Abstract
Atherosclerosis is a progressive inflammatory disease of the medium to large arteries that is the largest contributor to cardiovascular disease (CVD). B cell subsets have been shown in animal models of atherosclerosis to have both atherogenic and atheroprotective properties. In this review we highlight the research that developed our understanding of the role of B cells in atherosclerosis both in humans and mice. From this we discuss the potential clinical impact B cells could have both as diagnostic biomarkers and as targets for immunotherapy. Finally, we recognize the inherent difficulty in translating findings from animal models into humans given the differences in both cardivascular disease and the immune system between mice and humans, making the case for greater efforts at addressing the role of B cells in humans atherosclerosis.
Keywords: B cells, Atherosclerosis, Natural antibodies, Rituximab, Immune modulation
Introduction
Cardiovascular disease (CVD) remains the leading global cause of death, accounting for at least 17.3 million deaths annually [1]. The primary contributor to CVD is atherosclerosis, a progressive inflammatory disease of large and medium sized arteries defined by accumulation of lipids in the vascular wall and progressive expansion of vascular lesions [2,3]. Traditional risk factors for atherosclerosis include obesity, hypertension, dyslipidemia, diabetes, and smoking status. While these risk factors frequently present in patients that develop clinical manifestations of atherosclerosis, they fall short at predicting the majority of acute events which occur in asymptomatic individuals [4–6]. The Framingham Heart Study demonstrated that half of patients present with unheralded myocardial infarction or sudden cardiac death as their first manifestation of coronary artery disease [7]. Approximately two-thirds of acute coronary syndromes occur following rupture of atherosclerotic plaque initially less than 50% stenosed which may explain the development of acute events in asymptomatic individuals [8–10]. This suggests that our current means of testing for and treatment of CVD is missing a large portion of the population at risk for atherothrombotic events and requires us to look beyond the contributions of traditional risk factors for our answers.
Early lesions, called ‘fatty streaks’, form as a result of LDL aggregation in the vascular wall which consequently becomes oxidized producing a mix of immunogenic lipid species [3]. Oxidized LDL (oxLDL) drives the inflammatory response by activating endothelial cells and macrophages to express adhesion molecules and inflammatory cytokines that recruit other leukocytes to the lesion. Macrophages use scavenger receptors to take up oxLDL converting them to lipid laden foam cells [2]. When foam cells undergo apoptosis they are poorly cleared which causes the formation of a necrotic core with an overlying collagen rich fibrous cap [11]. Expansion of the lesion over time causes stenosis of the artery and ischemia of the surrounding tissue. Erosion or rupture of the fibrous cap exposes thrombotic material from the necrotic core to the circulation leading to thrombus formation and downstream tissue infarction. The innate and adaptive immune systems have been implicated in modulating these vessel wall responses. Numerous leukocyte subsets have been linked to atherosclerosis both through correlative autopsy findings and in rodent models of the disease and have been reviewed thoroughly elsewhere [2,12–14], We have previously published a more detailed review of the roles of B cells in murine atherosclerosis [15]. This review will highlight key aspects of emerging findings in the field of B cells and atherosclerosis focusing on their potential clinical implications.
The Role of B Cells in Atherosclerosis
Lymphocytes have long been known to locate at sites of plaque formation. Gerlis described “foci of inflammatory cells” within the adventitia of coronary arteries in patients that suffered acute MI in 1956 [16]. Development of targeting antibodies allowed for characterization of cells via immunohistochemistry which definitively showed these foci to contain B cells [17–19]. Importantly, a recent study by Hamze and colleagues used laser capture microdissection to analyze individual lymphocytes in diseased coronaries finding that the majority of B cells were present in the adventitia of these arteries and that they primarily expressed an activated plasmablast phenotype suggesting the cells were active at the sites of disease [20]. These observations have been supported with a wealth of work in murine models of atherosclerosis. Indeed, Habenicht and colleagues showed that aged atherogenic mice (Apoe−/−) develop tertiary lymphoid organs containing mature B cell follicles within the adventitia of the aorta [21,22]. A number of elegantly performed studies over the last few decades have implicated a role for B cells in atherosclerosis. In particular, two papers from 2002 were published describing an atheroprotective role for B cells by manipulating B cell numbers in mouse models. Hansson’s group showed that splenectomy, which depletes B and T cells, dramatically increased atherosclerosis and that protection was provided by adoptive transfer of B cells into the splenectomized mice [23]. This finding was reinforced by Major and colleagues through work that showed bone marrow transplant from B cell deficient mice (μMT) into lethally irradiated Ldlr−/− mice led to the development of significantly more atherosclerosis compared with mice receiving wild type bone marrow [24]. The atheroprotective function of B cells was hypothesized to be, in part, due to their production of protective IgM antibodies against oxidized phospholipids [23]. This notion was previously proposed by the findings of Witztum and others [25–27] and supported in subsequent studies demonstrating that mice unable to secrete IgM (sIgM) develop significantly greater atherosclerosis than control mice [28].
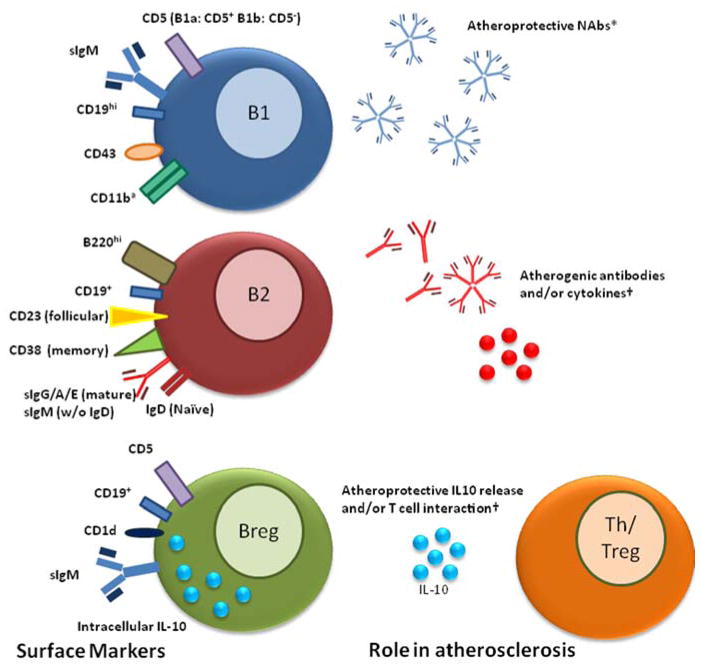
The idea that all B cells are atheroprotective has been revised by recent publications showing that B cells can also be atherogenic. Evidence for this was found through the specific depletion of B2 B cells with a monoclonal antibody against CD20 [29,30] or by using B cell activating factor (BAFF) receptor deficient mice which are also depleted for B2 cells [31,32]. In both cases, the B2 depleted mice had attenuated diet-induced atherosclerosis suggesting that this subset has atherogenic properties. Additionally, the adoptive transfer of 5 million splenic B2 cells taken from C57BL/6 mice into atherogenic lymphocyte deficient (Rag2−/−γc−/−Apoe−/−) and atherogenic B cell deficient (μMT) mice led to significantly increased atherosclerosis compared to PBS or peritoneal B1 B cell transfer [29]. Alternatively, adoptive transfer of innate B1 B cells into splenectomized Apoe−/− mice was shown to attenuate atherosclerosis suggesting that these cells were atheroprotective and demonstrating subset specific differences in B cell function in mice [33]. Figure 1 shows the surface markers used to differentiate murine B cell subsets and the possible roles they have in atherosclerosis. Potential equivalent human B cell subsets are discussed below.
