Abstract
Aims
to specify ophthalmic findings in multi systemic smooth muscle dysfunction syndrome, recently clarified as due to a R179H mutation in the ACTA2 gene encoding smooth muscle cell α-actin.
Methods
An observational clinical series of three children who underwent full ophthalmic evaluation.
Results and conclusions
a) congenital mydriasis with wisps from the iris collarette appears as the primary marker of the rare disorder. b) the main fundus finding is arteriolar tortuosity from infancy, and with a trend of slight progression. One case had vessel loops and minute breaks of the blood-retina barrier in a temporal sector. c) defective accommodation was documented in 2 of the 3 cases. d) the poor imaging condition from day one after delivery was not amblyopiogenic and apparently did not hamper subsequent visual development. e) the paediatric ophthalmologist should be part of a multi-specialist team due to the risk of systemic smooth muscle related complications, among them primarily serious cardiac and cerebrovascular pathology.
Keywords: Fixed dilated pupil, Patent duct, Tortuous retinal vessels, ACTA2 gene
INTRODUCTION
Multi systemic smooth muscle dysfunction syndrome (OMIM # 613834) is a recently described genetic disease. Clinically, it is characterized by dysfunction of smooth muscle throughout the body leading to congenital fixed dilated pupils with defective accommodation, persistent ductus arteriosus, aortic and cerebrovascular disease, hypotonic bladder, intestinal hypoperistalsis and pulmonary hypertension. It is due to a p.R179H mutation in the ACTA2 gene, chromosome 10q23.3 [1] that is predicted to express a mutant α-actin in smooth muscles. The original paper on the mutation presented 7 patients with the congenital iris anomaly but their retinal vascular findings were not reported.
Congenital mydriasis is an extremely rare pupil anomaly and is very important in helping with the early diagnosis of this condition. Furthermore, regular ophthalmic follow-up is important as well as recognition of associated cardiac and cerebrovascular pathology and severe neurological sequelae.
CASE STORIES
Case No 1
A full term baby born after an uneventful pregnancy and delivery, had tachypnoe from day 1. A few weeks old echocardiography and heart catheterization demonstrated hypertrophy of the right ventricle, grossly dilated pulmonary arteries, a patent foramen ovale, and a wide patent ductus arteriosus which was ligated. As a toddler the ascending aorta was markedly enlarged.
Psychomotor development was normal. As a young school child a transient left hemiparesis occurred. MR imaging and MR angiography (MRI/MRA) demonstrated a right hemisphere infarction considered non-acute with bilateral internal carotid artery dilatation proximal to the occlusion starting at the terminal portion of the internal carotid artery. The child underwent a two-step bilateral neurosurgical bypass for cerebral revascularization, complicated by an extensive non-haemorrhagic infarction of the right hemisphere. After partial recovery, the patient unexpectedly died 2 years later. At autopsy there was no new brain pathology, nor dissection or rupture of greater vessels. (Case A). [1]
Eye findings
Ophthalmic exam a few weeks old showed fixed dilated 6 mm pupils, unresponsive to light and cycloplegic eye drops. There was no iris tissue centrally to the collarette, where remnants of the pupillary membrane were prominent as a delicate rete. Fundus vessels were somewhat tortuous. Glasses correcting a +3 D hyperopia were used as a toddler. Distance acuity of around 0.8 was recorded throughout. The patient could read fine print with bifocals; the accommodative capacity was not specified.
Case No 2
A full term baby born after an uneventful pregnancy and delivery presented with tachypnoe at day 4. Echocardiography disclosed a wide patent ductus arteriosus, which was ligated. Psychomotor development was normal until a few years old when headaches and impulsive/hyperactive behaviour began. MRI showed white matter abnormalities characterized by an increased T2 signal intensity, particularly in the frontal white matter. One year later transient left hemiparesis occurred, and MRI revealed a right hemisphere infarction. Direct angiography showed bilateral internal carotid artery occlusion. The patient recovered after bilateral by-pass surgery.
