“The cure for the headache was a kind of a leaf, which required to be accompanied by a charm, and if a person would repeat the charm at the same time that he used the cure, he would be made whole; but that without the charm the leaf would be of no avail.”
Socrates, according to Plato 1
Placebo response rates are known to be high in pediatric migraine trials.2,3 Although this constitutes a major burden for clinical trials that struggle to find effective drugs for the treatment of pediatric migraine, the placebo effect has an important but overlooked potential in clinical care. As captured in our opening quote, a healer’s capacity to stimulate positive expectations was fundamental in ancient medicine. Unfortunately this lesson seems to be undervalued and its potential underutilized in modern clinical practice. From a clinical perspective, placebo responses are likely one of the best allies of good clinical care if they can be effectively, efficiently, and ethically harnessed. Migraine, due to its susceptibility to stress and allostatic load4 is an excellent paradigm to examine the potential analgesic and clinical benefits resulting from positive and motivational therapeutic interventions taking advantage of the placebo effect.
Expectations of clinical benefits seem to be at the heart of the placebo effect.5 Understanding how expectancies of improvement (triggered by verbal suggestions, or learning procedures) interact with distinct biological systems to shape therapeutic outcomes has been the focus of past pharmacological and neuroimaging studies in the field of placebo.6 These studies have highlighted the importance and clinical relevance of these responses. However, little has been done to translate the accumulated knowledge into improved clinical care. This might be due partially to the lack of a defined model guiding the implementation of these complementary processes in clinical practice but also due to the need for more clinical studies showing their benefit. In pediatrics, the opportunities for using methods that decrease the use of medication that might have long-term side effects on the child’s brain makes this approach even more salient.
Here we focus on how physicians may take advantage of high pediatric placebo responsivity in the migraine clinic to optimize treatment outcomes and to provide patients with an additional therapeutic placebo benefit. We begin by reviewing current pediatric migraine treatments, summarize candidate mechanisms underlying clinical relevant placebo effects, and conclude by suggesting ways to maximize the clinical value of this psychobiological response in pediatric migraine practice.
Pediatric Migraine
Frequently starting in childhood and extending into adulthood, migraine is a central nervous system disorder affecting nearly 15% of the population worldwide.7 According to the World Health Organization (WHO), migraine is among the most prevalent health conditions and it is in the top 20 causes of global disability.8 Despite the high prevalence, and the negative personal and societal impact, migraine is considered to be both underdiagnosed and undertreated, especially in children.9 The estimated cumulative prevalence of pediatric migraine is about 8%, increasing with age.10 However, the prevalence of pediatric headaches is estimated at approximately 60%,10 and migraine is thought to be a common underdiagnosed cause behind some of these recurrent headaches in children.11 The spectrum of migraine symptoms varies as a function of age.12 When compared with the clinical manifestation of migraine in adults, pediatric migraine attacks tend to be shorter and bilateral. Besides, children may often display a wider variety of gastro-intestinal, autonomic and non-nociceptive symptoms, characterized as migraine variants.2
Treating Pediatric Migraine
Like many disorders of the central nervous system (CNS), there are no therapies that are fully effective across patients with migraine. The therapeutic approach in pediatric migraine usually involves a multimodal approach combining pharmacotherapy, which can be abortive or prophylactic, with biobehavioral, and psychoeducational interventions that address the long-term management of the disorder.13 Therefore, depending on the degree of disability and impaired quality of life resulting from migraine, successful management of this disorder entails identifying triggering factors, providing pain relief, and considering prophylaxis.
With regard to pharmacotherapy, abortive and prophylactic options for pediatric populations have been largely based on evidence originating from adult studies. In the past decade, however, there has been a growing awareness that children are not merely small adults. Studies have shown that when comparing pediatric and adult populations, one fifth of the studied drugs have important differences with regard to effectiveness, dosing, or safety.14 Such data suggest that the efficacy and safety established for adults cannot be inferred to children without further research. Placebo-controlled clinical trials have been performed to assess the effectiveness of candidate migraine pharmacological treatments for children.2 However, the majority of these trials have failed to demonstrate effectiveness of active drugs over placebo. Only two triptans (almotriptan and rizotriptan) have been approved by the Food and Drug Administration (FDA) to be safe and effective for the abortive treatment of pediatric migraine.15 With regards to prophylactic treatments, only one antiepileptic drug (topiramate) and one antidepressant (trazodone) have been shown to be more effective than placebo.2 However, the evidence supporting these drugs is limited, which constitutes a challenge for physicians when trying to prescribe effective drugs. Importantly, the most frequent reason for the lack of positive results in trials of both acute and prophylactic pediatric migraine pharmacotherapy is the high placebo analgesia response rates.
