Abstract
Background
Elevated immune activation is associated with an increased risk of HIV acquisition. Tenofovir (TFV) has immunomodulatory properties in vitro, but how this extends in vivo remains unknown.
Methods
HIV-negative adults received daily co-formulated tenofovir-disoproxil-fumarate (TDF) 300 mg/emtricitabine (FTC) 200 mg for 30 days followed by a 30-day washout. Markers of T-cell activation, inflammation and cytokines were measured before drug and on days 30 (on drug) and 60 (30-day washout). Data were analyzed using one-way ANOVA/pairwise comparisons. Intracellular disposition of TFV-diphosphate (TFV-DP) and FTC-triphosphate (FTC-TP) in CD4+ and CD8+ T-cells and monocytes was characterized, and the relationship with immune activation was evaluated using Pearson correlation coefficient.
Results
T-cell activation was available in 19 participants. CD38/HLA-DR co-expression on CD8+ T-cells decreased from baseline to day 30 (3.97 vs. 2.71%;P=0.03) and day 60 (3.97 vs. 2.41%;P=0.008). Soluble CD27 decreased from baseline to day 60 (184.1 vs. 168.4 pg/ml;P=0.001). Cytokines and inflammation markers were not significantly different. TFV-DP and FTC-TP were approximately 4-fold higher in monocytes vs. CD4+ and CD8+ T-cells, but neither correlated with activation markers.
Conclusions
TDF/FTC therapy was associated with decreased T-cell activation in HIV-negative adults, which could contribute to the antiviral effect of pre-exposure prophylaxis.
Keywords: immune activation, tenofovir, pre-exposure prophylaxis, clinical pharmacology, intracellular nucleoside analog
Background
T-cell immune activation and inflammation are well-known to accelerate the progression of HIV/AIDS in chronically infected individuals [1,2,3]. Recently, an emerging body of literature has also identified a direct association between immune activation and the susceptibility to HIV infection. Studies in macaques [4,5] and humans [6,7,8,9] have reported that an immune quiescent phenotype, manifested by decreased cytokine production, low CD4+ T-cell activation marker expression, suppression of cellular alloimmune responses and decreased CD4+ T-cell gene expression, may confer protection against HIV infection in highly exposed-seronegative individuals. The CAPRISA 004 trial identified that women who became infected with HIV had higher concentrations of proinflammatory cytokines, activated natural killer (NK) cells and CD8+ T-cell degranulation compared with uninfected women, irrespective of tenofovir gel use [10]. Based on these findings, it has been postulated that immune activation may be a modifiable risk factor for HIV acquisition and that promotion of immune quiescence in high-risk individuals could become a potential strategy to reduce HIV infection [10,11]. To date, no proven interventions aimed at promoting immune quiescence to reduce the risk of HIV infection are available.
Tenofovir (TFV) is a nucleotide reverse transcriptase inhibitor (NRTI) widely used for the treatment of HIV infection and recently approved in the United States as pre-exposure prophylaxis (PrEP) against HIV acquisition. In addition to its inhibition of reverse transcriptase to elicit antiviral activity, TFV has diverse immunomodulatory effects in various human and animal immune cell lines. Previous in vitro research has identified an anti-inflammatory effect of TFV and other acyclic nucleoside phosphonates in macrophages, lymphocytes and murine hepatocytes [12,13,14], while other studies have reported a pro-inflammatory effect [12,15,16]. Recent studies have also associated immune quiescence with topical TFV exposure in the murine vagina and the human rectum [17,18]. However, it remains unclear how (or if) these in vitro and in vivo topical findings extend to systemic immune-modulation or cytokine concentrations in HIV-negative individuals.
In addition to cytokines and cell activation marker expression, circulating soluble biomarkers such as soluble CD14 (sCD14) and CD27 (sCD27) and markers of inflammation such as C-reactive protein (hs-CRP) are important correlates of immune activation and inflammation. In HIV infection, sCD14, sCD27 and hs-CRP have been associated with disease progression, morbidity and mortality [19,20,21,22,23,24]. For example, soluble CD14 (sCD14), a marker of monocyte and macrophage activation, is highly predictive of mortality and neurocognitive impairment in HIV infection [20,25]. Likewise, soluble CD27 (sCD27), which is secreted by activated T lymphocytes (particularly CD4+ T-cells) [26,27] has been associated with T-cell decline and virologic response to antiretroviral therapy in HIV-infected patients [28,29]. In terms of HIV prevention, sCD14 was recently evaluated in the CAPRISA 004 trial, where plasma concentrations of sCD14 did not correlate with the risk of HIV infection, but more information is needed regarding correlations between sCD14 (and sCD27) and HIV prevention [30]. Additionally these soluble biomarkers and hs-CRP may serve as another correlate to evaluate systemic immune-modulation in HIV-negative individuals receiving TFV.