Repeated echocardiograms were normal until the early teens when ascending aorta was dilated; there also was a hypotonic bladder. A few years later MRI and MRA showed no further changes. (Case B). [1]
Eye findings
Except for fixed dilated pupils, eye examination including ophthalmoscopy during the newborn period was normal. As a toddler visual acuity was >0.7 (−1.75 D), and markedly tortuous retinal arterioles were observed. At school age the patient had 6 mm mydriasis which showed no change after cyclopentolate. Blood-filled thin vessels crossing the lens surface originated from irregular wisps at the iris collarette. (Figure 1 &2) The myopia insidiously increased to the present teenage level of −5 D; near vision without spectacles was normal (0.3/0.3).
Figure 1.
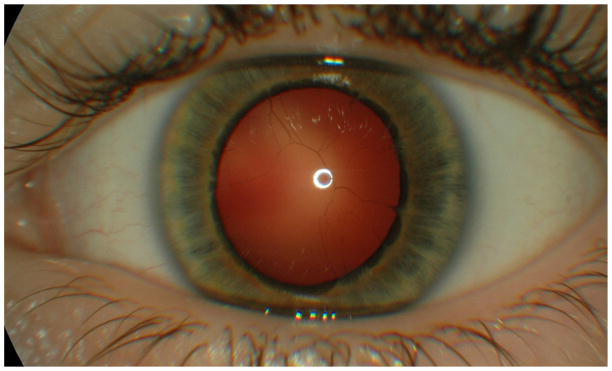
Blood filled vessels in the congenitally dilated pupil. Irregular wisps originating from the collarette
Fig 2.
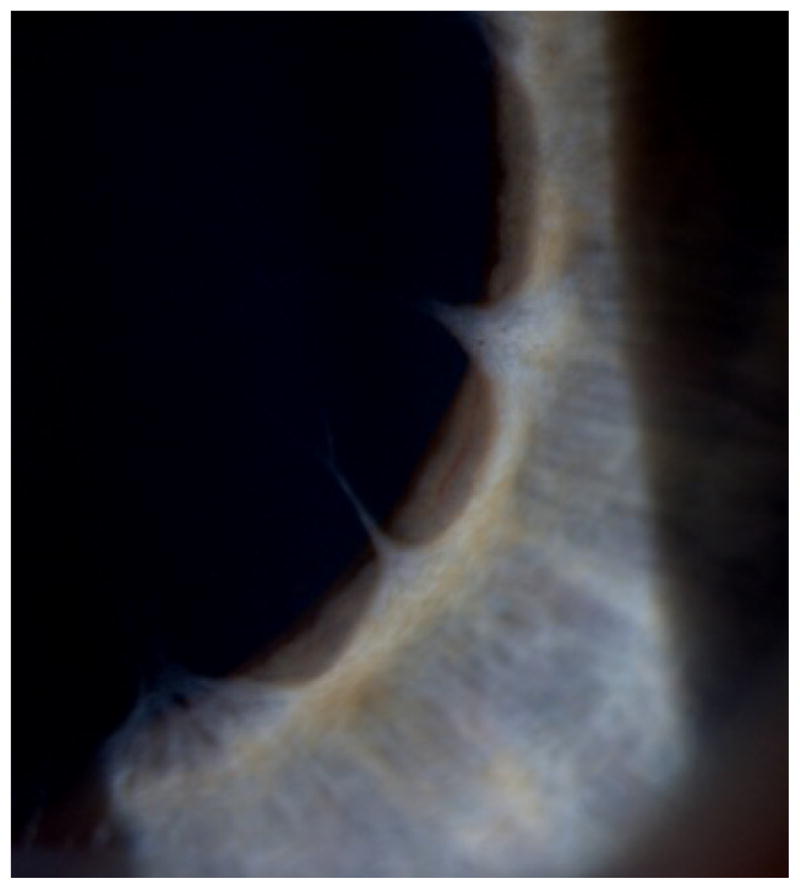
Irregular wisps originating from the collarette in high magnification
As a young teenager, the patient presented dilated tortuous arterioles and precapillary vessels loops, (Figure 3). Later occasional minute hemorrhages were observed. Fluorescein angiography disclosed minor late leakage points, (Figure 4) and focal laser burns were applied. Angiographies repeated with a one year interval still showed vessel loops but no leakage.
