Abstract
Background
Despite the improvement in survival of pediatric patients with rhabdomyosarcoma, the outcome of patients with sinonasal rhabdomyosarcoma is poor and has not significantly changed. Since few institutions have extensive experience with sinonasal rhabdomyosarcoma in children and adults, our objective was to determine prognostic factors and treatment outcomes for this rare malignancy.
Methods
A retrospective review was performed of consecutive patients with sinonasal rhabdomyosarcoma treated at our institution from 1992 to 2012. Kaplan-Meier estimates and the log rank test were performed to determine factors associated with disease recurrence and disease specific survival.
Results
Initial remission was achieved in 12 of the 16 patients. Age younger than 18 years (n=9) was a positive prognostic factor, as there were no recurrences (p<0.01) and no deaths (p<0.01). The alveolar subtype was a poor prognostic factor, as four of the five patients with this histology died of disease (p<0.01), and both patients with initial remission developed recurrence (p<0.01). Presentation with later TNM stage was also significant for poorer survival, as two of the three patients with stage IV died of disease (p=0.05). Patient sex and treatment modality were not significant.
Conclusions
Although the sinonasal region is an unfavorable site for rhabdomyosarcomas, in our series patients younger than 18 years and those with embryonal or botryoid subtypes responded very well to current multimodality treatment. However, a poor prognostic trend is evident in patients with sinonasal alveolar rhabdomyosarcomas, as they appear to present more often with regional and distant metastases, have increased recurrence, and decreased survival.
Keywords: sinonasal, rhabdomyosarcoma, prognostic factors, skull base, parameningeal
Introduction
Rhabdomyosarcomas are rare soft tissue malignancies which arise from myogenic cells and most commonly occur in the pediatric population. Although it is the most common soft tissue malignancy in children, the incidence is only 0.4414 per 100,000 children per year.1 Despite this low incidence, the head and neck region is one of the most commonly affected sites, accounting for nearly one third of the cases.1-3 In patients with head and neck rhabdomyosarcoma, over two-thirds occur in patients younger than 20 years of age.4
In the head and neck, parameningeal rhabdomyosarcoma carries a significantly worse prognosis compared to the orbit or non-parameningeal subsites and has a propensity for skull base infiltration and intracranial involvement. Locations for parameningeal rhabdomyosarcoma include the paranasal sinuses, nasal cavity, nasopharynx, infratemporal fossa, pterygopalatine fossa, middle ear, and mastoid.5 Five-year survival decreases from nearly 85% for patients with orbital rhabdomyosarcoma to 50% for those with the parameningeal tumors. 4
Over the last forty years, the Intergroup Rhabdomyosarcoma Study Group (IRSG) has conducted considerable research into optimal treatment regimens for pediatric patients with rhabdomyosarcoma that include chemotherapy, radiation therapy, and surgery. With multimodality treatment, overall survival for patients with any type of rhabdomyosarcoma has improved from 55% to 71% over the study period from 1972 to 1997.6
Despite the improvement in overall survival of pediatric patients with rhabdomyosarcoma, few institutions have extensive treatment experience with parameningeal rhabdomyosarcoma in children and adults due to the rarity of these tumors. A recent retrospective review of the Surveillance, Epidemiology, and End Results (SEER) database did not shown an improvement in survival over the study period of 1973 to 2007.4 Our objective was to review both pediatric and adult patients with parameningeal rhabdomyosarcoma that involved the sinonasal region treated at our tertiary academic institution over the last 20 years in order to provide further information on the prognostic factors and treatment outcomes of this rare malignancy.
Methods
Our study was approved by the Institutional Review Board of the University of California at Los Angeles. We retrospectively reviewed the clinical data of all consecutive patients with head and neck parameningeal rhabdomyosarcoma that were treated and followed at a single tertiary academic medical center from January 1992 to August 2012. Sixteen patients were included in the study, and the follow-up after diagnosis varied from 7 to 231 months (median: 24.5 months). The main outcome measures were disease recurrence and disease specific survival over the study period.