TFV may also decrease uric acid concentrations in HIV-infected individuals, as was shown in subjects previously treated with didanosine [31]. This is of interest since uric acid has been associated with systemic inflammation and is highly correlated with CRP, tumor necrosis (TNF)-α and interleukin (IL)-6 [32]. To date, the effect of TFV on uric acid in HIV-negative individuals has not been fully elucidated.
Given previous in vitro observations, we hypothesized that exposure to TFV would have systemic immunomodulatory effects in vivo. We sought to test this hypothesis in HIV-negative individuals who do not exhibit confounding influences from HIV replication. In this study, we evaluated the changes in T-cell immune activation, cytokine production, soluble biomarkers and uric acid in HIV-negative individuals exposed to tenofovir-disoproxil-fumarate/emtricitabine (TDF/FTC) therapy.
Methods
Participants and study design
HIV-negative, healthy volunteers were enrolled in an intensive pharmacokinetic study of daily co-formulated oral TDF/FTC for 30 days followed by 30-day washout (NCT01040091; www.clinicaltrials.gov). Individuals were eligible if they were between the ages of 18–55 years, were able to provide informed consent and to comply with study procedures. All participants were tested for HIV and hepatitis B virus infection upon screening and were excluded if they tested positive. Individuals who were pregnant (or planning to become pregnant), breastfeeding, had a history of pathologic bone fractures, a body weight <110 pounds, an estimated glomerular filtration rate by MDRD <60 mL/min/1.73 m2, albuminuria:creatinine >30:1 or were taking any concomitant nephrotoxic medication were also excluded. After completing 30 days of daily TDF/FTC, the participants were followed for an additional 30 days off-drug and re-evaluated at day 60. Blood samples for cellular immune activation, cytokines, soluble biomarkers, hs-CRP and uric acid were collected at baseline (before TDF/FTC initiation) and at days 30 and 60. Additionally, purified CD3+CD4+ and CD3+CD8+ T-cells and monocytes (CD14+) were obtained once per participant at various time intervals (days 1, 3, 7, 20 and 30) for intracellular TFV-diphosphate (TFV-DP) and FTC-triphosphate (FTC-TP) measurement. Approval was obtained by the Colorado Multiple Institutional Review Board and informed consent was obtained from all participants.
T-cell immune activation analysis
HLA-DR and CD38 co-expression in CD4+ and CD8+ T-cells was analyzed at baseline, day 30 and day 60 from whole blood stained with fluorescent anti-CD3/CD4/CD38/HLA-DR or anti-CD3/CD8/CD38/HLA-DR monoclonal antibodies (BD Biosciences, San Jose, CA). Cells were incubated for a total of 30 minutes at room temperature and red blood cells were lysed. The remaining cells were fixed with 450µL of FACS lysing solution (BD Biosciences) prior to flow cytometry analysis, which was performed using a FACSCalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA). CD38 and HLA-DR expression was analyzed using settings determined by FACSComp by using CaliBRITE beads (BD Biosciences, San Jose, CA). The proportion of HLA-DR+/CD38+ CD4+ and CD8+ T-cells was automatically determined using the HLA-DR/CD38 Multiset Algorithm (BD Biosciences, San Jose CA).
Cytokine, soluble marker, hs-CRP and uric acid analysis
Concentrations of IL-1a, IL-1b, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-15, IL-17, IL-23, interferon-gamma (IFN-γ), TNF-α, TNF-β, sCD14, sCD27, hs-CRP and uric acid were measured in EDTA plasma collected at baseline, day 30 and day 60, which was stored at −80°C and thawed for analysis. For the cytokine analysis, thawed plasma was diluted 1:1 and cytokine concentrations were measured using the 15-plex high-sensitivity Quansys Multiplex cytokine array ELISA (Quansys Biosciences, Logan, UT) as per the manufacturer recommended protocol. Samples were run in duplicate and the wells were analyzed using Q-view image software; F-stop 2.8, ISO 400, Exposure Times (sec) of 30/60/180 sec. High-sensitivity IL-6 was measured using Quantikine ELISA (R&D #HS600B). The wells were analyzed at 490nm with wavelength correction at 650nm on a Coulter Microplate Reader. sCD14 and sCD27 concentrations were measured in freshly thawed EDTA plasma diluted 1:500 in calibration diluent (R&D #DY140) and 1:20 in dilution buffer (Pelikine #M1960), respectively. hs-CRP analysis was performed in plasma diluted 1:100 in calibration diluent. As necessary, samples were further diluted (1:300, 1:400 and 1:500) and rerun. For all sCD14, sCD27 and hs-CRP, ELISA was performed as per the manufacturer recommended protocol and the wells were analyzed at 450nm on a Coulter Microplate Reader. For uric acid, samples were thawed to 37°C in a water bath for 10 minutes followed by vortexing vials for 15 seconds and centrifuged at room temperature at 1000g for 10 minutes. Supernatants were loaded into well cups and analyzed using an ACE clinical autoanalyzer (Alpha Wasserman, West Caldwell, NJ).
