Abstract
While most drugs of abuse increase dopamine neurotransmission, rapid neurochemical measurements show that different drugs evoke distinct dopamine release patterns within the nucleus accumbens. Rapid changes in dopamine concentration following psychostimulant administration have been well studied; however, such changes have never been examined following opioid delivery. Here, we provide novel measures of rapid dopamine release following intravenous infusion of two opioids, morphine and oxycodone, in drug naïve rats using fast-scan cyclic voltammetry and rapid (1 min) microdialysis coupled with mass spectrometry. In addition to measuring rapid dopamine transmission, microdialysis HPLC-MS measures changes in GABA, glutamate, monoamines, monoamine metabolites, and several other neurotransmitters. Although both opioids increased dopamine release in the nucleus accumbens, their patterns of drug-evoked dopamine transmission differed dramatically. Oxycodone evoked a robust and stable increase in dopamine concentration and a robust increase in the frequency and amplitude of phasic dopamine release events. Conversely, morphine evoked a brief (~ 1 min) increase in dopamine that was coincident with a surge in GABA concentration and then both transmitters returned to baseline levels. Thus, by providing rapid measures of neurotransmission, this study reveals previously unknown differences in opioid-induced neurotransmitter signaling. Investigating these differences may be essential for understanding how these two drugs of abuse could differentially usurp motivational circuitry and powerfully influence behavior.
Keywords: reward, motivation, addiction, opioid
INTRODUCTION
Opioids, particularly μ-opioid receptor (MOR) agonists, are commonly prescribed for pain management. However, opioids can cause molecular and cellular adaptations which promote physical and behavioral dependencies and the development of addiction is therefore a major concern (Williams et al., 2001). Indeed, prescription opioid abuse has increased dramatically over the past decade (Compton & Volkow, 2006). More recent generations of opioids (e.g., oxycodone, hydrocodone, and fentanyl) are tremendously effective for pain management and while it was initially suggested that oxycodone is unlikely to be more addictive than morphine (Davis et al., 2003), oxycodone is widely abused and is among the fastest growing drugs of abuse (Compton & Volkow, 2006). While this is certainly influenced by greater availability due to prescription use, recent studies suggest that oxycodone may have a higher potential to promote addiction-related behaviors compared to other opioids, such as morphine (Stoops et al., 2010, Comer et al. 2013). However, little is known about how oxycodone and morphine differentially influence changes in brain function associated with drug abuse and addiction.
While the mechanisms underlying drug addiction are complex and still intensely debated, dopamine (DA) neurotransmission is incontrovertibly involved in the development, maintenance, and compulsive intake of abused drugs (Phillips et al., 2003a; Robinson & Berridge, 2003; Hyman et al., 2006). Despite different mechanisms (Luscher & Ungless, 2006), all abused drugs increase DA transmission within the nucleus accumbens (NAc) (Di Chiara & Imperato, 1988; Nestler & Malenka, 2004). Techniques that allow for rapid measures of DA release have revealed that different drugs elicit distinct DA signaling dynamics that better inform our understanding of the neurochemical consequences of drug intake (Cheer et al., 2007; Aragona et al., 2008; Daberkow et al., 2013). Further, additional neurotransmitter systems, such as glutamate and GABA, within the NAc are critical for reward and motivation (Kelley 2004; Shen et al J Neurosci 34: 16, 5649-57); (Wydra et al Addition Biology 2, 307-24). Given the importance of these neurotransmitter systems in the regulation of behaviors associated with drug abuse and that there is currently no information regarding rapid neurotransmission following opioid delivery, we aimed to determine if rapid changes in these systems within the NAc differ following morphine or oxycodone delivery.
Given the explosive rise in prescription opioid abuse (Compton & Volkow, 2006), the speculation that oxycodone may more potently promote drug addiction (Comer et al., 2008), and the important role for increased DA transmission in behaviors associated with drug abuse and addiction, we hypothesized that oxycodone, compared to morphine, would more robustly enhance extracellular DA concentrations ([DA]) within the NAc. Here, we employed two advanced in vivo measurement technologies, fast-scan-cyclic voltammetry (FSCV) and rapid-sampling microdialysis with high performance liquid chromatography coupled with mass spectrometry (HPLC-MS) to provide a sub-second (phasic) and minute-to-minute comparison of morphine- and oxycodone-evoked alterations in DA release. Additionally, microdialysis with HPLC-MS allowed the determination if these drugs differentially alter neurotransmission patterns across a wide range of signaling molecules known to be important for both modulating DA signaling and mediating reward, motivation, and drug addiction.
METHODS
Subjects
Thirty-seven male Sprague Dawley rats (~300 g) were obtained with indwelling jugular vein catheters (Charles River Laboratories, Raleigh, NC) and were used as subjects (FSCV morphine: n = 8; FSCV oxycodone: n = 8; FSCV naloxone-morphine or oxycodone: n = 9; HPLC-MS Dialysis morphine: n = 4; HPLC-MS Dialysis oxycodone: n = 5). Rats were maintained on 12:12 reverse light/dark cycle (lights off 0800). Rats were housed individually with ad libitum access to food and water. All procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Michigan Committee on the Use and Care of Animals, and all efforts were made to minimize animal suffering and reduce the number of subjects used.
Surgery
Rats were anesthetized with intra-muscular ketamine hydrochloride (90 mg/kg) and xylazine hydrochloride (10 mg/kg). For FSCV subjects, a guide cannula (Bioanalytical Systems, West Lafayette, IN) was secured dorsal to the NAc core (AP: +1.3; ML: ± 1.3; DV: -2.5; mm relative to bregma) or shell (AP: +1.8; ML: ± 0.8; DV: -2.5 mm) and a Ag/AgCl reference electrode was placed in the contralateral cortex (AP: -0.8; ML: ±3.0; DV: -2.5; mm relative to bregma). A bipolar stimulating electrode (Plastics One, Roanoke, VA) was lowered into the ventral tegmental area (VTA) (AP: -5.2; ML: ± 0.8; mm relative to bregma) until electrically-evoked DA release within the striatum was detected, as described previously (Wightman et al., 2007). Similarly, microdialysis subjects were implanted with a guide cannula (CMA, Holliston, MA) positioned 1 mm above the NAc (AP: +1.7; ML: ± 0.9; DV: -6.0 mm). All implants were permanently secured to the skull with stainless steel surgical screws and dental acrylic. Following surgery, rats recovered for ~5 days until they reached pre-operative weight.
Experimental Procedures - FSCV
On the day of the pharmacological experiment, a recording cable was anchored to the skull cap and connected to a rotating commutator (Crist Instruments, Hagerstown, MD) suspended from the top of the test chamber allowing rats to move freely about the Plexiglas recording chamber. Vehicle-loaded tubes were threaded through the rotating commutator and along the headstage cable and attached to the i.v. catheter and a syringe pump outside of the test chamber. Carbon-fiber microelectrodes (~ 120 μm in length) were lowered into the NAc core or shell and secured in locations where both electrically-evoked and naturally occurring phasic DA release events were detected (Wightman et al., 2007).
Similar to our previous FSCV studies (Aragona et al., 2008; Porter-Stransky et al., 2011), experiments began with a 15-min baseline recording interval followed by another 15-min recording that controlled for i.v. vehicle administration (0.2 ml of sterile saline; 5 s). Rats were then administered, at 15-min intervals, cumulative doses of morphine sulfate (Sigma, St. Louis, MO) or oxycodone hydrochloride (Sigma, St. Louis, MO) dissolved in sterile saline (0.2 ml; 5 s). Following saline injections, separate groups of animals received infusions of either oxycodone or morphine according to the following doses: low dose = 0.1 mg/kg, medium dose = 0.5 mg/kg, and high dose = 1.0 mg/kg; i.v. Drug doses were chosen based on what rats self-administer with the lowest dose under morphine self-administration thresholds (Mierzejewski et al., 2003). The clinical morphine-tooxycodone potency ratio has been shown to be between 1:1 to 1:1.5, with large individual differences in bioavailability (Davis et al., 2003). As such, oxycodone doses were kept equivalent to morphine doses.
Another control, using the MOR preferring antagonist naloxone, was conducted to test the involvement of MORs in the opioid-evoked increases in DA transmission identified in the present study. A separate group of rats received baseline and saline recording periods followed by a pre-exposure of naloxone 3.0 mg/kg; i.v. (Tocris, Bristol, UK). 15 minutes after the naloxone infusion, either morphine or oxycodone (0.5 mg/kg) was administered because this dose consistently elicited a similarly initial increase in [DA]. For all subjects, the final recording session was followed by administration of a DA autoreceptor blocker, the D2-type receptor antagonist raclopride (Sigma, St. Louis, MO; 1.0 mg/kg; i.v.; 5s), as a positive control. This dose of raclopride blocks somatodendritic and terminal autoreceptors (Andersson et al., 1995) and robustly increases DA transients and [DA] (Aragona et al., 2008).
FSCV-Data acquisition
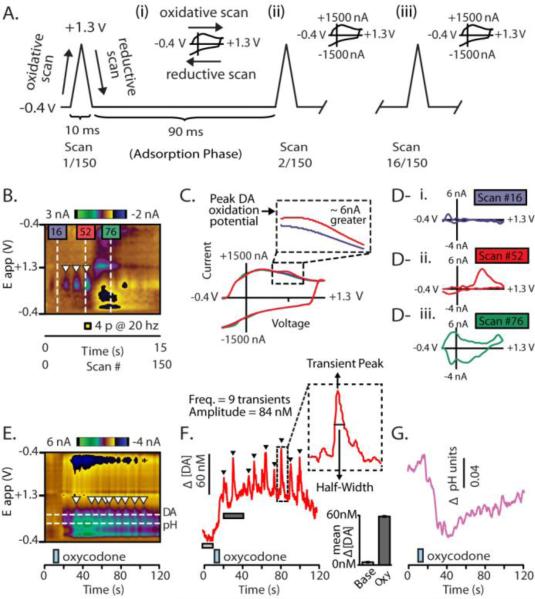
Carbon-fiber microelectrodes were lowered into the NAc core or shell and a negative holding potential of -0.4V was applied. Then, at 10Hz, a triangular voltage ramp was applied (Figure 1A; oxidative scan, -0.4 V to 1.3 V; reductive scan, 1.3 V to -0.4 V at a rate of 400 V/s) (Figure 1A) which results in a large and highly consistent change in current that is recorded from the carbon surface (Robinson et al., 2003) (Figure 1A, insets i – iv). Since the charging current resulting from these voltage ramps are stable (Robinson et al., 2003), they are continually background subtracted in order to examine the changes in current associated with changes in concentration of electroactive species surrounding the carbon fiber (Figure 1B-D) (Robinson et al., 2003). As such, FSCV data are presented as a delta, and background subtracted data are the norm for measuring phasic changes in neurotransmission, including DA release (Garris & Wightman, 1994; Phillips et al., 2003b; Willuhn et al., 2012). Current changes are detected at the surface of the carbon fiber micro-electrodes, and changes beyond those caused by the charging current are plotted in false color against time and the applied voltage ramp (Figure 1B) (Michael et al., 1999). This allows convenient visualization of neurochemical changes across the entire applied voltage ramp. With this particular optimization, FSCV robustly detects changes in [DA] as well as changes in local pH (Figure 1 D,E) (Venton et al., 2003; Roitman et al., 2004; Takmakov et al., 2010; Badrinarayan et al., 2012). Here, we show current changes measured at the carbon fiber measured during electrical stimulation of DA neurons with and without background subtraction (Figure 1C) and one can easily see that the DA release by the stimulation causes a increase in current beyond that produced by the charging voltage of the applied potential needed to measure phasic DA with FSCV.