Figure 1.
Surface markers used to distinguish murine B cell subsets and the potential functions of B cell subsets in atherosclerosis. *Established in the literature. †Proposed in the literature. a
B2 B cells
Conventional B2 B cells are associated with adaptive immunity. These cells develop in the bone marrow from common lymphoid progenitors and migrate to secondary lymphoid organs such as the spleen and lymph nodes, going through a number of transitional stages before becoming naïve mature B cells in the follicular regions of lymphoid organs. B2 B cells respond to antigen presentation in a T cell dependent manner undergoing proliferation, affinity maturation, and isotype class switching to produce large amounts of highly specific antibodies against foreign pathogens. This process can be maladaptive in the setting of autoimmunity when these antibodies react to auto-antigens. It is hypothesized that B2 B cells may promote atherosclerosis in mice through their ability to produce inflammatory cytokines that can activate Th1 T cells and monocyte/macrophages [29]. Alternatively, this could be due to the presence of immune complexes involving IgG auto-antibodies within atherosclerotic plaques [25], or yet undiscovered mechanisms. That B2 B cells may have atheroprotective properties under certain conditions was suggested by findings that adoptive transfer of 30 or 60 million splenic B2 B cells from Apoe−/− mice significantly reduced Western diet-induced atherosclerosis in μMTApoe−/− mice [34]. This apparent contradiction with findings of Kyaw that 5 million B2 B cells from B6 mice promoted atherogenesis may suggest that prior B cell exposure to lipid antigen may impact on the effect of B cells on atherosclerosis. Indeed, we have shown that transfer of 60 million B2 B cells derived from C57BL/6 mice into μMTApoe−/− mice did not have an atheroprotective effect [35] suggesting that hypercholesterolemia may induce an atheroprotective phenotype in B2 B cells.
B1 B cells
B1 B cells serve an integral role in the innate immune system. In mice, they develop from specific precursors in the fetal liver, reside in serosal cavities, and self-replicate in a T-independent manner [36]. B1 B cells spontaneously produce antibodies, with few nucleotide inclusions, that are primarily IgM [37–39]. Their protective role in atherosclerosis is thought to be due to the production of natural antibodies (NAbs) that bind modified self epitopes such as oxidation specific epitopes (OSEs) found on OxLDL and apoptotic cells [40]. One class of NAbs in particular, called E06, binds to phosphorylcholine on the surface of OxLDL and apoptotic cells. It has been established that E06 is atheroprotective both through the inhibition of OxLDL uptake by resident macrophages which slows their conversion to foam cells and increased clearance of apoptotic bodies which decreases necrotic core formation [41]. Indeed, Binder and colleagues showed that immunization of atherogenic Ldlr−/− mice with Streptococcus pneumonia, which also expresses the phrosphorylcholine antigen recognized by E06, was found to greatly increase production of circulating E06 IgM and decrease the extent of atherosclerosis [42]. The same group showed that Ldlr−/− mice that were lethally irradiated and received IL-5−/− bone marrow had significantly reduced circulating titers of E06 and developed greatly increased atherosclerosis compared to IL-5+/+ bone marrow donor controls [43], supporting the notion that B1a B cells have atheroprotective functions due at least in part to the production of NAb such as E06. Similar to the question of whether all B2 B cells are atherogenic, recent identification of a novel subset of B1 B cells called innate response activating (IRA) B cells by Rauch and colleagues [44] raised the question of atherogenic B1 B cells. They showed that IRA B cells, derived from peritoneal B1a B cells that migrate to the spleen, produce granulocyte-macrophage colony-stimulating factor (GM-CSF) in response to LPS stimulation. This could be important to atherosclerosis as GM-CSF induces an inflammatory phenotype in macrophages and treatment of mice with GM-CSF aggravates atherosclerosis [45]. This example of a potentially atherogenic B cell derived from “atheroprotective” B1a B cells further underscores the notion that the simple dichotomy that B1 B cells attenuate and B2 B cells aggravate atherosclerosis is likely too simplistic.
Regulatory B cells
A final classification of B cells are regulatory B cells (Bregs) and are functionally defined by their ability to suppress the immune response primarily through the production of the anti-inflammatory cytokine IL-10 or through direct interaction with other leukocytes [46]. Several types of Bregs exist, including B1a-derived and B2-derived Bregs as well as a possibly distinct B cell subset termed B10 cells due to their primary function of IL-10 production [47,48]. While it is unknown how Bregs contribute to atherosclerosis, it is realistic to hypothesize that they would be atheroprotective given our knowledge that Bregs have anti-inflammatory properties and that depletion of IL-10 in mice is atherogenic [49]. Furthermore, there has been extensive research on Bregs in human autoimmunity showing that functional or numerical deficits in Bregs appear to play a role in disease pathogenesis [46,50]. Moreover, helminth induction of IL-10 expression from B cells appears to protect from multiple sclerosis further suggesting that Bregs may be important in protecting against immune mediated diseases [51].
B Cells in human vascular disease
A recent study of cases taken from the Framingham Heart study used genome wide association (GWAS) to develop gene co-expression networks and identify independent genes that associate with CHD [52]. This study found that genes associated with B cell activation were the most strongly enriched in controls but not in CHD cases suggesting B cells may play an important protective role in human atherosclerosis. However, few studies have compared B cells and B cell subsets to clinical measures of CHD. One study showed that circulating CD80+ late phase activated B cell numbers correlate with carotid intimal medial thickness (cIMT), an established risk factor for adverse cardiovascular events, suggesting that this subset contributes to atherosclerosis [53]. Adding to the difficulty in this field is our poor understanding of human B cell subsets. The B1a B cell subset in mice is recognized by its surface expression of the T cell marker CD5 and its spontaneous production of NAbs. This does not carry over to humans as multiple B cell subsets are known to express CD5 [54]. In 2011, Griffin and colleagues used a clever approach whereby they identified a subset of circulating human B cells that shared behaviors characteristic of murine B1 cells. These cells spontaneously produce NAbs, stimulate T cells to proliferate, and demonstrate tonic intracellular signaling [55]. The subset was present in human umbilical cord blood as well as adult peripheral blood, and has a surface phenotype that is CD19, CD20, CD27, and CD43 positive. The same group has recently reported the existence of a Breg-like human B cell population they termed “Orchestrator” B cells (B1orc) which are CD11b+ B1 cells that spontaneously secrete IL-10 and suppress T cell activation [56]. There have been challenges to this group’s findings arguing that the CD19, CD20, CD27, and CD43 positive B cells in humans are not the equivalent of murine B1 cells but instead may be pre-plasmablasts [57–62], highlighting the difficulties in identifying the human equivalents of murine subsets. Nonetheless, as CD19, CD20, CD27, and CD43 positive B cells spontaneously produces IgM, further study is required to determine if these cells produce NAb to OSE, determine if numbers of circulating CD19, CD20, CD27, and CD43 positive B cells are associated with circulating levels of NAb to OSE and atherosclerosis. It has been well established that leukocytosis is independently associated with CAD [63] and poor outcomes after cardiovascular events [64–66] suggesting that circulating leukocyte counts can act as biomarkers for disease progression and outcome. Though less established, it has been shown that circulating lymphocyte counts show an inverse relationship to recurrent ischemic events and cardiovascular mortality [63]. Indeed, the ratio of neutrophils to lymphocytes is an indicator of acute vascular events and associates with CAD severity and 3 year outcome [67]. Importantly, increased neutrophil to lymphocyte ratio has been shown to have independent prognostic value of cardiovascular events [68]. A better understanding of individual B cell subset association with atherosclerosis could provide a clearer picture of the functional relevance of lymphocytes to cardiovascular disease in humans and possibly provide better prognostic measures than neutrophil to lymphocyte ratio. Importantly, characterization of human B cell subsets may help identify individuals at higher risk of acute atherothrombotic events.
