Abstract
Background
Human papillomavirus (HPV) infections in Thailand are a public health concern but information on HPV infection in sex workers and men who have sex with men (MSM) is limited. The aim of this study was to measure the prevalence and genotype distribution of HPV among low- and high-risk, HIV-negative populations.
Methods
A total of 300 participants were categorized as general women, female sex workers, MSM, and MSM sex workers. HPV infections were identified by the Papanicolaou (Pap) test and nested-PCR. A phylogenetic analysis of partial HPV L1 genes was performed.
Results
Abnormal cytology was found in 5% of general women, 10% of female sex workers, 24% of MSM and 28% of MSM sex workers. HPV was detected in 9% of general women, 13% of female sex workers and 30% in both MSM and the MSM sex workers. The prevalence of HPV high-risk genotypes was significantly higher in female sex workers and MSM while low-risk genotypes and genital warts were significantly higher in MSM sex workers. Significantly more patients with genital warts and CIN I/AIN I harbored low-risk genotypes while those with CIN II/AIN II harbored high-risk genotypes.
Conclusion
High- and low-risk HPV genotypes persist in high-risk groups in Bangkok. Some genotypes infecting at-risk populations are not vaccine-preventable. These findings may help to elucidate the prevalence of HPV infections in Thailand and serve as the basis for additional investigations into risk factors for these populations.
Keywords: Thailand, human papillomavirus (HPV), men who have sex with men (MSM), sex workers, HPV genotyping
Introduction
Human papillomavirus (HPV) infection is considered to be a sexually transmitted disease causing approximately 600,000 cases of cancer of the cervix, vulva, vagina, anus and oropharynx annually, as well as benign diseases such as genital warts.(1) HPV infections, most of which are vaccine preventable, are thought to preclude the development of cervical intraepithelial lesions and subsequent invasive carcinoma in women and anal cancer in men. Even though the incidence of cervical cancer has been decreasing globally over recent decades, the incidence of anal carcinoma, for which effective screening programs and data are lacking, appears to have been rising over the last two decades.(2) The risks of HPV infection depend on sexual behaviors including the number of partners and age of first sexual activity.(3) Compared to general male and female populations, sex workers including men who have sex with men (MSM) are at higher risk for HPV infection.(4) As such, MSM sex workers have a high risk for developing HPV associated anal intraepithelial neoplasia (AIN) or anal squamous cell cancer. Also, both male and female sex workers may be at high risk of HPV infection due to multiple sexual partners and unprotected sexual activities, which can lead to the HPV transmission from person to person.(5)
HPV has more than 100 genotypes of which 40 can be found in the anogenital region.(6) Each can be classified as a low-risk or a high-risk genotype depending on their oncogenic characteristics. Low-risk genotypes include but are not limited to HPV 6, 11, 40, 42, 43, 44, 53, 54, 61, 72, 73 and 81: genotypes 6 and 11 are associated with genital warts. High-risk genotypes include HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 and 69: genotypes 16 and 18 primarily causing cervical and anal cancers. High-risk HPV genotypes have been detected by others in a number of high-risk groups such as sex workers; for example, in Austrian female sex workers (7) as well as Peruvian MSM and MSM sex workers.(4)
HPV prevalence data in HIV-negative heterosexual men in developing countries is emerging and varies considerably depending on geography and anatomical site sampled.(8) Still, the HPV prevalence data for HIV-negative MSM (9) and MSM sex workers (4) appear to be substantially higher than heterosexual men. Among 305 heterosexual men screened in Hangzhou, China, 14% were HPV positive: oncogenic HPV types were found in 4% and non-oncogenic HPV types in 9%.(10) While in a study based in Shenzhen, China demonstrated that 34% of HIV-negative MSM were HPV positive.(11) Another recent study demonstrated that of 105 Peruvian MSM examined, 77.1% were infected with HPV and almost half (47.3%) were infected by a oncogenic type; high-risk HPV type was associated with sex work.(4)
In Thailand, HPV testing and screening have not yet been widely adopted systematically, much less in high-risk groups such as MSM and MSM sex workers. As such, HPV prevalence and genotype distribution data are limited or reported with significant variation. HPV prevalence in general women in Thailand has been reported ranging from approximately 9–20% (12) to 83% (13) depending on the study location or sample population assessed. One consistency reported in Thai patients has been that the oncogenic HPV genotypes 16 and 18 have been detected more frequently in high-risk groups.
The aim of this study was to measure the prevalence and genotype distribution of HPV infection among low- and high-risk, male and female groups. A total of 300 participants from the Sexually Transmitted Infections (STI) cluster in Bangkok’s Bangrak Hospital were categorized into one of four groups: general women, female sex workers, MSM and MSM sex workers. The initial study design included general women and MSM populations as low-risk groups and female sex worker and MSM sex worker populations as high-risk groups. Participants were tested for HPV using the Papanicolaou (Pap) test and nested-PCR; phylogenetic analysis was done on sequenced partial L1 HPV genes.
Materials and methods
Study design and sample size
A cross-section observational study was designed and implemented with the Bangrak Hospital STI cluster in Bangkok, Thailand. All enrollees were HIV negative and categorized into one of four groups: general women, female sex worker, general MSM and MSM sex worker. Sample size estimations for each gender group were determined based on estimates from the ICO HPV online information system,(14) specifying in search fields either “women with normal cervical cytology” or “anogenital HPV infection in men” from “South-Eastern Asia countries”. Estimates were based on a two-tailed analysis with 99% significance resulting in a minimum enrollment of 79 participants for each women group and 35 participants for each men group. Actual enrollment was 100 general women, 100 female sex workers, 50 MSM and 50 MSM sex workers. Institutional Review Board approvals were obtained from Bangrak Hospital and Faculty of Tropical Medicine, Mahidol University.
HPV detection by Pap test and nested-PCR
Both cervical and anal tissues from all 300 participants were collected for Pap tests and nested-PCR assays. All Pap tests were performed and read at the Central Laboratory of Cytologists at the Thai National Cancer Institute in Bangkok as per standard institutional procedures developed from the 2001 Bethesda System.(15) Corresponding specimens were frozen at −80°C until further testing. HPV DNA isolation and detection by nested-PCR were performed at the Virology laboratory, Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University as per standard laboratory procedures. Briefly, the HPV DNA was extracted using the commercial viral nucleic acid extraction kit (Geneaid Biotech Ltd, Taiwan) and the viral DNA template was used for PCR, modified as previously done.(16) An initial PCR reaction of 40 cycles (annealing at 55°C for 1 minute each cycle) amplified a 450 base pair (bp) fragment of the HPV L1 gene with the forward primer MY09 (CGTCCMARRGGAWACTGATC) and reverse primer MY11 (GCMCAGGGWCATAAYAATGG). The initial PCR products were used as templates for a second PCR reaction of 30 cycles (annealing at 53°C for 1 minute each cycle) to detect the shorter 150 bp fragment using the forward primer GP05 (TTTGTTACTGTGGTAGATACTAC) and reverse primer GP06 (GAAAAATAAACTGTAAATCATATT). The nested-PCR amplicons were separated by electrophoresis and displayed by ethidium bromide under ultraviolet light (Supplemental Figure 1).
