Abstract
Rationale
Bone marrow (BM) cell therapy for ischemic heart disease (IHD) has shown mixed results. Before the full potency of BM cell therapy can be realized, it is essential to understand the BM niche following acute myocardial infarction (AMI).
Objective
To study the BM composition in patients with IHD and severe left ventricular dysfunction (LVD).
Methods & Results
BM from 280 patients with IHD and LVD were analyzed for cell subsets by flow cytometry and colony assays. BM CD34+ cell percentage was decreased 7 days after AMI (mean of 1.9% vs. 2.3-2.7% in other cohorts; p< 0.05). BM-derived endothelial colonies were significantly decreased (p< 0.05). Increased BM CD11b+ cells associated with worse left ventricular ejection fraction (LVEF) after AMI (p< 0.05). While increased BM CD34+ percentage associated with greater improvement in LVEF (+9.9% vs. +2.3%, p=0.03, for AMI patients; and +6.6% vs. -0.02%, p=0.021 for chronic IHD patients), decreased BM CD34+ percentage in chronic IHD patients correlated with decrement in LVEF after cell therapy (-2.9% vs. +0.7%, p=0.0355).
Conclusions
In this study we show a heterogeneous mixture of BM cell subsets, decreased endothelial colony capacity, a CD34+ cell nadir seven days after AMI, a negative correlation between CD11b percentage and post-infarct LVEF, and positive correlation of CD34 percentage with change in LVEF after cell therapy. These results serve as a possible basis for the small clinical improvement seen in autologous BM cell therapy trials and support selection of potent cell subsets and/or reversal of co-morbid BM impairment.
Keywords: Myocardial infarction, blood cells, angiogenesis, bone marrow, stem and progenitor cells
Introduction
Bone marrow cell (BMC) therapy after acute myocardial infarction (AMI) improves left ventricular (LV) function in experimental models of disease.1, 2 In patients, autologous BMC therapy for ischemic heart disease (IHD) has shown a small efficacy signal.3 Before the full potency of BMC therapy can be realized, we must understand and appreciate the bone marrow (BM) niche in the setting of IHD.
The BM contains stem and progenitor cells capable of generating neovessels in a variety of tissues in response to ischemia and inflammation.4, 5 A number of experimental and observational clinical studies have shown increased percentage of endothelial progenitor cells (EPCs) in the peripheral blood (PB) after AMI and correlation with improved left ventricular systolic function following AMI.6-8 It is possible that the BM is a source of circulating EPCs following infarction; however, a paucity of information is available that describes the BM niche in patients with IHD.
Of particular interest following AMI are CD34+ cells in the BM. These stem and progenitor cells, when injected in the ischemic or infarcted myocardium, reduce the number of angina pectoris episodes in a dose-dependent manner and improve myocardial perfusion after AMI.9, 10
In the absence of understanding the BM niche after AMI, some have raised the possibility that earlier BMC therapy trials showing improved left ventricular ejection fraction (LVEF) were either “red herrings” or that later studies due to cell processing procedures vitiated BMC potency.11, 12 Others have called for abandoning BMCs, altogether.13 To improve upon these early clinical investigations, we must understand the composition and functional status of the delivery agent (bone marrow).
Therefore, we chose to study the BM of a large cohort of patients with IHD and severe left ventricular dysfunction (LVD) with particular respect to BMC surface expression and vasculogenic colony capacity. The goal of this report is to describe the detailed composition of the BM obtained at different times after AMI.
Methods
Study populations and sources of cells
BM and PB were obtained from consenting patients enrolled in the CCTRN TIME, LateTIME and FOCUS trials. The TIME trial randomized 120 AMI patients with severe LVD to intracoronary injection of BM mononuclear cells (MNCs) versus placebo at 3 versus 7 days after AMI.14 The LateTIME trial randomized 87 AMI patients to intracoronary injection of BM MNCs versus placebo 14-21 days after AMI.15 The FOCUS trial randomized 92 chronic IHD patients with severe LVD not amenable to surgical revascularization to intramyocardial injection of BM MNCs versus placebo.16 Among the three studies, 299 study participants were recruited at five clinical centers and their satellites under IRB approvals. Of the 299 subjects, 291 consented to donate to the biorepository. Due to insufficient volume in 11 samples, the final evaluable dataset consisted of samples from 280 patients. An automated closed-system density gradient centrifugation separation protocol using Ficoll was used to separate BMCs from whole BM (Sepax® device, Biosafe Group, Switzerland). Within 12 hours of the BM harvest, a prescribed number of autologous BMCs were administered in the hearts of subjects after myocardial infarction (MI). Extra aliquots of BMCs were shipped overnight to a central biorepository for rapid assessment of cell phenotype, evaluation of cell function, and cryopreservation.17 Immediately upon receipt in the central biorepository, BMCs were separated by Ficoll and density gradient centrifugation.
Cell phenotyping and flow cytometry
BMC phenotyping was performed by immunostaining (BD Biosciences) and flow cytometry (BD LSRII) using antibody-fluorochrome conjugates (BD Biosciences) for 30 minutes on ice. Appropriate isotype controls were also used (BD Biosciences). Stained cells were washed, resuspended in Dulbecco's phosphate-buffered saline (DPBS) plus 2% fetal bovine serum (FBS) containing Via-Probe (BD Biosciences) and analyzed using a Becton Dickenson LSRII flow cytometer. ISHAGE protocols were used for enumerating CD34+ and CD133+ cells. FlowJo software (TreeStar, Inc., Oregon, USA) was used to analyze the flow cytometry data. Confocal imaging of fluorescently labeled BM cells was performed to confirm labeling (Supplemental Figure I).