Figure 1.
Measuring real-time neurochemical transmission dynamics with background subtracted FSCV. A. The carbon-fiber microelectrode is held at -0.4V for 90 ms between voltage ramps. This is ‘adsorption phase’ attracts positive electroactive analytes because of the negative potential applied and causes them to ‘adsorb’ to the carbon surface. At a rate of 10 Hz, the holding potential is rapidly ramped (400 V/s) to a positive voltage (1.3V; ‘oxidative scan’) and then back down to the negative holding potential (-0.4V; ‘reductive scan’). This triangular scan produces a highly robust increase in current at the carbon surface, called the ‘charging current’ (insets A - i to - iv). Each scan (which has a corresponding cyclic voltammogram (CV), see below) is represented along the x-axis (150 measures – 150 CVs - in 15 s). Highlighted are several representative scans and the resulting current changes which they cause. B. Background subtracted changes in current measured during the triangular ramp are plotted in false color across the change in voltage associated with the ramp (-0.4V to 1.3V back to -0.4V; plotted on a straight line along the y-axis). C. Each voltage ramp generates a robust charging current. The charging current is extremely stable which allows for FSCV data to be background subtracted. This process permits for measures in acute current surges beyond that of the charging current to be detected. D. Unique CVs from three different ramps (from scans 16, 52, and 76) are shown (D - i to - iii). These demonstrate how small the acute changes in current caused by changes in neurochemical concentration are relative to the magnitude of the charging current (as the inset in Fig 1C shows, the red scan is slightly larger than the background scan). CVs from the 3 selected ramps the approximate difference in current is shown for each CV (16, 52, and 76). The blue scan (D – i) shows a typical charging current, after background subtraction, (taken from a location in the color plot where there was not an obvious change in color). Conversely, the red scan (D – ii) shows the current at scan 52 which is 200 ms after the DA neurons in the VTA received electrical stimulation (yellow box). The CV reveals an increase in current caused by an increase in DA concentration at the recording site. Electrical stimulation also elicited an expected, delayed decrease in current attributed to a basic shift in pH. The CV for this is shown by scan 76 in Fig 1 Diii. E. Color plot resulting from a representative infusion of oxycodone (blue box). Two reliable neurochemical events are evoked by opioid infusion: an increase in DA concentration and an acidic shift in pH. F. [DA] trace (red trace), which corresponds to the upper vertical dotted line in Fig 1E and DA ‘transients’ are clearly visible (triangles). Mean [DA] change (bar graph inset) can be determined by averaging a period of time prior to the infusion (light gray bar) and a time period after the infusion (dark gray bar). The inset shows that further transient analysis (amplitude and half-width) provides further resolution underpinning DA transmission. G. Phasic changes in acidic pH following oxycodone delivery (blue box).
Specific chemical species are identified by their characteristic change in current and this is identified by the shape of its cyclic voltammogram (CV; Figure 1D) (Robinson et al., 2003; Heien et al., 2005). CVs are current by voltage traces that provide electrochemical ‘fingerprints’ that identify each electroactive species because of their unique oxidation and reduction reactions during the voltage ramp (Phillips et al., 2003a). Following background subtraction, since minimal changes in current occurred before the representative electrical stimulation shown, the CV just prior to the stimulation shows very low changes in current (Figure 1D-i, scan 16). However, electrical stimulation of the VTA caused a rapid increase in current at the peak oxidation potential for DA (Figure 1B and D-ii, scan 52). As reliably shown in previous studies, electrical stimulation of DA neurons was also followed by a slow and long-lasting change in basic pH (Figure 1E,G) compared to evoked DA. To convert current to [DA] and pH units, electrodes were calibrated in a microfluidic flow system (Sinkala et al., 2012) with solutions of known DA concentration and differing pH values to generate calibration curves (Badrinarayan et al., 2012). Recorded CVs were converted from current into [DA] using principle component analyses using electrically-evoked DA and pH CVs acquired before and after each experiment as training sets for principle component analysis (Heien et al., 2005; Keithley et al., 2009; Keithley & Wightman, 2011). This allows the separation of DA from pH and the conversion of current changes into nanomolar concentation (nM) for DA and pH units (Heien et al., 2005; Keithley, Carelli, & Wightman, 2010; Keithley, Heien, & Wightman, 2009). Electrical stimulations of the VTA were used to optimally place FSCV electrodes within the NAc, to verify electrode fidelity throughout the experiment, and to generate training sets.
FSCV Analysis
As in our previous experiments (Porter-Stransky et al., 2011; Badrinarayan et al., 2012), carbon fiber electrodes were constructed in all aspects as described previously (Robinson et al., 2003) except that epoxy was applied to the seal where the carbon meets the glass encasement. Waveform generation, data collection, filtering, and analysis were performed as previously described (Wightman et al., 2007). A DA transient was defined as a five-fold or greater phasic surge of [DA] relative to the root-mean-square noise that is approximately 1s in duration (Figure 1F) (Phillips et al., 2003a). Data were collected in 30 s files and background subtracted at approximately the lowest current value. Current was converted to [DA] using principle component analysis (Keithley & Wightman, 2011) and transient detection and quantification was achieved using MATLAB (code provided by Dr. Richard Keithley) and MINIANALYSIS (Synaptosoft, Decatur, GA), respectively. As established in previous studies (Aragona et al., 2008; Aragona et al., 2009; Badrinarayan et al., 2012), transients below 20 nM were not included in the analysis because events below this magnitude are not reliably detected across electrodes.
Microdialysis with HPLC-MS
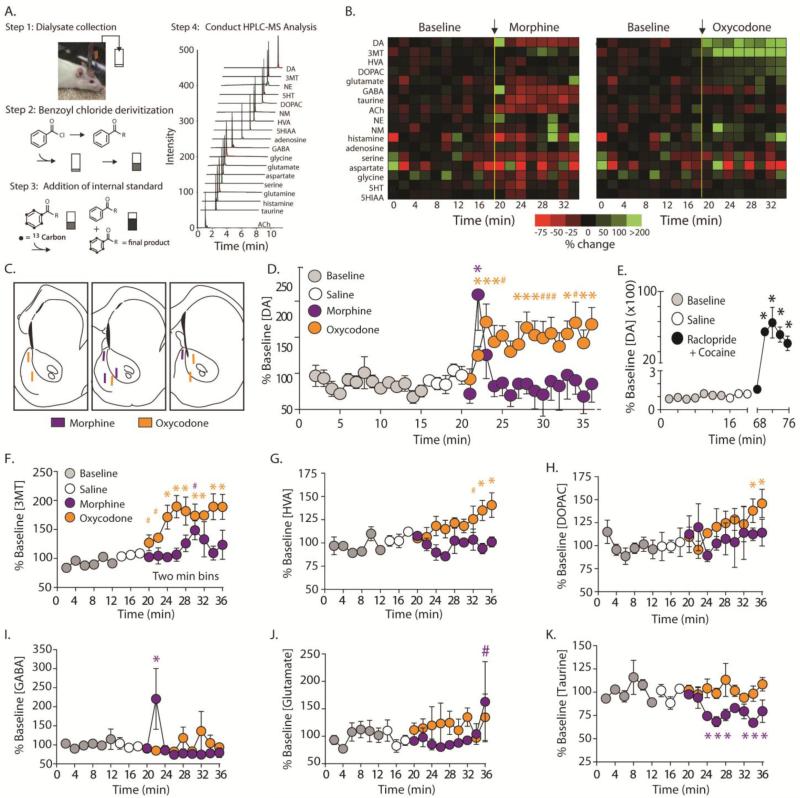
As the FSCV studies used here are highly optimized to measure changes in DA and pH, we employed rapid microdialysis coupled with a novel HPLC-mass spectrometry (HPLC-MS) method (Song et al., 2012) to measure 1 min changes in [DA], as well as changes in 15 additional neurochemical species with high sensitivity. Dialysate samples were collected and prepared as previously described (Song, Mabrouk, Hershey, & Kennedy, 2012). Briefly, at the beginning of microdialysis experiments, a 1 mm probe was inserted into a guide cannula to target the NAc. Microdialysis probes were perfused at 2 μl/min for 1.5 hours prior to sample collection using a Fusion 400 syringe pump (Chemyx, Stafford, TX). Samples were then collected every 60 s yielding 2 μL samples. Immediately after dialysate collection, the following reagents were added to each 2.0 μL dialysate fraction: 1.5 μL of borate buffer (sodium tetraborate, 100 mM), 1.5 μL benzoyl chloride in 2% acetonitrile, 1.5 μL of stable-isotope labeled internal standard solution, and 1.5 μL d4-acetylcholine (ACh) internal standard solution (Song et al., 2012) (Figure 9A). Resulting fractions were analyzed using a nanoAcquity HPLC system (Waters, Milford, MA) equipped with a Waters 1 mm × 100 mm HSS T3 reverse-phase HPLC column operated at 100 μL/min. Eluting analytes were detected using an Agilent 6410 triple quadrupole MS (Agilent, Santa Clara, CA) operating in positive mode performing dynamic multiple-reaction-monitoring (dMRM). The following compounds were monitored in dialysate by the HPLC-MS method: DA and its metabolites 3-methoxytyramine (3-MT), homovanillic acid (HVA), and DOPAC; norepinephrine (NE) and its metabolite normetanephrine (NM); serotonin (5-HT) and its metabolite 5-hydroxyindole-3-acetic acid (5-HIAA); adenosine; histamine; aspartate; serine; taurine; glutamate; and GABA (Figure 9A,B). ACh, which cannot be labeled with benzoyl chloride, was detected directly.
Figure 9.