The high number of patients reporting stress as a precipitating factor for migraines, together with the high comorbidity of migraine with stress related psychiatric disorders,4 and the success rates of stress management therapies emphasizes the importance of the non-pharmacological interventions as successful alternatives to pharmacologic treatment in managing pediatric migraine. Adding a non-pharmacological treatment approach like cognitive behavioral therapy (CBT) (including pain coping training and biofeedback), seems to successfully boost the therapeutical benefits of pharmacotherapy (ie, amitriptyline).16 Moreover, comparing a psychological intervention, such as stress management training, with a pharmacological intervention (ie, the β-blocker metoprolol), greater clinical improvement has been reported with the psychological intervention.17
Widely used for migraine prophylaxis, studies have reported that acupuncture may be as effective, or possibly more effective than, prophylactic pharmacological migraine treatments, and with fewer adverse effects.18 Furthermore, homeopathic therapies, also commonly used in the treatment of migraine have been also suggested to result in a significant decrease in frequency, severity, and duration of pediatric migraine attacks.19 Notably, however, both acupuncture and homeopathic interventions do not seem to perform better than placebos in controlled clinical trials.20,21 The observable beneficial responses resulting from these interventions most likely are a reflection of the placebo effect, perhaps enhanced by a more elaborate administration ritual bordering on spiritual beliefs in the efficacy of the remedy, a close patient-practitioner interaction, and the practitioner’s belief in the treatment.
Placebo Responses in Clinical Trials of Pediatric Migraine
Placebo analgesia is traditionally viewed as the reduction in pain following the administration of an inert/sham treatment. Due to the increased interest in medical research and clinical practice, current definitions of placebo effects have become more comprehensive.22 It is known that placebo effects or responses do not depend on placebo administration. Placebo responses, translated into genuine psychobiological events, are attributable to the overall therapeutic context of any intervention, which is why placebos (ie, inert/sham treatments) are used as controls in clinical trials.
As reported above, placebo effects seem to underlie a substantial portion of the therapeutic effects observed after non-pharmacological and pharmacological pediatric migraine interventions. From a methodological perspective, high placebo responsivity represents a major burden in clinical trials as significant differential outcomes between active interventions and placebos become more difficult to detect.2,3 Whereas pharmacological placebo effects have been estimated around 35% in adult migraine trials, pediatric trials suggest placebo response rates of 50% or higher,23 indicating an even greater challenge for pediatric trials. Moreover, an inverse relationship between age and placebo response rates has been reported in migraine.24 This inverse relationship has been suggested to continue into adulthood. Younger adults appear to be more likely to respond to placebo as compared with older adults who are more likely to respond to pharmacotherapy.25
Such findings have sparked debates on ways to address and minimize placebo responses in pediatric migraine trials.26 However, from a clinical perspective, one cannot ignore the fact that migraine symptoms significantly improve after placebo administration in more than half of the children.23 Placebo pills decrease the average occurrence of headaches to fewer than three a month from a starting point of nearly six a month.2 Moreover, as stated above, the non-pharmacological and alternative interventions considered to be driven by placebo mechanisms, seem to be particularly effective in pediatric populations.19 Therefore, instead of focusing on eliminating placebo responses in clinical migraine trials, the focus should be redirected towards understanding the underlying mechanism responsible for high placebo response rates in children with migraine (after excluding confounding factors such as spontaneous remission) in order to maximize that mechanism and use it therapeutically.