Progenitor cell analyses
BMCs were evaluated for clonogenic capacity by assays for hematopoietic and EPC activity, as previously described. Colony forming cell (CFC) assay (Methocult, Stem Cell Technologies) was performed at all five study sites to evaluate hematopoietic progenitor cell activity. Endothelial colony formation assays were performed in the centralized biorepository core laboratory using methods previously described to evaluate for vasculogenic and pro-angiogenic progenitor cell activity.17 In brief, BMCs were plated in Endocult (Stem Cell Technologies) or endothelial growth media-2 (EGM-2) (Stem Cell Technologies) according to manufacturer guidelines, and incubated at 37°C in a fully humidified atmosphere with 5% CO2. Colony formations were enumerated weekly for four weeks and the maximum number of colonies per plate were used for analyses. BM and PB from healthy individuals (Lonza, Walkersville, MD, USA) were used to demonstrate viable progenitor cell assays. BM and PB from healthy individuals were processed using the same mononuclear cell (MNC) preparation (i.e., overnight shipment, Sepax MNC separation) as the IHD patients.
Statistical analysis
Summary statistics are tabulated as percentages for discrete variables for TIME, LateTIME, and FOCUS. Summarizations of baseline characteristics are compared across studies, with differences between continuous variables assessed using the general linear model, while differences between dichotomous variables were evaluated using chi-square testing. Therapy groups were combined due to the absence of differences for the Table 1 baseline characteristics across therapy groups in each of the studies. Bone marrow and peripheral blood characteristics were assessed for congruency with Pearson correlation coefficients.
Table 1. Patient Characteristics.
Baseline demographics and co-morbidities of patients enrolled in CCTRN cell therapy trials.
| Characteristic | TIME, Day 3 N=67 | TIME, Day 7 N=53 | LateTIME N=87 | FOCUS N=92 | P |
|---|---|---|---|---|---|
| Male/Female | 88%/12% | 87%/13% | 83%/17% | 89%/11% | 0.6382 |
| Age, mean ± SD | 56.1 ± 11.3 | 57.8 ± 10.3 | 56.6 ± 11.3 | 63.4 ± 10.1 | < 0.001 |
| White | 87% | 87% | 86% | 96% | 0.0892 |
| Hispanic | 6% | 7.5% | 4.6% | 4% | 0.8504 |
| Diabetes mellitus | 27% | 7.5% | 21% | 40% | 0.0001 |
| Hypertension | 51% | 68% | 53% | 79% | 0.0002 |
| Hyperlipidemia | 64% | 72% | 70% | 93% | < 0.0001 |
| Angina pectoris | 13% | 21% | 22% | 36% | 0.0086 |
| Smoker | 67% | 57% | 59% | 72% | 0.1668 |
| Weight (kg), mean ± SD | 93.7 ± 20.4 | 93.8 ± 19.2 | 85.3 ± 16.1 | 95.1 ± 23.2 | 0.0061 |
| Height (cm), mean ± SD | 175.8 ± 8.4 | 175.2 ± 10.9 | 175.0 ± 9.7 | 175.5 ± 9.1 | 0.9358 |
| Body Mass Index (kg/m2), mean ± SD | 30.2 ± 5.9 | 30.3 ± 4.9 | 27.8 ± 5.1 | 30.7 ± 6.3 | 0.0035 |
| LVEF, mean ± SD | 36.7% ± 6.3% | 36.5% ± 5.6% | 35.9% ± 6.9% | 31.7% ± 8.8% | < 0.0001 |
| Infarct size | |||||
| Time (Days) between PCI and Autologous BM-MNC Injection, mean ± SD | 3 ± 1 | 7.5 ± 1 | 17 ± 4 | NA | < 0.0001 |
| Medications | |||||
| Aspirin | 98.5% | 94.3% | 95.4% | 83.7% | 0.0024 |
| Beta-blocker | 98.5% | 96.2% | 88.5% | 94.6% | 0.0523 |
| Clopidogrel | 97.0% | 94.3% | 96.6% | NA | 0.7418 |
| Statin | 95.5% | 98.1% | 79.3% | 68.5% | < 0.0001 |
| ACE-Inhibitor | 83.8% | 86.8% | 73.6% | NA | 0.1139 |
Kg = kilogram, cm = centimeter, m2 = meter squared, SD = standard deviation, PCI = percutaneous intervention, BM-MNC = bone marrow mononuclear cell, NA = not available
After correction for multiplicity, any p value < 0.003 is considered statistically significant.
Results
Patient characteristics
Between July 8, 2008 and November 15, 2011, BM from 280 patients with acute and chronic IHD and LVD (LVEF ≤ 45%) were collected. The majority of subjects were older, obese white men with a history of smoking, hypertension, and hyperlipidemia (Table 1). After multiplicity correction, p values of < 0.003 were deemed as statistically significant differences among the proportions of patients. As expected, there was a greater proportion of patients with chronic IHD that also had cardiovascular disease relevant co-morbidities (i.e., diabetes mellitus, hypertension, hyperlipidemia) and angina pectoris. BM from nine healthy volunteers aged 20 to 40 years (median, 36 years) were recruited during this same time period and their BM was processed using the same MNC isolation methods as the IHD patients.