HPV sequencing and genotyping by phylogenetic analysis
The HPV partial L1 gene DNA amplicons from the nested-PCR were purified using the PCR Clean-up Extraction Kit (Macherey-Nagel Inc., Germany) and subjected to DNA sequencing with the GP05 forward primer on an automated fluorescent sequencing PRISM Reader and ABI 377 System (Macrogen Co., Ltd, Seoul). The nucleotide sequences were aligned with ClustalX 2.1 (University College Dublin, Ireland) and compared with GenBank reference sequences: HPV 6 (GU344803.1), HPV 11 (GU344763.1), HPV 16 (FJ797753.1), HPV 18 (GU344774.1), HPV 31 (HM596539.1) and HPV 81 (GQ288793.1). The human herpesvirus 2 (JN415125.1) was used as the outgroup reference gene. A phylogenetic tree was constructed using the 44 HPV partial L1 gene sequences and was based on a maximum likelihood method and kimura 2-parameter model for distance estimate. The percentages of replicate trees, in which the associated taxa clustered in the bootstrap method, were performed for values representing 1000 replicates. Confidence values were calculated by bootstrap test and a consensus tree was drawn by MEGA version 6.0 (Arizona, USA).
Statistical analysis
Demographic, clinical and laboratory results were analyzed for significant differences using Pearson Chi-square and Fisher’s Exact tests using SPSS statistics 18.0 (IBM Corp., USA). A p-value of < 0.05 was considered to be statistically significant.
Results
Study demographics and prevalence of genital warts
General demographics of the study are shown in Table 1. The MSM sex worker group was slightly younger having a median age of 26 compared to a median age of 32 for the other 3 groups. Of note, a significant proportion of the samples collected derived from those of reproductive age (21–40 age group). Among the 300 study participants, 88 (29%) presented with genital warts, which could be found in the vaginal area of 19 (19%) of the general women group and 23 (23%) of female sex worker cases. Genital warts found in the MSM group included 21 (42%) on the anus area and 2 (4%) on both the anus and penis areas. Genital warts found in the MSM sex worker group included 15 (30%) on the anus area, 3 (6%) on the penis area, 4 (8%) on both the anus and penis areas and 1 (2%) on the anus and rectum. Approximately 75% of both female groups and the MSM sex worker group had not received a Pap test in their medical history whereas 90% of MSM participants had no Pap test. There was a slightly higher trend of both female and MSM sex workers who had a Pap test within the previous year compared to their gender non-sex worker counterparts (Table 1).
Table 1.
Study population demographics, genital wart distribution and Pap test history
| Demographic Characteristic | General Women (n=100) |
Female Sex Worker (n=100) |
MSM1 (n=50) |
MSM Sex Worker (n=50) |
Total (n=300) |
|---|---|---|---|---|---|
| Age | |||||
| <= 20 years | 5 (5%) | 2 (2%) | 4 (8%) | 7 (14%) | 18 (6%) |
| 21–40 years | 64 (64%)† | 87 (87%)† | 38 (76%)† | 43 (86%)† | 232 (77%)† |
| 41–60 years | 31 (31%) | 11 (11%) | 8 (16%) | 0 | 50 (17%) |
| Median | 33 | 32 | 33 | 26 | 31 |
| Genital wart | |||||
| Vagina | 19 (19%) | 23 (23%) | N/A | N/A | 42 (21%)2 |
| Anus | 0 | 0 | 21 (42%) | 15 (30%) | 36 (12%) |
| Penis | N/A | N/A | 0 | 3 (6%) | 3 (3%)3 |
| Anus and penis | N/A | N/A | 2 (4%) | 4 (8%) | 6 (6%)3 |
| Anus and rectum | 0 | 0 | 0 | 1 (2%) | 1 (0.3%) |
| Total | 19 (19%) | 23 (23%) | 23 (46%) | 23 (46%) | 88 (29%) |
| Pap test history | |||||
| No Pap test | 73 (73%) | 75 (75%) | 45 (90%) | 39 (78%) | |
| 1 year | 12 (12%) | 19 (19%) | 3 (6%) | 4 (8%) | |
| >1 year to 5 years | 15 (15%) | 6 (6%) | 2 (4%) | 7 (14%) |
Table adopted from previous publication: Bamrungsak, et al. Thai AIDS J 2013, 25:105–113
Significantly different number of enrollees compared to other age categories (p<0.0001)
Men who have sex with men
Denominator = 200 (female groups only)
Denominator = 100 (male groups only)
N/A – not applicable
Cytological results based on Pap test
Abnormal cytology was found among 41 (13.6%) enrollees (Table 2), 36 (87%) of which were of reproductive age, from 21–40 years old. Of the 5 (5%) general women having abnormal cervical cytology, 2 (2%) were classified as having atypical squamous cells of undetermined significance (ASC-US), 1 (1%) had low-grade squamous intraepithelial lesion (LSIL) and HPV change, and 2 (2%) cervical intraepithelial neoplasia grade I (CIN I). Of participants in the female sex worker group, 10 (10%) had abnormal cervical cytology: 3 (3%) as having ASC-US, 4 (4%) as LSIL and HPV change, 2 (2%) as CIN I and 1 (1%) CIN II. While 54 (54%) female sex workers were negative for intraepithelial neoplasia, 27 (27%) of general women were negative, demonstrating a significant difference between these groups.
Table 2.
Cytology results based on Pap test
| Cytology Results by Pap test | General Women (n=100) |
Female Sex Worker (n=100) |
MSM1 (n=50) |
MSM Sex Worker (n=50) |
Total (n=300) |
|---|---|---|---|---|---|
| Negative for intraepithelial neoplasia | 27 (27%) | 54 (54%)† | 38 (76%) | 34 (68%) | 153 (51%) |
|
| |||||
| Inflammation | 44 (44%) | 28 (28%)† | 0 | 0 | 72 (24%) |
| Candida spp. | 16 (16%) | 7 (7%)† | 0 | 0 | 23 (7.6%) |
| Trichomonas vaginalis | 1 (1%) | 0 | 0 | 0 | 1 (0.3%) |
| Herpes simplex virus | 1 (1%) | 0 | 0 | 2 (4%) | 3 (1%) |
| Atrophy | 6 (6%) | 1 (1%)† | 0 | 0 | 7 (2.3%) |
| Sub-total | 68 (68%) | 36 (36%)† | 0 | 2 (4%) | 106 (35.3%) |
|
| |||||
| ASC-US2 | 2 (2%) | 3 (3%) | 5 (10%) | 4 (8%) | 14 (4.6%) |
| LSIL and HPV change3 | 1 (1%) | 4 (4%) | 3 (6%) | 2 (4%) | 10 (3.3%) |
| CIN4I/AIN5I | 2 (2%) | 2 (2%) | 1 (2%) | 7 (14%)†† | 12 (4%) |
| CIN II/AIN II | 0 | 1 (1%) | 3 (6%) | 1 (2%) | 5 (1.6%) |
| Sub-total | 5 (5%) | 10 (10%) | 12 (24%) | 14 (28%) | 41 (13.6%) |
|
| |||||
| Total | 100 | 100 | 50 | 50 | 300 (100%) |
Table adopted from previous publication: Bamrungsak, et al. Thai AIDS J 2013, 25:105–113
Men who have sex with men
Atypical squamous cells with undermined significance
Low-grade squamous intraepithelial lesion and human papillomavirus change
Cervical intraepithelial neoplasia
Anal intraepithelial neoplasia
Significant difference compared to general women (p=0.001)
Significant difference compared to MSM (p=0.001)
Of the MSM group, 12 participants (24%) had abnormal anal cytology: 5 (10%) had ASC-US, 3 (6%) had LSIL and HPV change, 1 (2%) had anal intraepithelial neoplasia grade I (AIN I) and 3 (6%) had AIN II. Of the MSM sex worker group, 14 (28%) had abnormal cytology: 4 (8%) had ASC-US, 2 (4%) had LSIL and HPV change, 7 (14%) had AIN I and 1 (2%) AIN II. We observed significantly more MSM sex workers (14%) positive for AIN1 compared to MSM (2%) (Table 2).