Figure 3.
Minute hemorrhages and minor leakage points in fluorescens angiography
Figure 4.
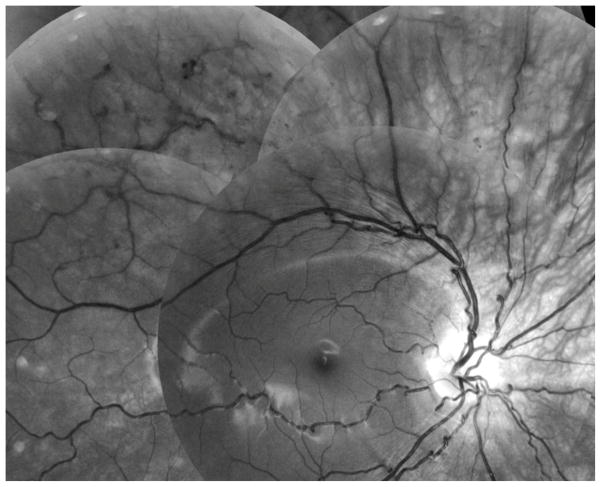
Fundus vessels showing loopy arterioles, case 2
As a teenager corrected visual acuity remained 1.0 and 0.8 (−5D). Wearing myopia correction, the patient could only decipher larger print, but not the small N 4 1/4 standard print within the 50 cm range of the Royal Air Force (R.A.F) near vision ruler. A near add of 2 D however allowed such fine reading at a distance of 26 cm, suggesting an accommodative capacity close to 2 D. Ishihara and contrast sensitivity (Pelli-Robson) tests were normal, and semidarkness did not impair the distance visual chart reading. Kinetic Goldman perimetry was normal except when tested for a small object (I, 4e) which showed an inferior altitudinal relative defect in the left eye whereas the laser treated right eye had only marginal visual field loss. Right and left eye axial lengths were 24.0 mm (IOL-Master) and corneal curvature radius averaged 7.69 mm.
Case No 3
A baby born full term, with an uneventful pregnancy and delivery, on day 1 had tachypnoe and tachycardia. A few weeks old the patient suffered a cardiac arrest related to intravenous amiodarone medication (administered for a transient cardiac arrhythmia) and was resuscitated. A patent ductus arteriosus was ligated a few weeks old. After extubation a left vocal cord paralysis was demonstrated. Subsequent psychomotor development appeared normal.
As a young school child, on suspicion of the present syndrome, MRI of brain, heart and great vessels was performed, as well as ultrasonography of the urinary tract. White matter abnormalities of brain were described, without frank ischemic lesions. The ascending aorta was dilated, and there was hypotonic bladder dysfunction. Genetic testing revealed a de novo ACTA2 missense mutation p.R179L.