Circulating immunoglobulins and B cell homing receptors as possible biomarkers of atherosclerosis
Clinicians and researchers have invested significant effort and resources in an attempt to identify biomarkers which can predict risk of adverse cardiovascular events in individuals with subclinical atherosclerosis as there are currently no reliable means to identify unstable atherosclerotic plaques that are prone to rupture. Biomarkers such as CRP, Lp-PLA2, or MPO and non-invasive testing such as coronary calcium scoring, coronary computed tomographic angiography, carotid ultrasound to assess intima-media thickness, and assessment of endothelial function with flow-mediated vasodilation, along with traditional risk factors, have been proposed to aid clinicians in risk-stratifying patients. Given our rapidly growing knowledge regarding the role of B cells in atherosclerosis perhaps novel biomarkers of disease can be developed from circulating B cells or their products.
Immunoglobulins
Studies involving circulating antibodies have shown associations between immunoglobulin isotype and atherosclerosis. In patients with prior assessment of coronary artery disease burden with coronary angiography, patients with higher levels of IgG had greater burden of CAD whereas IgM had an inverse relationship with CAD burden [69]. This could suggest that IgG and IgM are atherogenic and atheroprotective respectively. Additionally, it was reported that circulating IgA associates with disease burden through a multivariate analysis from patients assessed by carotid and femoral angiography. In this cohort IgA levels independently associated with atherosclerosis suggesting IgA could have an atherogenic function [70]. Alternatively, these findings could be purely associative wherein circulating antibodies could serve as novel biomarkers of disease. Two recent studies looked at the response of oxidative biomarkers, including oxidized phospholipid epitopes on circulating apolipoprotein B-100 (apoB) and IgG and IgM apoB immune complexes, to lipid-lowering statin therapies in patients with stable CAD or ACS. Both cohorts demonstrated that apoB levels increase in response to statins whereas immune complexes decrease [71,72] suggesting that circulating antibodies could also potentially serve as indicators for efficacy of therapy.
B cell homing markers
The idea of modifying leukocyte homing as a means of treating atherosclerosis has become popular based on findings that monocyte subsets differentially regulate the chemokine receptors CCR2 and CX3CR1. Classical monocytes (CD14+CD16−) which associate with cardiovascular events express high levels of CCR2 whereas non-classical (CD14+CD16+) express CX3CR1 and negatively associate with carotid atherosclerosis [73] suggesting homing factors could be used as targets or diagnostic markers for cardiovascular disease. Our group recently demonstrated that splenic B cells isolated by CD43 negative selection traffick to the aorta and provide atheroprotection in Apoe−/− mice. [34]. Moreover, adoptive transfer of CCR6−/− B cells reduced B cell trafficking to the aorta and did not provide atheroprotection. This data suggests that the trafficking of B cells to the aorta plays an important role in B cell-mediated atheroprotection in mice. Other factors implicated in B cell homing to sites of atherosclerosis include L-selectin [74] and CCR7 [21]. Though these findings remain to be established in humans. This raises the question of whether homing factors such as chemokine receptors, selectins, and integrins could play an important role in the function of human B cells in atherosclerosis. Furthermore, as with CD14 and CD16 expression on monocytes, perhaps surface homing receptors on B cells could be used to assess outcomes in cardiovascular disease. Further research is required to confirm these murine findings and to establish whether differential expression of chemokines on B cell subsets correlate with atherosclerosis in humans.
Novel Treatment Strategies
Immune Modulation
Alteration of normal immune function through use of therapies targeting specific cells or molecules carries the potential to have a profound impact on patients. In some circumstances, the disease of interest may be positively affected with minimal adverse side effects. However, there remains the potential that disruption of normal immune function may result in serious adverse effects such as increasing the risk for severe infusion reactions during administration, increasing risk of infection or increasing the risk of malignancy. Our expanding knowledge of B cell function in atherosclerosis holds great potential for developing treatment options that could induce the atheroprotective, or block the atherogenic, functions of B cells. One aspect to pursuing this line of treatment may be unraveling the mechanism by which B cells modulate atherosclerosis from the adventitia [75]. Further research is necessary to clarify whether the presence of B cells within ATLOs are playing an atheroprotective or atherogenic role [21]. Following clarification of B cell function within the adventitia of the arterial wall, small molecules could be developed to either enhance or inhibit B cell function in these regions. Furthermore, immune modulation could be attempted which would promote the development of B cells shown to have beneficial effects. For instance, local treatment with IL-5 or an agonist, which in mice induces B1a proliferation [43] and in humans is associated with levels of NAbs against oxLDL [76], could serve a protective role by increasing B1 B cell numbers and protective antibody titers. Alternatively, targeted therapies can be developed to specifically inhibit or induce programmed cell death in atherogenic B cells.
B cell subset depletion – Treatment with biologics
With the common use of immunosuppressive biologics for the treatment of autoimmunity and the expanding evidence that parts of the immune system serve atheroprotective capacities, it is important that we determine the potential implications of immunosuppressive biologics on cardiovascular disease. It is well established that there is a strong association between autoimmune rheumatic disease and atherosclerosis [77]. Indeed, acute coronary disease is the primary cause of premature mortality in patients with autoimmune diseases and patients with autoimmunity have been shown to have greater inflammation and plaque instability in their coronaries at autopsy [78,79]. In particular to B cells, rheumatoid arthritis is associated with the presence of auto-antibodies such as rheumatoid factor that form immune complexes found in affected joints [80]. Furthermore, B cells are known to contribute to rheumatoid arthritis through antigen presentation to T cells and cytokine production [81]. The use of the monoclonal anti-CD20 antibody Rituximab (RTX) has become a common clinical practice in the treatment of rheumatoid arthritis, vasculitis, system lupus erythematosus, and other rheumatologic diseases refractory to anti-TNFα treatment or immunosuppresive therapy [82]. Long-term B cell depletion using RTX is thought to improve outcomes through the reduction in autoreactive antibodies in comparison to long lasting protective antibodies [83]. These authors hypothesized that this is due to preferential depletion of short lived auto-reactive cells compared to long-lived plasma cells. While monoclonal anti-CD20 antibody delivery attenuates atherosclerosis development in mice, whether RTX treatment in humans is associated with reduced subclinical atherosclerosis or CV events is poorly understood. Early findings from several cohort studies suggest RTX treatment could be beneficial for cardiovascular outcomes but they are limited in size and scope. Thirty-eight patients receiving RTX treatment showed improved flow mediated dilation after 24 months and slightly improved carotid intima-media thickness [84]. These findings were supported by a study that reported five patients treated with RTX showed improved flow mediated dilation of the brachial artery, resulted in a transient decrease in carotid intima-media thickness, as well as improved lipid profiles [85]. Another study reported improved lipid profiles after six months in patients whose RA positively responded to RTX treatment compared with those that responded poorly [86]. In contrast, a study of rheumatoid arthritis patients measuring arterial stiffness after 12 months of RTX therapy found no difference in arterial stiffness and decreased inflammatory markers such as C-reactive protein but increased LDL with HDL levels unchanged [87]. These studies are small in size and use surrogate markers rather than acute cardiovascular events making conclusions hard to draw however they raise the interesting possibility that RTX treatment could be beneficial for CV outcomes.