Based on the Pap test, the cytology results from cervical and anal tissue (2 of which were MSM sex workers) demonstrated infection with several microorganisms including Candida spp., Trichomonas vaginalis and herpes simplex virus. The cytology reports also revealed that general women had significantly more inflammation or infection with other microorganisms as compared to female sex workers: 68 (68%) in general women and 36 (36%) in the female sex worker group (Table 2). All 7 (100%) of the atrophy cases were older, between 41–60 years.
Detection of HPV by nested-PCR and sequencing
Two hundred cervical tissue samples (100 from general women and 100 from female sex workers) and 100 anal tissue samples (50 from MSM and 50 from MSM sex workers) were subjected to HPV testing by nested-PCR. Due to initial unsuccessful attempts to amplify an L1 gene fragment by PCR alone, nested-PCR was employed to detect HPV DNA resulting in a 150 bp fragment of the L1 gene (Supplemental Figure 1). HPV DNA was detected in 52 samples (17.3%): 9 (9%) in the general women group, 13 (13%) in the female sex worker group, 15 (30%) in the MSM group, and 15 (30%) in the MSM sex worker group (Table 3). The prevalence of nested-PCR positive results and abnormal cytology within each group was similar. However HPV prevalence in MSM and MSM sex workers was significantly higher than the general women and female sex workers respectively (Table 3). Approximately 30% of participants in both the MSM and MSM sex worker groups demonstrated HPV positivity as compared to 10% and 13% of participants in the general women and female sex worker group.
Table 3.
Prevalence of HPV genotypes by PCR and sequencing in each at-risk group
| HPV Type | General Women (n=100) |
Female Sex Worker (n=100) |
MSM (n=50) |
MSM Sex Worker (n=50) |
Total (n=300) |
|---|---|---|---|---|---|
| Nested-PCR | 9 (9%) | 13 (13%) | 15 (30%)† | 15 (30%)†† | 52 (17.3%) |
|
| |||||
| Sequencing* | |||||
| Low-risk | |||||
| HPV 6 | 2 (2%) | 2 (2%) | 3 (6%) | 3 (6%) | 10 (3.3%) |
| HPV 11 | 2 (2%) | 1 (1%) | 1 (2%) | 5 (10%)††† | 9 (3%) |
| HPV 81 | 1 (1%) | 1 (1%) | 1 (2%) | 1 (2%) | 4 (1.3%) |
| Sub-total | 5 (5%) | 4 (4%) | 5 (10%) | 9 (18%)††† | 23 (7.6%) |
|
| |||||
| High-risk | |||||
| HPV 16 | 2 (2%) | 3 (3%) | 2 (4%) | 1 (2%) | 8 (2.6%) |
| HPV 18 | 1 (1%) | 0 | 2 (4%)†††/† | 0 | 3 (1%) |
| HPV 31 | 0 | 4 (4%)† | 3 (6%) | 3 (6%) | 10 (3.3%) |
| Sub-total | 3 (3%) | 7 (7%)† | 7 (14%)†††/† | 4 (8%) | 21 (7%) |
| Sequence negative | 1 (1%) | 2 (2%) | 3 (6%) | 2 (4%) | 8 (2.7%) |
|
| |||||
| Total | 9 (9%) | 13 (13%) | 15 (30%)†† | 15 (30%)†† | 52 (17.3%) |
44 of 52 nested-PCR positive results sequenced (see NCBI accession numbers in phylogenetic analysis)
Significantly different to general women of female sex worker (p < 0.05)
Significantly different to female sex worker (p < 0.05)
Significantly different to MSM or male sex worker (p < 0.05)
HPV genotyping
Forty-four samples (of 52 nested-PCR positive tested) were found to be HPV positive by DNA sequencing (Table 3). The HPV low-risk genotypes 6, 11 and 81 were detected in 3.3%, 3% and 1.3% of samples respectively and high-risk genotypes 16, 18 and 31 were found in 2.6%, 1% and 3.3%, respectively. Two distinct patterns of HPV genotype distribution emerged across the four tested populations. First the prevalence of the low-risk genotype HPV 11 was significantly higher in MSM sex workers than the other three groups contributing to a significantly higher prevalence of low-risk genotypes in this group. The second is the prevalence of the high-risk genotype 18 was found significantly higher in the MSM group than the other three groups (Table 3). Otherwise, a fairly equal distribution of both low- and high-risk genotypes could be found in each population. We then crosschecked detected genotypes with Pap test results. Only high-risk genotypes 16 and 18 were found in cases having inflammation (Table 4). Of the 27 cases infected with Candida spp., Trichomona vaginalis or Herpes simplex virus, one (3.7%) was found to have high-risk genotype HPV 31 and 2 (7.4%) harbored low-risk genotypes. In general, both HPV low- and high-risk genotypes were found equally related across Pap test results. Interestingly, patients diagnosed as having CIN I/AIN I had a significantly higher prevalence of infection with low-risk HPV genotypes (50%) compared to high-risk genotypes (25%); conversely of the patients having CIN II/AIN II, a significantly higher prevalence of infection with high-risk genotypes (80%) was observed (Table 4). As expected, the prevalence of patients with genital warts was significantly higher in those infected with the low-risk genotypes than those infected high-risk genotypes (Table 4).
Table 4.