The clinical relevance of placebo responses and its underlying mechanisms
With evidence based medicine, the development of effective pharmacotherapies, increased emphasis on informed consent, and the use of placebos (ie, inert treatments) unbeknown to the patients is considered deceptive and ethically controversial. This ethical dilemma has hindered the implementation of placebos in the practice of medicine. However, whereas in the past it was believed that deception was essential to obtain successful placebo responses, recent research on open-label placebo (ie, patients are aware that a placebo is being administered) suggests that deception is no longer needed to achieve the desired therapeutic outcomes. In a study for the treatment of irritable bowel syndrome (IBS), the transparent administration of placebos (ie, open-label placebo), resulted in clinically successful responses.27 Notably, placebos produced symptom relief when patients were fully aware they were taking a placebo pill. This suggests an ethical way to use placebos in the clinic (ie, with informed consent and assent from the patients), consistent with evidence based medicine. In the same line, in a pediatric study of attention deficit hyperactivity disorder medication, the administration of open-label placebo was successfully used in the reduction of pharmacotherapy by as much as 50%.28 These data provide further support to the previous IBS findings27 advocating that placebos can be used in a transparent way without compromising the therapeutic effect. Another obvious advantage was the reduction of pharmacological load and fewer pharmacological side effects. Whereas in the IBS study noted above27 expectancies induced via verbal suggestions might have played a key role in inducing the placebo effect (ie, due to therapeutic instructions linked to the placebo administration), in the latter study,28 learning via classical conditioning was likely the prominent factor enhancing treatment expectations. It has also been shown that placebo analgesia can be induced by observational learning.29 Hence, different forms of learning seem to underlie placebo responsivity. Importantly, verbally induced suggestions, conditioning, and modeling can be all seen as vehicles through which expectations are acquired. Expectancies can also induce noxious outcomes, particularly negative expectancies. In fact, nocebo effects (ie, noxious effects that cannot be attributed to the active component of the drug) are a common and important phenomenon in medicine.30 Both placebo and nocebo responses are integral elements of any therapeutic intervention, which validates the significance of contextual and psychological factors in the healing process.
The open-hidden paradigm has proven to be extremely valuable in showing the relevance of the psychological component in pharmacological treatments.31 By eliminating treatment expectations through the hidden administration of the drug, the magnitude of the placebo effect in pharmacological treatments can be examined without ethical constrains. Studies comparing open and hidden drug administrations across conditions indicate that the needed dose to improve these conditions is significantly higher with hidden as compared with open infusions.32 Being transparent about the possible benefits of the administered treatment enhances therapeutic expectations that in turn result in benefits during both drugs32 and placebo27,28. It has also been reported that expecting an inert treatment abolishes the beneficial effects of an analgesic drug.33 Hence, when the placebo component is absent in active medication, the therapeutic efficacy is significantly reduced or even abolished. On the other hand, the effectiveness of pharmacotherapies might double by enhancing positive treatment expectancies.34 The stronger the ritual surrounding the treatment, the better it works. Factors such as type, form, color and quantity, surrounding therapeutic interventions, have been shown to influence the magnitude of the placebo response by shaping the significance and meaning of the treatment.35,36 Taken together these findings further support the general idea that beliefs, feelings, and meaning of treatments are important and should be taken into consideration in therapeutical settings.
Notably, when it comes to expectancies and belief systems, children seem to have the ability to approach a situation from an unbiased or naive perspective due to lack of experience and perhaps the absence of prefrontal control. Delayed prefrontal maturation seems to be a necessary adaptation for basic learning37 which might constitute an advantageous evolutionary tradeoff.38 A mature prefrontal cortex might hinder flexible thinking, due to prior experience that could affect and bias expectations. This makes children’s belief system more flexible and easier to shape by experience.39 Hence, they might be easily influenced by authority figures, and more open to follow confident and secure models, such as clinicians and parents.
Doctors seem willing to introduce placebo treatments and take advantage of placebo responses in clinical practice,40 perhaps more so in the pediatric population. Surveys on patients’ perspectives regarding the use of placebos also suggest that patients are open towards the use of placebos.41 These surveys on patients’ attitudes underscore the importance of honesty, transparency, and trust in the prescription of placebo treatments. This suggests a preferred transparent model of shared decision-making, in which the decision and the benefits of administering placebo are deliberated among the parts. Recent progress in placebo research is starting to provide clinicians with the tools to introduce placebos and maximize placebo effects in the clinic in an ethically acceptable way, and both doctors and patients seem ready to embrace it.