Heterogeneous BMC phenotypes with quantitative variation in patients with IHD
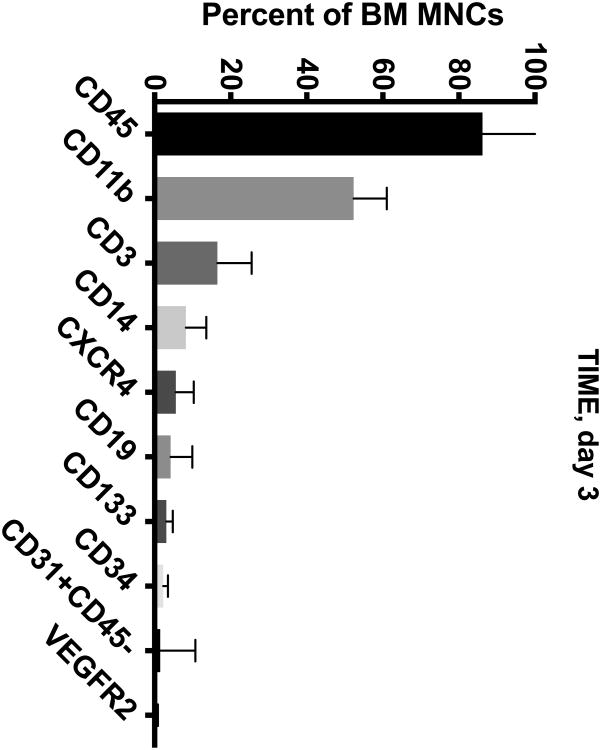
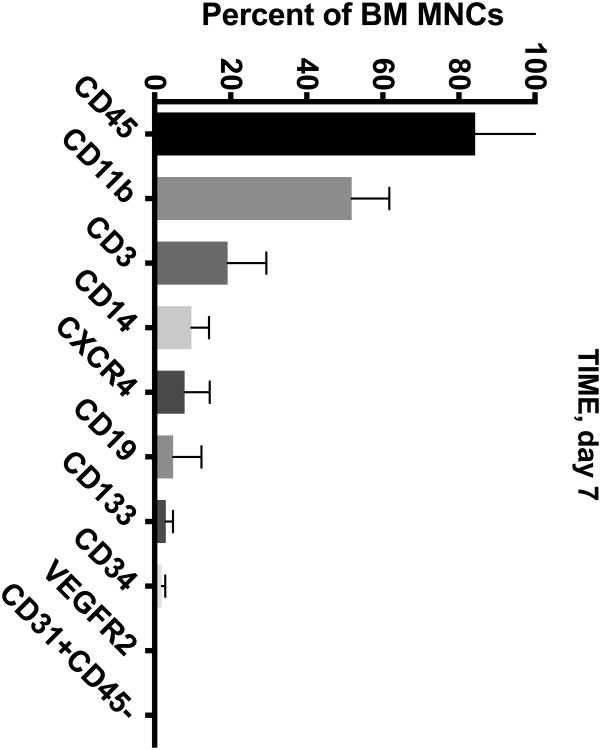
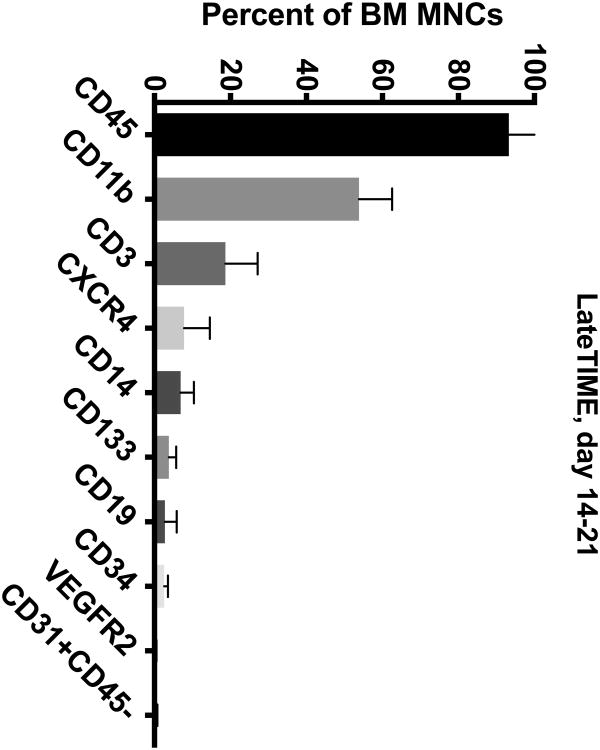
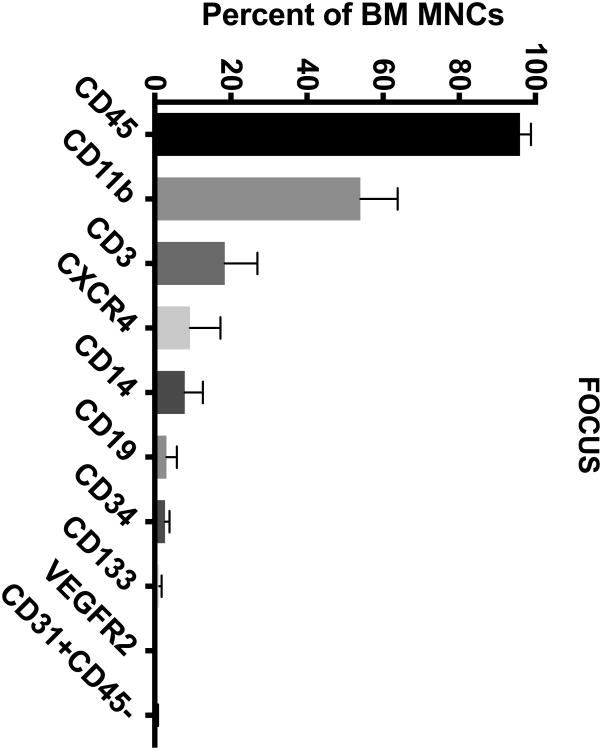
The BM from IHD patients was predominately (> 50%) composed of CD45+ and CD11b+ cells (Figure 1A-D). To a lesser extent (5-20%), the BM contained cells expressing CD3+, CD14+, and CXCR4+. In addition, the BM contained minor populations (< 5%) of cells expressing CD19+, CD133+, CD34+, CD31+CD45-, and VEGFR2+.