Rapid sampling microdialysis coupled with HPLC-MS reveals differential changes in dopamine transmission within the NAc between morphine and oxycodone. A. Dialysate was collected every min (step 1) and immediately derivatized with benzoyl chloride (step 2). Internal standards were added to improve quantification (step 3), and fractions were analyzed with HPLC-MS (step 4). A representative total ion chromatogram of a dialysate sample reveals that a tremendous numbers of analytes can be measured using this technology compared to traditional microdialysis. B. Heatmap representing mean percent changes under baseline conditions and changes following i.v. morphine (0.5 mg/kg; n=4) and oxycodone (0.5 mg/kg; i.v.; n=5) delivery. C.. Histological representation of all probe placements for subjects receiving morphine (purple) and oxycodone (orange). D. Normalized changes in [DA] within the NAc (core and shell combined). Baseline levels are shown in gray and control i.v. infusions of saline (white circles) did not significantly increase [DA] above basal levels. Infusion of morphine (0.5 mg/kg i.v.; purple circles) significantly increased [DA] for 1 min and returned to baseline levels. Consistent with FSCV data, oxycodone (orange circles), caused a significant increase in [DA] immediately and remained elevated for the entire sampling period. E. As a positive control, a drug cocktail known to robustly increase [DA] (the D2 antagonist – raclopride; and the DAT blocker – cocaine) evoked an extremely robust increase in [DA] (~5,000%). F. Consistent with changes in [DA], the DA metabolite 3MT trended toward significance in the first 2 recording blocks following oxycodone administration, and was significantly increased during the remainder of the recording session. However, following morphine, 3MT was largely unchanged following morphine delivery with the exception of a trend toward significance in the sixth recording session. G. Following oxycodone, the DA metabolite HVA showed a trend toward significance in the seventh session and was significantly increased in the final two recording bins. However, HVA levels remained unchanged following morphine delivery. H. The DA metabolite DOPAC was significantly elevated in the final two recording bins following oxycodone delivery, but showed no change following morphine infusion. I. The only neurotransmitter that showed a similar pattern of transmission that was similar to DA was GABA transmission following morphine delivery. GABA was significantly elevated following morphine, but not oxycodone, infusion and this increase was only significant during the first time point following drug infusion. J. There was a trend toward a significant increase in glutamate concentration in the final recording session following morphine but not oxycodone delivery. K. Morphine infusion significantly decreased taurine concentration beginning at the third time bin and remained decreased for the duration of the recording period. Conversely, oxycodone infusion had no effect on taurine levels. Error bars indicate SEM. * indicates statistically significant increases (p < 0.05). # indicates trending increases (p < 0.09).
We collected 15 baseline samples and 5 samples following an i.v. saline infusion. Next, subjects received an i.v. infusion (200 μL) of 0.5 mg/kg of either morphine or oxycodone, and fractions were collected for 15 min. These doses were chosen because FSCV studies revealed that these doses evoked similar magnitudes in peak increases in [DA] evoked by drug infusion and caused similar durations in behavioral immobility (data not shown). Since cocaine in the presence of an autoreceptor blocker (D2-type antagonist raclopride) has been shown to synergistically increase [DA] (Rouge-Pont et al., 2002; Aragona et al., 2008), co-infusions of raclopride and cocaine were administered as a positive control in a subset of subjects. Specifically, 1 mg/kg, i.v. raclopride was administered at the end of the experiment and was followed 10 min later by 3 mg/kg, i.v. cocaine infusion. Dialysate was collected for 10 min following these infusions.
Statistics
To compare mean changes in [DA] measured by FSCV across all drug doses to pre-infusion basal levels, 120 s sampling periods were averaged into 10 or 1 s bins and a linear mixed-model was utilized because of its ability to handle repeated-measures data in which observations are not independent (Aragona et al., 2008). Comparisons of transient frequency, amplitude, and half-width following drug treatment compared to mean saline values were also determined using linear mixed-model regressions (data binned into 1 min bins for the duration of the 15-min recording period). Potential core/shell differences were assessed using a liner mixed-model by including the region as a fixed-effect variable. To compare mean changes in DA transients across entire recording sessions, transient frequency, amplitude, and half-width per min were compared across drug doses to control infusions of saline using one-way ANOVAs with Bonferroni post-test corrections for multiple comparisons. For microdialysis data, neurotransmitter concentrations were normalized and converted to percentage of baseline. To statistically determine drug-induced changes in neurotransmitter and metabolite levels, a linear mixed-model was utilized. The linear mixed-models calculated specific t and p values for each time point as well as conducted an omnibus F test. F values were reported to examine overall main effects, whereas t values were reported to assess differences for specific time points and are corrected for multiple comparisons. Significance of each time point was determine when calculated t values crossed the threshold of the critical t value, which corresponded to an α level of 0.05. Degrees of freedom were adjusted based on the distance between comparisons in this model. All statistical analyses were conducted using SPSS version 19 for Windows, graphs were generated in Graph Pad Prism and Adobe Illustrator. All data are presented as mean ± SEM and an α level of 0.05 was used for determining statistical significance (*) for all analyses. An α level of 0.09 was used to indicate trending significance (#).
Histology
Following experiments, carbon-fiber FSCV electrodes were removed and rats were euthanized with ketamine (0.2 ml; i.v.). In FSCV subjects, an electrolytic lesion was made to mark the location of the recording electrode using a tungsten electrode at the location of the recording site through the guide cannula at the microdrive setting used during testing (Aragona et al., 2008). Probe placement for microdialysis studies could be determined easily with light microscopy upon removal of the probe and the same histological preparations. Brains were removed and post-fixed in formalin. Brains were sectioned into 40-50 μm coronal sliced on a cryostat and stained with cresyl violet to verify electrode placement.
RESULTS
Dramatic differences in dopamine concentration evoked by morphine and oxycodone
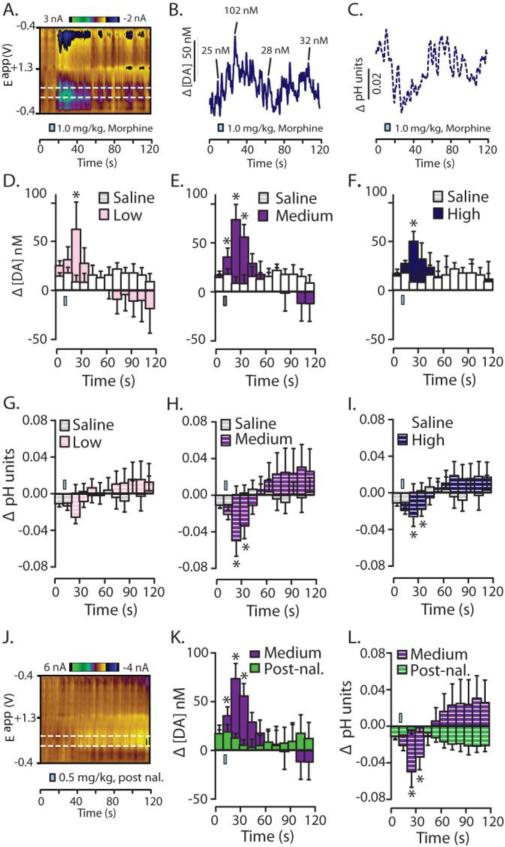
Detection of naturally-occurring transients is necessary for accurate FSCV measurements of pharmacologically-induced changes in DA transmission (Wightman et al., 2007); therefore, carbon-fiber electrodes were lowered into the NAc core or shell to locations where both electrically-stimulated DA release and naturally-occurring phasic release events (i.e., ‘DA transients’). A representative color plot (Figure 2A) and corresponding traces (Figures 2B and C) demonstrate that i.v. infusion of morphine results in a rapid increase in [DA] (Figures 2B) and an acidic shift in pH (Figures 2C).
Figure 2.
Average acute changes in dopamine concentration and local pH in the NAc evoked by i.v. morphine. A. A representative color plot shows increased DA transmission (top white line) and an acidic shift in local pH (bottom white line) following i.v. administration of the high dose of morphine (1.0 mg/kg). B. Current was converted into [DA] and trace is representative of the peak DA oxidation potential. Select DA transients were identified and their respective amplitudes are given. C. Similarly, current from the representative color plot was converted into changes in pH units and the pH trace was taken from the lower dotted white line on the color plot. D. – I. Change in [DA] and pH units were binned every 10 s across a 120 s sampling window for statistical comparison to pre-infusion values. D – F. Changes in [DA] during infusions of cumulatively increasing doses of morphine compared to control infusions of saline. D. Following the low morphine dose (0.1mg/kg; i.v.), [DA] was increased in the second time bin. E. Following the medium morphine dose (0.5 mg/kg; i.v.) [DA] was increased in the first, second, and third time bin. F. As with the low dose, following the high morphine dose (1.0 mg/kg; i.v.) [DA] was only increased during the second time bin. G. – I. Changes in pH units following infusions of cumulatively increasing doses of morphine compared to infusions of saline. G. The low dose of morphine did not significantly alter pH units. H. Following the medium dose of morphine there was a significant acidic shift in pH during the first, second, and third time bin following morphine infusion. I. Additionally, high dose morphine infusion also caused an acidic shift in local pH during the first, second, and third time bins. J. – L. To examine if MORs were dependent on the rapid neurochemical changes following morphine delivery, the most effective dose of morphine was chosen (0.5 mg/kg) to examine rapid changes [DA] and pH following blockade of MORs via pre-treatment with the MOR-preferring antagonist, naloxone (3.0 mg/kg; i.v.; given 15 min prior to morphine infusion). J. A representative color plot demonstrating that following naloxone pre-treatment, morphine-evoked increases in DA transmission and the acidic shift in pH are eliminated. K. Quantitatively, while the medium dose of morphine (0.5 mg/kg) significantly increased [DA] following a saline pre-treatment, this effect was abolished if morphine infusion was preceded by naloxone. L. Naloxone pretreatment also abolished morphine-evoked changes in pH. Error bars indicate SEM. * indicates statistically significant increases (p < 0.05). The i.v. infusion is indicated by a blue rectangle which is accurate according to the time and duration of saline or drug infusion.
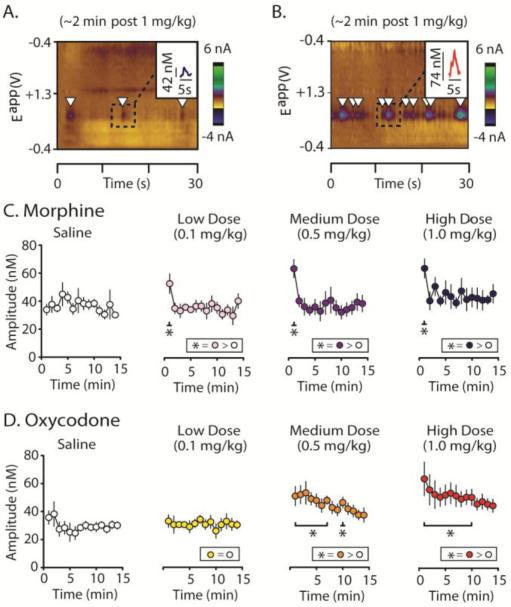
To quantify these data, [DA] was binned every 10 s and drug-evoked changes were compared to those following control infusions of saline. The low dose (0.1 mg/kg) infusion of morphine significantly elevated [DA] only within the first bin immediately following drug infusion (Figure 2D; t(79.149) = 2.133, p = 0.036). Following the medium dose (0.5 mg/kg), morphine evoked significant increases in [DA] for the first (t(76.088) = 2.491, p = 0.015), second (t(81.698) = 5.591, p < 0.001), and third (t(83.963) = 3.229), p = 0.002) bins following drug infusion (Figure 2E). As with the low dose, the high dose (1.0 mg/kg) of morphine evoked increases in [DA] only within the second bin following drug infusion (Figure 2F; t(69.705) = 3.587, p = 0.001).