Patient characteristics that were analyzed included age, sex, histopathologic subtype, pre-treatment TNM clinical stage, and treatment regimen, which consisted of primary chemoradiation with or without surgery. A distinction was made between patients younger and older than age 18, since that is the age at which patients transition from pediatric to adult primary medical care at our institution. The TNM classification was based on pre-treatment imaging, including computed tomography, magnetic resonance imaging, and positron emission tomography. The definitive treatment regimens for every patient included in our study were based on the IRSG recommendations.6-8 The “gold standard” treatment since the early 1990s for parameningeal rhabdomyosarcoma includes multiagent chemotherapy and external beam radiation. Both of these treatment modalities were used in all of our patients.
The Kaplan-Meier estimates of the survival and recurrence probabilities were visualized in graphical displays (Figures 1-3). Differences in survival distributions such as age (< 18), sex, TNM stage (II, III, IV), treatment, and alveolar subtype were tested with the log-rank test. Follow-up time was from the date of the start of treatment for the primary tumor to date of last contact or death. Remission was defined as the disappearance of the tumor on imaging studies and/or physical exam.
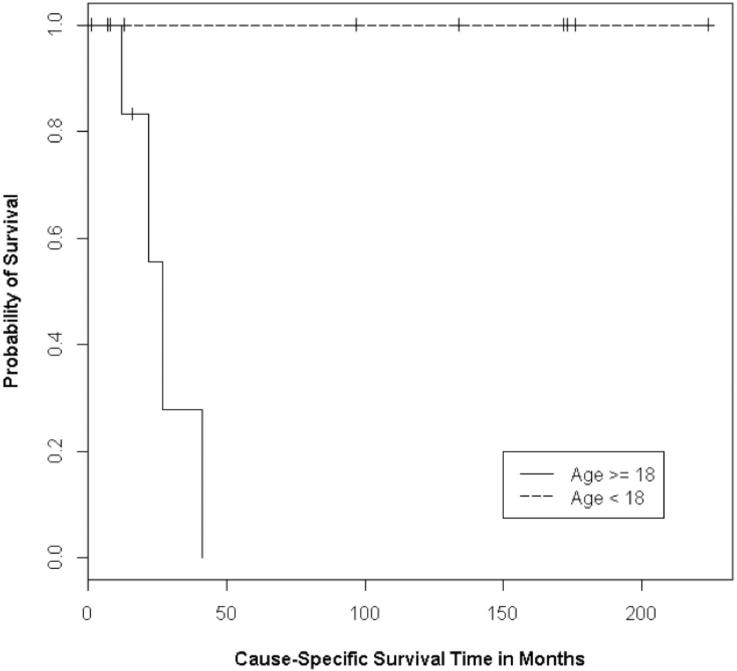
Figure 1.
Kaplan-Meier curves for the survival of patients younger than 18 years and older than 18 years at time of rhabdomyosarcoma diagnosis (P<0.01)
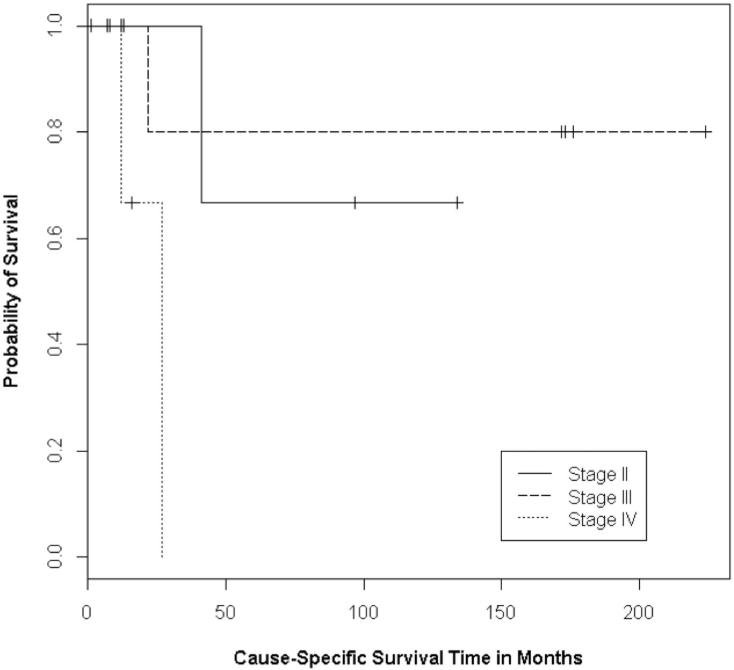
Figure 3.