Figure 1. Cell Subsets in the BM of CCTRN Patients.
BM-MNCs from subjects enrolled in CCTRN cell therapy trials were immunostained and enumerated by flow cytometry. Lineages are presented in histograms showing cell subsets from most prevalent to least with bars representing mean±SD. In the TIME trial, patients were randomized 1:1 to undergo BM aspirations either (A) 3 days or (B) 7 days after AMI. (C) In the LateTIME trial, all subjects underwent BM aspirations 2-3 weeks after AMI. (D) In the FOCUS trial, BM aspiration was performed on patients with chronic myocardial
Decreased colony formations generated from BM of patients with IHD and LVD
BM from all IHD patients and healthy controls showed hematopoietic progenitor activity by generating CFC colonies in Methocult media (Table 2). However, shortly after AMI the number of individuals whose BM showed pro-angiogenic and vasculogenic activity by colony forming unit Hill (CFU-Hill) assay (Endocult) and endothelial colony-forming cell (ECFC) assay was significantly reduced (CFU Hill 55% vs. 100%; p< 0.001 and ECFC 43% vs. 100%: p< 0.001). Even in the subacute period (2-3 weeks) after AMI significantly fewer patients generated CFU-Hill (74% vs. 100%; p< 0.0001) and ECFC colonies (78% vs. 100%: p< 0.0001). Although fewer Late TIME patients grew colonies, the number of CFU Hill colonies was not reduced in BM where colonies grew (Figure 2A). However, ECFC colony number was significantly decreased in the patients at 2-3 weeks after AMI (Figure 2B). Interestingly, in heart failure (HF) patients from the FOCUS group, which had higher proportion of patients with co-morbid factors like diabetes, hyperlipidemia, and hypertension, BM was more likely to generate CFU-Hill and ECFC colonies than BM from the TIME group.
Table 2. Frequency of BM Derived Progenitor Cell Colony Outgrowth Among Patients with IHD Compared to Healthy Controls.
BM-MNCs from healthy control subjects (n=9), TIME, LateTIME, and FOCUS patients were grown in Methocult (CFC assay), Endocult (CFU-Hill assay), and EGM-2 (ECFC assay). Outgrowth of hematopoietic progenitor cell colonies (CFC) was observed in all patients. However, BM from patients with IHD enrolled in TIME, LateTIME, and FOCUS trials were less likely to generate endothelial cell colonies (CFU-Hill and ECFC) compared to healthy individuals.
| Healthy | TIME | Comparison to Control | LateTIME | Comparison to Control | FOCUS | Comparison to Control | |
|---|---|---|---|---|---|---|---|
| CFC | 100% | 100% | NS | 100% | NS | 100% | NS |
| CFU-Hill | 100% | 55% | p< 0.001 | 74% | p< 0.0001 | 74% | p< 0.0001 |
| ECFC | 100% | 43% | p< 0.001 | 78% | p< 0.0001 | 65% | p< 0.0001 |
Figure 2. Colony Growth from BM-MNCs of CCTRN Subjects with IHD.

(A) A representative phase-contrast micrograph of a CFU-Hill colony. (B) A representative phase-contrast micrograph of an ECFC colony. (C) In subjects whose BM generated CFU-Hill colonies, there was no difference in number of colonies between healthy controls (N=9) and IHD patients enrolled in TIME, LateTIME, and FOCUS trials. (D) In patients whose BM generated ECFC colonies, there was a significant decrease in maximum number of ECFC colonies in the LateTIME group, approximately 14 days after AMI.
Decreased BM CD34+ Cells 7 Days after AMI
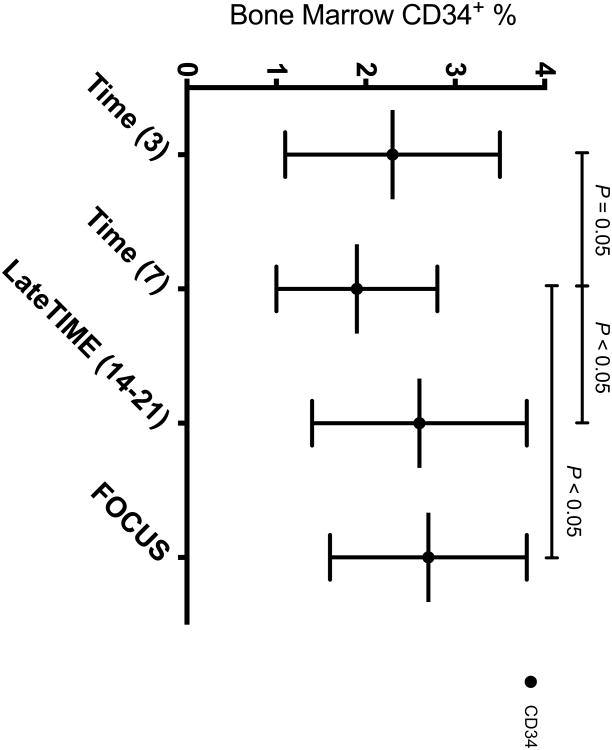
Out of the ten BMC subsets enumerated (Figure 1), only CD34+ cells differed according to time from MI, with study participants 7 days from AMI showing the lowest percentage of CD34+ cells (1.9%) compared to subjects 3 days after AMI (2.3%; p=0.05), 14-21 days from AMI (2.6%; p< 0.05), and chronic IHD patients (2.7%; p< 0.05) (Figure 3).
