Abstract
Objectives
Tumor burden and invasiveness establish a microenvironment that surgery could alter. This study shows a comprehensive analysis of size, dynamics, and function of peripheral lymphocyte subsets in pancreatic cancer patients before and at different times after duodenopancreatectomy.
Methods
Lymphocyte frequency and natural cytotoxicity were evaluated by flow cytometry and in vitro assay on peripheral blood from initial and advanced-stage pancreatic cancer patients before (BS), at day 7 (PS7), and at day 30 (PS30) after surgery.
Results
An increase in natural killer (NK) cells and the diminution of B-cells occurred at PS30, whereas cytotoxicity decreased at PS7. The positive correlation between NK frequency and cytotoxicity at BS and PS7 revealed an altered NK behavior. The elevation of NK cell frequency at PS30, an initial defect in CD56bright NK, and the aberrant correlation between NK frequency and cytotoxicity remained significant in advanced-stage patients, whereas the diminution of NK cytotoxicity only affected initial stage patients.
Conclusions
The NK cell functional ability is altered in presurgery patients; duodenopancreatectomy is associated with short-term impairment of NK function and with a long-term NK cell augmentation and reversion of the aberrant NK behavior, which may impact on immunosurveillance against residual cancer.
Key Words: pancreatic cancer, duodenopancreatectomy, natural killer cells
Pancreatic cancer is one of the most common causes of cancer-related death in Western countries. In particular, pancreatic ductal adenocarcinoma represents more than 80% of all pancreatic neoplasms diagnosed.1 At present, surgical resection represents the best curative treatment for pancreatic cancer, with approximately 15% of patients considered suitable.2 The outcome of pancreatic tumor resection has been associated with several histological factors,3–7 including tumor size, histopathological grading,8 and cleared margin of resection.9
In the last few years, a great debate arose on the best surgical procedure for tumor eradication to obtain long-term results with significant survival benefit.10 In particular, the duodenopancreatectomy (DP) associated with extended lymphadenectomy and mesopancreas en bloc resection is deemed necessary for an adequate retropancreatic clearance of the tumor.11
The mesopancreas consists in a perineural lymphatic layer located dorsally to the pancreas, extending from the posterior surface of the pancreatic head to behind the mesenteric vessels (superior mesenteric vein and superior mesenteric artery [SMA]).12 Other authors have defined the mesopancreas as the soft tissue between the pancreatic parenchyma and the SMA that contains vascular, lymphatic, and nervous structures.11 Overall, the retropancreatic area can be considered as not a single entity with well-defined boundaries but an anatomical site of embryological fusion of peritoneal layers that are continuous and connected through the contained vessels, LNs, and nerves to the para-aortic area.13 The lack of anatomical boundaries of the mesopancreas renders difficult to obtain a microscopically negative retropancreatic resection margin and to control the neoplastic spread along the lymphovascular and neural structures; thus, it is highly recommended to perform an extended dissection of the retropancreatic and para-aortic areas to achieve an almost complete posterior clearance.11,13 This total mesopancreas excision should decrease the recidive rate (R1) and improve survival.12,14–17 Duodenopancreatectomy associated with extended lymphadenectomy and mesopancreas exeresis, by removing a sizeable portion of lymphatic tissue, most probably affects immune system status significantly.
The role that the immune system exerts in recognizing and eliminating continually arising transformed cells is well established. This host protection process, named immunosurveillance, is aimed at maintaining physiological tissue homeostasis and controlling the initiation, growth, and progression of tumor.18 Both innate and adaptive effector populations such as natural killer (NK) cells, dendritic cells, and CD4+ and CD8+ T-cells contribute to immunosurveillance; in particular, NK cells, phenotypically defined by the absence of CD3 and the expression of CD56 surface antigen (CD3-CD56+), are a heterogeneous lymphocyte population composed of CD3-CD56dim and CD3-CD56bright subsets, which participates to antitumor responses by exhibiting cytotoxic function (CD3-CD56dim NK cells) and secreting a number of cytokines (CD3-CD56bright NK cells). Natural killer cells are resident in the secondary lymphoid tissue, such as lymph nodes and tonsils, where they are almost totally CD3-CD56bright, which circulate in the peripheral blood (PB) and rapidly accumulate in the parenchyma of several organs during inflammation, tumor growth, and invasion. Circulating NK cells consist for about 90% of CD3-CD56dim subset, whose “natural” cytotoxicity is regulated by a balance of signals deriving from inhibitory and activating receptors that recognize ligands either normally expressed or stress-induced on target cells. Malignant transformation often induces alteration in the expression pattern of the ligands of NK receptors, which in turn results in NK cell loss of inhibition and induction of activation, being both events essential to achieve NK cell-mediated cytotoxicity.19
Natural killer cell subset distribution, trafficking, and functions, including proliferation, differentiation, cytotoxicity, and cytokine production, are also controlled by a great variety of cytokines, such as IL-2, IL-15, IL-18, IL-21, and chemokines, whose specific receptors are differentially expressed on CD3-CD56dim and CD3-CD56bright subsets.20
It is becoming increasingly clear that the establishment of an immunosuppressive local and systemic environment represents an important mechanism for tumor capability to circumvent both innate and adaptive immune defenses.21 Indeed, several immunosuppressive cell populations (such as regulatory T-cells and myeloid-derived suppressor cells) and cytokines (such as IL-10, TGF-β, and the tumor-associated IL-18 that exerts an indirect negative regulation on NK and effector T-cell cytotoxic activity) have been found in tumor lesions and in the PB of pancreatic cancer patients.22–26
In this complex context, the study of the impact of surgical tumor mass eradication on patient immune status is gaining particular importance. Surgery can induce a rapid and transient inflammatory status and also modify, both locally and systemically, immune cell distribution and/or activation and release of soluble factors27; this results in a long-lasting alteration of patient’s immune system and, ultimately, of immunosurveillance.28,29
In this study, we analyzed the dynamics of the main PB lymphocyte subsets (CD4+ and CD8+ T-cells, B-cells, and CD56dim and CD56bright NK cell subsets) of the NK cell cytotoxic activity and of neutrophil count as an important systemic inflammation marker in a cohort of pancreatic cancer patients before and at different time points after DP with extended lymphadenectomy and mesopancreas resection. The effect of tumor stage on presurgery immune status and on postsurgery phenotypic and functional modifications was also evaluated.