Intracellular quantification of TFV-DP and FTC-TP in CD4+ and CD8+ T-cells and monocytes
Blood samples for purified CD4+ and CD8+ T-cells and monocytes were collected at 2 hours post-dose from one study visit for each participant. Briefly, PBMCs were isolated from CPT tubes according to manufacturer’s instructions and labeled with directly fluorescent-conjugated antibodies (anti-CD3-APC, anti-CD4-APC-Cy7, anti-CD8 Alexa Fluor 405, anti-CD14-phycoerythrin [PE]) by incubation on ice for 30 minutes. After incubation, the labeled PBMC sample was washed with 1% BSA/PBS and suspended in 1% BSA/PBS for sorting by FACSAria flow cytometry, version 6.1.3 (BD). All sorted cell fractions (CD4+ and CD8+ T-cells and monocytes) were counted by an Automated Cell Counter (Countess, Invitrogen) using tryptan blue exclusion to assess cell viability. Cell fractions were then lysed with 500µL of 70:30 methanol:water for storage at −80°C until analysis. TFV-DP and FTC-TP in CD4+ and CD8+ T-cells and monocytes cells was quantified by a validated liquid chromatography/tandem mass spectrometry (LC-MS/MS) method, as described previously [33].
Intracellular drug concentrations were evaluated with a naïve-pooled approach by fitting a mono-exponential equation to the concentration time data with GraphPad version 6.00 for Windows (GraphPad Software, La Jolla California USA, www.graphpad.com); Ct= Css*(1−exp(−k*t)) where t is time on therapy, Css is the fitted steady-state concentration, and k is the fitted elimination rate constant. Individual Css concentrations were estimated by adjusting the observed concentration according to time on therapy as follows: Css= Ct/(1−exp(−k*t)), where Ct was the observed concentration at time t and k was the fitted value from the pooled approach.
Statistical analysis
The primary outcome was the change in CD4+ and CD8+ T-cell immune activation as a result of TDF/FTC therapy. All data were loge transformed and analyzed using one-way ANOVA for repeated measures (assuming sphericity) and pairwise comparisons for significant results between baseline and day 30 and baseline and day 60, using Fisher’s protected least significant difference. The relationship between cell activation and intracellular TFV-DP and FTC-TP in CD4+ and CD8+ T-cells and monocytes was assessed using the Pearson correlation coefficient. Baseline sex and race differences in T-cell activation, soluble markers, hs-CRP and uric acid were analyzed using unpaired t-test. T-cell immune activation, cytokines and intracellular TFV-DP and FTC-TP measurements below the lower limit of quantification (LLOQ) of their respective assays were given a value of the midpoint between zero and the LLOQ and were included in the analysis. A P value ≤0.05 was considered significant. Data are presented as geometric mean (95% CI) unless specified otherwise. All statistical analyses were performed using GraphPad Prism version 6.00 for Windows.
Results
Study population
A total of 19 participants were enrolled in the study. T-cell immune activation and intracellular TFV-DP and FTC-TP concentrations were available for 19 participants, whereas cytokines, soluble markers, hs-CRP and uric acid concentrations were available for 18 participants. The demographic characteristics of the study population are summarized in Table 1.
Table 1.
Demographic characteristics of the study population (N=19).
| Characteristic | Number |
|---|---|
| Men | 9 |
| Women | 10 |
| African American | 9 (5 Women) |
| Non-African American | |
| White | 9 (5 Women) |
| Hispanic | 1 (Man) |
| Median age (range) years | 30 (20–47) |
T-cell immune activation
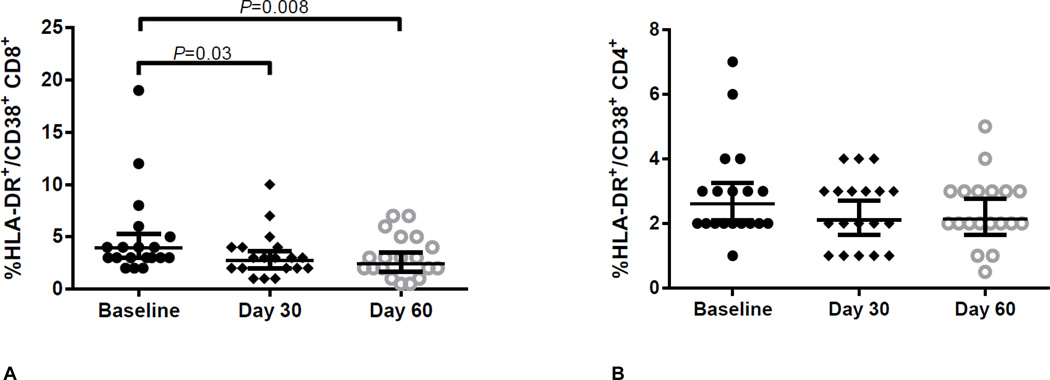
The overall HLA-DR/CD38 co-expression in CD8+ T-cells decreased significantly (ANOVA P=0.02) from baseline to day 30 (3.97% vs. 2.71%; P=0.03; Table 2, Figure 1) and remained decreased 30 days after drug discontinuation (3.97% vs. 2.41%; P=0.008; Table 2 and Figure 1). CD4+ T-cell activation also decreased from baseline to days 30 and 60, although these changes were not statistically significant (Table 2; ANOVA P=0.25). For both CD8+ and CD4+ T-cells there were no differences in HLA-DR/CD38 co-expression between day 30 and day 60 (P≥0.51). CD8+ T-cell activation was reported at 0% for two participants at day 60 (for which a value of 0.5% was imputed). No sex or race differences in baseline CD4+ and CD8+ T-cell immune activation were observed (P≥0.18).