Eye findings
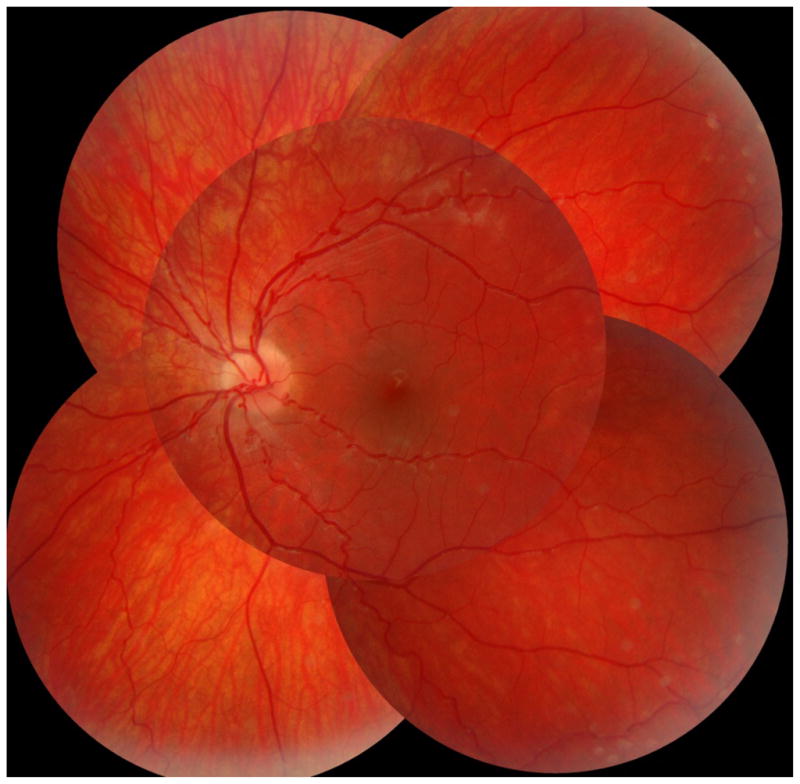
At age 4 months, the 5 mm pupils were non-reactive to light as well as to pilocarpine and epinephrine. Slight retinal vessel tortuousity was first noted as a young toddler and increased since then. (Figure 5) A few years old, tinted glasses with + 2D were prescribed, and a visual acuity of 0.8 recorded, but the child rejected using spectacles. As a young school child visual acuities were 0.8 and 1.0, irrespective of correction (+2.0 D and +0.75 D). Near points of accommodation (NPA) on the R.A.F ruler could only be recorded using a reading add of +2 D; near vision was 0.3/0.75, which means lower than corresponding to his distance acuities. Eyes were straight and eye movements normal. Even gross Titmus stereopsis appeared absent. Ishihara testing normal. Visual fields for finger movements and on tangent screen (white 5/1000) were normal. Visual problems in darkness were not observed. Nidek autokeratorefractometry readings were identical before and after cyclopentolate. Fundus photos showed markedly tortuous arterioles, but no indication of microvascular changes. For ethical reasons (age), a fluorescein angiography was not done. Axial lengths and mean corneal curvature radius were about 21.8 mm (IOL-Master) and 7.79 mm, respectively.
Figure 5.
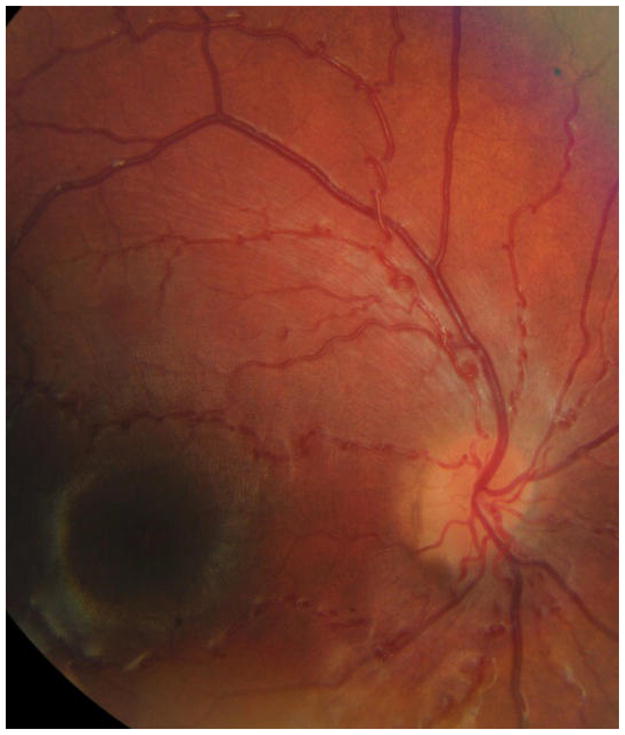
Fundus vessels showing loopy arterioles, Case 3
DICUSSION
Recently, we reported that de novo mutation in ACTA2 (R179H) causes a syndrome characterized by dysfunction of smooth muscle throughout the body, leading to aortic and cerebrovascular disease, fixed dilated pupils, hypotonic bladder, malrotation and hypoperistalsis of the gut, and pulmonary hypertension (Multi Systemic Smooth Muscle Dysfunction Syndrome; OMIM # 613834). Two of the present cases were included in the original paper describing this syndrome, [1] and case 3 has a de novo ACTA2 missense mutation R179L responsible for quite similar clinical manifestations.