Kaplan-Meier curves for the survival of patients with TNM stage II, III, and IV rhabdomyosarcomas (P=0.05)
Results
Over the 20-year study period, we identified 16 patients with head and neck parameningeal rhabdomyosarcoma who were treated and followed at our tertiary academic medical center from January 1992 to August 2012. All 16 patients had tumors primarily located in or had extension to the sinonasal region. There were no patients with primary middle ear or mastoid tumors. The distribution of patient clinical characteristics and the treatment outcomes are shown in Table 1. Our cohort included five males (31%) and 11 females (69%) with an age range at time of diagnosis of 2 to 70 years (median: 17 years). Nine patients were younger than 18 years.
Table 1.
Patient Characteristics and Treatment Outcomes
| Patient Age;Sex | Primary Location | Tumor Extension | TNM stage | Histologic Type | Details of Primary Surgery & Pathology | Site and Time to Recurrence (months) | Outcome | Survival from diagnosis (months) |
|---|---|---|---|---|---|---|---|---|
| 9;M | NP | Mastoid, ME, PA, SS | II (T2aN0M0) | Botryoid | Remission | 134 | ||
| 26;F | NC | ES, SS, pterygoids | II (T2aN0M0) | Alveolar | Pre-CR (debulking); margins positive | Regional (4) | Deceased | 41 |
| 28;F | PPF | SS, ES, ITF | IV (T2bN1M1) | Alveolar | Deceased | 12 | ||
| 2;F | NP | ES | III (T2bN1M0) | Botryoid | Remission | 172 | ||
| 3;F | NC | None | II (T2aN0M0) | Embryonal | Pre-CR (wide local excision); margins positive | Remission | 13 | |
| 9;M | NC | ES, SS, intracranial | III (T2bN0M0) | Embryonal | Remission | 173 | ||
| 21;M | ES | MS & PPF | III (T2aN1M0) | Embryonal | Local (4) | AWD | 12 | |
| 14;F | SS | ES, intracranial | II (T2aN0M0) | Embryonal | Post-CR (excision of residual SS tumor); margins negative | Remission | 105 | |
| 4;F | ITF | MS and ptyergoids | III (T2bN0M0) | Embryonal | Post-CR (open surgery for 5cm ITF tumor); margins negative | Remission | 231 | |
| 70;F | MS | ES | III (T2bN1M0) | Embryonal | Remission | 7 | ||
| 6;F | MS | None | III (T2bN0M0) | Embryonal | Remission | 176 | ||
| 26;M | MS | ES & ITF | IV (T2bN0M1) | Embryonal | Post-CR (Caldwell Luc for residual MS tumor); margins negative | AWD | 16 | |
| 17;M | ES | SS | II (T2aN0M0) | NOS | Post-CR (FESS); no viable tumor in pathology | Remission | 8 | |
| 17;F | SS | SS | II (T2aN0Mo) | Alveolar | AWD | 10 | ||
| 23;F | MS | ES, SS, PPF, intracranial | IV (T2bN1M1) | Alveolar | Deceased | 27 | ||
| 51;F | ES | NP | III (T2bN1M0) | Alveolar | Local, distant (6) | Deceased | 22 | |
Abbreviations: AWD: alive with disease; CR: chemoradiation; ES: ethmoid air cells; FESS: functional endoscopic sinus surgery; ITF: infratemporal fossa; MS: maxillary sinus; NC: nasal cavity; NOS: not otherwise specified; NP: nasopharynx; PA: petrous apex; PPF: pterygopalatine fossa; SS: sphenoid sinus
Regarding histopathological subtype of rhabdomyosarcoma, eight patients had embryonal (50%), five had alveolar (31%), two had botryoid (13%), and one was not otherwise specified (6%). Of the 16 patients, six were classified as TNM stage II (38%), seven as stage III (44%), and three as stage IV (19%).
