Seeman TE, Tinetti ME, Nevitt MC. Berkman LF. Validation and use of performance measures of functioning in a non-disabled older population: MacArthur studies of successful aging. Aging (Milano) 1994;6:410–419. doi: 10.1007/BF03324272. [DOI] [PubMed] [Google Scholar]
- Haren MT, Malmstrom TK, Miller DK, Patrick P, Perry HM, 3rd, Herning MM, Banks WA. Morley JE. Higher C-reactive protein and soluble tumor necrosis factor receptor levels are associated with poor physical function and disability: a cross-sectional analysis of a cohort of late middle-aged African Americans. J Gerontol A Biol Sci Med Sci. 2010;65:274–81. doi: 10.1093/gerona/glp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. Muscle mass and strength: relation to function in population studies. J Nutr. 1997;127:1004S–1006S. doi: 10.1093/jn/127.5.1004S. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ. Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R. Fiatarone MA. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol. 2001;56:B209–217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- Kimball SR. Jefferson LS. Control of protein synthesis by amino acid availability. Curr Opin Clin Nutr Metab Care. 2002;5:63–67. doi: 10.1097/00075197-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Nairn AC, MacLean DA. Vary TC. TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab. 2002;282:E336–E347. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- Levinger I, Howlett KF, Peake J, Garnham A, Hare DL, Jerums G, Selig S. Goodman C. Akt, AS160, metabolic risk factors and aerobic fitness in middle-aged women. Exerc Immunol Rev. 2010;16:98–104. [PubMed] [Google Scholar]
- Marzetti E, Landi F, Marini F, Cesari M, Buford TW, Manini TM, Onder G, Pahor M, Bernabei R, Leeuwenburgh C. Calvani R. Patterns of circulating inflammatory biomarkers in older persons with varying levels of physical performance: a partial least squares–discriminant analysis (PLS–DA) approach. Front Med. 2014 doi: 10.3389/fmed.2014.00027. doi: 10.3389/fmed.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S, Nayak US. Isaacs B. Balance in elderly patients: the "get-up and go" test. Arch Phys Med Rehabil. 1986;67:387–389. [PubMed] [Google Scholar]
- Mosoni L, Valluy MC, Serrurier B, Prugnaud J, Obled C, Guezennec CY. Patureau Mirand P. Altered response of protein synthesis to nutritional state and endurance training in old rats. Am J Physiol Endocrinol Metab. 1995;268:E328–E333. doi: 10.1152/ajpendo.1995.268.2.E328. [DOI] [PubMed] [Google Scholar]
- Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A. Sieber CC. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) ‘cachexia-anorexia in chronic wasting diseases’ and ‘nutrition in geriatrics’. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Norton LE, Layman DK, Bunpo P, Anthony TG, Brana DV. Garlick PJ. The leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. J Nutr. 2009;139:1103–1109. doi: 10.3945/jn.108.103853. [DOI] [PubMed] [Google Scholar]
- Papet I, Dardevet D, Sornet C, Béchereau F, Prugnaud J, Pouyet C. Obled C. Acute phase protein levels and thymus, spleen and plasma protein synthesis rates differ in adult and old rats. J Nutr. 2003;133:215–219. doi: 10.1093/jn/133.1.215. [DOI] [PubMed] [Google Scholar]
- Rémond D, Machebeuf M, Yven C, Buffière C, Mioche L, Mosoni L. Patureau- Mirand P. Postprandial whole-body protein metabolism after a meat meal is influenced by chewing efficiency in elderly subjects. Am J Clin Nutr. 2007;85:1286–1292. doi: 10.1093/ajcn/85.5.1286. [DOI] [PubMed] [Google Scholar]
- Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L. Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidemia. J Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, Combaret L. Dardevet D. Prevention of low grade inflammation restored postprandial muscle anabolism and improved muscle mass in old rats. J Physiol. 2009;587:5483–5492. doi: 10.1113/jphysiol.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg IH. Summary comments. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, Debeer J. Tarnopolsky MA. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS One. 2010;245:e10778. doi: 10.1371/journal.pone.0010778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez M, El-Khoury AE, Castillo L, Chapman TE. Young VR. Phenylalanine and tyrosine kinetics in young men throughout a continuous 24-h period, at a low phenylalanine intake. Am J Clin Nutr. 1995;61:555–570. doi: 10.1093/ajcn/61.3.555. [DOI] [PubMed] [Google Scholar]
- Santos ML, Gomes WF, Pereira DS, Oliveira DM, Dias JM, Ferrioli E. Pereira LS. Muscle strength, muscle balance, physical function and plasma interleukin-6 (IL-6) levels in elderly women with knee osteoarthritis (OA) Arch Gerontol Geriatr. 2011;52:322–6. doi: 10.1016/j.archger.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SMF, Deeg DJH. Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:U82–U90. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Tavassoli N, Guyonnet S, Abellan Van Kan G, Sourdet S, Krams T, Soto ME, Subra J, Chicoulaa B, Ghisolfi A, et al. Description of 1108 older patients referred by their physician to the ‘Geriatric Frailty Clinic (G.F.C) for Assessment of Frailty and Prevention of Disability’ at the gerontopole. J Nutr Health Aging. 2014;18:457–464. doi: 10.1007/s12603-014-0462-z. [DOI] [PubMed] [Google Scholar]
- Thiele JR, Habersberger J, Braig D, Schmidt Y, Goerendt K, Maurer V, Bannasch H, Scheichl A, Woollard KJ, von Dobschütz E, Kolodgie F, Virmani R, Bjoern Stark G, Peter K. Eisenhardt SU. Dissociation of pentameric to monomeric C-reactive protein localizes and aggravates inflammation. In vivo proof of a powerful proinflammatory mechanism and a new anti-inflammatory strategy. Circulation. 2014;130:35–50. doi: 10.1161/CIRCULATIONAHA.113.007124. [DOI] [PubMed] [Google Scholar]
- Tiainen K, Hurme M, Hervonen A, Luukkaala T. Jylhä M. Inflammatory markers and physical performance among nonagenarians. J Gerontol A Biol Sci Med Sci. 2010;65:658–663. doi: 10.1093/gerona/glq056. [DOI] [PubMed] [Google Scholar]
- Toth MJ, Matthews DE, Tracy RP. Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J Physiol Endocrinol Metab. 2005;288:E883–E891. doi: 10.1152/ajpendo.00353.2004. [DOI] [PubMed] [Google Scholar]
- Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M. Harris TB. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Wolf SE. Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- Wilson GJ, Layman DK, Moulton CJ, Norton LE, Anthony TG, Proud CG, Rupassara SI. Garlick PJ. Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am J Physiol Endocrinol Metab. 2011;301:E1236–1242. doi: 10.1152/ajpendo.00242.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