Harnessing the Placebo effect in the Pediatric Migraine Clinic
Although migraine represents a substantial burden to the affected children and their families,10 there is currently limited evidence of effective acute or prophylactic pharmacotherapy.2,3 Furthermore, especially in pediatrics, where placebo responses are high23 and there is little known about the noxious impact of chronic use of drugs on pediatric development, the affected children might greatly benefit from these pharmacologically inert but otherwise successful interventions. Based on our present knowledge regarding pediatric migraine, the mechanisms underlying placebo-induced therapeutic effects, and the possible advantage of a developing/pediatric brain,38,39 we provide some recommendations of how to maximize these therapeutically relevant psychobiological mechanisms and apply them clinically.
It is well known that patients’ cognitions and emotions influence the therapeutic outcome. Regarding migraine, which is often triggered and aggravated by external factors,4 it is especially important to avoid the historical dichotomy between biology and psychology. In the past decades with the technological advances in medicine, a patient’s emotional needs, beliefs and perceptions have, at times, not been included in treatment plans. However, recent studies are enhancing awareness of this topic by emphasizing that the doctor-patient encounter may have significant beneficial therapeutic effects in itself.42 This encounter constitutes a unique psychosocial interaction in which the physician embedded in a particular context exerts an influence on the child expectancies, beliefs, and confidence regarding the therapy.43 Through this unique psychosocial encounter, physicians have a powerful tool to optimize placebo responses and avoid nocebo responses.
In a clinical setting of pediatric migraine, the child, commonly accompanied by its parents, seeks help from the physician to decrease the pain and discomfort caused by migraine that is usually exacerbated by anxiety and stress.4 The doctor and the therapy are seen as possible rewards with the ability to suppress the discomfort caused by migraine.42 In other words, a positive doctor-patient interaction has the potential to reduce anxiety and stress and enhance expectations of analgesia which might activate the brain reward circuitry known to play a central role in placebo-induced pain relief.
The physician has the primary role of relieving the discomfort caused by migraine and his or her communication skills are central in boosting the desired therapeutic effects.44 Studies have consistently shown that specific aspects of the doctor-patient communication influence patients’ well-being such as treatment satisfaction, adherence to treatment, coping with the disease, quality of life and ultimately state of health.44,45 On the other hand, it has been reported that 50% of the patients in the USA do not have a clear understanding of what the physician told them after an appointment.46 Such misunderstanding will likely affect patients’ satisfaction, adherence, and consequently the therapeutic outcome. To improve the therapeutic outcome physicians are advised to make sure that the information delivered is positive, motivational, and understandable, reaching both the patient and the parents. Moreover, it is important to empower patients (ie, patient-centered care) and increase their self-confidence in their ability to follow the therapy and overcome the possible associated side effects. Patient centered care focuses on patients’ needs, wants and preferences, so that patients can make choices that best fit their needs. This type of care has been associated with improved healthcare outcomes, particularly in chronic disease patients,47 which can be achieved by allowing both the child and parents to take an active part of the decisions when choosing the adequate therapy.
Because psychological factors play a central role in placebo responsivity and children belief systems seem to be easily shaped by secure models, such as the physician, it becomes crucial that the physician behaves empathically to set in motion the adequate psychological mechanisms to optimize the placebo response and avoid nocebo responses. The therapeutic relevance of an empathic physician has been elegantly demonstrated in a sham acupuncture treatment study of IBS.48 In that study, patients that received the sham acupuncture treatment from an empathic, warm, and interested researcher experienced the same level of improvement as they would from effective active drugs. On the other hand, patients receiving sham acupuncture from a disengaged researcher who rushed into the treatment and was not interested in interacting resulted in a significant drop in improvement.48 Accordingly, a strong doctor-patient interaction based on care and interest might double the therapeutic placebo response.
Another important aspect known to affect the doctor-patient relationship and consequently the therapeutic outcome is the nonverbal communication. For instance, the simple act of holding the hand of a loved one has been shown to decrease the unpleasantness of electric shocks.49 Accumulating evidence also supports the general notion that the physical surroundings can increase or reduce stress50 which can interfere with the doctor-patient relationship and affect the well-being of patients. Hence, health care organizations should consider this knowledge to create uplifting, playful environments ultimately setting the stage for a comfortable interaction that will enhance placebo responsivity and maximize healing.