The combination of congenital fixed dilated pupil and patent ductus arteriosus was present in all patients known with this syndrome. As this pattern recognition should give a definite diagnostic clue, we find it important to describe the ophthalmologic findings in detail.
Anterior eye segment
In reported cases of congenital mydriasis, a grey or pale iris is usually described as hypoplastic with a flat contour devoid of crypts, and absent pupil contraction to light and convergence.[2] There is also no pharmacological response to pilocarpine and epinephrine. Filiform strands or wisps as the outer part of a persistent pupillary rete may convey a scalloping borderline contour to the collarette, (Figure 1 & 2) and the pupillary sfincter muscle appears absent. Such features may not be specific for the ACTA2 mutation, but should be a strong reminder of this diagnostic possibility when observed in neonates.
Less is known about the ciliary muscle function in the few patients published with large fixed pupils. The functional state shows similarity to what is observed after anticholinergic eyedrops, i.e. fixed mydriasis and markedly reduced or abolished accommodation. With regard to pupillotonia, the tonic pupil of Adie’s syndrome is not invariably large, and accommodation may be reduced, or recover/remain unaffected, respectively.[3] In our cases No. 2 and 3, a markedly reduced accommodation was confirmed by relevant testing. The blood filled vessels of the pupil are remnants of non regressed fetal vessels – like the patent duct.
Posterior eye segment
Based primarily on own data, retinal vessels may be normal in the neonatal period, but arteriolar tortuousity soon develops (Figure 3–5), a pattern also reported elsewhere.[4] In our oldest patient (No. 2) we observed focal loops at the junction between arteriole and capillary in a 40° mid-periphery sector. (Figure 3–4) In the photos, if directed towards the camera the loops imitated aneurysmal dilatations. Venules generally appeared unaffected.
Early retinal tortuousity is mainly considered an occasional finding on routine fundus examination. When slight or moderate, it is regarded ‘constitutional’ and unrelated to eye pathology. An exception is the general retinal tortuousity sometimes observed as a permanent neonatal sequel to advanced retinopathy of prematurity (ROP), making up a contrast to the later onset and progression as in those with the ACTA2 R179H or R179L mutation under study.[5] Later onset of tortuosity has been observed in various hereditary lysosymal storage diseases, considered due to accumulation of abnormal metabolites in endothelial and smooth muscle cells, and is observed also in Loeys-Dietz syndrome.[6–7] To our knowledge, there are no other reports of infant or childhood tortuousity associated with congenital mydriasis manifesting during the first year(s) of life and probably increasing over time.
Most related clinical reports are not specific regarding visual parameters and details of the ocular fundus. Funduscopy was merely recorded as normal in the few children published in the past, who also exhibited congenital mydriasis and surgery for patent ductus arteriosus. [2,8–10] Brain imaging data and genetic testing were not given, except in the case of Ades, [8] which was included in the original description of the present syndrome.[1]
Eye size and stature
Except for the findings mentioned above, the eyes and ophthalmic regions were without dysmorphology. Axial eye lengths were measured in two of our cases. Both were within normal range, though in the short tail of the distribution according to refraction and age.