Table 2.
Immune changes after 30 days of daily TDF/FTC therapy and after 30 days in “wash out” period (day 60).
| Baseline | ANOVA P value | Day 30 | P value* | Day 60 | P value* | |
|---|---|---|---|---|---|---|
| %HLA-DR+/CD38+ CD4+ | 2.61 (2.10 to 3.24) | 0.25 | 2.11 (1.64–2.70) | - | 2.13 (1.64 to 2.76) | - |
| %HLA-DR+/CD38+ CD8+ | 3.97 (2.98 to 5.29) | 0.02 | 2.71 (2.01 to 3.66) | 0.03 | 2.41 (1.65 to 3.53) | 0.008§ |
| IL-10 (pg/ml) | 1.88 (1.55 to 2.29) | 0.37 | 2.00 (1.65 to 2.44) | - | 1.74 (1.40 to 2.15) | - |
| IFN-γ(pg/ml) | 0.76 (0.48 to 1.20) | 0.93 | 0.73 (0.49 to 1.09) | - | 0.81 (0.50 to 1.32) | - |
| TNF-α (pg/ml) | 8.21 (6.74 to 10.02) | 0.26 | 8.32 (6.84 to 10.13) | - | 7.38 (5.77 to 9.43) | - |
| IL-6 (pg/ml) | 1.04 (0.75 to 1.45) | 0.48 | 0.88 (0.69 to 1.13) | - | 0.83 (0.58 to 1.18) | - |
| sCD27 (pg/ml) | 184.1 (163.4 to 207.6) | 0.001 | 184.1 (165.9 to 204.2) | 0.98 | 168.4 (149.3 to 190) | 0.001§ |
| sCD14 (mcg/ml) | 1.63 (1.51 to 1.74) | 0.06 | 1.51 (1.38 to 1.64) | - | 1.51 (1.42 to 1.61) | - |
| hs-CRP (mcg/ml) | 1.91 (1.25 to 2.92) | 0.16 | 1.24 (0.76 to 2.00) | - | 1.59 (0.95 to 2.66) | - |
| Uric Acid (mg/dl) | 4.30 (3.38 to 5.48) | 0.79 | 4.00 (3.09 to 5.17) | - | 4.45 (3.51 to 5.64) | - |
Data are geometric mean (95% CI). Cytokines, soluble markers, hs-CRP and uric acid available in N=18 participants.
Compared to baseline, only results with a significant ANOVA P values are reported. Statistical tests were conducted on loge transformed data.
Remained significant after Bonferroni correction. Abbreviations: TDF/FTC, tenofovir-disoproxil-fumarate/emtricitabine; IL-10, interleukin-10; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor α; IL-6, interleukin-6; sCD27, plasma soluble CD27; sCD14, plasma soluble CD14; hs-CRP, highly-sensitive C-reactive protein.
Figure 1.
CD8+ T-cell (Panel A) and CD4+ T-cell (Panel B) immune activation (CD38/HLA-DR co-expression) at baseline (before drug), day 30 (after 30 days of TDF/FTC) and day 60 (30 days of washout). CD8+ T-cell ANOVA P=0.02. CD4+ T-cell ANOVA P=0.25. Bars and error bars represent geometric means and 95% confidence intervals, respectively. Statistical tests were conducted on loge transformed data. Abbreviations: TDF/FTC, tenofovir-disoproxil-fumarate/emtricitabine.
Cytokines
Of the 15 cytokines analyzed using the Quansys Multiplex, quantifiable values at baseline, day 30 and day 60 were observed for IL-10, IFN-γ and TNF-α, but no differences were found (Table 2). High-sensitivity IL-6 measured with assay-specific ELISA did not exhibit any changes after 30 days of TDF/FTC or after 30 days off-drug (Table 2). TNF-α and IFN-γ concentrations were below the LLOQ of the assay in four and one participants, respectively. No sex or race differences in baseline cytokine levels were observed (P≥0.08).