MATERIALS AND METHODS
Patients and Controls
Heparinized PB samples of 24 pancreatic cancer patients (median age, 65 years; 11 females and 13 males) were collected at 3 different time points: before (BS), at day 7 (PS7), and at day 30 (PS30) after surgery; 16 age-matched and sex-matched subjects, which referred to our structure for nononcological ambulatory surgery, were used as control. Blood samples were processed for routine immunological parameter monitoring. Pancreatic cancer patients were stratified in initial (12) and advanced (12) tumor stage, according to Union for International Cancer Control TNM 2009. Initial cancer stage includes patients in which tumor is localized to the pancreas, adjacent tissues, and organs but has not invaded the lymph nodes (stage 0, IA, IB, and IIA). Advanced cancer stage includes conditions in which the tumor has invaded the surrounding lymph nodes (stage IIB) and/or is extended to the celiac trunk and the SMA (stage III).30 No one among pancreatic cancer patients enrolled in this study was subject to neoadjuvant or adjuvant chemotherapy. The study protocol was defined in accordance with the Declaration of Helsinki.
In Vitro Cytotoxicity Assay
Cytotoxic activity of PB mononuclear cells (PBMCs) was determined toward the erythroleukemia cell line K562, as previously described.31 Target cells were labeled with 51Cr (Perkin-Elmer, Monza, Italy), 100 μCi/1 × 106 cells for 1 hour at 37°C. Serial dilutions of PBMC were plated together with 5000/well 51Cr-labeled target cells in U-bottom wells of 96-well plates in 200 μL RPMI supplemented with 10% fetal calf serum and 10 mM Hepes. After 4 hours at 37°C, 30 μL of supernatants was collected and counted in a microplate scintillation counter (TopCount, PerkinElmer Life and Analytical Sciences, Monza, Italy). Spontaneous 51Cr release was evaluated by incubating target cells with medium alone, whereas total release was evaluated by incubating target cells with 10% sodium dodecyl sulfate. Percent-specific 51Cr release was calculated according to the following formula: percent-specific release = (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. Lytic units (LU)/106 cells at 10% cytotoxicity were calculated using D Coggin’s software.
Surgical Technique
All patients were subjected to DP with mesopancreas removal and extended lymphadenectomy. The operative procedure used was a personal reconstruction technique32 (DP according to V. Stipa, P. Chirletti), with more attention to the functional aspects—DP with gastric preservation and reconstruction with double jejunal loop. The first jejunal loop was anastomosed to the stomach by T-L gastrojejunal anastomosis; the second jejunal loop was used for a T-T (end-to-end) pancreaticojejunal anastomosis with invagination of the pancreatic stump and stenting of the Wirsung duct by Silastic catheter and a T-L (end-to-side) hepatojejunostomy. The gastrointestinal transit was restored by jejunojejunostomy below the biliary anastomosis.32
In this surgical technique, the extended lymphadenectomy includes the skeletonization of the hepatic pedicle, the celiac trunk, the SMA, and the preaortic lymphatic tissue between the celiac trunk, as well as the territory and lymphatic preaortic and interaortocaval districts (lymph node areas according to classification of the Japan Pancreas Society, published in 1996: all 8, 9, all 12, all 13, 14a, 14b and 14c, 14v, 16a2).33–35
Immunostaining and Multiparameter Cytofluorometric Analysis and Complete Blood Cell Counts
The frequency of the main PB lymphocyte subpopulations was evaluated with BD Multitest IMK kit (BD Biosciences, San Jose, Calif), according to the manufacturer’s procedure. In brief, 50 μL of anticoagulated whole PB was collected in BD Trucount tubes.
Each sample was stained with multitest CD3FITC/CD8PE/CD45PerCP/CD4APC or CD3FITC/CD16+CD56PE/CD45PerCP/CD19APC reagent for 15 minutes at room temperature in the dark and then incubated with erythrocyte lysing solution to remove red blood cells. Lymphocyte region was defined on the basis of low side scatter physical parameter and CD45PerCP fluorescence. CD45+ lymphocyte absolute count and the frequency of cytotoxic (CD3+CD8+) T, helper/inducers (CD3+CD4+) T, NK (CD3-CD16+CD56+), and B (CD3-CD19+) lymphocyte subsets were measured with a FACScalibur (BD Biosciences, San Jose, Calif), using BD Multiset software (BD Biosciences, San Jose, Calif). CD56dim and CD56bright NK cell subsets were quantified with CellQuest software (BD Biosciences).
Absolute neutrophil and lymphocyte counts were obtained with automated hematology instrument (ADVIA; Siemens Healthcare, Germany).
Statistical Analysis
Differences between groups were analyzed with Mann-Whitney U and Wilcoxon nonparametric tests as appropriate; correlation analysis was performed using Pearson correlation test. Statistical analysis was performed with PRISM version 5 software (GraphPad Software; San Diego, Calif).