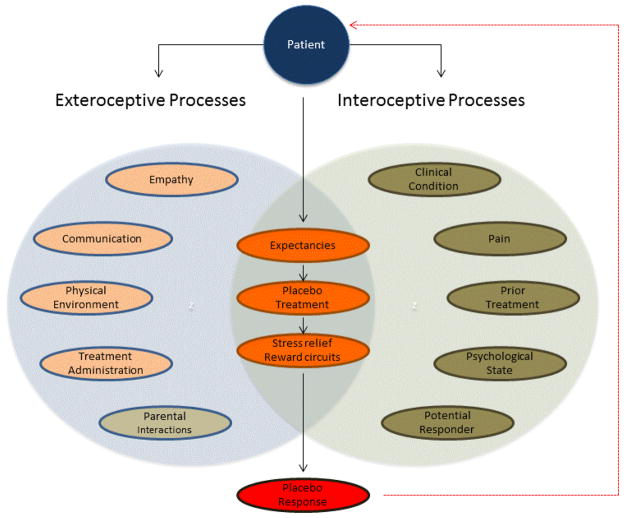
Because factors such as stress, sleep deprivation, and fasting may exacerbate migraine attacks,4 basic lifestyle modifications have the potential to reduce pain frequency and duration. Moreover, as children seem to have more flexible belief systems and higher learning capacities,38,39 the patient-practitioner interaction is crucial in increasing treatment compliance and facilitating learning of new healthy behaviors which might lead to a decrease of migraine attacks. Overall, if children and their parents feel confident and believe in the therapy they are receiving, they will focus their attention on more positive life experiences and their anxiety and stress levels will likely decrease,51 thus augmenting the placebo response. As seen with empathy, stress and anxiety may moderate expectations and their effect on clinical improvement.51 Positive expectations and beliefs lead to motivation and the adoption of assertive beneficial responses, whereas negative expectancies lead to inhibition and augmented anxiety.52 Therefore, it is important to optimize these psychological factors to reduce stress, empower the patient, and enhance treatment adherence to consequently maximize treatment efficacy (Figure).
Figure.
Diagram of exteroceptive and interoceptive components affecting placebo responsivity in pediatric migraine. Both clinical related and individual factors are represented as influencing therapeutic placebo responses in pediatric migraine clinic. Right, In pink, under exteroceptive processes are the clinical related factors that during the doctor-patient relationship are thought to modulate placebo responses (empathy, patient-centered communication, physical environment, treatment administration or therapeutic procedure). Parents are also thought to play an important role during the doctor-patient interaction as a source of positive expectations outside the specific medical context thus providing continued psychological support. Left, Represented in brown are the individual factors (ie, the interoceptive processes) known to influence the formation of placebo responses. These are related to the specifics of the clinical condition (eg, intensity and duration of pain) and clinical history (prior treatment, psychological state, potential responder) that is known to affect patients’ therapeutic expectancies. Middle, Illustrated in orange both the individual and contextual factors come together and interact with each other affecting patients expectancies, which in turn will affect the placebo treatment that might determine the overall responsivity (in red) via anxiety reduction and the reward circuitry.
Because they do not involve any type of deception and are centered on the doctor-patient relationship, these general recommendations are ready to be implemented. Notably, however, due to the fact that individual factors influence patients’ expectations, these strategies should be adapted and personalized to the medical history of the child. Ultimately the goal is to take advantage and enhance the placebo effect known to be present in any clinical intervention.
When there is limited evidence supporting the therapeutic effectiveness of pharmacotherapies, and when the placebo response rates are high as in pediatric migraine trials, the usage of placebos is not so unethical. Especially if administered openly with the parent’s permission and patient’s assent.27 Moreover, because overuse of abortive headache medications has been shown to lead to chronic daily headache,53 placebos might be useful as adjuncts to pharmacotherapies with significant dose related side-effects, issues that are perhaps even more important in the pediatric population. While taking advantage of learning processes such as classical conditioning, the intake of pharmacological drugs can be reduced by alternating these drugs with placebo pills.28 Based on previous studies in other domains, this partial reinforcement method is predicted to result in reduced side effects without affecting the success of the analgesic treatment. Another factor shown to induce successful analgesic outcomes is observational learning or modeling.29 Considering that modeling is central in the acquisition of behavior in children, the recruitment of social learning mechanisms as placebos might produce significant analgesic benefits in this pediatric population. This could be achieved with the creation of support groups for children with migraine in which they would meet migraineurs that have enrolled in successful therapies and describe how these therapies worked for them. Even though these learning strategies have been reported successful previously,28 further research is still needed to evaluate if these therapeutic benefits are sustained, especially in the field of pediatric migraine. Hence, while controlling for confounding factors, future studies should investigate successful mechanisms underlying therapeutic pediatric placebo treatments in migraine to further guide the implementation of these strategies that hold promising clinical effects. Notably, our knowledge in this area is still in its infancy and is limited by the lack of studies specifically designed to investigate the placebo effect in pediatric migraine. To harness the placebo effect in pediatric migraine practice, in its full potential, experimental and translational research is needed.