Prevalence of low- and high-risk HPV cases by cytology result and overall genital wart prevalence
| Pap Test Result | HPV Genotype*
|
|
|---|---|---|
| Low-risk | High-risk | |
|
| ||
| HPV 6, 11, 81 | HPV 16, 18, 31 | |
| Negative for intraepithelial neoplasia (n=153) | 3 (1%/2%)** | 3 (1%/2%) |
| Inflammation (n=72) | – | 3 (1%/4.2%) |
| Candida spp./Infection (n=27) | 2 (0.6%/7.4%) | 1 (0.3%/3.7%) |
| Atrophy (n=7) | – | – |
| ASC-US (n=14) | 6 (2%/43%) | 3 (1%/21%) |
| LSIL/HPV change (n=10) | 5 (1.6%/50%) | 4 (1.3%/40%) |
| CIN I/AIN I (n=12) | 6 (2%/50%)† | 3 (1%/25%) |
| CIN II/AIN II (n=5) | 1 (0.3%/20%) | 4 (1.3%/80%)† |
|
| ||
| Total (N=300) | 23 (7.5%) | 21 (7%) |
|
| ||
| Genital Warts | 18 (6%)† | 6 (2%) |
Of 300 samples, 44 of 52 nested-PCR positive results sequenced
The denominators to calculate the percentages in parentheses (a/b) are a) 300 for the total number of patients and b) the number of patients in each Pap test result category as shown (from Table 2)
Significant difference comparing low- and high-risk groups (p < 0.05)
Given the significant difference found between low-and high-risk HPV infected patients having genital warts, we wanted to determine the prevalence of low- and high-risk genotypes in each study group stratified by cytology results. Of the 5 general women HPV cases having vaginal warts, 4 (80%) had low-risk genotype. These 4 cases were spread equally among the 4 lower risk cytology results (Table 5). Interestingly, the female sex worker group demonstrated a lower prevalence of warts than general women, having only 2 patients infected with low-risk HPV 6 and 11, respectively. In the men, both MSM and MSM sex workers had significantly higher prevalence of high-risk HPV infections than both women groups. The cases were equally spread among ASC-US and LSIL and HPV change cytology results. The MSM sex worker group infected with low-risk genotypes demonstrated a significantly higher prevalence of AIN I (8%) compared to MSM (2%) and CIN I in both women groups. Also, the prevalence of MSM sex workers infected with low-risk genotypes (16%) was significantly more than MSM sex workers infected with high-risk genotypes (4%). The overall HPV prevalence, both low- and high-risk genotypes, in patients having warts was significantly higher in MSM (14%) and MSM sex workers (30.4%) than female sex workers (8.6%) (Table 5).
Table 5.
Prevalence of low- and high-risk HPV genotypes in patients with genital warts by cytology results
| HPV Type | General Women (n=100) |
Female Sex Worker (n=100) |
MSM (n=50) |
MSM Sex Worker (n=50) |
Total (n=300) |
|---|---|---|---|---|---|
| Low-risk* | |||||
| Inflam/Infection | 1 (1%) | – | – | – | 1 (0.3%) |
| ASC-US | 1 (1%) | 1 (1%) | 2 (4%) | 2 (4%) | 6 (2%) |
| LSIL/HPV change | 1 (1%) | 1 (1%) | 1 (2%) | 1 (2%) | 4 (1.3%) |
| CIN I/AIN I | 1 (1%) | – | 1 (2%) | 4 (8%)† | 6 (2%) |
| CIN II/AIN II | – | – | – | 1 (2%) | 1 (0.3%) |
| Sub-total | 4 (4%)††† | 2 (2%) | 4 (8%) | 8 (16%)†/††† | 18 (6%)††† |
|
| |||||
| High-risk** | |||||
| Inflam/Infection | 1 (1%) | – | – | – | 1 (0.3%) |
| ASC-US | – | – | 1 (2%) | 1 (2%) | 2 (0.6%) |
| LSIL/HPV change | – | – | 1 (2%) | 1 (2%) | 2 (0.6%) |
| CIN I/AIN I | – | – | – | – | – |
| CIN II/AIN II | – | – | 1 (2%) | – | 1 (0.3%) |
| Sub-total | 1 (1%) | 0 (0%) | 3 (6%)†† | 2 (4%)†† | 6 (2%) |
|
| |||||
| Total | 5 (5%) | 2 (2%) | 7 (14%)†† | 10 (20%)†† | 24 (8%) |
|
| |||||
| HPV prevalence in wart patients*** |
5 of 19 (26%) |
2 of 23 (8.6%) |
7 of 23 (14%)†† |
10 of 23 (30.4%)†† |
24 of 88 (27.2%) |
Low-risk: HPV 6, 11, 81
High-risk: HPV 16, 18, 31
Denominator to calculate HPV prevalence in wart patients used from Table 1, “Genital wart” totals
Significantly different compared to MSM and female groups (p < 0.05)
Significantly different compared to general female and/or female sex worker groups (p < 0.05)
Significantly different compared to sub-total high-risk genotype group (p=0.001)
Phylogenetic analysis based on HPV L1 gene
A Maximum Likelihood phylogenetic tree was generated from the analysis of 44 nucleotide sequences of a partial segment of the HPV L1 gene detected in patient samples (Figure 1). Six allele clusters were found based on HPV 6, 11, 16, 18, 31 and 81 with individual HPV genotypes closely related. HPV 16, 18 and 31, classified as high-risk genotypes, were found to cluster closely together and similarly, the HPV low-risk genotypes 6, 11 and 81 clustered closely. Accession numbers for HPV sequences from clinical samples are listed on the phylogenetic tree (Figure 1).
Figure 1.
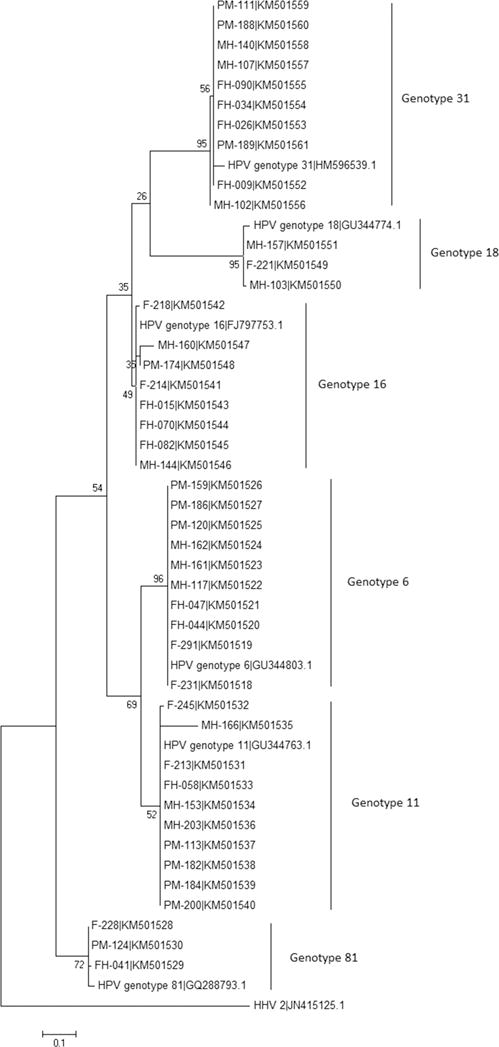
Maximum Likelihood phylogenetic tree. The tree was generated using 44 nucleotide sequences of the HPV L1 gene. Six allele clusters comprised of HPV 6, HPV 11, HPV 16, HPV 18, HPV 31 and HPV 81 genotypes. NCBI accession numbers are listed with sample number. Reference sequences include HPV 6 (GU344803.1), HPV 11 (GU344763.1), HPV 16 (FJ797753.1), HPV 18 (GU344774.1), HPV 31 (HM596539.1) and HPV 81 (GQ288793.1). The outgroup sequence is the human HPV 2 (JN415125.1).