RESULTS
We have analyzed the impact of DP with extended lymphadenectomy and mesopancreas exeresis on the dynamics of PB cell populations in a cohort of patients affected by pancreatic cancer.
Modulation of NK and B-Lymphocyte Subsets in Pancreatic Cancer Patients Undergoing DP With Complete Mesopancreas Removal and Extended Lymphadenectomy
The evaluation of the relative abundance of the main circulating leukocyte subsets may represent a starting point to assess cancer patient clinical response after curative surgery.36,37 Here we evaluated, by immunostaining and flow cytometric analysis, the frequency of the main lymphocyte subsets in the PB of pancreatic cancer patients, before and after DP.
Before intervention, the frequencies of the major lymphocyte populations (namely, CD3+ T-cells and its main CD4+ and CD8+ subsets, CD3-CD56+ NK cells, and CD19+ B-lymphocytes) were comparable between patients and age-matched controls (Fig. 1). Interestingly, patient NK cell percentage (and absolute concentration, not shown) significantly augmented at day 30, but not at day 7, after surgical intervention (Fig. 1B). In addition, the frequency of circulating B-cells was significantly reduced at the same time point (Fig. 1D). The elevation of NK cells and the diminution of B-cells 30 days after surgery were statistically significant also when compared with control subjects. Differently, the abundance of total T-cells, and of CD4+ and CD8+ subsets, remained comparable to preintervention values (Fig. 1A). Restricting the analysis to patients followed for an overall 2-year period after surgery, survivors show a higher median NK cell frequency at PS30 time point, when compared with deceased patients (Fig. 1C). Thus, surgical removal of the tumor selectively induces the modulation of NK cells and B-lymphocytes, both deeply involved in the immune host defense against cancer. In addition, observational data seem to suggest difference in postsurgery NK cell frequency values among deceased and survivor patients.
FIGURE 1.
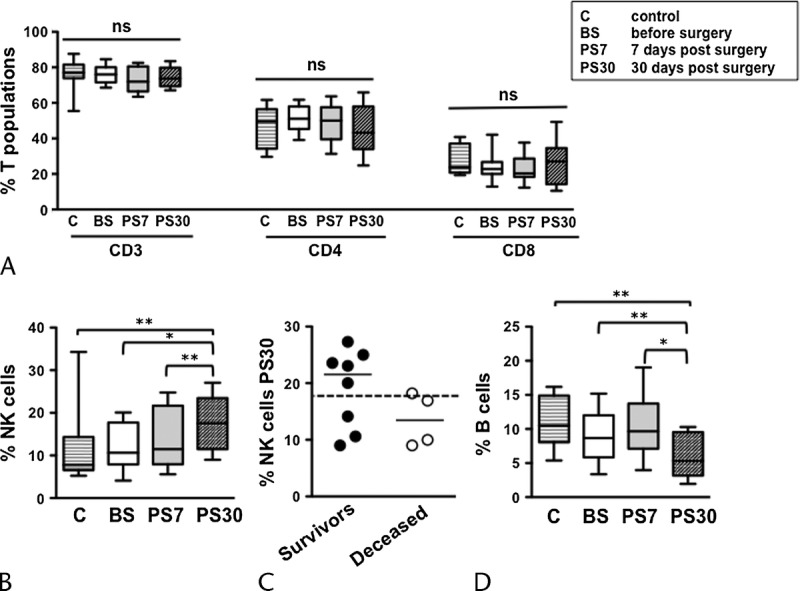
Modulation of the main lymphocyte subsets in pancreatic cancer patients undergoing DP with extended lymphadenectomy and mesopancreas exeresis. Frequency of circulating CD3+, CD4+, and CD8+ T (A), NK (B), and B-lymphocyte (D) subsets, in pancreatic cancer patients before surgery (BS), at day 7 (PS7), and at day 30 (PS30) postsurgery, as well as age-matched controls (C). Bars show median and interquartile range. *P < 0.05; **P < 0.005. Distribution and median of NK cell frequency at PS30 in 2-year survivors (black circles) and deceased (white circles) patients, compared with the median of all patients (C, dashed line).
Dynamics of NK Cell Frequency and Cytotoxic Activity in Pancreatic Cancer Patients Undergoing DP
Natural killer cells represent an important effector of antitumor immunosurveillance, by exerting a direct cytotoxicity against tumor cells. Their increase in PB samples of pancreatic cancer patients at 30 days postsurgery prompted us to investigate NK cell cytolytic function. The NK cell’s natural cytotoxicity depends on activating receptors that interact with “stress-induced” class I-like molecules that are frequently up-regulated on malignant cells, such as in pancreatic cancer.38,39 Natural killer cell cytotoxic activity in PBMC of presurgery patients was comparable to that of controls, evaluated as the percentage of in vitro killing of a reference tumor cell line (K562) (Fig. 2A); a transient diminution occurred at day 7 postintervention and was reconstituted at 30 days after surgery (Fig. 2A); comparable results have been obtained when NK cytotoxic activity was expressed in lytic units (data not shown). Thus, the elevation of NK cell frequency at day 30 after surgery does not quantitatively correlate with the transient diminution of NK cell cytotoxicity observed at an earlier (day 7 postsurgery) time point. We thus analyzed more in depth the dynamic relationship between the abundance of PB NK cells and the in vitro cytotoxic activity in pancreatic tumor patients; at presurgery, the frequency of NK cells and the extent of PBMC cytotoxic activity were positively correlated in each individual. This relationship was maintained at day 7 time point, whereas it was absent at day 30 postintervention (Fig. 2B). Interestingly, NK cell frequency and PBMC cytotoxic activity showed no significant correlation in control subjects; this evidence has been previously reported in the literature and explained by the large variability in individual immune cell state of activation.40,41 Collectively taken, these data suggest that although present in a normal percentage, the lytic behavior of presurgery patients’ NK cells is altered; moreover, their cytotoxic activity is reduced at a short time point after surgery. Later on after intervention (30 days), the elevation of frequency, the recovery of NK cytotoxic activity, and the loss of the correlation between NK cell frequency and cytotoxicity collectively suggest a normalization of NK cell behavior.