In our analysis of the prognostic factors on treatment outcomes (Table 2), age less than 18 years at diagnosis was significant for both decreased recurrence and improved survival (Figure 1). Eight of the nine pediatric patients had initial remission after primary treatment, and none have developed recurrent disease (p<0.01). There have been no deaths among these nine patients, and only one is alive with disease undergoing further treatment (p<0.01).
Table 2.
Prognostic Factors for Recurrence and Survival
| Prognostic Factor | # of patients (n=16) | # of Recurrences (p-value) | # of Deaths (p-value) |
|---|---|---|---|
| Age | (<0.01) | (<0.01) | |
| <18 years | 9 | 0 | 0 |
| >18 years | 7 | 3 | 4 |
| Histological subtype | (<0.01) | (<0.01) | |
| Alveolar | 5 | 2 | 4 |
| Non-alveolar | 11 | 1 | 0 |
| TNM stage | (0.78) | (0.05) | |
| II | 6 | 1 | 1 |
| III | 7 | 2 | 1 |
| IV | 3 | 0 | 2 |
| Sex | (0.97) | (0.23) | |
| Male | 5 | 1 | 0 |
| Female | 11 | 2 | 4 |
| Treatment Modality | (0.79) | (0.59) | |
| Chemoradiation | 10 | 2 | 3 |
| Chemoradiation + Surgery | 6 | 1 | 1 |
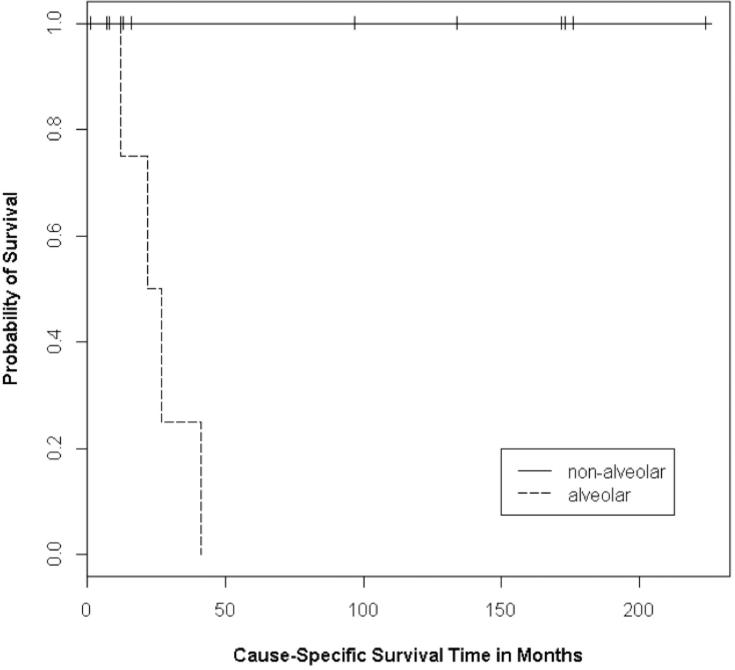
Regarding the specific histologic subtype, patients with alveolar tumors have poorer outcomes. Four of the 16 patients (25%) in our cohort died of disease, all of whom had alveolar rhabdomyosarcoma (p<0.01) (Figure 2). No patients with embryonal or botryoid tumors died of disease. Furthermore, of the two patients with alveolar rhabdomyosarcoma who had initial remission, both developed recurrence (p<0.01). Using Cox proportional hazard univariate analysis, the alveolar subtype had a hazard ratio of 13.29 (95% CI: 1.19, 148.9, p=0.036) for disease recurrence, suggesting the possibility of a large effect.