Discussion
Because pharmacological and non-pharmacological therapies are administered into a complex living being and in a particular context, it is not surprising that expectations and beliefs play a substantial role in shaping the outcome of these therapies, especially in pediatric populations. High placebo response rates, as noted in pediatric migraine trials, can adversely impact the evaluation of new treatments,2,3 but they may also provide welcome therapeutic benefit in clinical practice. In pediatric migraine, it is well documented but often overlooked that both environmental factors and endogenous physiological mechanisms play a central role in controlling pediatric migraine attacks.4 Hence, disregarding physicians’ power in inducing analgesic states during the therapeutic encounter can be considered suboptimal care. When providing care, the physician should take advantage of all the available knowledge, including that on placebo and nocebo responses, to maximize the therapeutic outcome.43 Taking advantage of these psychobiological therapeutic responses holds promising therapeutic effects that might translate into better treatment compliance, optimization of treatment efficacy, and reduce intake of pharmacological treatments and the corresponding adverse effects. As awareness of the role of placebos in pediatric migraine increases, we expect that new research especially in clinical practice will positively contribute to our understanding of the therapeutic benefits of placebo in pediatric population. Further development on methods of inducing, and more specifically of maintaining, the placebo response in this clinical population are open challenges for the future.
Acknowledgments
Supported by National Institute of Neurological Disorders and Stroke (K24NS064050 and R01NS0750182 to D.B) and National Center for Complementary and Alternative Medicine (R21 AT007530-01 to D.B.). V.F. supported by the Ryochi Sasakawa Young Leaders Fellowship Fund.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jowett B. The dialogues of Plato. London, UK: Oxford University Press; 1892. pp. 11–12. [Google Scholar]
- 2.El-Chammas K, Keyes J, Thompson N, Vijayakumar J, Becher D, Jackson JL. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250–258. doi: 10.1001/jamapediatrics.2013.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun H, Bastings E, Temeck J, Smith PB, Men A, Tandon V, et al. Migraine therapeutics in adolescents: a systematic analysis and historic perspectives of triptan in adolescents. JAMA Pediatr. 2013;167:243–249. doi: 10.1001/jamapediatrics.2013.872. [DOI] [PubMed] [Google Scholar]
- 4.Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73:219–34. doi: 10.1016/j.neuron.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93:77–84. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 6.Faria V, Fredrikson M, Furmark T. Imaging the placebo response: A neurofunctional review. Eur Neuropsychopharmacol. 2008;18:473–85. doi: 10.1016/j.euroneuro.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonardi M, Steiner TJ, Scher AT, Lipton RB. The global burden of migraine: measuring disability in headache disorders with WHO’s Classification of Functioning, Disability and Health (ICF) J Headache Pain. 2005;6:429–40. doi: 10.1007/s10194-005-0252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winner P, Hershey AD. Diagnosing migraine in the pediatric population. Curr Pain Headache Rep. 2006;10:363–369. doi: 10.1007/s11916-006-0061-8. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Arafeh I, Razak S, Sivaraman B, Graham C. Prevalence of headache and migraine in children and adolescents: a systematic review of population-based studies. Dev Med Child Neurol. 2010;52:1088–97. doi: 10.1111/j.1469-8749.2010.03793.x. [DOI] [PubMed] [Google Scholar]
- 11.Winner P, Martinez W, Mate L, Bello L. Classification of pediatric migraine: proposed revisions to the IHS criteria. Headache. 1995;35:407–10. doi: 10.1111/j.1526-4610.1995.hed3507407.x. [DOI] [PubMed] [Google Scholar]