FIGURE 2.
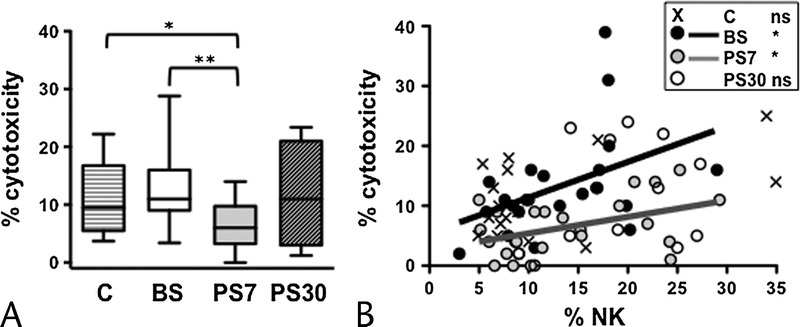
Modulation of NK cell cytotoxicity and dynamics of its relationship with NK cell frequency in pancreatic cancer patients undergoing DP. A, Percentage of NK cytotoxic activity in PB of pancreatic cancer patients before surgery (BS), at day 7 (PS7), and at day 30 (PS30) postsurgery, as well as age-matched controls. Bars show median and interquartile range. B, Scatter plots representing the percentage of PBMC cytotoxicity with respect to NK cell frequency; NK cells before surgery (BS, black symbols and line), at day 7 (PS7, gray symbols and line), and at day 30 (PS30, white symbols) postsurgery, as well as age-matched controls (x symbols). Lines represent the linear regression for any given pair of variables. *P < 0.05, **P < 0.005.
Initial and Advanced Cancer Stage Patients Undergoing DP Show Opposite Dynamics of NK Cell Frequency and Cytotoxic Activity
Tumor burden and invasiveness may impact on the immune system of the host. Our data reveal a complex defect in NK cell frequency and lytic capability in pancreatic tumor patients. We asked whether the NK cell frequency, PBMC cytotoxic activity, and the correlation between the 2 were influenced by tumor stage (initial vs advanced, according to the Union for International Cancer Control TNM 2009 Classification of Malignant Tumors, Seventh Edition).30 Interestingly, the significant elevation of NK cell frequency at 30 days after surgery was more markedly observed in advanced-stage patients, with respect either to presurgery time point and to controls (Figs. 3A, D); in this context, survivors at 2 years presented a higher median value than deceased ones (Fig. 3E); differently, a transient diminution of NK cytotoxicity at day 7 postsurgery only affected early-stage patients (Figs. 3B, F). Accordingly, NK cell percentage and the extent of PBMC cytotoxicity significantly correlated only in advanced but not in early-stage patients, at presurgery and day 7 postsurgery time points (Figs. 3C, G). Collectively taken, these data suggest that NK cell frequency alteration, and its aberrant correlation with individual patient cytotoxic activity, more deeply affect patients with advanced-stage tumor; conversely, the transient diminution of PBMC cytotoxicity at an early time point after surgery distinctly characterizes patients with early-stage tumor.
FIGURE 3.
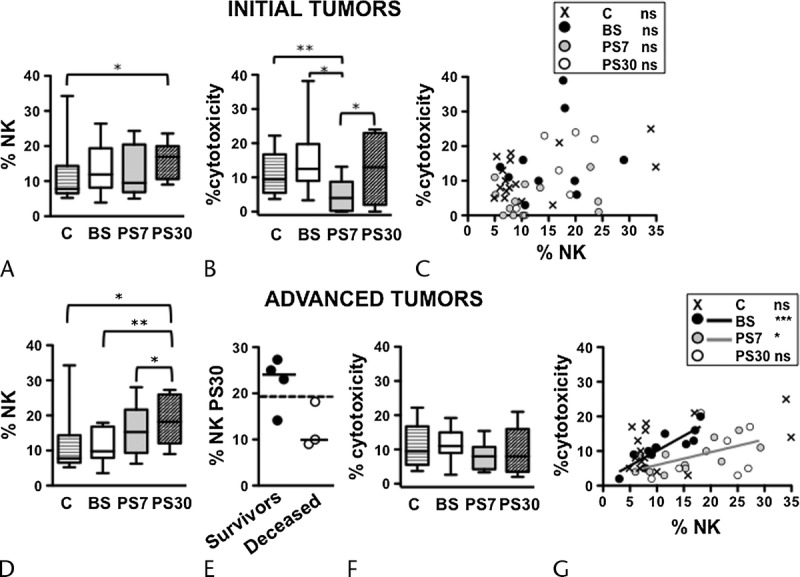
Distinct alterations in the dynamics of NK cell compartment of initial and advanced-stage cancer patients, at presurgery and postsurgery. Frequency of NK cells (A and D) and percentage of cytotoxicity (B and F) in PB of initial (A and B) and advanced (D and F) cancer stage patients, before surgery (BS), at day 7 (PS7), and at day 30 (PS30) postsurgery, as well as age-matched controls. Bars show median and interquartile range. Scatter plots representing the percentage of cytotoxicity versus the NK cell frequency of each initial (C) and advanced (G) cancer stage patient, before surgery (BS, black symbols and line), at day 7 (PS7, gray symbols and line), and at day 30 (PS30, white symbols) postsurgery, as well as age-matched controls (x symbols). Lines in the plots represent the linear regression for any given pair of variables. *P < 0.05, **P < 0.005, ***P < 0.0001. Distribution and median of NK cell frequency at PS30 in 2-year survivors (black circles) and deceased (white circles) patients, compared with the median of all advanced-stage patients (E, dashed line).