Figure 2.
Kaplan-Meier curves for the survival of patient with alveolar rhabdomyosarcoma and non-alveolar rhabdomyosarcoma (P<0.01)
The only other significant prognostic factor was TNM stage at diagnosis. Patients with stage IV disease had poorer survival, as two of the three patients with stage IV disease died (p=0.05) (Figure 3). There were only two deaths among the other 13 patients, including one of six with stage II disease, and one of seven with stage III disease. However, among the patients who achieved initial remission, TNM stage was not significant for recurrence (p=0.78). Similarly, patient sex and whether surgery was used in addition to chemoradiation were not significant for recurrence or survival.
Multiagent chemotherapy and external beam radiation were used in all patients. The exact chemotherapy treatment records were available for 15 of the 16 patients. In our cohort, patients most commonly received vincristine (n=15), actinomycin D (n=14), cyclophosphamide (n=10), ifosfamide (n=6), and etoposide (n=4). All patients survived long enough to complete a definitive course of radiation therapy to the primary tumor site. Patient specific radiation treatment summaries were available for 10 of our patients, and the range of radiation dose was 3600 to 6600 cGy (median= 5040 cGy), over approximately a six-week period.
Surgery was included in the primary treatment regimen in six patients. Two patients had prechemoradiation procedures. In one case, wide local excision was performed of an isolated 1.3x1 cm well-encapsulated nasal cavity mass for diagnostic purposes in a 3 year-old female. The second patient was a 26 year-old male who had an endonasal endoscopic debulking procedure for diagnostic and therapeutic purposes for an acute decrease in vision after presenting with orbital compression from tumor invasion through the lamina papyracea. Surgical margins were positive in both of these patients. In the case of the 3 year-old, she completed chemotherapy with vincristine and actinomycin D, as well as radiation with a total dose of 3600 cGy, and is disease free 13 months since diagnosis. The 26 year-old male had positive margins at the skull base after his endoscopic debulking procedure. He subsequently underwent chemoradiation and was disease free on imaging and physical exam immediately after treatment. However, he then developed regional recurrence and died 41 months after diagnosis.
Four other patients had surgery at the end of chemoradiation. Indication for surgery after chemoradiation was suspected residual tumor on imaging. Three of these four cases were endoscopic sinus surgeries or a Caldwell-Luc procedure for persistently opacified sinuses and suspected tumor. A fourth case was an open procedure for a 5cm tumor in the infratemporal fossa. Surgical specimens had negative margins or were negative for viable tumor in all cases. None of these four patients have evidence of persistent rhabdomyosarcoma in the sinonasal region. Three patients are in remission, and one has persistent lung metastases.