To test if these increases in DA release were mediated by activation of MORs, we conducted an additional experiment in which subjects were pretreated subjects with the MOR antagonist naloxone (3.0 mg/kg) prior to morphine infusion. For this test, the medium dose of morphine (i.e., the dose that evoked the most robust effects, 0.5 mg/kg) was used. Administration of the MOR preferring antagonist, naloxone, eliminated the morphine-evoked increases in [DA] as demonstrated in the color plot (Figure 2J) as well as quantification of the data using the same 10 s binning (Figure 2K; F(11, 53.572) = 1.264, p = 0.270)
In addition to measuring real-time DA changes, these voltammetric recording conditions also measure local changes in pH (See Figure 1E and G) (Takmakov et al., 2010; Badrinarayan et al., 2012). Our FSCV studies show that opioids, including oxycodone (below), morphine, and remifentanil (Vander Weele et al., 2011), all evoke an immediate acidification in local pH. This novel observation may reflect an increase in carbon dioxide blood concentration due to opioid-induced respiratory depression (Thompson et al., 1995; Takmakov et al., 2010). The low dose drug infusion did not alter pH (Figure 2G; p > 0.05 all time points). However, following both the medium and high doses, morphine caused significant acidification during the second and third time bin following drug infusion (Figure 2H; medium dose, t(81.234) = -3.681, p < 0.001 and Figure 1I; t(81.416) = -2.137, p = 0.001). Similar to its effects on DA, pre-exposure to naloxone eliminated acidic shifts in pH (Figure 2J and Figure 2L; F(11, 36.642) = 0.950, p = 0.506).
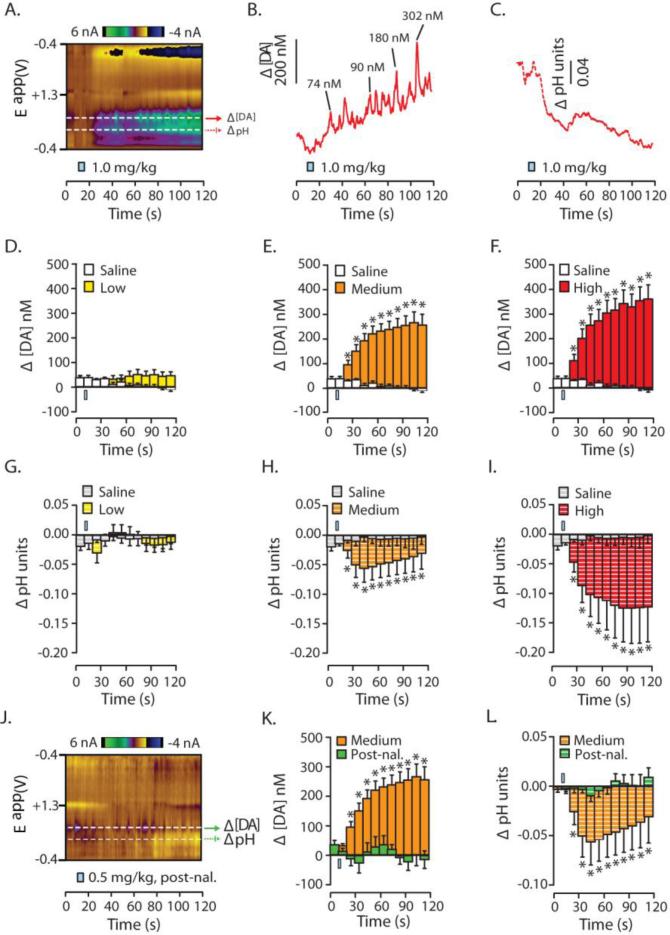
In contrast to morphine, oxycodone caused more robust acute changes in [DA] and acidic shifts in pH as can be seen by inspection of the representative color plot and corresponding traces (Figure 3A-C). The dose of oxycodone that evoked the greatest response (1 mg/kg) caused a dramatic increase in [DA], that is composed of both a gradual rise in concentration and a robust increase in DA transients superimposed upon this rise (Figure 3B). These representative examples also show the robust pH acidification (Figure 3C). Unlike morphine, responses following oxycodone were highly dose-dependent. Low dose administration of oxycodone did not significantly elevate [DA] (Figure 3D; F(11, 73.847) = 1.495, p = 0.152). However, both medium (Figure 3E; F(11, 75.752) = 14.653, p < 0.001) and high (Figure 3F; F(11, 75.752) = 14.653, p < 0.001) doses of oxycodone significantly increased [DA] in the second time bin following drug infusion and [DA] continued to rise throughout the recording period (Figure 3E,F).
Figure 3.
Average acute changes in dopamine concentration and local pH in the NAc evoked by i.v. oxycodone. A. A representative color plot shows increased DA transmission and an acidic shift in local pH following i.v. administration of the high dose of oxycodone (1.0 mg/kg). B. Current was converted into [DA] and the trace shown represents data gathered from the peak DA oxidation potential (see top white dotted line in 6A). Select DA transients are identified and their respective amplitudes given. C. Similarly, current from this color plot was converted into changes in pH units and the corresponding trace (taken from the lower dotted white line in 6A) is shown. D – I. [DA] and pH were binned every 10 s across the 120 s sampling window for comparison to pre-infusion values. D – F. Changes in [DA] during infusions of cumulatively increasing doses of oxycodone compared to control infusions of saline. Oxycodone-evoked increases are dose-dependent and doses that caused increases, resulting in robust and long lasting changes: D. The low oxycodone dose (0.1mg/kg; i.v.; yellow), caused no change in [DA]. E. The medium oxycodone dose (0.5 mg/kg; i.v.; orange) caused a significant increase in [DA] beginning in the second time bin and the increase lasted for the entire recording period. F. The high oxycodone dose (1.0 mg/kg; i.v.; red) caused the same significant increase in [DA] as the medium dose, from the second time bin to the end of the recording period. G – I. Changes in pH following infusions of cumulatively increasing doses of oxycodone compared to infusions of saline (doses; low to high = same colors except with white stripes): G. The low oxycodone dose (0.1mg/kg; i.v.) caused no change in local pH levels. H and I. Conversely, the medium (0.5 mg/kg; i.v.) and high (1.0 mg/kg; i.v.) oxycodone doses caused a significant acidic shift in pH from the first time bin through the remainder of the sampling period. J – L. The effects of oxycodone following pretreatment of either control infusions of saline or infusions of the MOR-preferring antagonist, naloxone (3.0 mg/kg; i.v.). J. A representative color plot demonstrating that following naloxone pre-treatment, oxycodone-evoked increases in DA transmission and the acidic shift in pH are eliminated. K. Quantitatively, while the medium dose of oxycodone (0.5 mg/kg; i.v.) significantly increased [DA] following a saline pre-treatment. However, when oxycodone delivery was preceded by naloxone it no longer increased [DA]. L. Similar to DA, naloxone pretreatment prevented oxycodone-induced changes in acidic pH. Error bars indicate SEM. * indicates statistically significant increases (p < 0.05). The i.v. infusion is indicated by a blue rectangle which is accurate according to the time and duration of saline or drug infusion.
Similar to its effects of DA, the low dose infusion of oxycodone did not alter pH (Figure 3G; p > 0.05 all time points). However, both medium and high doses of oxycodone caused robust acidic pH shifts beginning at the second recording bin and lasting throughout the recording period (Figure 3H,I; p < 0.05 at the aforementioned time points). As with morphine, naloxone pre-treatment eliminated oxycodone-induced increases [DA] and acidic shifts in pH, which is evident from the representative color plot (Figure 3J) and time averaged data (Figure 3K,L; F(11, 20.194) = 1.615, p = 0.169).
Morphine and oxycodone differentially alter phasic dopamine release events
Thus far we have shown that morphine and oxycodone cause very different changes in [DA] immediately following drug infusion. In addition to overall changes in [DA], FSCV allows for examination of distinct neurotransmission components that contribute to overall changes [DA] which are approximated by analyzing DA ‘transients’ (phasic surges in [DA]). Specifically, 1) the occurrence of phasic DA release events can be approximated by measuring transient frequency, 2) the magnitude of DA released per phasic event is estimated by transient amplitude, and 3) the duration of increases of DA into the extracellular space is measured by transient half-width. Therefore, we next analyzed transient frequency, amplitude, and half-width during the 15 min following each infusion to elucidate the contribution of these specific neurotransmission components for opioid-evoked increases in [DA].
Transient Frequency
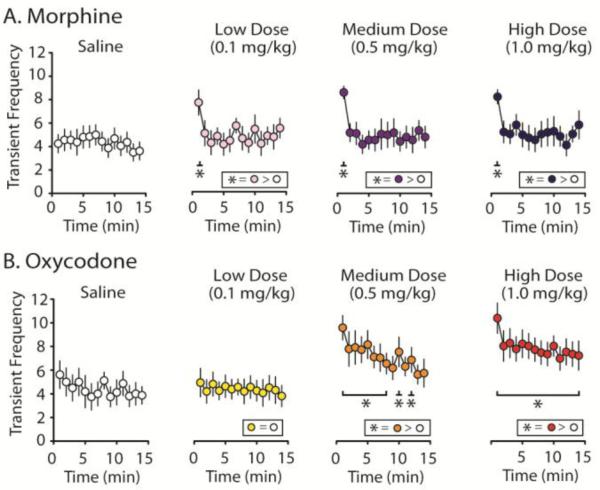
Similar to previous studies, control infusions of saline did not produce changes in transient frequency for morphine (Figure 4A; F(14, 82.910) = 1.095, p = 0.374) or oxycodone (Figure 4B; F(14, 76.585) = 1.806, p = 0.053). However, following morphine delivery, transient frequency was significantly increased above saline levels for the first minute after drug infusion, but not thereafter, for all doses tested (Figure 4A; main effect of time: low dose, F(14, 63.338) = 4.774, p < 0.001; medium dose, F(14, 68.048) = 7.297, p < 0.001; high dose, F(14, 58.068) = 4.895, p < 0.001; first min after infusion: low dose, t(76.067) = 2.762, p = 0.007; medium dose, t(81.234) = 6.772, p < 0.001; high dose, t(71.550) = 5.099, p < 0.001). In contrast to morphine, min-by-min analysis of transient frequency following oxycodone delivery shows a dose-dependent increase in transient frequency with the low dose causing no increase (Figure 4B; F(14, 65.414) = 0.541, p = 0.899), the medium dose increased transient frequency for most time points (Figure 4B; F(14, 62.633) = 8.453, p < 0.001), and the high dose significantly increased transient frequency over saline controls for the entire 15-min recording period (Figure 4B; F(14, 60.880) = 8.103, p < 0.001). Thus, given that transient frequency is an approximation of the number of phasic DA release events, these data strongly suggest that oxycodone, but not morphine, causes an increase in the occurrence of phasic surges of high concentration DA release events in the NAc.