Discussion
Prevalence of HPV and genital warts in female and MSM groups
This study has supported data from others and established the prevalence of HPV infection and the relationship of HPV genotypes in general women, female sex workers, MSM and MSM sex workers in Thailand. It is the first report in Thailand demonstrating HPV prevalence in MSM sex workers. Our prevalence data for general women (5%) support previous studies that reported between 5% and 10% in Thai women.(17, 18) Further, we have summarized cytology, PCR and HPV sequencing and genital warts results from our study populations. The overall prevalence of abnormal cytology detected by the Pap test (13.6%) was slightly lower than HPV positivity found by nested-PCR (17.3%) but not significantly different. Comparisons of low-and high-risk HPV prevalence in patients with abnormal cytology results are discussed below and data are shown in Table 4. The results found from each population group demonstrated unique patters that deserve further discussion.
Female sex workers worldwide are considered a high-risk population for abnormal cytology and most likely have a higher risk for HPV infection from having multiple sexual partners and unprotected sexual activities.(19, 20) Thai female sex workers have been shown to have significantly higher HPV prevalence than control women (21) which is in line with our data demonstrating a higher trend of abnormal cervical cytology among the female sex worker group (10%) compared to general women (5%). Further supporting these findings was a report demonstrating significant increased odds ratio (OR = 6.18) in Belgium female sex workers having low-grade squamous intraepithelial lesions compared to controls.(22) In the region, a China population-based study of 4,215 sexually active women demonstrated that 4% presented with CIN I (23) compared to 2% in our study, slightly lower but comparable. Interestingly, we observed that significantly more female sex workers (54%) were negative for intraepithelial neoplasia compared to general women (27%), who also had a significantly higher prevalence of inflammation (44% compared to 28% in female sex workers) and atrophy (6% compared to 1% in female sex workers). Given that the prevalence of HPV infection and genital warts in female sex workers (13% and 23%) were similar to general women (9% and 19%), one possible contribution to increased intraepithelial neoplasia in general women may be the role of Candida infection, as significantly more general women (16%) were infected with Candida compared to female sex workers (7%). However data supporting this possibility are inconclusive. While some have demonstrated that the presence of Candida is not associated with an increased risk for squamous abnormalities,(24) others have suggested that CIN can be promoted by vaginal microorganisms, including Candida, in conjunction with HPV infections.(25) Although not assessed in our study, factors independently associated with HPV infection in female sex workers include the use of non-barrier contraception, cytological abnormalities and the number of non-paying sexual partners.(26) Further assessment will need to verify these social behavioural patterns in Thai female sex workers and whether secondary vaginal infections may contribute to squamous abnormalities.
Inflammation, atrophy and infections with Candida and Trichomonas vaginalis were found primarily in general women group. These findings suggest that female sex workers, as required by their employer, may more frequently use condoms whereas general women may not, resulting in increased susceptibility to HPV infection, inflammation and infection by other microorganisms. As part of employment requirements, female sex workers should attend follow-up Pap tests every 6 months. Our data suggest general women and female sex workers rarely receive Pap tests: 73% of the general women and 75% of the female sex workers screened had not previously had a Pap test. Pap coverage is rather poor in Thailand and others have found that women interviewed avoided Pap test screening due to either fear of vaginal examination (27.6%), embarrassment (26.3%) or lack of any symptoms (22.4%).(27) Limited Pap test coverage in Thailand is potentially a very important public health issue that, although improving recently, may be overlooked to some degree in the health care system. Still, it is possible that female sex workers may attend clinics for education and screening as part of their employment requirements, reducing their risks to HPV infection and infection with other microorganisms, but these data are not consistent. The limited HPV screening practices currently employed in Thailand may result in under-diagnosis of HPV in all females with an abnormal smear who may be best suited for further testing. It has been proposed that female sex workers be screened when they enter prostitution regardless of their age.(22) But it appears that general women would also benefit from education and more frequent screening.
Globally, MSM populations are disproportionately affected by high-risk oncogenic HPV infections and increased incidence of HPV-associated anal cancers.(28) In 2003 in the US, the average annual incidence of anal cancer was 1.0 and 1.5 per 100,000 among men and women, respectively while the incidence of anal cancer among MSM was estimated to be as high as 37 per 100,000.(29, 30) However, there is a gap in knowledge regarding the HPV and anal cancer prevalence in MSM sex workers worldwide. In Thailand, MSM and MSM sex worker populations are not regularly screened for HPV, genital warts or anal carcinoma. A study from Peru found that 77% of MSM sex workers were positive for HPV.(4) Our data demonstrated that 30% of both MSM and MSM sex workers were HPV positive and that the prevalence of AIN I was significantly higher in MSM sex workers (14%) than MSM (2%). One possible explanation of the observed increased prevalence among MSM sex workers may be high-risk sexual behaviour such as multiple partners or sex without condoms, increasing physical disturbance of the epithelial lining of the anal canal or possibly HPV exposure; however data to support this claim are unavailable. Initially, the MSM group in our study was assigned as a low-risk population but clearly both MSM and MSM sex workers were equally at-risk for HPV infection.
Unique to MSM sex workers among all male cases was the significantly higher prevalence of low-risk HPV and anogenital warts: low-risk HPV was diagnosed in 18% of MSM sex workers compared to 10% of MSM and warts were found in 16% of MSM sex workers compared to 8% of MSM. Thirty percent of MSM sex workers with warts harbored any HPV type, significantly higher than MSM (14%) or women females sex workers (8.6%) with warts. While genital warts are the most common clinical manifestation of HPV infection (31) they are most frequently associated with low-risk HPV genotypes and found to be benign and not associated with mortality. However, genital warts are a source of psychosocial distress and can cause physical discomfort including pain, bleeding and itching. Moreover, genital warts are highly infectious. Approximately 65% of people whose sexual partner has genital warts will develop warts themselves, appearing between 3 weeks and 8 months after an HPV infection.(32) Moreover, shame, emotional and physical troubles and embarrassing sexual experiences were reported by MSM with genital warts in Peru.(33) Stigma and apparent health services’ inability to deal with genital warts and anal carcinoma in MSM populations limited their access to effective medical care. Although these issues have not yet been addressed in depth in Thailand, community outreach efforts to promote knowledge and discussions with providers regarding HPV and anorectal warts and cancer in MSM and MSM sex worker populations should not be overlooked. These social aspects merit further study in Thailand.
Genotype and phylogenetic analysis
The sequencing results and phylogenetic analysis of 44 HPV sequences revealed 6 HPV genotypes, 3 low-risk (6, 11, 81) and 3 high-risk (16, 18, 31). Our findings align with a general consensus that non-oncogenic HPV genotypes, particularly HPV 6 and 11, are responsible for the majority of genital warts in HPV infected patients. The 6 genotypes detected in this study have been reported previously in Thai women and of particular interest are the oncogenic types HPV 16 and 18.(18, 34, 35) A prospective case-control study in Northeast Thailand concluded that 66% of cervical cancer cases was caused by HPV 16 or 18, which increased the risk of cervical cancer with an odds ratio of 130.(36) Others have reported that the majority of HPV positive female sex workers in Thailand harbored the oncogenic genotypes HPV 16 or 18.(21) Additionally, female sex workers in Bangkok have been shown to be reservoirs of oncogenic HPV and it was suggested that cervical cancers in low-risk Thai women develop in part as a result of transmission of these viruses by their husbands from female sex workers.(37) In terms of additional regional data, of 802 female sex workers tested in a China based study, 24% harbored high-risk HPV types 16 (24%) or 18 (10%), which was similar to prevalence data among female sex workers in East Asia—HPV 16 (24%), 18 (11%) and 58 (9%)—but higher than female sex workers in Southeast Asia—HPV 52 (13%), 16 (9%) and 58 and 18 (5%).(38) In Vietnam female sex workers, high risk HPV was found in 89% of enrollees and the most common type was HPV 52 followed but HPV 16 and 18.(39) In our study, 13% of female sex workers harbored HPV, of which 7% were high-risk HPV genotypes. There was no significant difference of HPV prevalence among female sex workers and general women, suggesting that both groups have equal opportunities for screening and HPV detection and treatment.