Dynamics of NK Cell Subsets and Relationship With Cytotoxic Ability in Advanced and Initial Pancreatic Tumor Patients
To investigate in depth the NK cell compartment in initial and advanced-stage pancreatic cancer patients, we analyzed the 2 main functionally and phenotypically different NK cell subsets, namely, CD56bright (equipped with a low cytotoxic potential) and CD56dim (highly cytotoxic and representing the vast majority of PB NK cells). Strikingly, in the percentage of CD56bright NK cells in advanced-stage, but not in early-stage, patients was basally lower than controls and attained normal levels early after surgical intervention (Figs. 4A, D). No statistically significant differences could be found in the representativeness of CD56dim NK cells, in either group of patients (Figs. 4B, E). In addition, the extent of cytotoxic activity in individual early-stage patients bore no correlation with the frequency of CD56dim NK cells at any time points, similarly to what occurred in controls (Fig. 4C); at variance, the frequency of CD56dim NK cells significantly correlated with the levels of PBMC cytotoxicity in advanced-stage patients, both at presurgery and at day 7 postsurgery (Fig. 4F).
FIGURE 4.
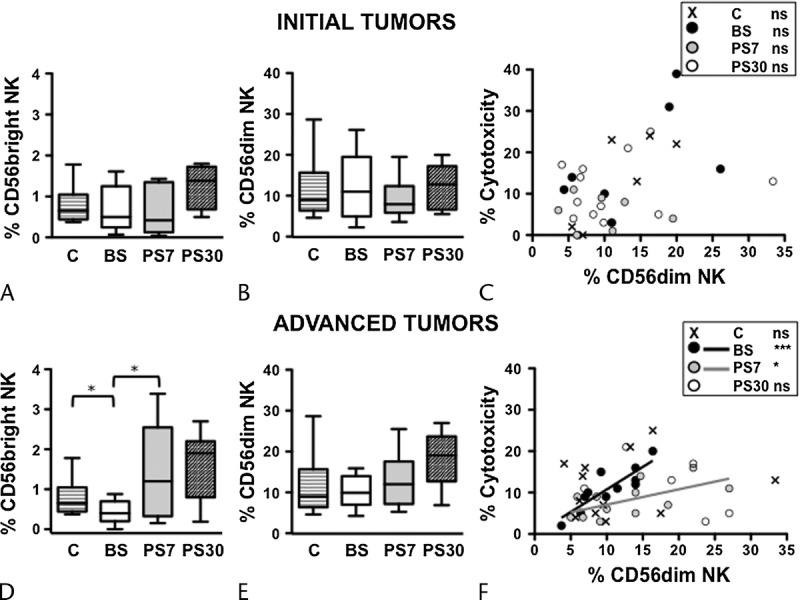
Distinct alterations in the dynamics of CD56dim and CD56bright NK cell subsets of initial and advanced-stage cancer patients, at presurgery and postsurgery. Frequency of CD56bright (A and D) and CD56dim (B and E) NK cell subsets in PB of initial (A and B) and advanced (D and E) cancer stage patients, before surgery (BS), at day 7 (PS7), and at day 30 (PS30) postsurgery, as well as age-matched controls. Bars show median and interquartile range. Scatter plots representing the percentage of cytotoxicity versus CD56dim NK cell frequency in each initial (C) and advanced (F) cancer stage patient, before surgery (BS, black symbols and line), at day 7 (PS7, gray symbols and line), and at day 30 (PS30, white symbols) postsurgery, as well as age-matched controls (x symbols). Lines in the plots represent the linear regression for any given pair of variables. *P < 0.05, **P < 0.005, ***P < 0.0001.
Collectively taken, these results support the notion that CD56dim and CD56bright NK cell subsets are more deeply altered in advanced-stage than in early-stage pancreatic cancer patients before surgery; it is conceivable that the different basal state also impacts on the NK cell behavior at postsurgery time points in the 2 groups of patients.
Dynamics of Neutrophil Count in Pancreatic Tumor Patients
Finally, we also assessed the impact of surgical intervention on systemic inflammation biomarkers, to better evaluate the role of the inflammatory microenvironment in the alteration of NK cell biology. Data reported in Figure 5 show that blood neutrophil concentration, an inflammation marker, and the neutrophil/lymphocyte ratio (NLR), which integrates information on the inflammatory milieu and physiological stress, dramatically increase 7 days after surgery and are close to normalization 30 days after surgery.
FIGURE 5.