Fluorescence angiography of retinal vessels
Patient No. 2 had a fluorescence angiography performed at age 14 years. It depicted abnormally tortuous arterioles, in contrast to the straight and normal venules. (Figure 4) Arterio-venous transit time was slightly prolonged (18–21 seconds), and the larger vessels were tight. However, microvascular changes were observed in an upper sector of the right eye. Laser burns were applied to the retinal mid-periphery, with reference to small-vessel loops and a few late fluorescein leakage points and minute haemorrhages; these lesions had disappeared at follow-up angiographies one and two years later.
The laser treatment was inspired from current strategies for diabetic retinopathy aiming at reducing metabolism and oxygen demand of the retinal photoreceptor layer. However, as an important difference, the angiographic appearance in our patient did not indicate capillary loss and dark areas of focal ischemia, which is typical when diabetic retinopathy has progressed to microvascular abnormality with precapillary loss of pericytes and basement membrane pathology.[11,12] With some support from mouse model studies, we regard the microvascular changes as most likely related to the abnormal α-actin in the pericytes and the smooth muscle cells of the retinal vessel walls.[13,14]
Timing of the ocular events
Regarding the congenital mydriasis, the reports uniformly specify the pupillary dysfunction as an early event and probably present from birth, all to suggest a general state of hypoplasia of the smooth muscles of the eye. Interestingly, iris hypoplasia with irregular wisps at the papillary margin was also reported in three children with aorticopulmonary septal defect,[15] a congenital heart disease originating from a failure of fusion of the two conotruncal ridges normally occurring in the 5th gestational week. This coincides with the gestational age where the ductus arteriosus is formed from the left 6th aortic arch. The etiology of the age-dependent tortuousity of the retinal vessels is unknown, but probably is related to morphological vessel wall changes including loss of contractility.
Optical imperfections due to large pupil and accommodative failure
A clear and crisp retinal image is considered the driving force during infancy and childhood for developing and refining visual resolution and discrimination, and also for tuning the refraction, usually as emmetropisation.[16–19] Early correction of significant ametropia is generally considered important in order to avoid amblyopia. The good visual acuities in our cases, however, suggest that optical aberrations due to large pupil and defective accommodation have not been real obstacles to visual development. However, it calls for careful description of visual acuity and accommodative capacity in more cases of this syndrome as this may teach us something about the role (lacking) accommodation in visual development.
CONCLUSION
A de novo mutation of the ACTA2 gene encoding the smooth muscle cell α-actin has been established in patients with multi systemic smooth muscle dysfunction syndrome associated with patent ductus arteriosus and mydriasis present at birth. Based on three cases with congenital mydriasis and a patent ductus arteriosus, this study focused on the ophthalmic manifestations and their impact on visual development and refraction. Tortuousity of retinal arterioles was the main posterior pole finding, apparent during the first year of life and with a tendency to increase by age. One of our cases had minute focal intraretinal microvascular abnormalities in mid-periphery.
The typical unresponsive mydriatic pupils with scalloping wisps of persistent pupillary membrane from the iris collarette, which anatomically appears the free end of the iris tissue in such cases, is an early indicator of this rare genetic disorder. Retinal vessel tortuousity is usually regarded an occasional finding, but when combined with persistent ductus arteriosus and the extremely rare occurrence of congenital mydriasis, immediate attention should be drawn to the multi systemic smooth muscle cell dysfunction syndrome (OMIM #613834) due to an ACTA2 missense mutation leading to the production of mutant cellular α-actin.[1] Evidently, the ophthalmologist should act in close collaboration with other specialists due to the risk of aortic and cerebrovascular diseases, and of other smooth muscle cell complications associated with this disorder.
To safeguard relevant spectacles in due time and overall visual development, the children should be followed by pediatric ophthalmologists. Furthermore, retinal vasculopathy eventually may warrant vitreoretinal attention, if it progresses to breakdown of the blood-retinal barrier.