Soluble biomarkers, hs-CRP and uric acid
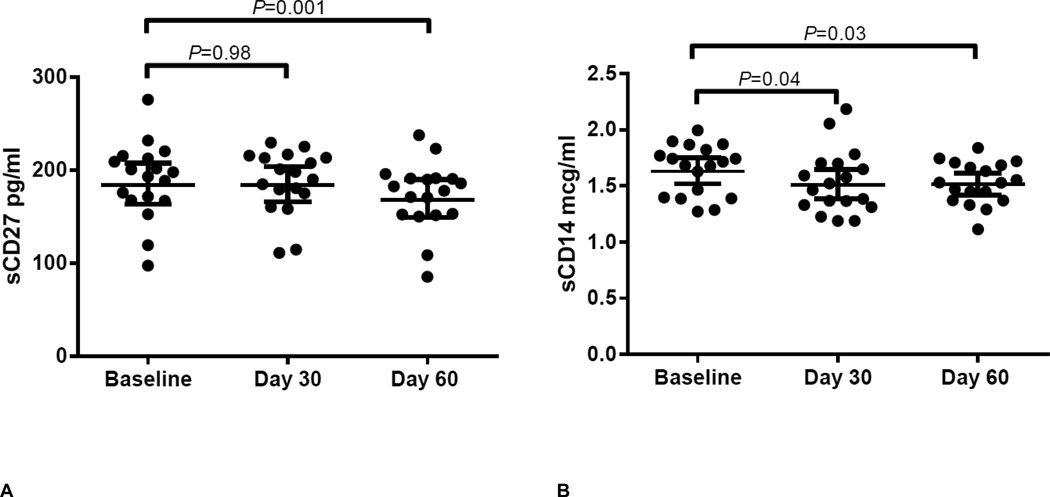
Plasma concentrations of sCD27 decreased significantly from baseline to day 60 (184.1 pg/ml vs. 168.4 pg/ml; P=0.001; Table 2 and Figure 2), but no change at day 30 was observed. Comparatively, there was a decline in sCD14 concentrations from baseline to days 30 and 60 (1.63 mcg/ml vs. 1.51 mcg/ml; P=0.03 and 1.63 mcg/ml vs. 1.51 mcg/ml; P=0.04; Table 2 and Figure 2), although the overall repeated measures ANOVA was not significant (P=0.06). Although a trend towards an initial decline from baseline to day 30 was observed in hs-CRP (1.91 mcg/ml vs. 1.24 mcg/ml; P=0.06), this was not significant, nor were changes in uric acid throughout the study (ANOVA P=0.79; Table 2). No baseline sex or race differences in soluble biomarkers or hs-CRP were observed (P≥0.20).
Figure 2.
Plasma sCD27 (Panel A) and plasma sCD14 (Panel B) concentrations at baseline (before drug), day 30 (after 30 days of TDF/FTC) and day 60 (30 days of drug washout. sCD27 ANOVA P=0.001. sCD14 ANOVA P=0.06. Bars and error bars represent geometric means and 95% confidence intervals, respectively. Statistical tests were conducted on loge transformed data. Abbreviations: TDF/FTC, tenofovir-disoproxil-fumarate/emtricitabine; sCD27 plasma soluble CD27; sCD14, plasma soluble CD14.
Intracellular TFV-DP in CD4+ and CD8+ T-cells and monocytes
The estimated median (range) Css for TFV-DP in CD4+ and CD8+ T-cells was 39 (16–82) fmol/106cells and 29 (4–54) fmol/106cells, respectively, vs. 137 (48–313) fmol/106 cells in monocytes. No significant correlation was identified between intracellular TFV-DP and CD38/HLA-DR expression at day 30 in CD4+ (r=0.19, 95% CI −0.28 to 0.59; P=0.42) or CD8+ (r=0.01, 95% CI, −0.44 to 0.46; P=0.95) T-cells, or with ΔCD38/HLA-DR between baseline and day 30 (P≥0.06). Similarly, there was no significant correlation between intracellular TFV-DP in monocytes and sCD14 at day 30 (r=0.13, 95% CI, −0.35 to 0.56; P=0.59) or with ΔsCD14 between baseline and day 30 (P=0.43). For FTC-TP, the estimated median (range) Css in CD4+ and CD8+ T-cells and monocytes was 5 (3–6) pmol/106cells, 4 (3–14) pmol/106cells and 13 (7–19) pmol/106cells, respectively. Similar to TFV-DP, no significant correlations were identified between intracellular FTC-TP and any activation measures or soluble marker measures (P≥0.48).
Discussion
This study identified a new relationship between decreased systemic immune-activation with TDF/FTC therapy in HIV-negative individuals. Assessing this relationship was a pre-specified secondary endpoint of this pharmacokinetic study. This novel biologic finding in vivo extends previous in vitro observations where TFV exposure resulted in immunomodulation in macrophages and lymphocytes [12,13,14,15,16]. Additional research is needed to replicate our findings in larger populations and to elucidate the clinical implications of decreased immune activation in HIV-negative persons receiving TDF/FTC therapy.