Figure 3. BM CD 34 + Percentage Among CCTRN Trial Cohorts.
Data represent mean ± SD. Percentage of CD34+ cells in the BM of patients 7 days after acute MI (1.9%) was decreased compared to other cohorts of IHD patients (TIME (3), 2.3%; LateTIME, 2.6%; FOCUS, 2.7%).
Post-infarct heart function and BM composition
To compare BM composition to post-infarct heart function, regression analyses were performed on the ten BMC subsets (Figure 1) and two endothelial assays (CFU-Hill and ECFC) compared to LVEF. Only CD11b+ cell (monocyte and macrophage) percentage significantly (and inversely) associated with post-infarct LVEF (p< 0.05): for every 1% greater in CD11b+, LVEF was lesser by 0.22%. These results support previous reports of increased innate immune cell activity following AMI and their importance in mediating myocardial remodeling.18-21
BM CD34+ cells as a biomarker for clinical outcome after cell therapy for IHD
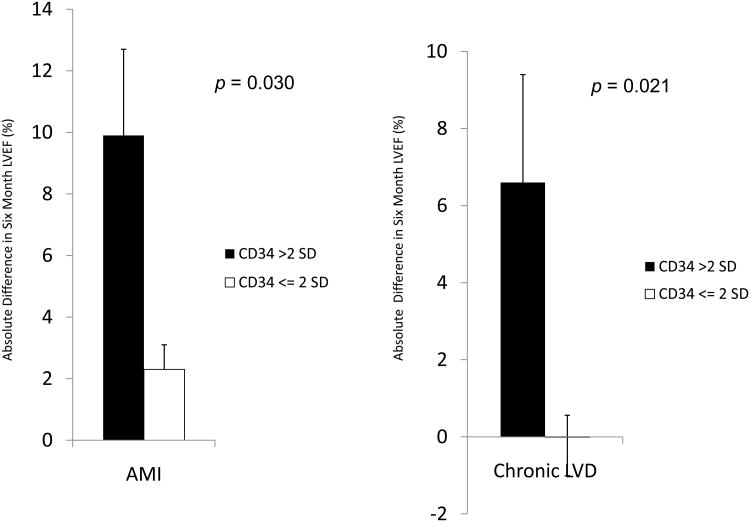
Given the importance of CD34+ stem/progenitor cells in various tissue repair processes, we scrutinized the BM CD34+ cell percentage of IHD patients in this study and found a distinct cohort of patients with elevated CD34+ cell percentage. Nine AMI patients showed a > 2 standard deviation (SD) increase in BM CD34+ cell percentage (mean, 5.7%) compared to the rest of the IHD patients (mean, 2.2%). Normally, in a resting state, human BM CD34+ cell percentage lies below 5%. Therefore, we hypothesized that increased CD34+ stem/progenitor cell percentage correlated with improvement in LVEF at 6 months follow-up. In fact, patients presenting with a high BM CD34+ percentage after AMI achieved greater increase in LVEF at 6 months compared with others (+9.9% absolute increase in LVEF vs. +2.32%, p=0.03) (Figure 4A). When applying this same analysis in patients with chronic IHD and severe LVD, three subjects with > 2 SD increase in BM CD34+ also showed a greater increase in LVEF compared with others (+6.6% vs. -0.02%, p=0.021) (Figure 4B).
Figure 4. BM CD34+ Cell Percentage and Change in LVEF at Six Months.
BM aspirations were performed in patients presenting with AMI and chronic LVD. CD34+ percentage was quantified by ISHAGE criteria and LVEF was examined at baseline and six months later. (A) Patients with AMI who presented with high BM CD34+ percentage (greater than 2 SD from the mean) showed significantly greater increase in LVEF at six-month follow-up. (B) Likewise, patients with chronic LVD and high BM CD34+ percentage (greater than 2 SD from the mean) also showed significantly greater increase in LVEF at six-month follow-up.
In complementary fashion, we hypothesized that lower BM CD34+ cell percentage after AMI indicated a suppression or lack of response in the BM resulting in a decrement in LVEF after AMI. To test this hypothesis the ten IHD patients with the lowest BM CD34+ percentages immediately following AMI were compared to the others. These individuals had no significant change in LVEF at 6-month follow-up (mean change in LVEF +2.55% vs.+2.68%, p=0.9671). However, the ten chronic LVD patients with the lowest BM CD34+ percentage demonstrated a significant decline in their LVEF at 6-month follow-up compared with others (mean change LVEF -2.93% vs. +0.69%, p=0.0355). Together, these results suggest that BM CD34+ stem/progenitor cell percentage may be a biomarker for response following AMI.
No correlation between peripheral blood and bone marrow cell subsets and progenitor activities
Given the minimal risk in obtaining PB and the higher risk of sampling BM, we examined whether PB measurements of cell subsets and progenitor activities correlated with BM. Nine cell lineages and progenitor outgrowth results were selected based on previous reports of repairing ischemic/infarcted myocardium (Supplemental Table I). None of the nine subsets and progenitor activities showed strong correlation between PB and BM. Of the nine, the strongest correlation between PB and BM was the CD34+CD133+ percentage, but its strength of correlation was weak (0.55, 95% CI 0.40-0.67).