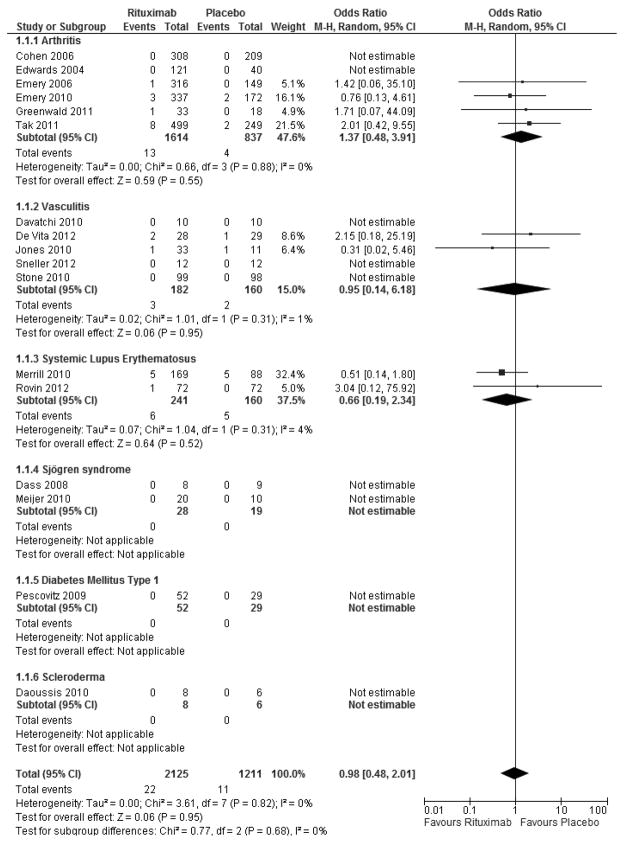
To further explore whether RTX has an impact on cardiovascular events, we performed a meta-analysis of all randomized controlled trials comparing Rituximab therapy with a standard medical therapy control in patients with rheumatologic disease. All included studies had to report the presence or absence of adverse cardiovascular events defined as cardiac death, myocardial infarction, stroke, or heart failure during the study period for inclusion in the analysis. Table 1 provides study and patient characteristics of all included studies. We included 17 studies [88–104] which randomized 3,336 patients to either RTX (n=2,125) or standard medical therapy (n=1,211) with follow-up ranging from 2 to 12 months. As seen by the Forest Plots in Figure 1, treatment with RTX compared with standard medical therapy does not increase nor decrease the short-term risk of adverse cardiovascular events (Odds Ratio 0.98, p=0.95) with very low heterogeneity between the studies (I2=0%). As atherosclerosis is a chronic, progressive inflammatory disease, longer time points will be required in order to determine if RTX induced B cell depletion alters cardiovascular outcomes in patients with rheumatologic disease. Other means of B cell depletion in the context of autoimmunity that have been proposed include neutralizing antibodies against BAFF or its ligand APRIL or neutralizing antibodies against the pan-B cell surface markers CD19 and CD22 [105]. Clinical trials using a monoclonal antibody against BAFF have made it through Phase II for the treatment of RA and SLE [106]. A second means of blocking BAFF and APRIL is being tested using a TACI-Fc fusion protein which is in phase II and III testing for various autoimmune disorders [107]. The possible effects these treatments will have on cardiovascular disease have not been addressed. Furthermore, depletion of BAFF could have unexpected complications as it was recently shown that BAFF regulates IL-10 production from MZ derived Bregs in mice [108].
Table 1.
Study and patient characteristics of studies included in meta-analysis
| Included Studies | Year Published | Disease | Study Design | Number of Patients (Ritux/ Control) | Rituximab Dosing | Mean Follow-up | Age (years) | Female | Disease duration (years) |
|---|---|---|---|---|---|---|---|---|---|
| Cohen [88] | 2006 | Rheumatoid Arthritis | RCT | 308 / 209 | 2 infusions of 1,000 mg | 24 weeks | 52 ± 12 | 81% | 12 ± 8 |
| Edwards [89] | 2004 | Rheumatoid Arthritis | RCT | 121 / 40 | 2 infusions of 1,000 mg | 48 weeks | 54 ± 11 | 78% | 11 ± 7 |
| Emery [90] | 2006 | Rheumatoid Arthritis | RCT | 316 / 149 | 2 infusions of 500 mg or 2 infusions of 1,000 mg | 24 weeks | 51 | 81% | 10 |
| Emery [91] | 2010 | Rheumatoid Arthritis | RCT | 340 / 172 | 2 infusions of 500 mg or 2 infusions of 1,000 mg | 24 weeks | 52 ± 13 | 82% | 7 ± 7 |
| Greenwald [92] | 2011 | Rheumatoid Arthritis | RCT | 33 / 18 | 2 infusions of 500 mg | 24 weeks | 50 | 88% | 10 ± 7 |
| Tak [93] | 2011 | Rheumatoid Arthritis | RCT | 499 / 249 | 2 infusions of 500 mg or 2 infusions of 1,000 mg | 52 weeks | 48 ± 13 | 81% | 0.9 ± 1.1 |
| Davatchi [94] | 2010 | Behcet’s disease | RCT | 10 / 10 | 2 infusions of 1,000 mg | 24 weeks | 30 ± 12 | 35% | NR |
| De Vita [95] | 2012 | Cryoglobulinemic Vasculitis | RCT | 28 / 29 | 2 infusions of 1,000 mg | 2 months | 63 ± 11 | 81% | NR |
| Jones [96] | 2010 | ANCA-Associated Renal Vasculitis | RCT | 33 / 11 | 375 mg per square meter per week for 4 weeks | 12 months | 68 | 47% | NR |
| Sneller [97] | 2012 | Cryoglobulinemic Vasculitis | RCT | 12 / 12 | 375 mg per square meter per week for 4 weeks | 6 months | 52 | 25% | NR |
| Stone [98] | 2010 | ANCA-Associated Vasculitis | RCT | 99 / 98 | 375 mg per square meter per week for 4 weeks | 6 months | 53 ± 15 | 50% | NR |
| Merrill [99] | 2010 | Systemic Lupus Erythematosus | RCT | 169 / 88 | 2 infusions of 1,000 mg and 2 repeat infusions of 1,000 mg at 24 weeks | 52 weeks | 40 | 91% | 9 ± 7 |
| Rovin [100] | 2012 | Proliferative Lupus Nephritis | RCT | 72 / 72 | 2 infusions of 1,000 mg and 2 repeat infusions of 1,000 mg at 24 weeks | 52 weeks | 31 ± 9 | 90% | 3 ± 4 |
| Dass [101] | 2008 | Sjögren Syndrome | RCT | 8 / 9 | 2 infusions of 1,000 mg | 6 months | 53 | NR | 8 |
| Meijer [102] | 2010 | Sjögren Syndrome | RCT | 20 / 10 | 2 infusions of 1,000 mg | 48 weeks | 43 ± 13 | 97% | 5 ± 5 |
| Pescovitz [103] | 2009 | Type 1 Diabetes Mellitus | RCT | 52 / 29 | 375 mg per square meter per week for 4 weeks | 12 months | 18 ± 8 | 38% | 0.22 |
| Daoussis [104] | 2010 | Scleroderma | RCT | 8 / 6 | 2 infusions of 375 mg per square meter per week and 2 repeat infusions of 375 mg per square meter per week at 24 weeks | 12 months | 54 | 86% | 7 |
Immunization
In addition to passive immunization where antibodies are administered to the individual, such as the use of biologics against autoimmunity described above, active immunization is a promising form of immune modulation as it could be used to cheaply induce a lasting immune response against common atherogenic antigens. Early evidence supporting this concept was provided by Palinski and colleagues when they showed that immunization of hyperlipidemic rabbits with malondialdehyde-modified LDL induced high titers of natural antibodies and protected against atherosclerosis [26]. Binder and colleagues corroborated these findings when they showed that immunization of Apoe−/− mice with Streptococcus pneumonia, which expresses the same phosphorylcholine epitope on its cell surface as that bound by T15-EO6 on Ox-LDL, induced high titers of ox-LDL reactive IgM and protected against atherosclerosis [42]. These findings suggest that immunization against oxidation specific epitopes (OSEs) could be an effective treatment option [109]. Another target for immunization is to induce tolerance against apoB-100 which has been shown to cause an atherogenic adaptive immune response [110]. Though tolerance is well established as a T cell response it is likely that Bregs are involved as they have been shown to suppress Th1 responses and induce Tregs in mice after immunization to induce auto-immune arthritis [111]. These findings suggest that immune modulation could be achieved through immunization to either induce a protective response against oxidized LDL or a tolerogenic response against the atherogenic component of the adaptive immune system. Further supporting this idea is the knowledge that natural antibodies against OSEs make up a large portion of total IgM in plasma of newborns showing that the human immune system strongly reacts against these conserved epitopes [41]. Though work in animals models strongly supports the use of immunization as a treatment option, more work is needed to fully describe the relevant epitopes involved in the human atherosclerotic immune response.