HPV prevalence data for MSM sex workers in Thailand are not available while HPV prevalence data for MSM in Thailand and Asia vary substantially depending on the country, anatomical area measured, and methodology employed. In Thailand, overall HPV prevalence in HIV-negative MSM was found to be 59% while high-risk HPV 16 was detected in 36% of HIV-negative MSM.(40) In both MSM and MSM sex worker groups, we observed a similar HPV prevalence of 30%. Interestingly, the prevalence of high-risk HPV in MSM (14%) was significantly higher than MSM sex workers (8%) while MSM sex workers demonstrated a significantly higher prevalence of low-risk genotypes (18%) and genital warts (16%) compared to MSM (10%) and (8%). Our data support others who suggest that MSM, and especially MSM who participate in sex work, are important groups to target with interventions to decrease or prevent HPV. Importantly, HPV infection has been associated with acquisition of HIV in MSM (41, 42) and high-risk HPV type was associated with sex work in MSM.(4) Also, male sex work in the developing world is understudied yet it is known to be an important factor perpetuating HIV infection globally.(43, 44) As such, MSM in Thailand, as others have noted regarding the developing world, represent a “hidden epidemic” of HIV,(45, 46) and the results of this study support expanding this epidemic to include HPV infection in MSM and MSM sex workers in Asia.
Our HPV genotyping results may add value regarding vaccine design and use in Thailand even though we did not investigate invasive cervical or anal cancer biopsy specimens. The current HPV vaccines available in Thailand include the quadrivalent vaccine having HPV 6, 11, 16 and 18 and the bivalent vaccine having HPV 16 and 18. Trials of both vaccines have shown nearly 100% efficacy against high-grade lesions of the cervix, vulva and vagina in uninfected women under 26 years of age.(47) The quadrivalent vaccine has also shown high efficacy against anogenital warts, reducing the risk of anal cancer and precancerous lesions in MSM without history of anal cancer or precancerous lesions and significantly reducing anal intraepithelial neoplasia recurrence among MSM.(48) The vaccine strategy is appropriate in the context of some studies. For example, as expected, HPV types 16 and 18 were the most common types in invasive cervical cancer cases in Thailand (13) as have been found in similar reviews.(49, 50) Further, a Thailand-based study aiming to determine the relationship between penile cancer and the prevalence of HPV genotypes found that penile cancer cases were most frequently (55%) infected with the high-risk HPV 18 while the low-risk HPV 6 was found in 43% of cases.(51) Although we do not measure cancer outcomes, our findings support previous data and similar studies that demonstrate the persistence of high-risk genotypes 16 and 18 in Thailand and in the region.(21, 39, 52) Unfortunately, neither vaccine used in Thailand is cross protective against nonspecific HPV genotypes. As such, a large proportion of people living in Thailand are susceptible to infection with HPV 31, particularly the MSM and female and MSM sex worker groups. Expanding availability and use of vaccines that have more comprehensive high- and low-risk genotypes (including HPV 31 and 81) for a Thai setting may improve disease outcomes and reduce the prevalence of genital warts, abnormal cytology or cancers. More epidemiological investigations are required.
Our study has several potential imitations including lack of generalizability and HPV detection method used. Given that our study employed the MY09/11 consensus primer pair, which is know to detect a wide range of HPV genotypes but is limited based on partial L1 gene amplification, our results may not be generalizable outside of Thailand. Our sequence results were genotype positive for 44 of 52 (85%) nested-PCR samples. Recent studies comparing the sensitivity and specificity of HPV typing using the Linear Array HPV Genotyping Test (Roche Molecular System, Inc., Branchburg, NJ) and other techniques including the Roche PGMY primer-based line blot assay (53) and PCR and sequencing (54, 55) demonstrated highly accurate results for HPV typing while confirming reproducibility in the detection of multiple infections while others have demonstrated 92% agreement of detected HPV types using the PGMY09/11 primer system compared to the MY09/11, demonstrating that the PGMY09/11 primer set was significantly more sensitive.(56) As such PCR detection and sequencing in our future studies may be replaced with the Roche Linear Array Test or PGMY system. Additionally, even though we did not include some relevant socio-economic status data, which may have strengthened the results and allowed for more accurate predictors of risk in Thailand, our results may be used as a baseline for further investigation.
General effectiveness of cervical and anal cancer screening may be improved by combining frequent cytology with HPV screening of all at-risk groups in Thailand, especially female sex workers, MSM and MSM sex workers, using validated assays including Pap test and PCR screening or more sensitive techniques such as Linear Array HPV Genotyping. Given the diaspora of HPV prevalence data in Thailand, especially for marginalized groups such as MSM sex workers, the results of this study may assist in the therapeutic management of patients and provide useful data for following up with patients having persistent HPV infection. These data may also be used to better determine type-specific HPV vaccines to be used. Additional efforts to vaccinate female sex workers, MSM and MSM sex workers with expanded HPV vaccines may reduce the burden of HPV-related diseases.
Supplementary Material
Supplemental Figure 1: Nested-PCR amplification products with GP05/GP06 primers from cervical and anal tissue samples. Lane M: 100 bp DNA ladder; Lane 1: HPV positive control; Lane 2: Negative control; Lane 3–12: patient samples.
Acknowledgments
We would like to thank Dr. Thiwaporn Thesawadwong of the Thailand National Cancer Institute, Department of Medical Sciences, Ministry of Public Health and Assist. Prof. Dr. Natthanej Luplertlop, Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University for their contribution and advice. We also acknowledge efforts made by Dr. Angkana Charoenwattanachokchai, Head of Sexually Transmitted Infections (STI) Cluster, Bangrak Hospital, Bureau of AIDS, TB and STIs, Department of Disease Control, Ministry of Public Health and Dr. Nisit Kongkergkiat for sample collection throughout this study. Also we thank Mrs. Usanee Promprakob and staff of the Cytology Department, National Cancer Institute, Department of Medical Sciences, Ministry of Public Health, for performing Pap tests. We thank Dr. Vivek R. Nerurkar and Dr. Richard Yanagihara from the University of Hawaii, Department of Tropical Medicine, Medical Microbiology and Pharmacology for their continued mentorship support and critical thinking.
Sources of financial support: Faculty of Graduate Studies, Mahidol University, Thailand; United States (U.S.) National Institutes of Health (NIH): Fogarty International Center (R25TW009345, Northern Pacific Global Health Fellows) and Division of Loan Repayment, National Institute on Minority Health and Health Disparities; and U.S. Public Health Service grants, including Centers of Biomedical Research Excellence (P20GM103516).