Discussion
In this detailed analysis of BM from IHD patients we show a heterogeneous mixture of cell subsets, decreased endothelial colony capacity, a CD34+ cell nadir seven days after AMI, inverse relation between CD11b percentage and LVEF immediately after AMI, and positive correlation of CD34 percentage with change in LVEF six months after autologous BMC therapy.
Since this is the first presentation of major and minor BMC subsets from IHD patients, no comparisons can be made to other studies. However, the proportions of major cell lineages (i.e., CD45+, CD3+) are consistent with the proportions observed in the standard clinical practice of BM transplant for patients with hematological malignancies.
In terms of BMC function, the data show that BM from patients with IHD and severe LVD contains hematopoietic progenitor cell activity (as measured by the CFC assay), but has reduced ability to form EPC colonies (as measured by ECFC and CFU-Hill assays). This study confirms a previous report showing no change in hematopoietic progenitor activity after MI,22 but extends prior knowledge by revealing impairment in BM-derived vascular precursors after AMI. When considering that the BM can be a source for circulating EPCs and that number of circulating EPCs correlates with LV function after AMI,8 the results suggest that the BM from IHD patients is a plentiful resource for hematopoiesis but potentially a finite reservoir of vasculogenic precursors. One consideration to make when evaluating these data is that the healthy control study participants were younger in age and without known cardiac disease. Although their BMCs were processed exactly like the IHD study patients, it is possible that age may have been a determinant in BM cell-derived endothelial colony formation. In a non-human primate model of age-related changes, decreased number and function of rhesus monkey circulating ECFCs were found in aged primates.23 Whether age affects BM-derived ECFC in humans has yet to be determined.
The only BMC subset that significantly differed according to time from AMI was the CD34+ cell fraction. Although a minor population in the BM, the percentage of BM CD34+ stem and progenitor cells was decreased in AMI patients seven days after percutaneous intervention (PCI), suggesting a temporary depression of CD34+ cells in the BM. In the setting of MI, possible instigators of this depression could include pro-inflammatory cytokines, angiogenic factors, and sympathetic nervous system signaling. In an experimental model of BMC therapy for AMI, regional MI led to systemic inflammation that triggered proliferation of activated myeloid cells in the BM.24 However, the BMCs showed a time-dependent depression in regenerative capacity with a nadir of activity at three days after AMI in mice. This relationship between the injured heart and BM mirrors what we observed in the current human study with particular respect to BM CD11b+ and CD34+ percentages. After AMI in experimental models, systemic cytokines that alter BM composition and depress regenerative function include IL-1α, IL-1β, IL-6, and GM-CSF, in addition to others,24 providing a possible explanation for the decrease in BM CD34+ percentage and impaired clonogenic capacity in IHD patients. Interestingly, in rodents, the cardiac regenerative capacity of BMCs can be recovered after treating BMCs ex vivo with immune suppression. Follow-up translational studies will include evaluations of BM and PB plasma for inflammatory cytokines and attempted recovery of BM function by inflammation antagonists.
Although our data are supported by experimental evidence, there are a few differences in relation to prior clinical reports from other groups. Only one other cell therapy group has reported CD34+ values over multiple time points after AMI. In contrast to our results, data from REPAIR-AMI subjects showed a small increase in BM CD34+ percentage at day 6-8 compared to days 2-6 after AMI with mean BM CD34+ increasing from 1.4% to 1.9%.22 Although the direction of change differed in our study, the absolute value seen at day seven was similar (1.9%). When comparing our BM CD34+ cell percentages with the prior report, absolute differences are ≤ 0.5%, which calls into question whether the differences are biologically significant. Moreover, differences in study populations between the CCTRN trials and the REPAIR-AMI trial could account for the differences in CD34 percentages. This fine point may bear further examination.
Although CD34+ stem and progenitor cells are a minor subset within the BM, they have multipotent potential and are closely enumerated in the standard practice of hematology, oncology, and BM transplantation. In IHD patients we found that BM CD34+ cell percentage after AMI correlates with change in LVEF at 6 months. Patients with AMI and severe LVD who had increased BM CD34+ cell percentage showed markedly improved LVEF (+ 10% absolute increase) at 6 months. These individuals represent an interesting cohort of enhanced responders. Whether their early LV improvement is sustained long-term remains to be determined. Another question of interest is the biological significance of increased BM CD34+ cells. Since four out of nine (44%) CD34+ enhanced responders were randomized to active cell therapy, it is possible that the high level of CD34+ cells may have served a direct role in heart regeneration. However, it is also possible that the high level of BM CD34+ cells is a biomarker for some other process, such as inflammation, angiogenesis, and/or catecholaminergic signaling instigated by an infarcted myocardium.
Data from this study show BM impairments in patients with IHD and severe LVD as a potential explanation for the mixed trial results in autologous BMC therapy trials. Rather than abandon BMCs as a therapeutic source, more investigation should occur into selecting potent cell subsets or reversing cell impairments prior to clinical use. First, these data confirm the importance of CD34+ cells in cardiac cell therapy. Leading up to this report, Losordo et al and Wang et al demonstrated reduced frequency of angina pectoris and improved exercise tolerance in IHD patients who received intramyocardial and intracoronary injections of autologous CD34+ cells compared to placebo.9, 25, 26 In patients with nonischemic dilated cardiomyopathy, transendocardial injection of autologous CD34+ cells was associate with higher myocardial retention rates and greater improvements in ventricular function compared to intracoronary route.27 Our data confirm the importance of CD34 number in BMC mediated repair after AMI but go on to show that even when cell number is intact, progenitor cell function can be decreased in these patients – reinforcing the concept that reversal of loss of function may be equally as important as improving cell number.