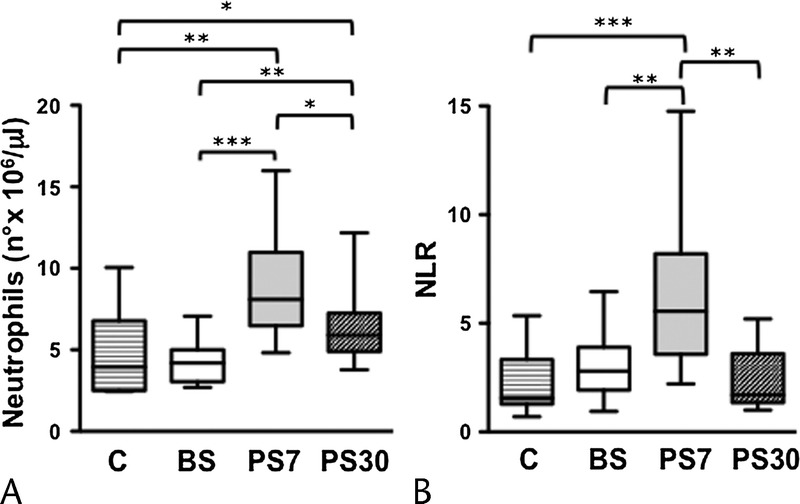
Dynamics of neutrophil absolute number and NLR in pancreatic cancer patients undergoing DP. Absolute neutrophil counts (A) and NLR (B) in whole blood were obtained in pancreatic cancer patients, at presurgery (BS), at day 7 (PS7), and at day 30 (PS30) postsurgery, as well as age-matched controls (C). *P < 0.05, **P < 0.005, ***P < 0.0001.
DISCUSSION
Because curative surgical resection still remains the only strategy of choice in the treatment of pancreatic cancer, it becomes of fundamental importance to study not only the basal immune status of pancreatic cancer patients,22 but also the effects of surgery on immune response. In this context, extensive surgery strategies may significantly impact on the asset of the immune system. We performed a phenotypical and functional immunomonitoring of a cohort of pancreatic cancer patients, before and at 2 different time points after a modified DP surgical technique, in which mesopancreas was resected en bloc together with the following lymph node areas: 8, 9, 12, 13, 14 (a, b, c, and v), and 16 (a2).33,35
We observed the selective modulation of PB NK cells and B-cells, characterized by an increase in NK cell and by a decrease in B-cell percentages, which become statistically significant 30 days after surgery. The frequency of total T-cells, and of CD4+ and CD8+ T-lymphocyte subpopulations were comparable to controls, before surgery, and did not show significant variations upon intervention.
Natural killer cells play a pivotal role in the immune response against tumor by exhibiting cytotoxic activity and secreting a number of immunomodulatory cytokines.19 Natural killer cells may exert their protective role by killing pancreatic cancer cells, which have lost major histocompatibility complex class I expression42 and/or express-induced ligands for NKG2D activating receptor,38 and their increased cell number may represent an important defense against residual cancer cells; actually, a positive correlation between pancreatic cancer patient survival and their PB NK cell absolute count has been previously described.43
On a functional point of view, a significant and transient diminution of NK cell cytotoxicity was observed in pancreatic cancer patients at day 7 postintervention, which did not depend on NK cell frequency, thus defining a transient postsurgery reduced NK cell immunosurveillance.
Some reports in literature have previously noted a postsurgery decline of NK cell function but not number, whose possible explanation may depend on the establishment of a postoperative inflammatory status, confirmed by the neutrophil counts (Fig. 5A), the NLR value (Fig. 5B), and the C-reactive protein concentration (not shown).44,45 In addition to inflammatory cytokines, the postsurgery elevation of glucocorticoids, catecholamines, and prostaglandins may also explain the short-term decrease in NK cell cytotoxicity, as these are all well-known factors that strongly affect the responsiveness of NK cells and represent a link between immune and neuroendocrine systems.46
A more detailed analysis of the dynamic relationship between PB NK cell number and function reveals a tumor-associated aberrant NK cell behavior, represented by the existence of a significant positive correlation, in each individual, between the abundance of NK cells and their cytotoxic activity at both presurgery and day 7 time points, which does not occur in the control population. The direct correlation between NK cell number and cytotoxic function has been already noted by Davis et al43 in pancreatic cancer patients and could derive from an alteration in NK cell proliferation and function, probably due to the profound tumor-dependent (at presurgery) and surgery-dependent (at 7 days postintervention) modulation of soluble factors. Indeed, the well-known deregulation of NK cell–modulating cytokines, such as IL-2, IL-12, and IL-18, and of CD4/CD25 regulatory T-cell subset in pancreatic cancer patients could set up an altered microenvironment, in which NK cells adopted aberrant proliferation, differentiation, and functional behavior.23,25,26,47 The hypothesis that tumor-dependent alterations could be responsible for the establishment of a pool of altered NK cells finds support in the observation that the positive correlation linking the percentage of NK cells (and of the more cytotoxic CD3-CD56dim NK cell subset in particular) with the extent of cytotoxic activity only affected patients with advanced but not initial stage cancer, at both presurgery and day 7 postsurgery time points; this different NK cell behavior could explain, despite the comparable cell number, the absence of a statistically significant postsurgery decline of NK cell function in advanced cancer patients.
Observational analysis in a 2-year follow-up, performed on a limited number of patients, also suggests the relevance of NK cell frequency because survivors (8/12 patients) showed a median of NK cell frequency distribution at PS30 considerably higher than that of deceased patients; this holds true also when advanced cancer patients are taken into consideration (4 survivors, 3 deceased). In addition, the decreased frequency of the lymph node–homing, cytokine-producing CD56bright NK cell subset, selectively observed in presurgery advanced cancer patients, could be related to the lymph node involvement by metastasis and normalized early after extended surgical procedure and tumor eradication (day 7).