One potential clinical implication of our findings relates to PrEP. With the introduction of PrEP, research has concentrated on the pharmacokinetics of TFV and FTC to determine the systemic, tissue and cellular thresholds of protective efficacy [34,35,36]. However, little focus has been placed on the pharmacodynamics of PrEP beyond the antiviral activity at the site of infection. It is clear that the protective effect provided by PrEP is primarily driven by the systemic and/or local antiviral effect of TDF and FTC at the time of HIV exposure [37,38,39]. However, given the relationship between baseline immune activation and the risk of HIV acquisition [6,8,10], systemic immune quiescence promoted by TDF/FTC exposure is a potentially attractive characteristic that could modify this biological risk factor and provide additional protection to the known antiviral effects of current and future HIV PrEP regimens. Among the proposed mechanisms by which immune quiescence could reduce the risk of HIV acquisition could be: a) reduced T-cell activation (which would lead to a smaller pool of HIV-susceptible target cells) [11,40], b) reduced expression of proinflammatory cytokines (which would prevent infiltration of activated target cells to propagate the infection) [40], and/or, c) concomitant antiviral activity against other pathogens (such as herpes simplex viruses), which would dampen immune activation at the local level [10,30].
Our analysis did not include HIV-infected individuals who often experience elevated immune activation that decreases during therapy. These fluctuations in immune activation create challenges for elucidating the independent effect of TDF/FTC in this population. Nevertheless, this is an important line of research given the potentially detrimental effects of elevated immune activation in these individuals.
The cellular immune activation findings observed in our HIV-negative population are supported by changes in soluble biomarkers. In our secondary analyses, we demonstrated a decrease both in sCD14 and sCD27 after exposure to TDF/FTC. For sCD14, there was an initial trend towards a decrease from baseline to day 30, which remained unchanged at day 60. Conversely, the plasma concentrations of sCD27 showed a delayed, but significant decrease from baseline to day 60. Collectively, these observations suggest promotion of immune quiescence after short-term exposure to TDF/FTC, with a possible initial effect on innate immune activation (sCD14) followed by a delayed modulation of the adaptive immunity (sCD27). In contrast to the changes observed in cell-associated immune activation, we did not observe significant effects on overall inflammation as measured by hs-CRP concentrations. This might be explained by the healthy nature of our population (as evidenced by low baseline inflammation), a small sample size, and/or a possible indication that the effect of TDF/FTC is mainly due to changes in T-cell activation with limited effect on inflammation.
The sustained effect of TDF/FTC on CD8+ T-cell activation and the delayed effect on sCD27 observed after drug discontinuation (30 days off-drug) are of particular interest. Among the possible explanations for this durable effect are the long half-life (~4.2 days) of intracellular TFV moieties in PBMCs [41] or a sustained immune quiescence phenotype that persists even after the active drug has been cleared from the cell. The latter explanation is supported by the lack of correlation between the intracellular concentrations of TFV-DP/FTC-TP with the cellular activation observed in our study. Further research is necessary to more fully understand the kinetics surrounding immune activation during TDF/FTC therapy.
To better understand the biology of TDF/FTC-associated immune modulation, we evaluated a wide array of cytokines, which were selected based on previous observations [12,13,15,42]. In our analysis, the vast majority of the measured cytokines were below the LLOQ at baseline and at days 30 and 60, which is not unexpected given the healthy population studied. This limited our analysis to those cytokines with measurable concentrations (IL-6, IL-10, TNF-α and IFN-γ), none of which demonstrated changes associated to TDF/FTC exposure. The absence of an effect on IL-6 secretion confirms our hs-CRP findings and suggests that TDF/FTC exposure has a minimal effect on this inflammatory pathway. However, these findings contrast with previous in vitro findings where TFV exposure resulted in a dose-dependent stimulation of IL-6, TNF-α and IL-10 in rat macrophages [12,13,14,42] and inhibition of IL-10 in human PBMCs [15]. Additionally, these findings are also distinct from recent data derived from topical TFV exposure where this drug decreased IL-2 and TNF-α in vaginal secretions [17] and IL-6 and IL-8 gene expression in rectal tissue biopsies [18]. These discrepancies highlight the need for more in vivo studies to bridge findings from in vitro and ex vivo settings.