Conclusion
Though our understanding of the role of B cells in atherosclerosis has come a long way in recent years, many more questions remain to be answered. For example, are natural antibodies the only means by which B1 B cells are atheroprotective or are there other factors, either secreted or interaction-dependent? What is the impact of regulatory B cells on atherosclerosis? Do atheroprotective effects of B cells occur at the local level of the atherosclerotic plaque or do B cells induce a systemic atheroprotective effect or both? Can a subset within the B2 B cell compartment be validated as an atherogenic cell type? Are the effects on atherosclerosis of B1 and B2 B cells context-dependent?
An important caveat is that the vast majority of the findings implicating B cells in atherosclerosis come from murine models which do not recapitulate thrombotic complications of human atherosclerosis [112]. Furthermore, distinct differences exist between the immune systems of humans and mice, as well as between various inbred mouse strains [113]. Of particular import, it is known that humans and mice express alternative subtypes of immunoglobulins as humans express IgG 1–4 and two subtypes of IgA, IgA1 and IgA2, whereas mice have an alternative IgG 2b, no IgG 4 and only IgA1 [113]. Furthermore, humans and mice also express different IgG receptors with different affinities and expression patterns [114]. This could have significant impact on potential therapeutics seeking to induce or target antibody production against atherosclerosis. Considering these fundamental differences between humans and mice, a stronger emphasis should be placed on assessing the impact of B cells on atherosclerosis in humans both through large cohort studies and basic research using human tissue and blood samples as well as plaque characterization with different imaging modalities. Though strong evidence exists that B cells both protect from and contribute to atherosclerosis in mice, it is of great importance that we determine the impact of B cells on atherosclerosis and CV events in humans. Only then will novel disease biomarkers or targets for treatment emerge.
Expert commentary
In this review we have touched on the seminal findings that were used to describe the potential role of B cells in atherosclerosis in animal models and humans. We addressed the complex contributions of different B cell subsets that are still being described and speculated on their clinical implications. Finally, we discussed possible clinical benefits that could be derived from an improved understanding of B cells in atherosclerosis in the development of novel risk factors and biomarkers as well as novel immunotherapies.
The field of B cells and atherosclerosis has taken several large steps forward recently with the findings that there are subset and context specific differences in B cell function in cardiovascular disease. However, as described in this review, there is still a great deal left to uncover pertaining to B cell subsets in humans and their function in conditions that lead to atherosclerosis. The two recent human studies that used GWAS and laser capture microdissection to assess important genes in CVD and B cells within human vasculature respectively are strong examples of where the field needs to move in the future [20,52]. With new high throughput genetic technologies it will be important to begin studying the genetic and epigenetic components of B cell subset development and whether there is a population of patients that have skewed B cell subsets that put them at higher risk for CVD. Furthermore, the wide-scale study of SNPs in genes important for B cell development and function could reveal novel targets for immune modulation. For instance, our lab previously published that a human SNP within the coding region of the id3 gene, which is expressed in approximately 25% of humans, associates with atherosclerosis as assessed by intimal-medial thickness [115]. Id3 is an important atheroprotective transcription factor and it regulates B cell trafficking to the aorta in mice [34]. It is our hypothesis that the human id3 SNP is important to human atherosclerosis, in part, through its function in B cells.
One of the greatest limitations in the field is our dependence on immune depleted mouse models such as SCID, Rag, or μMT which are deficient for various leukocytes. The advantage of these models is the ability to do adoptive transfer experiments without immune rejection. However, eliminating leukocytes from the model also eliminates interacting partners and severely modifies the complex network of regulation that makes up the immune system limiting what we are able to conclude from any of our findings and leading to disparate findings based on model, cell of origin, or prior cell stimulus. This is not to say these models are not useful or question the findings derived from them but it will be important for the field to embrace cell specific strategies and to commit ourselves to finding more immune-sufficient animal models.
The greatest potential treatment against CVD is immunization against atherogenic antigens. Considering scale and cost, a vaccine against atherosclerosis could potentially have great impact when added with statin therapy and lifestyle change to help deal with CVD, still the largest world-wide contributor to morbidity and mortality even with improved recognition and treatment [1]. At a time when health care costs have skyrocketed, it is important to focus on treatments that are both effective and cost-effective.
Five year view
Recognizing how much our knowledge has expanded in the last five years with the description of a possible human B1 cell equivalent and the recognition that B cells have subset and context dependent differences in how they respond in animal models of atherosclerosis, the next five years will potentially add a great deal more to our understanding of human and murine B cells subsets and their contributions to atherosclerosis. We believe through increased emphasis on studying human B cells and the discovery of additional surface markers that describe minor B cell populations, this will get us closer to revealing novel at-risk populations and developing targeted immune therapy for the treatment of atherosclerosis. Furthermore, a greater understanding of the target antigens that induce B cell response in CVD will lead to possible targets for immunization. We expect that early clinical trials of immunization will need to focus on high risk subgroups in order to show differences in clinical events. Additionally, more refined studies to determine the benefits garnered from immunotherapies will show whether there is a potential downside to B cell depletion or if this could be used as a viable treatment option in the future. These will be important steps as we continue to determine precisely how B cells contribute to CVD and how we can use them clinically as biomarkers or targets for therapy.
Figure 2.
Meta-analysis of RTX treatment in rheumatologic diseases shows no correlation with cardiovascular events. Forest plot showing RTX treatment vs. standard therapy looking at adverse cardiovascular events. Odds Ratio 0.98, p=0.95, I2=0%.