At 1 month upon surgical tumor eradication, NK cell behavior undergoes normalization, in that the frequency of circulating NK cells and the percentage of cytotoxic activity are independently regulated, similarly to what observed in control subjects; this could depend on a further remodeling of the microenvironment, as previous reports in the literature have shown a restored capability of stimulated PBMC and T-cells to produce Th1 cytokines, such as IL-12p70 and IFN-γ, at longer times after tumor eradication.29
Finally, we also documented that DP associates with a significant decrease in CD19+ B-cell frequency, occurring at 30 days after surgery. The role played by B-cells in cancer immunology is complex and controversial, often depending on their state of activation. In certain tumors, B-cells could represent a source of IL-10, whose role in immunosuppression is deeply known, and B-cell depletion enhances antitumor immune response in a B-cell knockout tumor-challenged animal model.48
Overall, our data demonstrate that upon extended DP occurs not only the transient reduction in NK cell cytotoxic function, but also the long-term modulation of NK cell and B-cell frequencies and the reversion of the aberrant positive correlation between NK cell count and function; such events seem to be deeply connected not only to surgery but also to tumor burden and invasiveness and could represent an attempt of host immune system to counteract the postsurgery loss of NK cell–mediated immunosurveillance.
ACKNOWLEDGMENTS
The authors thank Dr Antonella Ferri and Dr Monica Schiratti for the guidance in blood sample collection.
Footnotes
Francesco Iannone and Alessandra Porzia contributed equally to this work.
The authors declare no conflict of interest.
REFERENCES
- 1. Seufferlein T, Bachet JB, Van Cutsem E, et al. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012; 23: vii33– vii40. [DOI] [PubMed] [Google Scholar]
- 2. Loos M, Kleeff J, Friess H, et al. Surgical treatment of pancreatic cancer. Ann N Y Acad Sci. 2008; 1138: 169– 180. [DOI] [PubMed] [Google Scholar]
- 3. Connor S, Bosonnet L, Ghaneh P, et al. Survival of patients with periampullary carcinoma is predicted by lymph node 8a but not by lymph node 16b1 status. Br J Surg. 2004; 91: 592– 599. [DOI] [PubMed] [Google Scholar]
- 4. Sierzega M, Popiela T, Kulig J, et al. The ratio of metastatic/resected lymph nodes is an independent prognostic factor in patients with node-positive pancreatic head cancer. Pancreas. 2006; 33: 240– 245. [DOI] [PubMed] [Google Scholar]
- 5. Berger AC, Watson JC, Ross EA, et al. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2004; 70: 235– 240. [PubMed] [Google Scholar]
- 6. Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001; 234: 758– 768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bassi C, Stocken DD, Olah A, et al. Influence of surgical resection and post-operative complications on survival following adjuvant treatment for pancreatic cancer in the ESPAC-1 randomized controlled trial. Dig Surg. 2005; 22: 353– 363. [DOI] [PubMed] [Google Scholar]
- 8. Ueda M, Endo I, Nakashima M, et al. Prognostic factors after resection of pancreatic cancer. World J Surg. 2009; 33: 104– 110. [DOI] [PubMed] [Google Scholar]
- 9. Kure S, Kaneko T, Takeda S, et al. Analysis of long-term survivors after surgical resection for invasive pancreatic cancer. HPB (Oxford). 2005; 7: 129– 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Samra JS, Gananadha S, Hugh TJ. Surgical management of carcinoma of the head of pancreas: extended lymphadenectomy or modified en bloc resection? ANZ J Surg. 2008; 78: 228– 236. [DOI] [PubMed] [Google Scholar]
- 11. Gaedcke J, Gunawan B, Grade M, et al. The mesopancreas is the primary site for R1 resection in pancreatic head cancer: relevance for clinical trials. Langenbecks Arch Surg. 2010; 395: 451– 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gockel I, Domeyer M, Wolloscheek T, et al. Resection of the mesopancreas (RMP): a new surgical classification of a known anatomical space. World J Surg Oncol. 2007; 5: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peparini N, Chirletti P. Clearance of the retropancreatic margin in pancreatic carcinomas: total mesopancreas excision or extended lymphadenectomy? Eur J Surg Oncol. 2012; 38: 1146. [DOI] [PubMed] [Google Scholar]
- 14. Popescu I, Dumitrascu T. Total mesopancreas excision: key point of resection in pancreatic head adenocarcinoma. Hepatogastroenterology. 2011; 58: 202– 207. [PubMed] [Google Scholar]
- 15. Adham M, Singhirunnusorn J. Surgical technique and results of total mesopancreas excision (TMpE) in pancreatic tumors. Eur J Surg Oncol. 2012; 38: 340– 345. [DOI] [PubMed] [Google Scholar]
- 16. Kawabata Y, Tanaka T, Nishi T, et al. Appraisal of a total meso-pancreatoduodenum excision with pancreaticoduodenectomy for pancreatic head carcinoma. Eur J Surg Oncol. 2012; 38: 574– 579. [DOI] [PubMed] [Google Scholar]
- 17. Peparini N, Chirletti P. Mesopancreas: a boundless structure, namely R1 risk in pancreaticoduodenectomy for pancreatic head carcinoma. Eur J Surg Oncol. 2013; 39: 1303– 1308. [DOI] [PubMed] [Google Scholar]
- 18. Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007; 121: 1– 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caligiuri MA. Human natural killer cells. Blood. 2008; 112: 461– 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bernardini G, Gismondi A, Santoni A. Chemokines and NK cells: regulators of development, trafficking and functions. Immunol Lett. 2012; 145: 39– 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013; 39: 61– 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wörmann SM, Diakopoulos KN, Lesina M, et al. The immune network in pancreatic cancer development and progression. Oncogene. 2014; 33( 23): 2956– 2967. [DOI] [PubMed] [Google Scholar]