Acknowledgments
Dr. Eva Ottovay, Sønderborg, Denmark
University Eye Clinic, Odense, Denmark
Footnotes
No financial interests to disclose
References
- 1.Milewics DM, Østergaard JR, Ala-Kokko LM, et al. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet. 2010;152A:2437–43. doi: 10.1002/ajmg.a.33657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buys Y, Buncic JR, Enzenauer RW, et al. Congenital aplasia of the iris sphincter and dilator muscles and patent ductus arteriosus. Can J Ophthalmol. 1993;28:72–6. [PubMed] [Google Scholar]
- 3.Miller NR, Newman NJ. Neuro-ophthalmology. In: Walsh, Hoyt, editors. The Essentials. 5. Chapter 14. Philadelphia: Lippincott, Williams & Wilkins; 1999. pp. 450–5. [Google Scholar]
- 4.Gräf MH, Jungherr A. Congenital Mydriasis, Failure of Accommodation, and Patent Ductus Arteriosus. Arch Ophthalmol. 2002;120:309–10. [PubMed] [Google Scholar]
- 5.Fledelius HC. Pre-term delivery and subsequent ocular development. A 7–10 year follow-up of children screened 1982–84 for ROP. I. Visual function, slit-lamp findings, and fundus appearance. Acta Ophthalmol Scand. 1996;74:288–93. doi: 10.1111/j.1600-0420.1996.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 6.Libert J, Toussaint D. Tortuosities of retinal and conjunctival vessels in lysosomal storage disease. Birth Defects, Orig Artic Ser. 1982;18:347–58. [PubMed] [Google Scholar]
- 7.Sodi A, Ioannidis AS, Mehta A, et al. Ocular manifestations of Fabry’s disease: data from the Fabry Outcome Survey. Br J Ophthalmol. 2007;91:210–14. doi: 10.1136/bjo.2006.100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adès LC, Davies R, Haan EA, et al. Aortic dissection, patent ductus arteriosus, iris hypoplasia and brachytelephalangy in a male adolescent. Clin Dysmorphol. 1999;8:269–76. [PubMed] [Google Scholar]
- 10.Lemire BD, Buncic JR, Kennedy JS, et al. Congenital Mydriasis, Patent Ductus Arteriosus, and Congenital Cystic Lung Disease: New Syndromic Spectrum? Am J Med Genetics. 2004;131A:318–9. doi: 10.1002/ajmg.a.30341. [DOI] [PubMed] [Google Scholar]
- 11.Lindberg K, Brunvand L. Congenital mydriasis combined with aneurysmal dilatation of a persistent ductus arteriosus Botalli: a rare syndrome. Acta ophthalmol Scand. 2005;83:508–9. doi: 10.1111/j.1600-0420.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- 12.Newell FW. Ophthalmology, priciples and concepts. 8. St Louis: Mosby; 1996. pp. 507–14. [Google Scholar]
- 13.Kanski JJ. Clinical Ophthalmology, a systematic approach. 6. Edinburgh: Butterworth Heinemann; 2007. pp. 566–75. [Google Scholar]
- 14.Tomasek JJ, Haaksma CJ, Schwartz RJ, et al. Deletion of smooth muscle alfa-actin alters blood-retina barrier permeability and retinal function. Invest Ophthalmol Vis Sci. 2006;47:2693–2. doi: 10.1167/iovs.05-1297. [DOI] [PubMed] [Google Scholar]
- 15.Bergstrom CS, Saunders RA, Hutchinson AK, et al. Iris hypoplasia and aorticopulmonary septal defect: a neurocristopathy. J A APOS. 2005;9:264–7. doi: 10.1016/j.jaapos.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Raviola E, Wiesel TN. An animal model of myopia. N Engl J Med. 1985;312:1609–15. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- 17.Schaeffel F, Troilo D, Wallman J, et al. Developing eyes that lack accommodation grow to compensate for imposed defocus. Vis Neurosci. 1990;4:177–83. doi: 10.1017/s0952523800002327. [DOI] [PubMed] [Google Scholar]
- 18.Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1995;35:1175–94. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- 19.Brown NP, Koretz JF, Bron AJ. The development and maintenance of emmetropia. Eye. 1999;13:83–92. doi: 10.1038/eye.1999.16. [DOI] [PubMed] [Google Scholar]