Figure 4.
Dopamine transient frequency following administration of morphine or oxycodone. A. Histology for both morphine subjects (n = 8) recorded from the NAc core (n = 4) or shell (n = 4) and oxycodone subjects (n = 8) - NAc core (n = 4) or shell (n = 4) - were recorded from similar locations within the NAc. Phasic DA transmission data did not differ significantly between the core and shell so data were collapsed. B. The average number of transients per min across the 15 min recording interval are reported following saline controls and cumulatively higher doses of morphine or oxycodone (Low = 0.1 mg/kg, Medium = 0.5 mg/kg, High = 1.0 mg/kg; i.v.). C and D. Transient events were binned every min for the duration of the 15 min recording intervals following each drug dose and compared to the average post-saline frequency. C. Transient data per min following morphine infusions. While morphine failed to affect transient frequency across the entire 15 min recording period, all doses of morphine elicited a significant increase in transient frequency during the first minute following drug infusion compared to saline controls. D. Oxycodone-evoked increases in DA transient frequency were highly dose dependent. While the low dose infusion of oxycodone did not increase transient frequency, the medium dose increased DA transients for most time points and infusion of the high dose of oxycodone elevated transient frequency for the entire duration of the recording period. Error bars indicate SEM. * indicates statistically significant increases (p < 0.05).
Transient Amplitude
Representative color plots taken approximately 2 minutes following morphine (Figure 5A) and oxycodone (Figure 5B) infusions demonstrate the robust differences transient amplitudes following delivery of these different opioids with oxycodone evoking higher concentration transients compared to morphine and this is further illustrated by the insets of representative transients within these color plots (Figure 5A and 5B). Again, for statistical analysis, mean amplitudes were binned across 1-min time periods (Figure 5C and D). For both morphine (Figure 5C; F(14, 65.061) = 0.848, p = 0.616) and oxycodone subjects (Figure 5D; F(14, 65.107) = 1.394, p = 0.182), control saline infusions did not significantly alter mean transient amplitude compared to the baseline period. As with transient frequency, morphine caused an increase in transient amplitude within the first min immediately following drug infusion for all doses (low dose, t(61.269) = 2.237, p = 0.029; medium dose, t(89.211) = 1.314, p < 0.001; high dose, t(65.760) = 2.505, p = 0.015) but not thereafter (Figure 5C). However, oxycodone dose-dependently increased transient amplitude: the low dose of oxycodone failed to increase transient amplitude (Figure 5D; F(14, 58.642) = 0.608, p = 0.847), the medium increased amplitude for 7 min (Figure 5D; main effect, F(14, 70.005) = 2.268, p = 0.013), whereas the high dose significantly increased amplitude for 11 min (Figure 5D; main effect, F(14, 75.438) = 2.9905, p < 0.001).
Figure 5.
Dopamine transient amplitude following administration of morphine or oxycodone. A. Average transient amplitude over the 15 min recording periods following saline controls and groups receiving saline plus cumulative doses of either morphine or oxycodone (Low = 0.1 mg/kg, Medium = 0.5 mg/kg, High = 1.0 mg/kg; i.v.). While morphine does not evoke an overall change in transient amplitude during the entire 15 min recording intervals, both medium and high doses of oxycodone caused a significant increase in amplitude above saline controls. B. A representative color plot showing a recording ~2 min following a high dose morphine infusion within the NAc shell, and C. a similar recording following a high dose oxycodone infusion. B. and C. Insets show a zoomed in inspection of individual transients and provides their amplitudes. D. and E. Transient events were binned every min for the duration of the 15 min recording interval and compared to the average amplitude post-control saline infusion. D. As with transient frequency, morphine-evoked increases in DA transient amplitude measured across 1 min time bins shows that regardless of whether subjects received low, medium or high doses of morphine, transient amplitude was significantly increased above levels of those gathered from saline controls for only the first min following morphine infusion. E. Oxycodone-evoked increases in DA transient amplitude were dose-dependent. The low dose had no effect on transient amplitude; however, both medium and high doses of oxycodone elevated transient amplitude for the majority of the recording period. Error bars indicate SEM. * indicates statistically significant increases (p < 0.05).
Transient Half-Width
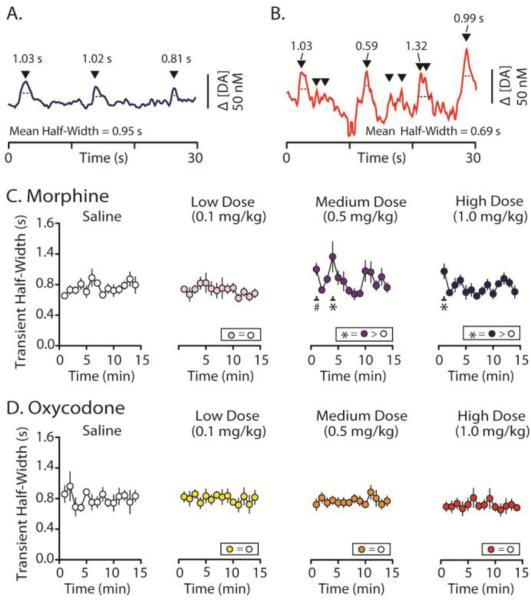
In contrast to transient frequency and amplitude, neither opioid robustly altered transient half-width (Figure 6). Example [DA] traces in Figure 6 are taken from the color plots shown in Figure 5. Inspection of individual traces following morphine (Figure 6A) and oxycodone delivery (Figure 6B), which include representative half-width values (as well as the mean for the trace), show that neither opioid substantially alters DA uptake, as determined by transient half-width values. For quantification of the data, data were binned min-by-min in the same manner as was done for transient frequency and amplitude. This analysis shows that while saline and low dose morphine infusions did not significantly increase transient half-width, the medium dose of morphine significantly increased half-width at the 1 and 4 min time point (t(104.945) = 2.787; p = 0.006). There was also a significant increase 1 minute following delivery of the high dose (Figure 6D; t(84.328) = 2.121, p = 0.037). Conversely, oxycodone administration did not alter transient half-width at any time point across all doses tested (Figure 6E; low dose, F(14, 60.054) = 0.483, p = 0.933; medium dose, F(14, 64.918) = 0.862, p = 0.602; high dose, F(14, 67.861) = 0.673, p = 0.793). Thus, in contrast to transient frequency and amplitude, transient half width showed not robust changes following infusion of either opioids, suggesting that slowing DA uptake is not a mechanism by which opioids increase [DA] within the NAc.
Figure 6.
Dopamine transient half-width following administration of morphine or oxycodone. A. Average transient half-width over the 15 min recording periods following saline controls and groups receiving saline plus cumulative doses (Low = 0.1 mg/kg, Medium = 0.5 mg/kg, High = 1.0 mg/kg; i.v.) of either morphine or oxycodone. Neither the low nor the high doses of morphine significantly evoked an overall increase in transient half-width during the entire 15 min recording intervals. However, the medium dose of morphine caused a significant increase in half-width above saline controls. Conversely, all doses of oxycodone failed to alter mean transient half-width. B. A representative [DA] trace (that corresponds to the color plot shown in Fig 4B) showing the recording ~2 min following high dose morphine infusion within the NAc shell, and C. a similar [DA] trace following a high dose oxycodone infusion. D and E. Mean transient half-width was binned every min for the duration of the 15 min recording interval and compared to the average half-width post-control saline infusion. D. Morphine-evoked increases in DA transient half-width measured across 1 min time bins shows that only subjects that received low dose morphine infusion failed to increase transient half-width. Following medium and high dose infusions of morphine, transient half-width was significantly increased at the 3 min mark following the medium dose of morphine and during the first min following the high dose. There was a trend towards significance during the first min following the medium dose of morphine. E. At all tested doses, oxycodone failed to increase transient half-width. Error bars indicate SEM. * indicates statistically significant increases (p < 0.05).
Core-shell differences following oxycodone but not morphine delivery
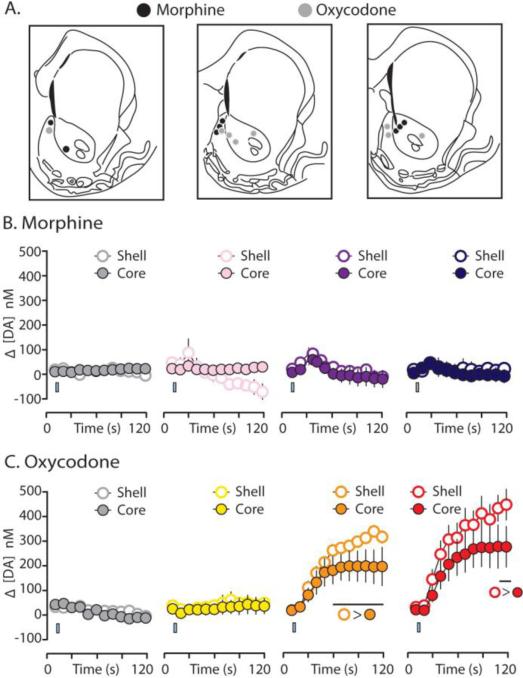
A common feature of the primary rewarding effects of drugs of abuse is that they evoke greater [DA] within the NAc shell compared to the NAc core (Pontieri et al., 1995; Ito et al., 2000; Frank et al., 2008). To determine whether oxycodone and morphine evoke greater [DA] in the shell than the core, we conducted one half of our FSCV measurements in the NAc core and the other half in the NAc shell (Figure 7A). While our previous studies using FSCV to examine cocaine-evoked DA transmission showed dramatic core/shell differences (Aragona et al., 2008; Aragona et al., 2009), the present study revealed only modest core/shell differences following opioid delivery. Indeed, morphine administration, at all doses tested, produced nearly equivalent increases in [DA] across the NAc core and shell (Figure 7A; main effect of subregion: low dose, F(1, 8.427) = 1.648, p = 0.233; medium dose, F(1,7.866) = 0.519, p = 0.492; high dose, F(1, 6.839) < 0.001, p = 0.984). However, oxycodone delivery resulted in a modest, but significantly higher, increase in [DA] within the NAc shell following delivery of the medium dose (from 60 to 120 s (Figure 7B; t(71.577) = -2.302, p = 0.024 for bin 6, and continuing p <0.001 for bins 7-12) and the high dose of oxycodone (during the last two time bins (100 to 120 s), t(65.738) = 2.060, p = 0.043, and t(63.350) = 2.197, p = 0.032). In addition to causing a greater increase in [DA] in the NAc shell, the medium and high (but not the low) doses of oxycodone caused greater increases in the frequency and amplitude of DA transients within the NAc shell compared to the core (Table 1).