Key Issues.
Recent findings in human studies have shown that B cell activation is strongly linked to cardiovascular disease
B cells aggregate at sites of lesion formation primarily in the adventitia
Research in animal models of atherosclerosis has demonstrated that B cell subsets have distinct roles with innate B1 B cells having atheroprotective functions and adaptive B2 B cells having atherogenic functions though this may be too simplistic of an understanding
Circulating immunoglobulins and surface markers on peripheral B cells have the potential to be used as novel biomarkers of CVD
A meta-analysis of B cell depletion therapy in rheumatological diseases does not associate with short-term cardiovascular events
There is great potential in the use of immunization therapy to vaccinate against atherogenic antigens such as those expressed on modified LDL
Cited Literature
- 1.Laslett Lj, Alagona P, Jr, Clark Ba, 3rd, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(25 Suppl):S1–49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Hansson Gk, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 3.Tabas I, Williams Kj, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116(16):1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 4.Wilson Pw. Established risk factors and coronary artery disease: the Framingham Study. Am J Hypertens. 1994;7(7 Pt 2):7S–12S. doi: 10.1093/ajh/7.7.7s. [DOI] [PubMed] [Google Scholar]
- 5.Canto Jg, Iskandrian Ae. Major risk factors for cardiovascular disease: debunking the “only 50%” myth. JAMA. 2003;290(7):947–949. doi: 10.1001/jama.290.7.947. [DOI] [PubMed] [Google Scholar]
- 6.Petretta M, Cuocolo A. In search of a marker of vulnerable carotid plaque: is the key in the heart? Atherosclerosis. 2012;223(1):95–97. doi: 10.1016/j.atherosclerosis.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Kannel Wb. Some lessons in cardiovascular epidemiology from Framingham. Am J Cardiol. 1976;37(2):269–282. doi: 10.1016/0002-9149(76)90323-4. [DOI] [PubMed] [Google Scholar]
- 8.Ambrose Ja, Winters Sl, Arora Rr, et al. Angiographic evolution of coronary artery morphology in unstable angina. J Am Coll Cardiol. 1986;7(3):472–478. doi: 10.1016/s0735-1097(86)80455-7. [DOI] [PubMed] [Google Scholar]
- 9.Ambrose Ja, Tannenbaum Ma, Alexopoulos D, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12(1):56–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 10.Little Wc, Constantinescu M, Applegate Rj, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78(5 Pt 1):1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 11.Libby P, Lichtman Ah, Hansson Gk. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38(6):1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8(10):802–815. doi: 10.1038/nri2415. Reviews innate and adaptive leukocytes and their contributions to atherosclerosis. [DOI] [PubMed] [Google Scholar]
- 13.Lichtman Ah, Binder Cj, Tsimikas S, Witztum Jl. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest. 2013;123(1):27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swirski Fk, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Perry Hm, Bender Tp, Mcnamara Ca. B cell subsets in atherosclerosis. Front Immunol. 2012;3:373. doi: 10.3389/fimmu.2012.00373. A detailed review of the literature on murine research into B cells in atherosclerosis. Thoroughly discusses the subset specific differences between B1 and B2 B cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerlis Lm. The significance of adventitial infiltrations in coronary atherosclerosis. Br Heart J. 1956;18(2):166–172. doi: 10.1136/hrt.18.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houtkamp Ma, De Boer Oj, Van Der Loos Cm, Van Der Wal Ac, Becker Ae. Adventitial infiltrates associated with advanced atherosclerotic plaques: structural organization suggests generation of local humoral immune responses. J Pathol. 2001;193(2):263–269. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH774>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 18.Ramshaw Al, Parums Dv. Immunohistochemical characterization of inflammatory cells associated with advanced atherosclerosis. Histopathology. 1990;17(6):543–552. doi: 10.1111/j.1365-2559.1990.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhou X, Hansson Gk. Detection of B cells and proinflammatory cytokines in atherosclerotic plaques of hypercholesterolaemic apolipoprotein E knockout mice. Scand J Immunol. 1999;50(1):25–30. doi: 10.1046/j.1365-3083.1999.00559.x. [DOI] [PubMed] [Google Scholar]
- 20•.Hamze M, Desmetz C, Berthe Ml, et al. Characterization of Resident B Cells of Vascular Walls in Human Atherosclerotic Patients. J Immunol. 2013 doi: 10.4049/jimmunol.1202870. Study of human coronary arteries that used laser capture microdissection to compare gene expression of lymphocytes in the intimal lesion and the adventitia. Showed adventitial B cells have a pre-plasmablast phenotype. [DOI] [PubMed] [Google Scholar]
- 21.Grabner R, Lotzer K, Dopping S, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206(1):233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moos Mp, John N, Grabner R, et al. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25(11):2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 23•.Caligiuri G, Nicoletti A, Poirier B, Hansson Gk. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109(6):745–753. doi: 10.1172/JCI07272. Study of atherogenic mice showing splenectomy caused significantly greater atherosclerosis to develop which was attenuated by adoptive transfer of B cells. Suggested B cells have atheroprotective function in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Major As, Fazio S, Linton Mf. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22(11):1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 25.Yla-Herttuala S, Palinski W, Butler Sw, Picard S, Steinberg D, Witztum Jl. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14(1):32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 26.Palinski W, Miller E, Witztum Jl. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci U S A. 1995;92(3):821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang Mk, Bergmark C, Laurila A, et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci U S A. 1999;96(11):6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis Mj, Malik Th, Ehrenstein Mr, Boyle Jj, Botto M, Haskard Do. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120(5):417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Kyaw T, Tay C, Khan A, et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185(7):4410–4419. doi: 10.4049/jimmunol.1000033. One of several papers to show that B2 B cell depletion attenuates the development of atherosclerosis. Also presents evidence that adoptive transfer of B2 B cells increases development of atherosclerosis. [DOI] [PubMed] [Google Scholar]
- 30.Ait-Oufella H, Herbin O, Bouaziz Jd, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2011;207(8):1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyaw T, Tay C, Hosseini H, et al. Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PLoS One. 2012;7(1):e29371. doi: 10.1371/journal.pone.0029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sage Ap, Tsiantoulas D, Baker L, et al. BAFF receptor deficiency reduces the development of atherosclerosis in mice--brief report. Arterioscler Thromb Vasc Biol. 2012;32(7):1573–1576. doi: 10.1161/ATVBAHA.111.244731. [DOI] [PubMed] [Google Scholar]