- 12.Bigal ME, Lipton RB. Migraine at all ages. Curr Pain Headache Rep. 2006;10:207–13. doi: 10.1007/s11916-006-0047-6. [DOI] [PubMed] [Google Scholar]
- 13.Eidlitz-Markus T, Haimi-Cohen Y, Steier D, Zeharia A. Effectiveness of nonpharmacologic treatment for migraine in young children. Headache. 2010;50:219–23. doi: 10.1111/j.1526-4610.2009.01534.x. [DOI] [PubMed] [Google Scholar]
- 14.Smith PB, Benjamin DK, Murphy MD, Johan-Liang R, Lyasu S, Gould B, et al. Safety monitoring of drugs receiving pediatric marketing exclusively. Pediatrics. 2008;122:e628–e633. doi: 10.1542/peds.2008-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis DW. Almotriptan for the acute treatment of adolescent migraine. Expert Opin Pharmacother. 2010;11:2431–6. doi: 10.1517/14656566.2010.517747. [DOI] [PubMed] [Google Scholar]
- 16.Powers SW, Kashikar-Zuck SM, Allen JR, LeCates SL, Slater SK, Zafar M, et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA. 2013;310:2622–30. doi: 10.1001/jama.2013.282533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sartory G, Müller B, Metsch J, Pothmann R. A comparison of psychological and pharmacological treatment of pediatric migraine. Behav Res Ther. 1998;36:1155–70. doi: 10.1016/s0005-7967(98)00081-3. [DOI] [PubMed] [Google Scholar]
- 18.Linde K, Allais G, Brinkhaus B, Manheimer E, Vickers A, White AR. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009:CD001218. doi: 10.1002/14651858.CD001218.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danno K, Colas A, Masson JL, Bordet MF. Homeopathic treatment of migraine in children: results of a prospective, multicenter, observational study. J Altern Complement Med. 2013;19:119–23. doi: 10.1089/acm.2011.0821. [DOI] [PubMed] [Google Scholar]
- 20.Shang A, Huwiler-Müntener K, Nartey L, Jüni P, Dörig S, Sterne JA, et al. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy. Lancet. 2005;366:726–32. doi: 10.1016/S0140-6736(05)67177-2. [DOI] [PubMed] [Google Scholar]
- 21.Colquhoun D, Novella SP. Acupuncture is theatrical placebo. Anesth Analg. 2013;116(6):1360–3. doi: 10.1213/ANE.0b013e31828f2d5e. [DOI] [PubMed] [Google Scholar]
- 22.Moerman DE, Jonas WB. Deconstructing the placebo effect and finding the meaning response. Ann Intern Med. 2002;136:471–6. doi: 10.7326/0003-4819-136-6-200203190-00011. [DOI] [PubMed] [Google Scholar]
- 23.Lewis DW, Winner P, Warren W. The placebo responder rate in children and adolescents. Headache. 2005;45:232–239. doi: 10.1111/j.1526-4610.2005.05050.x. [DOI] [PubMed] [Google Scholar]
- 24.Maas HJ, Danhof M, Della Pasqua OE. Analysis of the relationship between age and treatment response in migraine. Cephalalgia. 2009;29:772–80. doi: 10.1111/j.1468-2982.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 25.Ho TW, Fan X, Rodgers A, Lines CR, Winner P, Shapiro RE. Age effects on placebo response rates in clinical trials of acute agents for migraine: pooled analysis of rizatriptan trials in adults. Cephalalgia. 2009;29:711–8. doi: 10.1111/j.1468-2982.2008.01788.x. [DOI] [PubMed] [Google Scholar]
- 26.Arruda MA. No evidence of efficacy or evidence of no efficacy. JAMA Pediatr. 2013;167:300–2. doi: 10.1001/jamapediatrics.2013.1105. [DOI] [PubMed] [Google Scholar]
- 27.Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, Lembo AJ, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5:e15591. doi: 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandler AD, Glesne CE, Bodfish JW. Conditioned Placebo Dose Reduction: A new treatment in ADHD? Journal of developmental and behavioral pediatrics. J Dev Behav Pediatr. 2010;31:369–375. doi: 10.1097/DBP.0b013e3181e121ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. Pain. 2009;144:28–34. doi: 10.1016/j.pain.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 30.Hauser W, Hansen E, Enck P. Nocebo phenomena in medicine: their relevance in everyday clinical practice. Deutsches Arzteblatt international. 2012;109:459–465. doi: 10.3238/arztebl.2012.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedetti F, Carlino E, Pollo A. Hidden Administration of Drugs. Clin Pharmacol Ther. 2011;90:651–661. doi: 10.1038/clpt.2011.206. [DOI] [PubMed] [Google Scholar]