Although the mechanisms by which BMCs improve myocardial function are still unclear, the CD11b and CD34 data from this report suggest an important relationship between inflammatory cues and BMC response. In the ischemic/infarcted myocardial microenvironment, BMCs most likely act as paracrine regulators, mitigating toxic inflammation and triggering capillary re-growth, thereby preventing cardiomyocyte apoptosis or stimulating resident cardiac stem cells.1, 28 Therefore, selection of potent BMC subsets may best be defined in terms of homing (e.g., CXCR4 expression) and controlling inflammation and angiogenesis. If so, the optimal BMCs for cardiac regeneration after MI may require upregulation of chemokine receptors such as IL-1Rs or CXCR4. More simply, it may be possible to treat BMCs ex vivo prior to patient administration with agents that reverse BMC impairment(s). For example, given the upregulation of the pro-inflammatory cytokine, IL-1, in the bone marrow and hearts of IHD patients, pre-treating BMCs with IL-1R antagonists, such as anakinra, could reverse BMC impairment and improve cardiac outcomes.29 Ultimately, merging data about the milieu of the post-infarct myocardial microenvironment with data from reports such as this one will be necessary to select and engineer the most potent BMC subtype in the future.
Conclusions
In this study we show a heterogeneous mixture of BMC subsets, decreased endothelial colony capacity, a CD34+ cell nadir seven days after AMI, a negative correlation between CD11b percentage and post-infarct LVEF, and positive correlation of CD34 percentage with change in LVEF after cell therapy. These results serve as a possible basis for the small clinical improvement seen in autologous BMC therapy trials and support selection of potent cell subsets and/or reversal of co-morbid BM impairment.
Supplementary Material
Novelty and Significance.
What Is Known?
Bone marrow (BM) contains stem and progenitor cells capable of generating blood vessels in response to ischemia and inflammation.
After acute myocardial infarction (AMI), BM cell (BMC) therapy improves left ventricular (LV) function in experimental models but effects in AMI patients are minimal.
Limited information is available about the BM niche and links with LV function in patients with acute and chronic ischemic heart disease (IHD).
What New Information Does This Article Contribute?
A heterogeneous mixture of cell subsets is present in the BM of IHD patients.
Post-AMI endothelial colony capacity is decreased with a nadir in CD34+ cell number at 7 days and a negative correlation between CD11b percentage and LV function.
There is a positive correlation between CD34+ percentage and change in LV function after BMC therapy.
Experimental studies document BMC therapy improves LV function in AMI models. However, only very minimal LV functional improvement has been reported after BMC therapy in AMI patients or those with chronic IHD. We found that the BM niche of patients with LV dysfunction due to acute or chronic IHD, analyzed for cell subsets by flow cytometry and colony assays, contains a very heterogeneous mixture of cell subsets. Both the cell numbers and colony growth characteristics vary over time after AMI. The CD34+ cell percentage is significantly decreased 7 days after AMI compared with BM from less acute or chronic IHD patients. Also BM-derived endothelial colonies are significantly decreased. Increased CD11b+ cells are associated with significantly greater LV dysfunction after AMI. While increased CD34+ percentage is associated with greater improvement in LV function among the AMI patients compared with chronic IHD patients, a decreased CD34+ percentage in chronic IHD patients correlated with the decrease in LV function observed 6 months after BMC therapy. These findings may explain, in part, the only minimal and variable LV functional improvement observed in autologous BMC therapy trials and support selection of more potent cell types and/or attempts to reverse co-morbid BMC impairment.
Acknowledgments
The authors thank Raphael Bosse for his work in immunostaining and confocal microscopy.
Sources of Funding: NIH network grants (NHLBI U01HL087318-08)
NIH R01 to Pepine and Cogle (NHLBI R01 HL091005)
Nonstandard Abbreviations and Acronyms
- AMI
acute myocardial infarction
- BM
bone marrow
- BMC
bone marrow cell
- BM-MNC
bone marrow mononuclear cell
- CCTRN
Cardiovascular Cell Therapy Research Network
- CFC
colony forming cell
- CFU
colony forming unit
- DPBS
Dulbecco's phosphate-buffered saline
- ECFC
endothelial colony forming cell
- EGM-2
endothelial growth media-2
- EPC
endothelial progenitor cell
- FBS
fetal bovine serum
- IHD
ischemic heart disease
- LV
left ventricular
- LVD
left ventricular dysfunction
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- MNC
mononuclear cell
- NHLBI
National Heart, Lung, and Blood Institute
- PB
peripheral blood
- PCI
percutaneous coronary intervention
- SD
standard deviation
Footnotes
Clinical Trial Registrations: clinicaltrials.gov Identifiers: NCT00684021, NCT00684060, NCT00824005
Disclosure of Conflicts of Interest: None.