Our study has some limitations that reflect the need for additional research. First, due to the small, non-randomized nature of our study, these findings should be confirmed in other research and/or clinical settings which ideally would include a placebo group (e.g., PrEP clinical trials). Second, the mechanism behind the immune changes observed in our study remains poorly understood. Even though it was not observed in our healthy population, secretion of T-cell suppressive cytokines promoted by TFV exposure is still a plausible explanation that should be further evaluated. Other potential mechanisms such as immunomodulatory effects on endogenous nucleotide pools [43,44,45] should also be pursued. Additionally, it is possible that FTC contributes to these effects, but this could not be studied independently here. Finally, our study only focused on a small number of healthy individuals with low-risk for HIV infection and low levels of baseline T-cell immune activation and soluble biomarkers. Thus, we cannot generalize these findings to populations with higher baseline immune activation, or high risk of HIV infection [46].
In conclusion, short-term daily TDF/FTC therapy was associated with reduced immune activation in healthy HIV-negative subjects. This finding opens new research avenues to determine the mechanism for this effect; to evaluate the effect in HIV-positive individuals; and to elucidate its potential clinical implications.
Acknowledgements
We wish to thank the National Institutes of Health AIDS Research and Reference Reagent Program for the reference standards used for the assays; the study personnel and nursing who assisted with the clinical protocol; and the subjects who participated.
Sources of Funding:
This work was supported by grants from the National Institutes of Health; K23 AI104315 [JCM], U01 AI84735 [PLA] and UL1 RR025780 [University of Colorado Clinical and Translational Sciences Institute]. Colorado Multiple Institutional Review Board COMIRB# 08-0459.
Footnotes
Potential Conflicts of Interest (see separate forms):
Dr. Anderson reports donation of study drug from Gilead Sciences during the conduct of the study and Contract work with Gilead Sciences. All others authors: no conflict of interest.
Previous Presentations:
This work was presented in part at: Conference on Retrovirus and Opportunistic Infections 2014, Poster # 340 Boston, MA, March 2014.
REFERENCES
- 1.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 2.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu W, Chen S, Lai C, et al. Induction of CD8+ regulatory T cells protects macaques against SIV challenge. Cell rep. 2012;2:1736–1746. doi: 10.1016/j.celrep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Begaud E, Chartier L, Marechal V, et al. Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35. doi: 10.1186/1742-4690-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jennes W, Evertse D, Borget MY, et al. Suppressed cellular alloimmune responses in HIV-exposed seronegative female sex workers. Clin Exp Immunol. 2006;143:435–444. doi: 10.1111/j.1365-2249.2006.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaren PJ, Ball TB, Wachihi C, et al. HIV-exposed seronegative commercial sex workers show a quiescent phenotype in the CD4+ T cell compartment and reduced expression of HIV-dependent host factors. J Infect Dis. 2010;(Suppl 3):S339–S344. doi: 10.1086/655968. [DOI] [PubMed] [Google Scholar]
- 9.Pancino G, Saez-Cirion A, Scott-Algara D, et al. Natural resistance to HIV infection: lessons learned from HIV-exposed uninfected individuals. J Infect Dis. 2010;(Suppl 3):S345–S350. doi: 10.1086/655973. [DOI] [PubMed] [Google Scholar]
- 10.Naranbhai V, Abdool Karim SS, Altfeld M, et al. Innate immune activation enhances hiv acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J Infect Dis. 2012;206:993–1001. doi: 10.1093/infdis/jis465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Card CM, Ball TB, Fowke KR. Immune Quiescence: a model of protection against HIV infection. Retrovirology. 2013;10:141. doi: 10.1186/1742-4690-10-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zidek Z, Frankova D, Holy A. Activation by 9-(R)-[2-(phosphonomethoxy)propyl]adenine of chemokine (RANTES, macrophage inflammatory protein 1alpha) and cytokine (tumor necrosis factor alpha, interleukin-10 [IL-10], IL-1beta) production. Antimicrob Agents Chemother. 2001;45:3381–3386. doi: 10.1128/AAC.45.12.3381-3386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zidek Z, Potmesil P, Kmoniekova E, et al. Immunobiological activity of N-[2-(phosphonomethoxy)alkyl] derivatives of N6-substituted adenines, and 2,6-diaminopurines. Eur J Pharmacol. 2003;475:149–159. doi: 10.1016/s0014-2999(03)02110-1. [DOI] [PubMed] [Google Scholar]
- 14.Kostecka P, Holy A, Farghali H, et al. Differential effects of acyclic nucleoside phosphonates on nitric oxide and cytokines in rat hepatocytes and macrophages. Int Immunopharmacol. 2012;12:342–349. doi: 10.1016/j.intimp.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Melchjorsen J, Risor MW, Sogaard OS, et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. J Acquir Immune Defic Syndr. 2011;57:265–275. doi: 10.1097/QAI.0b013e3182185276. [DOI] [PubMed] [Google Scholar]