Footnotes
COI statement: Authors have no conflicts of interest.
References
- 1.IARC. Working Group on the Evaluation of Carcinogenic Risks to Humans. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–689. [PMC free article] [PubMed] [Google Scholar]
- 2.Arbyn M, de Sanjose S, Saraiya M, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer. 2012;131(9):1969–82. doi: 10.1002/ijc.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam E, Berkova Z, Daxnerova Z, Icenogle J, Reeves WC, Kaufman RH. Papillomavirus detection: demographic and behavioral characteristics influencing the identification of cervical disease. Am J Obstet Gynecol. 2000;182(2):257–64. doi: 10.1016/s0002-9378(00)70208-0. [DOI] [PubMed] [Google Scholar]
- 4.Quinn R, Salvatierra J, Solari V, Calderon M, Ton TG, Zunt JR. Human papillomavirus infection in men who have sex with men in Lima, Peru. AIDS Res Hum Retroviruses. 2012;28(12):1734–8. doi: 10.1089/aid.2011.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarkar K, Bhattacharya S, Bhattacharyya S, et al. Oncogenic human papilloma virus and cervical pre-cancerous lesions in brothel-based sex workers in India. J Infect Public Health. 2008;1(2):121–8. doi: 10.1016/j.jiph.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Haycox CL, Kuypers J, Krieger JN. Role of human papillomavirus typing in diagnosis and clinical decision making for a giant verrucous genital lesion. Urology. 1999;53(3):627–30. doi: 10.1016/s0090-4295(98)00351-3. [DOI] [PubMed] [Google Scholar]
- 7.Gitsch G, Kainz C, Reinthaller A, Kopp W, Tatra G, Breitenecker G. Cervical neoplasia and human papilloma virus infection in prostitutes. Genitourin Med. 1991;67(6):478–80. doi: 10.1136/sti.67.6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith JS, Gilbert PA, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of human papillomavirus infection in males: a global review. J Adolesc Health. 2011;48(6):540–52. doi: 10.1016/j.jadohealth.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Chin-Hong PV, Vittinghoff E, Cranston RD, et al. Age-Specific prevalence of anal human papillomavirus infection in HIV-negative sexually active men who have sex with men: the EXPLORE study. J Infect Dis. 2004;190(12):2070–6. doi: 10.1086/425906. [DOI] [PubMed] [Google Scholar]
- 10.Tang X, Xu AE, Dong XP, Sun XK, Shen H, Liu JF. Epidemiological investigation of human papillomavirus infection in men attending a sexually transmitted disease clinic in Hangzhou area. Biomed Environ Sci. 2006;19(2):153–7. [PubMed] [Google Scholar]
- 11.Zhang DY, Yin YP, Feng TJ, et al. HPV infections among MSM in Shenzhen, China. PLoS One. 2014;9(5):e96364. doi: 10.1371/journal.pone.0096364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sriamporn S, Khuhaprema T, Parkin M. Cervical cancer screening in Thailand: an overview. J Med Screen. 2006;13(Suppl 1):S39–43. [PubMed] [Google Scholar]
- 13.Chinchai T, Chansaenroj J, Swangvaree S, Junyangdikul P, Poovorawan Y. Prevalence of human papillomavirus genotypes in cervical cancer. Int J Gynecol Cancer. 2012;22(6):1063–8. doi: 10.1097/IGC.0b013e318259d904. [DOI] [PubMed] [Google Scholar]
- 14.The ICO Information Centre on HPV and Cancer [database online] 2012 [Google Scholar]
- 15.Solomon D, et al. The 2001 Bethesda System: Terminology for Reporting Results of Cervical Cytology. Journal of the American Medical Association. 2002;287(16):2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 16.Siriaunkgul S, Suwiwat S, Settakorn J, et al. HPV genotyping in cervical cancer in Northern Thailand: adapting the linear array HPV assay for use on paraffin-embedded tissue. Gynecol Oncol. 2008;108(3):555–60. doi: 10.1016/j.ygyno.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Chansaenroj J, Lurchachaiwong W, Termrungruanglert W, et al. Prevalence and genotypes of human papillomavirus among Thai women. Asian Pac J Cancer Prev. 2010;11(1):117–22. [PubMed] [Google Scholar]
- 18.Sukvirach S, Smith JS, Tunsakul S, et al. Population-based human papillomavirus prevalence in Lampang and Songkla, Thailand. J Infect Dis. 2003;187(8):1246–56. doi: 10.1086/373901. [DOI] [PubMed] [Google Scholar]
- 19.Joffe GP, Foxman B, Schmidt AJ, et al. Multiple partners and partner choice as risk factors for sexually transmitted disease among female college students. Sex Transm Dis. 1992;19(5):272–8. doi: 10.1097/00007435-199209000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Koutsky LA, Galloway DA, Holmes KK. Epidemiology of genital human papillomavirus infection. Epidemiol Rev. 1988;10:122–63. doi: 10.1093/oxfordjournals.epirev.a036020. [DOI] [PubMed] [Google Scholar]
- 21.Chandeying V, Garland SM, Tabrizi SN. Prevalence and typing of human papilloma virus (HPV) among female sex workers and outpatient women in southern Thailand. Sex Health. 2006;3(1):11–4. doi: 10.1071/sh05019. [DOI] [PubMed] [Google Scholar]
- 22.Mak R, Van Renterghem L, Cuvelier C. Cervical smears and human papillomavirus typing in sex workers. Sex Transm Infect. 2004;80(2):118–20. doi: 10.1136/sti.2002.003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R, Velicer C, Chen W, et al. Human papillomavirus genotype distribution in cervical intraepithelial neoplasia grades 1 or worse among 4215 Chinese women in a population-based study. Cancer Epidemiol. 2013;37(6):939–45. doi: 10.1016/j.canep.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Engberts MK, Vermeulen CF, Verbruggen BS, van Haaften M, Boon ME, Heintz AP. Candida and squamous (pre)neoplasia of immigrants and Dutch women as established in population-based cervical screening. Int J Gynecol Cancer. 2006;16(4):1596–600. doi: 10.1111/j.1525-1438.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- 25.Guijon F, Paraskevas M, Rand F, Heywood E, Brunham R, McNicol P. Vaginal microbial flora as a cofactor in the pathogenesis of uterine cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 1992;37(3):185–91. doi: 10.1016/0020-7292(92)90379-w. [DOI] [PubMed] [Google Scholar]
- 26.Tideman RL, Thompson C, Rose B, et al. Cervical human papillomavirus infections in commercial sex workers-risk factors and behaviours. Int J STD AIDS. 2003;14(12):840–7. doi: 10.1258/095646203322556192. [DOI] [PubMed] [Google Scholar]
- 27.Oranratanaphan S, Amatyakul P, Iramaneerat K, S S. Knowledge, attitudes and practices about the Pap smear among medical workers in Naresuan University Hospital, Thailand. Asian Pac J Cancer Prev. 2010;11(6):1727–30. [PubMed] [Google Scholar]
- 28.Ferlay J, Parkin DM, Curado MP. Cancer Incidence in Five Continents Vol I to IX, Lyon, France. IARC. 2010 [Google Scholar]