- 23. Yamamoto T, Yanagimoto H, Satoi S, et al. Circulating CD4+CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas. 2012; 41( 3): 409– 415. [DOI] [PubMed] [Google Scholar]
- 24. Von Bernstorff W, Voss M, Freichel S, et al. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001; 7( suppl 3): 925s– 932s. [PubMed] [Google Scholar]
- 25. Poch B, Lotspeich E, Ramadani M, et al. Systemic immune dysfunction in pancreatic cancer patients. Langenbecks Arch Surg. 2007; 392: 353– 358. [DOI] [PubMed] [Google Scholar]
- 26. Bellone G, Smirne C, Mauri FA, et al. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol Immunother. 2006; 55: 684– 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. González HD, Figueras J. Effect of surgical resection of metastatic disease on immune tolerance to cancer. How a systemic disease could be controlled by a local therapy. Clin Transl Oncol. 2007; 9: 571– 577. [DOI] [PubMed] [Google Scholar]
- 28. Alderton GK. Tumour immunology: suppressing the rejection of pancreatic tumours. Nat Rev Immunol. 2012; 12: 555. [DOI] [PubMed] [Google Scholar]
- 29. Bellone G, Novarino A, Vizio B, et al. Impact of surgery and chemotherapy on cellular immunity in pancreatic carcinoma patients in view of an integration of standard cancer treatment with immunotherapy. Int J Oncol. 2009; 34( 6): 1701– 1715. [DOI] [PubMed] [Google Scholar]
- 30. Sobin L, Gospodarowicz M, Wittekind C. UICC International Union Against Cancer TNM Classification of Malignant Tumours. Hoboken, NJ: Wiley-Blackwell Publication, 2009. [Google Scholar]
- 31. Palmieri G, Serra A, De Maria R, et al. Cross-linking of alpha 4 beta 1 and alpha 5 beta 1 fibronectin receptors enhances natural killer cell cytotoxic activity. J Immunol. 1995; 155: 5314– 5322. [PubMed] [Google Scholar]
- 32. Caronna R, Cardi M, Sammartino P, et al. Functional results of a personal technique of reconstruction after pancreaticoduodenectomy. J Exp Clin Cancer Res. 2003; 22: 187– 189. [PubMed] [Google Scholar]
- 33. Nimura Y. Extended surgery in bilio-pancreatic cancer: the Japanese experience. Semin Oncol. 2002; 29: 17– 22. [DOI] [PubMed] [Google Scholar]
- 34. Pedrazzoli S, Pasquali C, Sperti C. Role of surgery in the treatment of bilio-pancreatic cancer: the European experience. Semin Oncol. 2002; 29: 23– 30. [DOI] [PubMed] [Google Scholar]
- 35. Isaji S, Kawarada Y, Uemoto S. Classification of pancreatic cancer: comparison of Japanese and UICC classifications. Pancreas. 2004; 28: 231– 234. [DOI] [PubMed] [Google Scholar]
- 36. Moore MM, Chua W, Charles KA, et al. Inflammation and cancer: causes and consequences. Clin Pharmacol Ther. 2010; 87: 504– 508. [DOI] [PubMed] [Google Scholar]
- 37. Clark EJ, Connor S, Taylor MA, et al. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB (Oxford). 2007; 9: 456– 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu X, Rao GS, Groh V, et al. Major histocompatibility complex class I-related chain A/B (MICA/B) expression in tumor tissue and serum of pancreatic cancer: role of uric acid accumulation in gemcitabine-induced MICA/B expression. BMC Cancer. 2011; 11: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dambrauskas Z, Svensson H, Joshi M, et al. Expression of major histocompatibility complex class I-related chain A/B (MICA/B) in pancreatic carcinoma. Int J Oncol. 2014; 44: 99– 104. [DOI] [PubMed] [Google Scholar]
- 40. Gilman-Sachs A, DuChateau BK, Aslakson CJ, et al. Natural killer (NK) cell subsets and NK cell cytotoxicity in women with histories of recurrent spontaneous abortions. Am J Reprod Immunol. 1999; 41: 99– 105. [DOI] [PubMed] [Google Scholar]
- 41. Levy SM, Herberman RB, Simons A, et al. Persistently low natural killer cell activity in normal adults: immunological, hormonal and mood correlates. Nat Immun Cell Growth Regul. 1989; 8: 173– 186. [PubMed] [Google Scholar]
- 42. Pandha H, Rigg A, John J, et al. Loss of expression of antigen-presenting molecules in human pancreatic cancer and pancreatic cancer cell lines. Clin Exp Immunol. 2007; 148: 127– 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davis M, Conlon K, Bohac GC, et al. Effect of pemetrexed on innate immune killer cells and adaptive immune T cells in subjects with adenocarcinoma of the pancreas. J Immunother. 2012; 35: 629– 640. [DOI] [PubMed] [Google Scholar]
- 44. Pollock RE, Lotzová E, Stanford SD. Mechanism of surgical stress impairment of human perioperative natural killer cell cytotoxicity. Arch Surg. 1991; 126: 338– 342. [DOI] [PubMed] [Google Scholar]
- 45. Lukomska B, Olszewski WL, Engeset A, et al. The effect of surgery and chemotherapy on blood NK cell activity in patients with ovarian cancer. Cancer. 1983; 51: 465– 469. [DOI] [PubMed] [Google Scholar]
- 46. Rosenne E, Sorski L, Shaashua L, et al. In vivo suppression of NK cell cytotoxicity by stress and surgery: glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav Immun. 2013; 37: 207– 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roshani R, McCarthy F, Hagemann T. Inflammatory cytokines in human pancreatic cancer. Cancer Lett. 2013; 345: 157– 163. [DOI] [PubMed] [Google Scholar]
- 48. Inoue S, Leitner WW, Golding B, et al. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006; 66: 7741– 7747. [DOI] [PubMed] [Google Scholar]