Figure 7.
Oxycodone- and morphine-evoked changes in [DA] in the core and shell subregions of the NAc. A. Electrode placements in the core and shell. B. [DA] for morphine subjects were binned every 10s across the 120 s sampling window encompassing i.v. infusions (from left to right: saline; 0.1 mg/kg; 0.5 mg/kg; 1.0 mg/kg). Morphine did not significantly elevate [DA] in the NAc shell [open circles] compared to the core [closed circles]. C. Again, [DA] for oxycodone subjects were binned every 10s across the 120 s sampling window encompassing i.v. infusions (from left to right: saline; 0.1 mg/kg; 0.5 mg/kg; 1.0 mg/kg). Oxycodone did not significantly elevate [DA] in the NAc shell [open circles] compared to the core [closed circles] at the low 0.1 mg/kg dose. Conversely, oxycodone caused a modest, but significant increase in [DA] within the NAc shell compared to the core at the medium (0.5 mg/kg) and high (1.0 mg/kg) doses. Error bars indicate SEM. The i.v. infusion is indicated by a blue rectangle which is accurate according to the time and duration of saline or drug infusion.
Table 1.
Frequency and amplitude of DA transients in the NAc shell versus the core following i.v. oxycodone.
| Transient Frequency | Transient Amplitude | |||
|---|---|---|---|---|
| Shell | Core | Shell | Core | |
| Low | 5.6 ± 1.1 | 3.3 ± 1.1 | 34.2 ± 4.5 | 31.2 ± 2.1 |
| F(1,18) = 2.690, p = 0.118 | F(1,16) = 0.272, p = 0.609 | |||
| Medium | 9.5 ± 0.3 | 5.0 ± 1.1 | 54.3 ± 3.4 | 35.9 ± 2.0 |
| F(1,18) = 11.234, p = 0.004 | F(1,16) = 12.010, p = 0.003 | |||
| High | 10.2 ± 0.5 | 5.8 ± 4.0 | 65.3 ± 5.4 | 38.0 ± 4.0 |
| F(1,18) = 10.580, p = 0.004 | F(1,16) = 22.649, p < 0.001 | |||
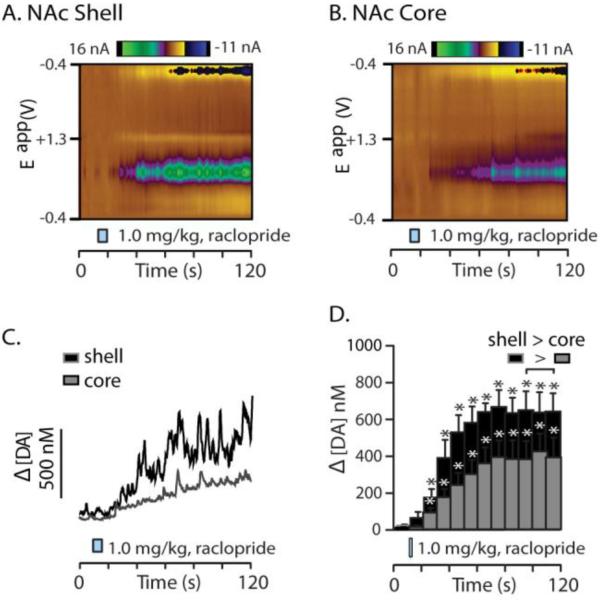
A positive control to test for electrode fidelity was the infusion of the DA autoreceptor blocker raclopride at a dose known to induce burst firing and forebrain DA release (1.0 mg/kg) (Andersson et al., 1995). Consistent with previous studies, [DA] levels were significantly higher within the NAc shell compared to the NAc core across multiple time points (Figure 8C,F; t(116.807) = -2.043, p = 0.043; t(107.583) = -2.112, p = 0.037; t(99.064) = -2.601, p = 0.011). Thus, these data demonstrate that drugs that cause DA neuron firing, presumably, equally into the NAc core and shell result in a more modest preferential enhancement of [DA] within the NAc shell which is likely due to reduced uptake in the NAc shell compared to the core (Aragona et al., 2008).
Figure 8.
Raclopride evokes greater dopamine concentration within the NAc shell compared to the NAc core. A. A representative color plot of increased DA transmission within the NAc shell following i.v. raclopride infusion (1.0 mg/kg; which followed the opioid infusions described above). B. The corresponding [DA] trace associated with the color plot in 7A (taken from the same point in the voltage ramp as the figures described above). Upon visual inspection of the trace, it is clear that there are many DA transients superimposed on the slower rise in [DA] following raclopride infusion. C. [DA] is significantly, and robustly, elevated in the second time bin following raclopride infusion and this lasted the entire recording period. D. A representative color plot is shown from the NAc core and E. the resulting [DA] trace associated with this color plot shows that autoreceptor blockade also causes an increase in DA transients superimposed on a gradual rise in [DA]. F. Quantitative data of raclopride-evoked increases in [DA] within the NAc core show that autoreceptor blockade also significantly increases [DA] in the core. Despite the similarities in the nature of raclopride-evoked increases in phasic DA release, increased [DA] in the shell is significantly higher compared to that evoked within the NAc core from 30 to 120 s following raclopride infusion. Error bars indicate SEM. * indicates statistically significant increases (p < 0.05).
Rapid microdialysis with mass spectrometry reveals coincident peaks in dopamine and GABA concentration following morphine, but not oxycodone, delivery, consistent with the FSCV results
The sub-second measurements provided by FSCV described above reveal that morphine and oxycodone evoke very different phasic patterns in DA transmission in the NAc. However, FSCV is optimized for measuring phasic changes in DA and pH. Therefore we employed rapid microdialysis coupled with HPCL-MS (1-min sampling resolution) to measure of 16 compounds following morphine and oxycodone delivery (Figure 9A,B). Another limitation of FSCV is that the background subtraction results in the inability to measure changes in [DA] over long periods of time (i.e., over many minutes). Again, microdialysis with HPLC-MS allows min-by-min changes after drug delivery for many chemical species (Figure 9A,B) (Song et al., 2012).
With respect to DA transmission within the NAc (Figure 9C) following opioid delivery, the microdialysis HPLC-MS data are consistent with the FSCV data shown above. While saline infusions (i.v.) did not significantly alter [DA] (Figure 9D), i.v. infusion of oxycodone produced a significant increase in [DA] that lasted the duration of the recording period compared to baseline and saline infusion levels (Figure 9D; main effect, F(40, 69.766) = 1.693, p = 0.027). In contrast, the significant morphine increase was brief: [DA] was elevated only during the first minute immediately following drug administration (t(107.996) = 5.303, p <0.001). Finally, we used a positive control to demonstrate the validity of these rapid dialysis measures. A combination of the D2 DA receptor antagonist raclopride and the DA transporter blocker (cocaine) which has been shown to cause a synergistic increase in [DA] (Rouge-Pont et al., 2002; Aragona et al., 2008) was administered. Consistent with these previous dialysis studies, cocaine in the presence of raclopride caused an extremely large increase in DA release (Figure 9E; p < 0.05 min 70-76).
Changes in concentrations of DA metabolites are consistent with the morphine/oxycodone differences observed for [DA] (Figure 9F,G,H). Specifically, oxycodone, but not morphine, caused increases in the DA metabolites 3MT (Figure 9F; oxycodone, F(9, 14.670) = 2.596, p = 0.051; morphine, F(9, 5.623) = 0.879, p = 0.589), HVA (Figure 9G; last two oxycodone bins, respectively: t(23.020) = 2.645, p = 0.014, t(22.939) = 3.079, p = 0.005; morphine, F(9, 11.182) = 1.030, p = 0.473), and DOPAC (Figure 9H; last two oxycodone bins respectively, t(29.887) = 2.080, p = 0.046, t(29.887) = 2.510, p = 0.018; morphine, F(9, 12.910) = 0.325, p = 0.951).
Microdialysis with HPLC-MS provides the opportunity to investigate simultaneous changes in multiple neurotransmitter concentrations following morphine and oxycodone. As described above, morphine, but not oxycodone, caused a brief increase in [DA] that rapidly returned to baseline and this result was observed using both FSCV and microdialysis (see above). It is therefore interesting that morphine, but not oxycodone, delivery produced a coincident increase in GABA concentration during the same time point of the initial peak in [DA] following drug delivery but not thereafter (Figure 9I; t(29.99) = 3.218, p = 0.003). Given GABA's inhibitory role in neurotransmission, this increase in GABA concentration following morphine may be responsible for DA returning to baseline immediately after its initial peak following delivery. This brief morphine-evoked increase in GABA within the NAc is likely involved in explaining how these two opioids differentially mediate reward.
In contrast to GABA, there was no significant change in glutamate concentration following delivery of either drug except for a trend for an increase toward the end of the sampling period following morphine delivery (Figure 9J). Additionally, there was a significant and sustained decrease in taurine concentration following morphine (F(9, 9.855) = 3.412, p = 0.035) but not oxycodone (F(9, 18.410) = 0.521, p = 0.840) delivery (Figure 9K). This decrease in taurine is interesting because it is consistent with previous studies showing that alcohol administration also alters accumbens taurine levels (Dahchour et al., 1994).
Regarding the remainder of the analytes detected, there were no statistically significant differences in neurotransmission between morphine and oxycodone delivery (data not shown), and neither drug caused statistically significant differences in detected dialysate levels of acetylcholine (oxycodone, F(9, 27.757) = 1.977, p = 0.081; morphine F(9, 10.732) = 0.953, p = 0.522), serotonin (oxycodone, F(9, 6.311) = 0.381, p = 0.908; morphine, F(9, 4.945) = 1.170, p = 0.455), the serotonin metabolite 5HIAA (oxycodone, F(9, 6.173) = 0.553, p = 0.797; morphine, F(9, 11.631) = 1.043, p = 0.463), norepinephrine (oxycodone, F(9, 17.258) = 0.658, p = 0.735; morphine, F(9, 11.545) = 0.429, p = 0.894), the norepinephrine metabolite normetanephrine (oxycodone, F(9, 17.326) = 0.964, p = 0.500; morphine, F(9, 6.143) = 0.805, p = 0.630), serine (oxycodone, F(9, 17.352) = 1.012, p = 0.467; morphine, F(9, 13.201) = 0.711, p = 0.691), adenosine (oxycodone, F(9, 16.407) = 0.297, p = 0.965; morphine, F(9, 11.320) = 1.253, p = 0.354), histamine (oxycodone, F(9, 5.829) = 1.509, p = 0.321; morphine, F(9, 4.971) = 0.838, p = 0.616), or aspartate (oxycodone, F(9, 18.268) = 1.217, p = 0.343; morphine, F(9, 12.052) = 0.943, p = 0.525) (data represented in heat maps in Fig. 9B).