- 29.Daling JR, Weiss NS, Klopfenstein LL, Cochran LE, Chow WH, Daifuku R. Correlates of homosexual behavior and the incidence of anal cancer. JAMA. 1982;247(14):1988–90. [PubMed] [Google Scholar]
- 30.Watson M, Saraiya M, Ahmed F, et al. Using population-based cancer registry data to assess the burden of human papillomavirus-associated cancers in the United States: overview of methods. Cancer. 2008;113(10 Suppl):2841–54. doi: 10.1002/cncr.23758. [DOI] [PubMed] [Google Scholar]
- 31.Anic GM, Giuliano AR. Genital HPV infection and related lesions in men. Prev Med. 2011;53(Suppl 1):S36–41. doi: 10.1016/j.ypmed.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–14. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 33.Nurena CR, Brown B, Galea JT, Sanchez H, Blas MM. HPV and genital warts among Peruvian men who have sex with men and transgender people: knowledge, attitudes and treatment experiences. PLoS One. 2013;8(3):e58684. doi: 10.1371/journal.pone.0058684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swangvaree SS, Kongkaew P, Ngamkham J. Frequency and type-distribution of human papillomavirus from paraffin-embedded blocks of high grade cervical intraepithelial neoplasia lesions in Thailand. Asian Pac J Cancer Prev. 2013;14(2):1023–6. doi: 10.7314/apjcp.2013.14.2.1023. [DOI] [PubMed] [Google Scholar]
- 35.Natphopsuk S, Settheetham-Ishida W, Pientong C, et al. Human papillomavirus genotypes and cervical cancer in northeast Thailand. Asian Pac J Cancer Prev. 2013;14(11):6961–4. doi: 10.7314/apjcp.2013.14.11.6961. [DOI] [PubMed] [Google Scholar]
- 36.Sriamporn S, Snijders PJ, Pientong C, et al. Human papillomavirus and cervical cancer from a prospective study in Khon Kaen, Northeast Thailand. Int J Gynecol Cancer. 2006;16(1):266–9. doi: 10.1111/j.1525-1438.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 37.Thomas DB, Ray RM, Kuypers J, et al. Human papillomaviruses and cervical cancer in Bangkok. III. The role of husbands and commercial sex workers. Am J Epidemiol. 2001;153(8):740–8. doi: 10.1093/aje/153.8.740. [DOI] [PubMed] [Google Scholar]
- 38.Peng RR, Li HM, Chang H, Li JH, Wang AL, Chen XS. Prevalence and genotype distribution of cervical human papillomavirus infection among female sex workers in Asia: a systematic literature review and meta-analysis. Sex Health. 2012;9(2):113–9. doi: 10.1071/SH11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoang HT, Ishizaki A, Nguyen CH, et al. Infection with high-risk HPV types among female sex workers in northern Vietnam. J Med Virol. 2013;85(2):288–94. doi: 10.1002/jmv.23456. [DOI] [PubMed] [Google Scholar]
- 40.Phanuphak N, Teeratakulpisarn N, Pankam T, et al. Anal human papillomavirus infection among Thai men who have sex with men with and without HIV infection: prevalence, incidence, and persistence. J Acquir Immune Defic Syndr. 2013;63(4):472–9. doi: 10.1097/QAI.0b013e3182918a5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chin-Hong PV, Husnik M, Cranston RD, et al. Anal human papillomavirus infection is associated with HIV acquisition in men who have sex with men. AIDS. 2009;23(9):1135–42. doi: 10.1097/QAD.0b013e32832b4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown B, Davtyan M, Galea J, Chow E, Leon S, Klausner JD. The role of human papillomavirus in human immunodeficiency virus acquisition in men who have sex with men: a review of the literature. Viruses. 2012;4(12):3851–8. doi: 10.3390/v4123851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padilla M, Castellanos D, Guilamo-Ramos V, Reyes AM, Sanchez Marte LE, Soriano MA. Stigma, social inequality, and HIV risk disclosure among Dominican male sex workers. Soc Sci Med. 2008;67(3):380–8. doi: 10.1016/j.socscimed.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mi G, Wu Z, Zhang B, Zhang H. Survey on HIV/AIDS-related high risk behaviors among male sex workers in two cities in China. AIDS. 2007;21(Suppl 8):S67–72. doi: 10.1097/01.aids.0000304699.85379.32. [DOI] [PubMed] [Google Scholar]
- 45.Caceres CF. HIV among gay and other men who have sex with men in Latin America and the Caribbean: a hidden epidemic? AIDS. 2002;16(Suppl 3):S23–33. doi: 10.1097/00002030-200212003-00005. [DOI] [PubMed] [Google Scholar]
- 46.Beyrer C. Hidden yet happening: the epidemics of sexually transmitted infections and HIV among men who have sex with men in developing countries. Sex Transm Infect. 2008;84(6):410–2. doi: 10.1136/sti.2008.033290. [DOI] [PubMed] [Google Scholar]
- 47.Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369(9574):1693–702. doi: 10.1016/S0140-6736(07)60777-6. [DOI] [PubMed] [Google Scholar]
- 48.Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54(7):891–8. doi: 10.1093/cid/cir1036. [DOI] [PubMed] [Google Scholar]
- 49.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88(1):63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith JS, Backes DM, Hoots BE, Kurman RJ, Pimenta JM. Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obstet Gynecol. 2009;113(4):917–24. doi: 10.1097/AOG.0b013e31819bd6e0. [DOI] [PubMed] [Google Scholar]
- 51.Senba M, Kumatori A, Fujita S, et al. The prevalence of human papillomavirus genotypes in penile cancers from northern Thailand. J Med Virol. 2006;78(10):1341–6. doi: 10.1002/jmv.20703. [DOI] [PubMed] [Google Scholar]
- 52.Zhao R, Zhang WY, Wu MH, et al. Human papillomavirus infection in Beijing, People’s Republic of China: a population-based study. Br J Cancer. 2009;101(9):1635–40. doi: 10.1038/sj.bjc.6605351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coutlee F, Rouleau D, Petignat P, et al. Enhanced detection and typing of human papillomavirus (HPV) DNA in anogenital samples with PGMY primers and the Linear array HPV genotyping test. J Clin Microbiol. 2006;44(6):1998–2006. doi: 10.1128/JCM.00104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woo YL, Damay I, Stanley M, Crawford R, Sterling J. The use of HPV Linear Array Assay for multiple HPV typing on archival frozen tissue and DNA specimens. J Virol Methods. 2007;142(1–2):226–30. doi: 10.1016/j.jviromet.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 55.Giuliani L, Coletti A, Syrjanen K, Favalli C, Ciotti M. Comparison of DNA sequencing and Roche Linear array in human papillomavirus (HPV) genotyping. Anticancer Res. 2006;26(5B):3939–41. [PubMed] [Google Scholar]
- 56.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38(1):357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Nested-PCR amplification products with GP05/GP06 primers from cervical and anal tissue samples. Lane M: 100 bp DNA ladder; Lane 1: HPV positive control; Lane 2: Negative control; Lane 3–12: patient samples.