Discussion
Parameningeal rhabdomyosarcoma is a rare malignancy with a historically poor response to treatment.2 Because of the low incidence, there is limited experience with the disease by single institutions and a need for reporting on the prognostic factors and treatment outcomes. Despite the improvement in relative survival for patients with all types of rhabdomyosarcoma, as reported by the Intergroup Rhabdomyosarcoma Study Group (IRSG), the five year survival for parameningeal tumors remains about 50% and does not appear to be significantly changing.2,4
Turner et al.4 recently published a retrospective review of head and neck rhabdomyosarcoma that included 248 patients with parameningeal tumors from the Surveillance, Epidemiology, and End Results (SEER) database, and the 5 year relative survival was 49.1%. The survival rate did not significantly change over the study period of 1973 to 2007.4
In our series of sinonasal parameningeal rhabdomyosarcoma patients, we found that patients younger than 18 years and those with embryonal or botryoid subtypes responded very well to current multimodality treatment including chemotherapy, radiation, and surgery. Of the 11 patients with histology other than alveolar, all are alive at last clinical visit, including only two with disease (median follow-up: 105 months). Of the nine patients younger than 18 years, eight are in remission after primary treatment and one is undergoing further treatment (median follow-up: 134 months). None of these pediatric patients have developed recurrence. The better prognosis in younger patients is consistent with an earlier case series from MD Anderson and may be attributed to the decreased incidence of the alveolar subtype in the pediatric population.9
Alveolar rhabdomyosarcomas are less common than the other histological subtypes in younger patients.9,10 Bisogno et al.10 analyzed pediatric patients with rhabdomyosarcoma of any location and found that adolescents 15 years of age and older were more likely to have alveolar tumors compared to children younger than 15 years (47.4% versus 32.6%). Additionally, the adolescents had poorer overall survival (57.2% versus 68.9%). In our study, specific to sinonasal rhabdomyosarcoma, adults not only had poorer outcomes, but were also more likely to have the alveolar subtype. Only one of the nine patients younger than 18 years (11%) had a tumor of the alveolar subtype, and she was 17 years at diagnosis. In contrast, four of the seven adults (57%) had alveolar rhabdomyosarcoma.
In addition to being more prevalent in adults, the alveolar subtype appears more aggressive at presentation and in our cohort had increased risk for metastases. Determining regional metastasis (Nx) in alveolar tumors has previously been shown to confer a significantly worse survival.11 Three of the five patients (60%) with the alveolar subtype presented with disease in the cervical lymphatics, including two (40%) also with distant metastases to the humerus or abdomen and retroperitoneum. Additionally, one more patient who was initially staged N0 developed regional recurrence after primary treatment. This aggressive clinical course was not as apparent in embryonal or botryoid rhabdomyosarcoma. Only three of the other 11 patients (27%) without the alveolar subtype presented with regional neck disease, and only a single patient (9%) presented with distant metastases to the lungs.
Treatment for rhabdomyosarcoma can include different combinations of surgery, chemotherapy, and radiation.6 Surgery alone at the primary site may be sufficient if negative margins can be obtained without causing significant morbidity. 6 However, for tumors near the skull base, definitive diagnosis is often made late in the disease process, since clinical presentation can be subtle and tumors are already locally invasive or have developed metastases; thus, obtaining negative margins may be difficult.9 In our study, surgery was performed in four patients after chemoradiation for suspected persistent tumor on imaging. A 26 year-old male and a 14 year-old female had excision of subcentimeter tumors in the sphenoid and maxillary sinuses, respectively. A 17 year-old male had endonasal endoscopic surgery for suspected tumor in the ethmoids and sphenoid due to opacified sinuses, but pathology was not significant for any viable tumor, and he is in remission. Lastly, a 4 year-old female had a partial mandibulectomy and parotidectomy, for a 5 cm residual tumor in the infratemporal fossa. Negative margins were obtained. She has had no further treatment and is disease free over 18 years after surgery. Although the number of cases is small, these results suggest that surgery has an important role in parameningeal rhabdomyosarcoma after chemoradiation therapy for improving outcomes of primary disease after tumor shrinkage. Local control of disease was obtained in all four of these patients.
The present study holds a number of shortcomings. The study design is a retrospective case series, which holds many inherent biases. However, the potential selection bias was limited by including only chronologically consecutive parameningeal cases of patients that were treated and followed at our institution. Ensuring the inclusion of all consecutive cases is an advantage of an institutional case series. Another limitation is that only univariate analysis was conducted. Multivariate models were attempted with stepwise or forward selection algorithms but did not produce stable or convergent models for both recurrence or survival models. With only 16 patients, the model cannot assess any relationships with more than one variable. However, the results of the current study indicate that although parameningeal rhabdomyosarcoma are unfavorable tumors, pediatric patients and patients with embryonal or botryoid subtypes respond very well to multimodality treatment. Older patients (age > 18 years of age) present with a more aggressive clinical course, which appears to correlate with the increased incidence of the alveolar histological subtype in older populations.