DISCUSSION
Prescription opioid abuse is a rapidly growing epidemic (Compton & Volkow, 2006; McCabe et al., 2013b). In particular, oxycodone is among the most commonly abused opioids (McCabe et al., 2013a; b). While there is no doubt that greater availability of this prescription drug has contributed to this rise in abuse, there is growing evidence suggesting that oxycodone may be more potent with respect to its ability to promote addictive behaviors (Stoops et al., 2010, Comer et al. 2013, Emery et al., 2013). For example, in the clinical realm, oxycodone administration to human heroin addicts, compared to other opioids, caused robust reinforcing effects with no increases in negative effects (Comer et al., 2008). Further, in the same study, a verbal report from a heroin-dependent individual stated that oxycodone is the ‘Rolls Royce’ of opioids and that it produces a ‘smooth’ high” (Comer et al., 2008). While recent studies have begun to investigate the influence of oxycodone on behavior and brain processes (Zhang et al., 2009; Niikura et al., 2013; Mayer-Blackwell et al., 2014; Zhang et al., 2014), it has remained unclear if this drug differentially alters neurotransmission associated with reward and motivation compared to other opioids. Here, we demonstrate that DA transmission within the NAc, a key neurobiological component of motivation, is starkly different following oxycodone compared to morphine delivery. Moreover, we reveal that morphine delivery is associated with a coincident surge in DA and GABA concentration immediately following drug delivery in the NAc. This increase in GABA concentration observed following morphine, but not oxycodone, may explain why [DA] quickly returns to baseline levels following delivery of morphine but not oxycodone.
Using two rapid neurochemical measurement technologies, we reveal robust oxycodone-evoked increases in [DA]. The present FSCV experiment detected that [DA]s achieve levels as high as 500nM in the NAc shell, and the microdialysis study shows that oxycodone-evoked increase in [DA] is stable and long lasting. Indeed, drug-evoked increases in [DA] as well as DA transients remain elevated throughout the 15-min recording period. Conversely, both the microdialysis and FSCV experiments reveal that morphine evokes an initial, rapid increase in [DA] only during the first min following drug infusion. Importantly, this morphine-evoked increase in [DA] was coincident with an increase in GABA concentration, which was also elevated only within the first 1 min time bin following drug infusion.
Greater increases in [DA] following oxycodone, versus morphine, delivery was also associated with a greater frequency and amplitude of phasic DA release events (DA transients) but was not associated with slowed reuptake as measured by transient half-width. DA transients originate from burst firing of DA containing neurons within the midbrain (Aragona et al., 2008; Sombers et al., 2009; Owesson-White et al., 2012). This finding that oxycodone caused a dramatic increase in DA transients is of interest because strong arguments continue to be made that the ability of a drug to potentiate DA transients is closely associated with a drug's potential risk for addiction (Covey et al., 2014).
Despite both being MOR agonists, morphine and oxycodone have distinct pharmacokinetic and pharmacodynamic properties that may contribute to their different effects on DA transmission (Nielsen et al., 2007; Lemberg et al., 2009). Although oxycodone has a lower affinity for the MOR than morphine (Chen et al., 1991; Lalovic et al., 2006), oxycodone crosses the blood-brain barrier more readily than morphine (Villesen et al., 2006), and unbound concentrations of oxycodone are higher in the brain than in the blood following i.v. administration, suggesting that oxycodone is actively transported across the blood-brain barrier (Boström et al., 2006; Villesen et al., 2006; Boström et al., 2008). Indeed, for the same unbound concentrations of oxycodone and morphine in the blood, unbound oxycodone in the brain is as much as six times higher than morphine (Boström et al., 2008). Additionally, ligand-directed signaling could contribute to differences in drug-evoked DA transmission, since morphine and oxycodone have different intrinsic efficacies and receptor internalization profiles (Lester & Traynor, 2006; Peckham & Traynor, 2006; Melief et al., 2010). Finally, morphine and oxycodone have different metabolic pathways, and the actions of their respective metabolites differ, which may potentially contribute to differential drug-evoked [DA]s (Kalso, 2007). Future studies are needed to investigate the roles of these different pharmacokinetic and pharmacodynamic properties of morphine and oxycodone on altering DA transmission.
Mechanistically, since the coincident increase in GABA concentration was associated with morphine, but not oxycodone, delivery, it is an intriguing possibility that the surge in GABA could be directly related to the rapid return of DA to its baseline. Meaning, GABA may mediate the inhibitory mechanism which rapidly shuts down the initial morphine-evoked increases DA transmission. Thus, the present neurochemical data provide a potential mechanism regarding differences in opioid-evoked changes in [DA] between morphine and oxycodone. There are at least two possible mechanisms whereby GABA inhibits DA transmission in the NAc. First, since local perfusion of morphine into the NAc increases GABAergic signaling (Sun et al., 2011), it is possible that increased GABA levels in the NAc may activate GABAA receptors on DA terminals and thereby reduce DA release (Aono et al., 2008; Saigusa et al., 2008). In contrast, oxycodone may promote greater MOR activation of GABAergic interneurons, reducing local GABA levels and subsequently disinhibiting DA release from terminals (Aono et al., 2008).
Second, the fact that morphine, but not oxycodone, increases GABA transmission within the NAc, may be due to increased morphine-induced firing of GABAergic neurons in the VTA projecting to the NAc (van Zessen et al., 2012). This is an intriguing possibility because previous studies have shown that a single injection of morphine can produce a conditioned place aversion in drug naïve rats (Parker et al., 2002) (but see (Fenu et al., 2006)). There are numerous anecdotal reports describing how opioids are initially aversive before they become rewarding, and this initial aversion may involve a GABAergic mechanism. Recent studies have carefully identified that place aversions are indeed mediated by activation of these GABAergic projection neurons from the VTA to the NAc (Tan et al., 2012). Regardless of the specific mechanism, the present neurochemical data provide novel explanatory power regarding why morphine has long been known to be initially aversive and may further explain why oxycodone is a preferred opioid (Comer et al., 2008).
Previous work has suggested that activation of MORs on GABAergic interneurons within the VTA deactivate these interneurons which results in the disinhibition of DA neuron firing, and increase in DA neuron burst firing, and elevated [DA] release from forebrain terminals (Gysling and Wang, 1983, Johnson and North, 1992, Matsui and Williams, 2011). However, there are additional mechanisms that remain to be elucidated since oxycodone, but not morphine, causes a substantial increase in DA transients, and previous studies have shown that morphine delivery causes an overall increase DA neuron firing without increasing burst firing (Gysling & Wang, 1983). Furthermore, local application of morphine into the VTA increases DA neuron firing rate that is subsequently followed by a decrease in firing rate (Matthews & German, 1984). These electrophysiology findings are consistent with the present data, except the aforementioned electrophysiology study did not report an initial morphine-induced burst of DA neuron activity that would coincide with the rapid morphine-evoked DA release observed in the first minute post-infusion. A likely explanation for this disconnect is that most of the electrophysiology experiments conducted decades ago only recorded from “traditional” DA neurons. Now, however, functionally and anatomically distinct sub-populations of DA neurons with different firing properties have been discovered (Ikemoto, 2007; Lammel et al., 2008; Lammel et al., 2011). Interestingly, a subset of DA neurons along the midline of the VTA are far more susceptible to bursting (Lammel et al., 2008) and therefore are a strong candidate to be the DA neurons responsible for this initial morphine-evoked DA release. Future studies are needed test this hypothesis.
As discussed above, it is possible that morphine-evoked increases in GABA concentration mediate early aversive processing upon initial morphine exposure. However, morphine can also be highly rewarding. Indeed, activation of MORs can promote consumption of palatable foods and has been linked to pleasure (Will et al., 2003; Kelley, 2004; Berridge & Kringelbach, 2013). Therefore, future studies are needed to determine how increases in GABA concentrations in the NAc differentially contribute to morphine reward compared to oxycodone reward.
CONCLUSION
This is the first study to compare the initial rapid neurochemical changes in the NAc core and shell following delivery of two highly abused opioids, morphine and oxycodone. FSCV and rapid microdialysis HPLC-MS revealed that oxycodone caused a greater increase in [DA] in the NAc compared to morphine. Oxycodone robustly increased [DA] in both sub-regions of the NAc, albeit greater within the NAc shell. These increases lasted the entire recording period and in addition to increasing [DA], oxycodone robustly increased the frequency and amplitude of DA transients. However, with the exception of increasing DA metabolites, rapid microdialysis showed that oxycodone caused no appreciable change to other signaling molecules. Conversely, morphine caused a coincident surge in DA and GABA concentration within the first min following drug delivery but not thereafter. Yet, despite these differences both opioids are highly addictive when abused. As stated above, there is growing evidence that oxycodone appears to be a preferred opioid and its use continues to increase tremendously. Thus, it is essential to understand drugs differentially alter neurochemistry if we hope to provide relief to addicts and this begins by using in vivo tools, such as those described herein, to reveal how different drugs differentially impact brain signaling systems that powerfully takeover behavior.
Acknowledgements
The authors would like to thank Brenann E. Couturier, Myranda Bryan, Arif Hamid, and Bianca Jenkins for technical assistance; Shanna L. Resendez for helpful discussion; and Richard B. Keithley for analysis consultation and the MATLAB transient detection program.
Funding
This work was supported by NIDA PO1DA031656 (B.J.A.), R37 EB00330 (R.T.K.), and the Martha Muenzer Memorial Award (C.M.V.W.), and K.A.P.S. was supported by NIH T32 DA007267.
Abbreviations
- CV
cyclic voltammogram
- DA
extracellular dopamine concentration
- DA
dopamine
- FSCV
fast-scan cyclic voltammetry
- HPLC-MS
high performance liquid chromatography coupled with mass spectrometry
- i.v.
intravenous
- MOR
μ-opioid receptor
- NAc
nucleus accumbens
- VTA
ventral tegmental area
Footnotes
Disclosure
The authors declare no competing financial interests.