- 16.Zidek Z, Potmesil P, Holy A. Cytostatic activity of antiviral acyclic nucleoside phosphonates in rodent lymphocytes. Toxicol Appl Pharmacol. 2003;192:246–253. doi: 10.1016/s0041-008x(03)00215-1. [DOI] [PubMed] [Google Scholar]
- 17.Vibholm L, Reinert LS, Sogaard OS, et al. Antiviral and immunological effects of tenofovir microbicide in vaginal herpes simplex virus 2 infection. AIDS Res Hum Retroviruses. 2012;28:1404–1411. doi: 10.1089/AID.2012.0078. [DOI] [PubMed] [Google Scholar]
- 18.McGowan I, Hoesley C, Cranston RD, et al. A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007) PLoS One. 2013;8:e60147. doi: 10.1371/journal.pone.0060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hileman CO, Longenecker CT, Carman TL, et al. C-reactive protein predicts 96-week carotid intima media thickness progression in HIV-infected adults naive to antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;65:340–344. doi: 10.1097/QAI.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 23.Leeansyah E, Malone DF, Anthony DD, et al. Soluble biomarkers of HIV transmission, disease progression and comorbidities. Curr Opin HIV AIDS. 2013;8:117–124. doi: 10.1097/COH.0b013e32835c7134. [DOI] [PubMed] [Google Scholar]
- 24.Boulware DR, Hullsiek KH, Puronen CE, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203:1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57:371–379. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J, Jochems C, Anderson AM, et al. Soluble CD27-pool in humans may contribute to T cell activation and tumor immunity. J Immunol. 2013;190:6250–6258. doi: 10.4049/jimmunol.1300022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atlas A, Thanh Ha TT, Lindstrom A, et al. Effects of potent antiretroviral therapy on the immune activation marker soluble CD27 in patients infected with HIV-1 subtypes A–D. J Med Virol. 2004;72:345–351. doi: 10.1002/jmv.20006. [DOI] [PubMed] [Google Scholar]
- 28.Messele T, Brouwer M, Girma M, et al. Plasma levels of viro-immunological markers in HIV-infected and non-infected Ethiopians: correlation with cell surface activation markers. Clin Immunol. 2001;98:212–219. doi: 10.1006/clim.2000.4958. [DOI] [PubMed] [Google Scholar]
- 29.De Milito A, Aleman S, Marenzi R, et al. Plasma levels of soluble CD27: a simple marker to monitor immune activation during potent antiretroviral therapy in HIV-1-infected subjects. Clin Exp Immunol. 2002;127:486–494. doi: 10.1046/j.1365-2249.2002.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naranbhai V, Samsunder N, Sandler NG, et al. Neither microbial translocation nor TLR responsiveness are likely explanations for preexisting immune activation in women who subsequently acquired HIV in CAPRISA 004. J Acquir Immune Defic Syndr. 2013;63:294–298. doi: 10.1097/QAI.0b013e31828e604b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Claas GJ, Jülg B, Goebel FD, et al. Metabolic and anthropometric changes one year after switching from didanosine/stavudine to tenofovir in HIV-infected patients. Eur J Med Res. 2007;12:54–60. [PubMed] [Google Scholar]
- 32.Lyngdoh T, Marques-Vidal P, Paccaud F, et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One. 2011;6:e19901. doi: 10.1371/journal.pone.0019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bushman LR, Kiser JJ, Rower JE, et al. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal. 2011;56:390–401. doi: 10.1016/j.jpba.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louissaint NA, Cao YJ, Skipper PL, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses. 2013;29:1443–1450. doi: 10.1089/aid.2013.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.García-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5:e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grant RM, Lama JR, Anderson PL, et al. iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. CAPRISA 004 Trial Group. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 41.Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29:384–390. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zídek Z, Holý A, Franková D. Antiretroviral agent (R)-9-(2-phosphonomethoxypropyl)adenine stimulates cytokine and nitric oxide production. Eur J Pharmacol. 1997;331:245–252. doi: 10.1016/s0014-2999(97)01004-2. [DOI] [PubMed] [Google Scholar]
- 43.Kakuda TN, Anderson PL, Becker SL. CD4 cell decline with didanosine and tenofovir and failure of triple nucleoside/nucleotide regimens may be related. AIDS. 2004;18:2442–2244. [PubMed] [Google Scholar]
- 44.Ray AS, Olson L, Fridland A. Role of purine nucleoside phosphorylase in interactions between 2',3'-dideoxyinosine and allopurinol, ganciclovir, or tenofovir. Antimicrob Agents Chemother. 2004;48:1089–1095. doi: 10.1128/AAC.48.4.1089-1095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biswas N, Rodriguez-Garcia M, Crist SG, et al. Effect of tenofovir on nucleotidases and cytokines in HIV-1 target cells. PLoS One. 2013;8:e78814. doi: 10.1371/journal.pone.0078814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen CR, Moscicki AB, Scott ME, et al. Increased levels of immune activation in the genital tract of healthy young women from sub-Saharan Africa. AIDS. 2010;24:2069–2074. doi: 10.1097/QAD.0b013e32833c323b. [DOI] [PMC free article] [PubMed] [Google Scholar]