References
- 1.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, Weissman NJ, Leon MB, Epstein SE, Kornowski R. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726–1732. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 3.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey AS, Willenbring H, Jiang S, Anderson DA, Schroeder DA, Wong MH, Grompe M, Fleming WH. Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:13156–13161. doi: 10.1073/pnas.0604203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madlambayan GJ, Butler JM, Hosaka K, Jorgensen M, Fu D, Guthrie SM, Shenoy AK, Brank A, Russell KJ, Otero J, Siemann DW, Scott EW, Cogle CR. Bone marrow stem and progenitor cell contribution to neovasculogenesis is dependent on model system with SDF-1 as a permissive trigger. Blood. 2009;114:4310–4319. doi: 10.1182/blood-2009-03-211342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 7.Massa M, Campanelli R, Bonetti E, Ferrario M, Marinoni B, Rosti V. Rapid and large increase of the frequency of circulating endothelial colony-forming cells (ECFCs) generating late outgrowth endothelial cells in patients with acute myocardial infarction. Exp Hematol. 2009;37:8–9. doi: 10.1016/j.exphem.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Suresh R, Chiriac A, Goel K, Villarraga HR, Lopez-Jimenez F, Thomas RJ, Terzic A, Nelson TJ, Perez-Terzic C. CXCR4+ and FLK-1+ identify circulating cells associated with improved cardiac function in patients following myocardial infarction. J Cardiovasc Transl Res. 2013;6:787–797. doi: 10.1007/s12265-013-9502-z. [DOI] [PubMed] [Google Scholar]
- 9.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA ACT34-CMI Investigators. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, Lerakis S, Sher S, Vaughan D, Perin E, Willerson J, Kereiakes D, Gersh BJ, Gregory D, Werner A, Moss T, Chan WS, Preti R, Pecora AL. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161:98–105. doi: 10.1016/j.ahj.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Marban E, Malliaras K. Bone marrow-derived cell therapy after myocardial infarction--reply. JAMA. 2013;309:1459–1460. doi: 10.1001/jama.2013.2604. [DOI] [PubMed] [Google Scholar]
- 12.Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 13.King A. Stem cells. Could it be TIME to abandon BMCs? Nat Rev Cardiol. 2013;10:8. doi: 10.1038/nrcardio.2012.170. [DOI] [PubMed] [Google Scholar]
- 14.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, Thomas JD, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Kappenman C, Westbrook L, Piller LB, Simpson LM, Baraniuk S, Loghin C, Aguilar D, Richman S, Zierold C, Spoon DB, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD Cardiovascular Cell Therapy Research Network (CCTRN) Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, Perin EC, Baran KW, Chambers J, Lambert C, Raveendran G, Simon DI, Vaughan DE, Simpson LM, Gee AP, Taylor DA, Cogle CR, Thomas JD, Silva GV, Jorgenson BC, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Smith DX, Baraniuk S, Piller LB, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD Cardiovascular Cell Therapy Research Network. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD Cardiovascular Cell Therapy Research Network (CCTRN) Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zierold C, Carlson MA, Obodo UC, Wise E, Piazza VA, Meeks MW, Vojvodic RW, Baraniuk S, Henry TD, Gee AP, Ellis SG, Moye LA, Pepine CJ, Cogle CR, Taylor DA. Developing mechanistic insights into cardiovascular cell therapy: Cardiovascular Cell Therapy Research Network Biorepository Core Laboratory rationale. Am Heart J. 2011;162:973–980. doi: 10.1016/j.ahj.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 19.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, Kitabata H, Okochi K, Arita Y, Ishibashi K, Komukai K, Kataiwa H, Nakamura N, Hirata K, Tanaka A, Akasaka T. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Zhao G, Wang S, Wang Z, Sun A, Yang X, Qiu Z, Wu C, Zhang W, Li H, Zhang Y, Zhao J, Zou Y, Ge J. CXCR6 deficiency ameliorated myocardial ischemia/reperfusion injury by inhibiting infiltration of monocytes and IFN-gamma-dependent autophagy. Int J Cardiol. 2013;168:853–862. doi: 10.1016/j.ijcard.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Assmus B, Iwasaki M, Schachinger V, Roexe T, Koyanagi M, Iekushi K, Xu Q, Tonn T, Seifried E, Liebner S, Kranert WT, Grunwald F, Dimmeler S, Zeiher AM. Acute myocardial infarction activates progenitor cells and increases Wnt signalling in the bone marrow. Eur Heart J. 2012;33:1911–1919. doi: 10.1093/eurheartj/ehr388. [DOI] [PubMed] [Google Scholar]
- 23.Shelley WC, Leapley AC, Huang L, Critser PJ, Zeng P, Prater D, Ingram DA, Tarantal AF, Yoder MC. Changes in the frequency and in vivo vessel-forming ability of rhesus monkey circulating endothelial colony-forming cells across the lifespan (birth to aged) Pediatr Res. 2012;71:156–161. doi: 10.1038/pr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Takagawa J, Lam VC, Haddad DJ, Tobler DL, Mok PY, Zhang Y, Clifford BT, Pinnamaneni K, Saini SA, Su R, Bartel MJ, Sievers RE, Carbone L, Kogan S, Yeghiazarians Y, Hermiston M, Springer ML. Donor myocardial infarction impairs the therapeutic potential of bone marrow cells by an interleukin-1-mediated inflammatory response. Sci Transl Med. 2011;3:100ra90. doi: 10.1126/scitranslmed.3002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, Olson RE, Flanagan J, Sodano D, Murayama T, Kawamoto A, Kusano KF, Wollins J, Welt F, Shah P, Soukas P, Asahara T, Henry TD. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Cui J, Peng W, Lu M. Intracoronary autologous CD34+ stem cell therapy for intractable angina. Cardiology. 2010;117:140–147. doi: 10.1159/000320217. [DOI] [PubMed] [Google Scholar]
- 27.Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, Cernelc P, Torre-Amione G, Haddad F, Wu JC. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128:S42–9. doi: 10.1161/CIRCULATIONAHA.112.000230. [DOI] [PubMed] [Google Scholar]
- 28.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, Oddi C, Roberts CS, Melchior RD, Mueller GH, Abouzaki NA, Rengel LR, Varma A, Gambill ML, Falcao RA, Voelkel NF, Dinarello CA, Vetrovec GW. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study. Am J Cardiol. 2013;111:1394–1400. doi: 10.1016/j.amjcard.2013.01.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.