- 32.Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol. 2004;3:679–84. doi: 10.1016/S1474-4422(04)00908-1. [DOI] [PubMed] [Google Scholar]
- 33.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 34.Kam-Hanse S, Jakubowski M, Kelley JM, Kirsh JM, Hoaqlin DC, Kaptchuk TJ, et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med. 2014;6:218ra5. doi: 10.1126/scitranslmed.3006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Branthwaite A, Cooper P. Analgesic effects of branding in treatment of headaches. Br Med J (Clin Res Ed) 1981;282:1576–8. doi: 10.1136/bmj.282.6276.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Craen AJ, Tijssen JG, de Gans J, Kleijnen J. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J Neurol. 2000;247:183–8. doi: 10.1007/s004150050560. [DOI] [PubMed] [Google Scholar]
- 37.Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–10. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Thompson-Schill SL, Ramscar M, Chrysikou EG. Cognition without control: When a little frontal lobe goes a long way. Curr Dir Psychol Sci. 2009;18:259–263. doi: 10.1111/j.1467-8721.2009.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.German TP, Defeyter MA. Immunity to functional fixedness in young children. Psychon Bull Rev. 2000;7:707–12. doi: 10.3758/bf03213010. [DOI] [PubMed] [Google Scholar]
- 40.Kermen R, Hickner J, Brody H, Hasham I. Family physicians believe the placebo effect is therapeutic but often use real drugs as placebos. Fam Med. 2010;42:636–42. [PubMed] [Google Scholar]
- 41.Hull SC, Colloca L, Avins A, Gordon NP, Somkin CP, Kaptchuk TJ, Miller FG. Patients’ attitudes about the use of placebo treatments: telephone survey. BMJ. 2013;347:f3757. doi: 10.1136/bmj.f3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benedetti F. Placebo and the new physiology of the doctor-patient relationship. Physiol Rev. 2013;93:1207–46. doi: 10.1152/physrev.00043.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benedetti F. How the doctor’s words affect the patient’s brain. Eval Health Prof. 2002;25:369–86. doi: 10.1177/0163278702238051. [DOI] [PubMed] [Google Scholar]
- 44.Verheul W, Sanders A, Bensing J. The effects of physicians’ affect-oriented communication style and raising expectations on analogue patients’ anxiety, affect and expectancies. Patient Educ Couns. 2010;80:300–6. doi: 10.1016/j.pec.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 45.Bensing JM, Verheul W. The silent healer: the role of communication in placebo effects. Patient Educ Couns. 2010;80:293–9. doi: 10.1016/j.pec.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 46.Bodenheimer T. The future of primary care: transforming practice. N Engl J Med. 2008;359:2086–2089. doi: 10.1056/NEJMp0805631. [DOI] [PubMed] [Google Scholar]
- 47.Levinson W. Patient-centered communication: a sophisticated procedure. BMJ Qual Saf. 2011;20:823–5. doi: 10.1136/bmjqs-2011-000323. [DOI] [PubMed] [Google Scholar]
- 48.Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inagaki TK, Eisenberger NI. Neural correlates of giving support to a loved one. Psychosom Med. 2012;74:3–7. doi: 10.1097/PSY.0b013e3182359335. [DOI] [PubMed] [Google Scholar]
- 50.Brown KK, Gallant D. Impacting patient outcomes through design: acuity adaptable care/universal room design. Crit Care Nurs Q. 2006;29:326–41. doi: 10.1097/00002727-200610000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Wager TD. Expectations and anxiety as mediators of placebo effects in pain. Pain. 2005;115:225–6. doi: 10.1016/j.pain.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 52.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–90. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 53.Lucas S. Initial abortive treatments for migraine headache. Curr Treat Options Neurol. 2002;4:343–350. doi: 10.1007/s11940-002-0044-y. [DOI] [PubMed] [Google Scholar]