REFERENCES
- Andersson JL, Nomikos GG, Marcus M, Hertel P, Mathe JM, Svensson TH. Ritanserin potentiates the stimulatory effects of raclopride on neuronal activity and dopamine release selectivity in the mesolimbic dopaminergic system. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:374–385. doi: 10.1007/BF00172774. [DOI] [PubMed] [Google Scholar]
- Aono Y, Saigusa T, Mizoguchi N, Iwakami T, Takada K, Gionhaku N, Oi Y, Ueda K, Koshikawa N, Cools AR. Role of GABAA receptors in the endomorphin-1-, but not endomorphin-2-, induced dopamine efflux in the nucleus accumbens of freely moving rats. European journal of pharmacology. 2008;580:87–94. doi: 10.1016/j.ejphar.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–1899. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ. Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. J Neurosci. 2012;32:15779–15790. doi: 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Neuroscience of affect: brain mechanisms of pleasure and displeasure. Current opinion in neurobiology. 2013;23:294–303. doi: 10.1016/j.conb.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström E, Hammarlund-Udenaes M, Simonsson US. Blood–brain barrier transport helps to explain discrepancies in in vivo potency between oxycodone and morphine. Anesthesiology. 2008;108:495–505. doi: 10.1097/ALN.0b013e318164cf9e. [DOI] [PubMed] [Google Scholar]
- Boström E, Simonsson US, Hammarlund-Udenaes M. In vivo blood-brain barrier transport of oxycodone in the rat: indications for active influx and implications for pharmacokinetics/pharmacodynamics. Drug metabolism and disposition. 2006;34:1624–1631. doi: 10.1124/dmd.106.009746. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZR, Irvine RJ, Somogyi AA, Bochner F. Mu receptor binding of some commonly used opioids and their metabolites. Life sciences. 1991;48:2165–2171. doi: 10.1016/0024-3205(91)90150-a. [DOI] [PubMed] [Google Scholar]
- Comer SD, Metz VE, Cooper ZD, Kowalczyk WJ, Jones JD, Sullivan MA, Manubay JM, Vosburg SK, Smith ME, Peyser D, Saccone PA. Comparison of a drug versus money and drug versus drug self-administration choice procedure with oxycodone and morphine in opioid addicts. Behav Pharmacol. 2013;24:504–516. doi: 10.1097/FBP.0b013e328363d1c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. 2008;33:1179–1191. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Covey DP, Roitman MF, Garris PA. Illicit dopamine transients: Reconciling actions of abused drugs. Trends in neurosciences. 2014;37:200–210. doi: 10.1016/j.tins.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daberkow DP, Brown HD, Bunner KD, Kraniotis SA, Doellman MA, Ragozzino ME, Garris PA, Roitman MF. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J Neurosci. 2013;33:452–463. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, Quertemont E, De Witte P. Acute ethanol increases taurine but neither glutamate nor GABA in the nucleus accumbens of male rats: a microdialysis study. Alcohol Alcohol. 1994;29:485–487. [PubMed] [Google Scholar]
- Davis MP, Varga J, Dickerson D, Walsh D, LeGrand SB, Lagman R. Normal-release and controlled-release oxycodone: pharmacokinetics, pharmacodynamics, and controversy. Support Care Cancer. 2003;11:84–92. doi: 10.1007/s00520-002-0385-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenu S, Spina L, Rivas E, Longoni R, Di Chiara G. Morphine-conditioned single-trial place preference: role of nucleus accumbens shell dopamine receptors in acquisition, but not expression. Psychopharmacology (Berl) 2006;187:143–153. doi: 10.1007/s00213-006-0415-2. [DOI] [PubMed] [Google Scholar]
- Frank ST, Krumm B, Spanagel R. Cocaine-induced dopamine overflow within the nucleus accumbens measured by in vivo microdialysis: a meta-analysis. Synapse. 2008;62:243–252. doi: 10.1002/syn.20489. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysling K, Wang RY. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983;277:119–127. doi: 10.1016/0006-8993(83)90913-7. [DOI] [PubMed] [Google Scholar]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalso E. How different is oxycodone from morphine? Pain. 2007;132:227–228. doi: 10.1016/j.pain.2007.09.027. [DOI] [PubMed] [Google Scholar]
- Keithley RB, Heien ML, Wightman RM. Multivariate concentration determination using principal component regression with residual analysis. Trends Analyt Chem. 2009;28:1127–1136. doi: 10.1016/j.trac.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley RB, Wightman RM. Assessing principal component regression prediction of neurochemicals detected with fast-scan cyclic voltammetry. ACS Chem Neurosci. 2011;2:514–525. doi: 10.1021/cn200035u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience & biobehavioral reviews. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen L-Y, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: Role of circulating active metabolites*. Clinical Pharmacology & Therapeutics. 2006;79:461–479. doi: 10.1016/j.clpt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberg KK, Heiskanen TE, Kontinen VK, Kalso EA. Pharmacology of oxycodone: does it explain why oxycodone has become a bestselling strong opioid? Scandinavian Journal of Pain. 2009;1:S18–S23. [Google Scholar]
- Lester PA, Traynor JR. Comparison of the in vitro efficacy of μ, δ, κ and ORL1 receptor agonists and non-selective opioid agonists in dog brain membranes. Brain research. 2006;1073:290–296. doi: 10.1016/j.brainres.2005.12.066. [DOI] [PubMed] [Google Scholar]
- Matthews RT, German DC. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience. 1984;11:617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- Mayer-Blackwell B, Schlussman SD, Butelman ER, Ho A, Ott J, Kreek MJ, Zhang Y. Self administration of oxycodone by adolescent and adult mice affects striatal neurotransmitter receptor gene expression. Neuroscience. 2014;258:280–291. doi: 10.1016/j.neuroscience.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, West BT, Boyd CJ. Leftover prescription opioids and nonmedical use among high school seniors: a multi-cohort national study. J Adolesc Health. 2013a;52:480–485. doi: 10.1016/j.jadohealth.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, West BT, Boyd CJ. Medical use, medical misuse, and nonmedical use of prescription opioids: results from a longitudinal study. Pain. 2013b;154:708–713. doi: 10.1016/j.pain.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proceedings of the National Academy of Sciences. 2010;107:11608–11613. doi: 10.1073/pnas.1000751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael DJ, Joseph JD, Kilpatrick MR, Travis ER, Wightman RM. Improving data acquisition for fast-scan cyclic voltammetry. Anal Chem. 1999;71:3941–3947. doi: 10.1021/ac990491+. [DOI] [PubMed] [Google Scholar]
- Mierzejewski P, Koros E, Goldberg SR, Kostowski W, Stefanski R. Intravenous self-administration of morphine and cocaine: a comparative study. Pol J Pharmacol. 2003;55:713–726. [PubMed] [Google Scholar]
- Nestler EJ, Malenka RC. The addicted brain. Sci Am. 2004;290:78–85. doi: 10.1038/scientificamerican0304-78. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Ross FB, Lotfipour S, Saini KS, Edwards SR, Smith MT. Oxycodone and morphine have distinctly different pharmacological profiles: radioligand binding and behavioural studies in two rat models of neuropathic pain. Pain. 2007;132:289–300. doi: 10.1016/j.pain.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Niikura K, Ho A, Kreek MJ, Zhang Y. Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. Pharmacol Biochem Behav. 2013;110:112–116. doi: 10.1016/j.pbb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Roitman MF, Sombers LA, Belle AM, Keithley RB, Peele JL, Carelli RM, Wightman RM. Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem. 2012;121:252–262. doi: 10.1111/j.1471-4159.2012.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Cyr JA, Santi AN, Burton PD. The aversive properties of acute morphine dependence persist 48 h after a single exposure to morphine: evaluation by taste and place conditioning. Pharmacology Biochemistry and Behavior. 2002;72:87–92. doi: 10.1016/s0091-3057(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Peckham EM, Traynor JR. Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. Journal of Pharmacology and Experimental Therapeutics. 2006;316:1195–1201. doi: 10.1124/jpet.105.094276. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods Mol Med. 2003a;79:443–464. doi: 10.1385/1-59259-358-5:443. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003b;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter-Stransky KA, Wescott SA, Hershman M, Badrinarayan A, Vander Weele CM, Lovic V, Aragona BJ. Cocaine must enter the brain to evoke unconditioned dopamine release within the nucleus accumbens shell. Neurosci Lett. 2011;504:13–17. doi: 10.1016/j.neulet.2011.08.028. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli E. Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J Neurosci. 2002;22:3293–3301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigusa T, Aono Y, Mizoguchi N, Iwakami T, Takada K, Oi Y, Ueda K, Koshikawa N, Cools AR. Role of GABAB receptors in the endomorphin-1-, but not endomorphin-2-, induced dopamine efflux in the nucleus accumbens of freely moving rats. European journal of pharmacology. 2008;581:276–282. doi: 10.1016/j.ejphar.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Sinkala E, McCutcheon JE, Schuck MJ, Schmidt E, Roitman MF, Eddington DT. Electrode calibration with a microfluidic flow cell for fast-scan cyclic voltammetry. Lab Chip. 2012;12:2403–2408. doi: 10.1039/c2lc40168a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Mabrouk OS, Hershey ND, Kennedy RT. In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography-mass spectrometry. Anal Chem. 2012;84:412–419. doi: 10.1021/ac202794q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Hatton KW, Lofwall MR, Nuzzo PA, Walsh SL. Intravenous oxycodone, hydrocodone, and morphine in recreational opioid users: abuse potential and relative potencies. Psychopharmacology (Berl) 2010;212:193–203. doi: 10.1007/s00213-010-1942-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JY, Yang JY, Wang F, Wang JY, Song W, Su GY, Dong YX, Wu CF. Lesions of nucleus accumbens affect morphine-induced release of ascorbic acid and GABA but not of glutamate in rats. Addiction biology. 2011;16:540–550. doi: 10.1111/j.1369-1600.2010.00244.x. [DOI] [PubMed] [Google Scholar]
- Takmakov P, Zachek MK, Keithley RB, Bucher ES, McCarty GS, Wightman RM. Characterization of local pH changes in brain using fast-scan cyclic voltammetry with carbon microelectrodes. Anal Chem. 2010;82:9892–9900. doi: 10.1021/ac102399n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PI, Joel SP, John L, Wedzicha JA, Maclean M, Slevin ML. Respiratory depression following morphine and morphine-6-glucuronide in normal subjects. Br J Clin Pharmacol. 1995;40:145–152. [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Michael DJ, Wightman RM. Correlation of local changes in extracellular oxygen and pH that accompany dopaminergic terminal activity in the rat caudate-putamen. J Neurochem. 2003;84:373–381. doi: 10.1046/j.1471-4159.2003.01527.x. [DOI] [PubMed] [Google Scholar]
- Villesen HH, Foster DJ, Upton RN, Somogyi AA, Martinez A, Grant C. Cerebral kinetics of oxycodone in conscious sheep. Journal of pharmaceutical sciences. 2006;95:1666–1676. doi: 10.1002/jps.20632. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Will M, Franzblau E, Kelley A. Nucleus accumbens μ-opioids regulate intake of a high-fat diet via activation of a distributed brain network. The Journal of neuroscience. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Everitt BJ, Phillips PE. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc Natl Acad Sci U S A. 2012;109:20703–20708. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mayer-Blackwell B, Schlussman SD, Randesi M, Butelman ER, Ho A, Ott J, Kreek MJ. Extended access oxycodone self-administration and neurotransmitter receptor gene expression in the dorsal striatum of adult C57BL/6 J mice. Psychopharmacology (Berl) 2014;231:1277–1287. doi: 10.1007/s00213-013-3306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology. 2009;34:912–922. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]