- 33.Kyaw T, Tay C, Krishnamurthi S, et al. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109(8):830–840. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 34.Doran Ac, Lipinski Mj, Oldham Sn, et al. B-cell aortic homing and atheroprotection depend on Id3. Circ Res. 2012;110(1):e1–12. doi: 10.1161/CIRCRESAHA.111.256438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipinski Mj, Perry Hm, Doran Ac, Oldham Sn, Mcnamara Ca. Comment on “Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis”. J Immunol. 2011;186(1):4. doi: 10.4049/jimmunol.1090119. author reply 6. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz Mj, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108(4):1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barber Cl, Montecino-Rodriguez E, Dorshkind K. Developmental relationships between B-1 and B-2 progenitors. Cell Cycle. 2011;10(22):3810–3811. doi: 10.4161/cc.10.22.18190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pennell Ca, Mercolino Tj, Grdina Ta, Arnold Lw, Haughton G, Clarke Sh. Biased immunoglobulin variable region gene expression by Ly-1 B cells due to clonal selection. Eur J Immunol. 1989;19(7):1289–1295. doi: 10.1002/eji.1830190721. [DOI] [PubMed] [Google Scholar]
- 39.Mercolino Tj, Locke Al, Afshari A, et al. Restricted immunoglobulin variable region gene usage by normal Ly-1 (CD5+) B cells that recognize phosphatidyl choline. J Exp Med. 1989;169(6):1869–1877. doi: 10.1084/jem.169.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binder Cj. Natural IgM antibodies against oxidation-specific epitopes. J Clin Immunol. 2010;30 (Suppl 1):S56–60. doi: 10.1007/s10875-010-9396-3. [DOI] [PubMed] [Google Scholar]
- 41.Chou My, Fogelstrand L, Hartvigsen K, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119(5):1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binder Cj, Horkko S, Dewan A, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9(6):736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 43.Binder Cj, Hartvigsen K, Chang Mk, et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114(3):427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rauch Pj, Chudnovskiy A, Robbins Cs, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335(6068):597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Gregoli K, Johnson Jl. Role of colony-stimulating factors in atherosclerosis. Curr Opin Lipidol. 2012;23(5):412–421. doi: 10.1097/MOL.0b013e328357ca6e. [DOI] [PubMed] [Google Scholar]
- 46.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 47.Dilillo Dj, Matsushita T, Tedder Tf. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 48.Vitale G, Mion F, Pucillo C. Regulatory B cells: evidence, developmental origin and population diversity. Mol Immunol. 2010;48(1–3):1–8. doi: 10.1016/j.molimm.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(5):969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 50.Blair Pa, Norena Ly, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol. 2008;64(2):187–199. doi: 10.1002/ana.21438. [DOI] [PubMed] [Google Scholar]
- 52•.Huan T, Zhang B, Wang Z, et al. A systems biology framework identifies molecular underpinnings of coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33(6):1427–1434. doi: 10.1161/ATVBAHA.112.300112. Study used genome wide association to develop gene expression families increased in human atherosclerosis. Showed B cell activation genes were greatly reduced in expression in CVD patients suggesting B cells have protective capacity in human atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanigawa T, Kitamura A, Yamagishi K, et al. Relationships of differential leukocyte and lymphocyte subpopulations with carotid atherosclerosis in elderly men. J Clin Immunol. 2003;23(6):469–476. doi: 10.1023/b:joci.0000010423.65719.e5. [DOI] [PubMed] [Google Scholar]
- 54.Lee J, Kuchen S, Fischer R, Chang S, Lipsky Pe. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009;182(7):4116–4126. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 55•.Griffin Do, Holodick Ne, Rothstein Tl. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208(1):67–80. doi: 10.1084/jem.20101499. First study to describe the human B1 B cell equivalent. Found B cells that spontaneously produced antibody, interacted with T cells, and self-replicated in umbilical cords and peripheral blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffin Do, Rothstein Tl. Human “orchestrator” CD11b(+) B1 cells spontaneously secrete interleukin-10 and regulate T-cell activity. Mol Med. 2012;18:1003–1008. doi: 10.2119/molmed.2012.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Descatoire M, Weill Jc, Reynaud Ca, Weller S. A human equivalent of mouse B-1 cells? J Exp Med. 2011;208(13):2563–2564. doi: 10.1084/jem.20112232. author reply 2566–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reynaud Ca, Weill Jc. Gene profiling of CD11b(+) and CD11b(−) B1 cell subsets reveals potential cell sorting artifacts. J Exp Med. 2012;209(3):433–434. doi: 10.1084/jem.20120402. author reply 434–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perez-Andres M, Grosserichter-Wagener C, Teodosio C, Van Dongen Jj, Orfao A, Van Zelm Mc. The nature of circulating CD27+CD43+ B cells. J Exp Med. 2011;208(13):2565–2566. doi: 10.1084/jem.20112203. author reply 2566–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griffin Do, Rothstein Tl. Human b1 cell frequency: isolation and analysis of human b1 cells. Front Immunol. 2012;3:122. doi: 10.3389/fimmu.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11(1):34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 62.Covens K, Verbinnen B, Geukens N, et al. Characterization of proposed human B-1 cells reveals pre-plasmablast phenotype. Blood. 2013;121(26):5176–5183. doi: 10.1182/blood-2012-12-471953. [DOI] [PubMed] [Google Scholar]
- 63.Coller Bs. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler Thromb Vasc Biol. 2005;25(4):658–670. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- 64.Lipinski Mj, Martin Re, Cowley Mj, et al. Effect of statins and white blood cell count on mortality in patients with ischemic left ventricular dysfunction undergoing percutaneous coronary intervention. Clin Cardiol. 2006;29(1):36–41. doi: 10.1002/clc.4960290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asadollahi K, Beeching Nj, Gill Gv. Leukocytosis as a predictor for non-infective mortality and morbidity. QJM. 2010;103(5):285–292. doi: 10.1093/qjmed/hcp182. [DOI] [PubMed] [Google Scholar]
- 66.Barron Hv, Cannon Cp, Murphy Sa, Braunwald E, Gibson Cm. Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction: a thrombolysis in myocardial infarction 10 substudy. Circulation. 2000;102(19):2329–2334. doi: 10.1161/01.cir.102.19.2329. [DOI] [PubMed] [Google Scholar]
- 67.Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225(2):456–460. doi: 10.1016/j.atherosclerosis.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Sbrana F, Cocci F, Papa A, et al. Routine laboratory tests to risk-stratify patients with chronic coronary artery disease. J Cardiol. 2013;61(2):132–137. doi: 10.1016/j.jjcc.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Tsimikas S, Brilakis Es, Lennon Rj, et al. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48(2):425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 70.Muscari A, Bozzoli C, Gerratana C, et al. Association of serum IgA and C4 with severe atherosclerosis. Atherosclerosis. 1988;74(1–2):179–186. doi: 10.1016/0021-9150(88)90204-3. [DOI] [PubMed] [Google Scholar]
- 71.Choi Sh, Chae A, Miller E, et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J Am Coll Cardiol. 2008;52(1):24–32. doi: 10.1016/j.jacc.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 72.Fraley Ae, Schwartz Gg, Olsson Ag, et al. Relationship of oxidized phospholipids and biomarkers of oxidized low-density lipoprotein with cardiovascular risk factors, inflammatory biomarkers, and effect of statin therapy in patients with acute coronary syndromes: Results from the MIRACL (Myocardial Ischemia Reduction With Aggressive Cholesterol Lowering) trial. J Am Coll Cardiol. 2009;53(23):2186–2196. doi: 10.1016/j.jacc.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 73.Bjorkbacka H, Fredrikson Gn, Nilsson J. Emerging biomarkers and intervention targets for immune-modulation of atherosclerosis - a review of the experimental evidence. Atherosclerosis. 2013;227(1):9–17. doi: 10.1016/j.atherosclerosis.2012.10.074. [DOI] [PubMed] [Google Scholar]