Conclusion
Although parameningeal rhabdomyosarcomas located in or involving the sinonasal region are unfavorable tumors, in our series patients younger than 18 years and those with embryonal or botryoid subtypes responded very well to current multimodality treatment. Patients with alveolar rhabdomyosarcoma, which in our study is more common in adults, appear to present more often with regional or distant metastases, have increased recurrence, and decreased survival. Close coordination between head and neck surgeons, medical oncologists, and radiation oncologists is critical to improve the treatment outcomes in these patients with alveolar rhabdomyosarcoma.
Acknowledgement
The corresponding author, CFT, had full access to all the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis. None of the authors have any conflicts of interest, financial, or otherwise. Statistical analyses were supported by the UCLA Clinical and Translational Science Institute (grant UL1TR000124 and UL1RR033176).
Footnotes
Oral Presentation at the American Head & Neck Society 8th International Conference on Head and Neck Cancer July 21-25, 2012; Toronto, Ontario, Canada
No financial funding or support
Conflict of interest: none
References
- 1.Perez EA, Kassira N, Cheung MC, Koniaris LG, Neville HL, Sola JE. Rhabdomyosarcoma in children: a SEER population based study. J Surg Res. 2011;170(2):e243–251. doi: 10.1016/j.jss.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Mauer HM, Beltangady M, Gehan EA, et al. The intergroup rhadomyosarcoma study-I. A final report. Cancer. 1988;61(2):209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Simon JH, Paulino AC, Ritchie JM, Mayr NA, Buatti JM. Presentation, prognostic factors and patterns of failure in adult rhabdomyosarcoma. Sarcoma. 2003;7(1):1–7. doi: 10.1080/1357714031000114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner JH, Richmon JD. Head and neck rhabdomyosarcoma: a critical analysis of population-based incidence survival data. Otolaryngol Head Neck Surg. 2011;145(6):967–973. doi: 10.1177/0194599811417063. [DOI] [PubMed] [Google Scholar]
- 5.Wurm J, Constantinidis J, Grabenbauer GG, Iro H. Rhabdomyosarcomas of the nose and paranasal sinuses: treatment results in 15 cases. Otolaryngol Head Neck Surg. 2005;133(1):42–50. doi: 10.1016/j.otohns.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Raney RB, Maurer HM, Anderson JR, et al. The intergroup rhabdomyosarcoma study group (IRSG): Major lessons from the IRS-I through IRS-IV studies as background for the current IRSV treatment protocols. Sarcoma. 2001;5(1):9–15. doi: 10.1080/13577140120048890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crist W, Gehan EA, Ragab AH, et al. The third intergroup rhabdomyosarcoma study. J Clin Oncol. 1995;13(3):610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 8.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19(12):3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 9.Callender TA, Weber RS, Janjan N, et al. Rhabdomyosarcoma of the nose and paranasal sinuses in adults and children. Otolaryngol Head Neck Surg. 1995;112(2):252–257. doi: 10.1016/S0194-59989570246-6. [DOI] [PubMed] [Google Scholar]
- 10.Bisogno G, Compostella A, Ferrari A, et al. Rhabdomyosarcoma in adolescents: a report from the AIEOP soft tissue sarcoma committee. Cancer. 2012;118(3):821–827. doi: 10.1002/cncr.26355. [DOI] [PubMed] [Google Scholar]
- 11.Rodeberg DA, Garcia-Henriquez N, Lyden ER, et al. Prognostic significance and tumor biology of regional lymph node disease in patients with rhabdomyosarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2011;29(10):1304–1311. doi: 10.1200/JCO.2010.29.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]