- 74.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock Ij, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203(5):1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campbell Ka, Lipinski Mj, Doran Ac, Skaflen Md, Fuster V, Mcnamara Ca. Lymphocytes and the adventitial immune response in atherosclerosis. Circ Res. 2012;110(6):889–900. doi: 10.1161/CIRCRESAHA.111.263186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sampi M, Ukkola O, Paivansalo M, Kesaniemi Ya, Binder Cj, Horkko S. Plasma interleukin-5 levels are related to antibodies binding to oxidized low-density lipoprotein and to decreased subclinical atherosclerosis. J Am Coll Cardiol. 2008;52(17):1370–1378. doi: 10.1016/j.jacc.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 77.Hollan I, Meroni Pl, Ahearn Jm, et al. Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev. 2013 doi: 10.1016/j.autrev.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 78.Zinger H, Sherer Y, Shoenfeld Y. Atherosclerosis in autoimmune rheumatic diseases-mechanisms and clinical findings. Clin Rev Allergy Immunol. 2009;37(1):20–28. doi: 10.1007/s12016-008-8094-x. [DOI] [PubMed] [Google Scholar]
- 79.Aubry Mc, Maradit-Kremers H, Reinalda Ms, Crowson Cs, Edwards Wd, Gabriel Se. Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol. 2007;34(5):937–942. [PubMed] [Google Scholar]
- 80.Moura Ra, Graca L, Fonseca Je. To B or not to B the conductor of rheumatoid arthritis orchestra. Clin Rev Allergy Immunol. 2012;43(3):281–291. doi: 10.1007/s12016-012-8318-y. [DOI] [PubMed] [Google Scholar]
- 81.O’neill Sk, Shlomchik Mj, Glant Tt, Cao Y, Doodes Pd, Finnegan A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol. 2005;174(6):3781–3788. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 82.Furst De, Breedveld Fc, Kalden, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2007. Ann Rheum Dis. 2007;66(Suppl 3):iii2–22. doi: 10.1136/ard.2007.081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teng Yk, Wheater G, Hogan Ve, et al. Induction of long-term B-cell depletion in refractory rheumatoid arthritis patients preferentially affects autoreactive more than protective humoral immunity. Arthritis Res Ther. 2012;14(2):R57. doi: 10.1186/ar3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benucci M, Saviola G, Manfredi M, Sarzi-Puttini P, Atzeni F. Factors correlated with improvement of endothelial dysfunction during rituximab therapy in patients with rheumatoid arthritis. Biologics. 2013;7:69–75. doi: 10.2147/BTT.S39182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kerekes G, Soltesz P, Der H, et al. Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin Rheumatol. 2009;28(6):705–710. doi: 10.1007/s10067-009-1095-1. [DOI] [PubMed] [Google Scholar]
- 86.Raterman Hg, Levels H, Voskuyl Ae, Lems Wf, Dijkmans Ba, Nurmohamed Mt. HDL protein composition alters from proatherogenic into less atherogenic and proinflammatory in rheumatoid arthritis patients responding to rituximab. Ann Rheum Dis. 2013;72(4):560–565. doi: 10.1136/annrheumdis-2011-201228. [DOI] [PubMed] [Google Scholar]
- 87.Mathieu S, Pereira B, Dubost Jj, Lusson Jr, Soubrier M. No significant change in arterial stiffness in RA after 6 months and 1 year of rituximab treatment. Rheumatology (Oxford) 2012;51(6):1107–1111. doi: 10.1093/rheumatology/kes006. [DOI] [PubMed] [Google Scholar]
- 88.Cohen Sb, Emery P, Greenwald Mw, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 89.Edwards Jc, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 90.Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54(5):1390–1400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 91.Emery P, Deodhar A, Rigby Wf, et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE)) Ann Rheum Dis. 2010;69(9):1629–1635. doi: 10.1136/ard.2009.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Greenwald Mw, Shergy Wj, Kaine Jl, Sweetser Mt, Gilder K, Linnik Md. Evaluation of the safety of rituximab in combination with a tumor necrosis factor inhibitor and methotrexate in patients with active rheumatoid arthritis: results from a randomized controlled trial. Arthritis Rheum. 2011;63(3):622–632. doi: 10.1002/art.30194. [DOI] [PubMed] [Google Scholar]
- 93.Tak Pp, Rigby Wf, Rubbert-Roth A, et al. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Ann Rheum Dis. 2011;70(1):39–46. doi: 10.1136/ard.2010.137703. [DOI] [PubMed] [Google Scholar]
- 94.Davatchi F, Shams H, Rezaipoor M, et al. Rituximab in intractable ocular lesions of Behcet’s disease; randomized single-blind control study (pilot study) Int J Rheum Dis. 2010;13(3):246–252. doi: 10.1111/j.1756-185X.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 95.De Vita S, Quartuccio L, Isola M, et al. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64(3):843–853. doi: 10.1002/art.34331. [DOI] [PubMed] [Google Scholar]
- 96.Jones Rb, Tervaert Jw, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363(3):211–220. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 97.Sneller Mc, Hu Z, Langford Ca. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64(3):835–842. doi: 10.1002/art.34322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stone Jh, Merkel Pa, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Merrill Jt, Neuwelt Cm, Wallace Dj, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62(1):222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rovin Bh, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 101.Dass S, Bowman Sj, Vital Em, et al. Reduction of fatigue in Sjogren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann Rheum Dis. 2008;67(11):1541–1544. doi: 10.1136/ard.2007.083865. [DOI] [PubMed] [Google Scholar]
- 102.Meijer Jm, Meiners Pm, Vissink A, et al. Effectiveness of rituximab treatment in primary Sjogren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(4):960–968. doi: 10.1002/art.27314. [DOI] [PubMed] [Google Scholar]
- 103.Pescovitz Md, Greenbaum Cj, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Daoussis D, Liossis Sn, Tsamandas Ac, et al. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology (Oxford) 2010;49(2):271–280. doi: 10.1093/rheumatology/kep093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8(6):348–358. doi: 10.1038/nrcardio.2011.62. [DOI] [PubMed] [Google Scholar]
- 106.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9(7):491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 107.Carbonatto M, Yu P, Bertolino M, et al. Nonclinical safety, pharmacokinetics, and pharmacodynamics of atacicept. Toxicol Sci. 2008;105(1):200–210. doi: 10.1093/toxsci/kfn105. [DOI] [PubMed] [Google Scholar]
- 108.Yang M, Sun L, Wang S, et al. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol. 2010;184(7):3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- 109.Leibundgut G, Witztum Jl, Tsimikas S. Oxidation-specific epitopes and immunological responses: Translational biotheranostic implications for atherosclerosis. Curr Opin Pharmacol. 2013 doi: 10.1016/j.coph.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herbin O, Ait-Oufella H, Yu W, et al. Regulatory T-cell response to apolipoprotein B100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32(3):605–612. doi: 10.1161/ATVBAHA.111.242800. [DOI] [PubMed] [Google Scholar]
- 111.Carter Na, Rosser Ec, Mauri C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res Ther. 2012;14(1):R32. doi: 10.1186/ar3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bentzon Jf, Falk E. Atherosclerotic lesions in mouse and man: is it the same disease? Curr Opin Lipidol. 2010;21(5):434–440. doi: 10.1097/MOL.0b013e32833ded6a. [DOI] [PubMed] [Google Scholar]
- 113•.Mestas J, Hughes Cc. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. Review of the immunological differences between humans and commonly used mouse models. [DOI] [PubMed] [Google Scholar]
- 114.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119(24):5640–5649. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 115.Doran Ac, Lehtinen Ab, Meller N, et al. Id3 is a novel atheroprotective factor containing a functionally significant single-nucleotide polymorphism associated with intima-media thickness in humans. Circ Res. 2010;106(7):1303–1311. doi: 10.1161/CIRCRESAHA.109.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]