Abstract
The present study utilized an acute bout of resistance exercise to examine the effects on the immune- and inflammatory-related genes in peripheral blood mononuclear cells (PBMCs). To date, only a limited number of gene transcripts related to the immune and inflammatory processes have been examined. Ten resistance-trained men (20-24 yrs), with at least 2 yrs resistance exercise training (RET) experience performed an acute bout of RET for ∼30 min following a 12 hr fast. The RET included the back parallel squat and leg press at 45% & 55% of 1-RM for 2 sets and 65% of 1-RM for the following 4 sets. All exercises were performed with a 2:2 cadence followed by 2 min rest periods. Venous blood was sampled at rest, immediately following exercise, and at 2 hr post-exercise and analyzed for total and differential leukocytes and global gene expression using Affymetrix Genechips. Results showed that leukocytes, monocytes, lymphocytes, and lactate values were elevated immediately post-exercise (p<0.05) over baseline. At 2 hr post-exercise, leukocytes and granulocytes remained elevated (p<0.05), whereas lymphocytes were lower than (p<0.05) baseline values. RET induced transient fluctuations in immune cells. Initial microarray results indicate that gene expression signatures are highly correlated with peripheral blood mononuclear counts and that differentially expressed genes supported the immunophenotyping results. At the 2 hr recovery time point, matrix metalloproteinase 9 (MMP 9), orosomucoid 1 (ORM 1) and arginase 1 (ARG 1) all showed significant up regulation, while the gene CD160 was down regulated. These results demonstrate that an acute bout of RET disrupts cellular homeostasis, induces a transient redistribution of certain leukocytes, and results in a transcriptional change in blood samples consistent with phenotyping results that differs from aerobic exercise.
Keywords: microarray, genomic response, immune response, resistance exercise
Introduction
Identifying global alterations in genomic factors as a result of various exercise stimuli has the potential to afford a better understanding of adaptive responses to exercise. Peripheral blood mononuclear cells (PBMCs) have been shown to increase in response to exercise and are thought to mediate stress via production of cytokines, chemokines and growth factors (11). There has been much investigation into changes in PMBC population and immunological changes in circulating cytokines in relation to acute endurance exercise (>75% VO2max) however, less is known about genomic response to resistance exercise training (RET). RET programs can cause muscle damage and soreness because of the workload or resistances utilized. In addition to soreness, muscle damage, as a result of RET, also contributes to inflammation in the surrounding tissue and alterations in immune cells in peripheral blood (9, 10, 20, 21, 28. The variations in leukocyte concentration during and after both endurance and resistance exercise may be the result of a transitory redistribution of immune cells between the peripheral lymphoid tissues and the circulation (28, 33). Part of this inflammation may also alter the circulating neutrophil and peripheral blood mononuclear cell (PBMC) gene expression (11).
The immune and inflammatory systems play key roles in health and disease processes. Therefore, determining the transcriptional changes in PBMCs that control these processes will provide more knowledge with regards to exercise induced stress response. Previous work has examined transcriptional changes in PBMCs in response to aerobic exercise, in healthy untrained subjects over a 30 min time course (11). This work demonstrates a dramatic increase in inflammatory cytokines and stress-related protein kinases, 1 hr post exercise. Given previous findings (9, 16, 20, 27), our investigation sought to examine the genomic responses to an acute bout of resistance exercise using Affymetrix microarrays (Santa Clara, CA) on peripheral blood. Results of these microarray data will help support whether or not any of the post-exercise differential immunological patterns coincide with gene expression responses. The current study was designed to address whether a different mode of exercise will impact the genomic response of PBMCs. We hypothesized that the RET insult would activate a transcriptional response involved in inflammation, cell growth, and tissue repair as compared to those activated by an acute bout of aerobic exercise.
Methods
Participants
Ten moderately trained male college athletes (n = 10) gave their written informed consent to participate in this study, which was approved by the University's Institutional Review board, and completed a medical-history questionnaire. All participants were members of NCAA Division III athletic teams (post-season) and had been training for at least 5 years, with a minimum of 2 years of weight-lifting experience. Medical history results showed that no subjects had significant medical issues that would interfere with the study. Participant characteristics for experiments are presented in Table 1.
Table 1. Participant Characteristics, mean ± SD.
| Variable | Mean/SD |
|---|---|
| Age (years) | 22.3 ± 1.3 |
| Height (cm) | 181.1 ± 6.0 |
| Body Mass (kg) | 90.0 ± 6.8 |
| Body Fat (%) | 10.9 ± 3.9 |
| 1-RM back parallel squat (kg) | 159.2 ± 18.0 |
| 1-RM leg press (kg) | 410.0 ± 33.3 |
| Years of training | 5.0 ± 2.3 |
To minimize influence on each subject's immune system, participants were asked to adhere to specific instructions before exercise testing. This included abstinence from caffeine, alcohol, and anti-inflammatory medications for 24 hr. Furthermore, participants agreed to abstain for 30 days from using large doses of vitamin/mineral supplements (>100% of recommended dietary allowances) until after the second exercise session. Participants were also instructed not to engage in exercise 24 hr prior to each exercise testing session.
Participants were excluded from the study if they had an autoimmune disease (i.e., lupus, multiple sclerosis, rheumatoid arthritis, or insulin-dependent diabetes mellitus), tested positive for Human Immunodeficiency Virus (HIV), or had been diagnosed with Acquired Immune Deficiency Syndrome (AIDS). Participants were also excluded if they were taking prescription medications, using steroids, using ergogenic supplements (e.g., creatine) within 30 days prior to testing, or had indicated that they experienced high psychological stress. Before each testing session, participants completed a second questionnaire to establish if they met the above pre-testing criteria and identify if they displayed any symptoms associated with upper respiratory tract infection (URTI) illness that would alter immune-cell parameters.
Procedures
Strength Assessment and Resistance-Exercise Protocol
One week before experimental testing, several baseline measurements were obtained which measured physical and strength parameters. This included measurements of baseline height, body weight, and body composition using the Jackson and Pollock's (1985) skinfold method, and strength assessment by performing one-repetition maximums (1-RMs) using the 1-RM testing protocol (4) for both the leg press (Hoist Fitness Systems, San Diego, CA) and parallel back squat (Hoist, Fitness systems, San Diego, CA).
On the day of experimental testing, volunteers reported to the laboratory after 12 hr of fasting. The temperature of the laboratory was ∼21°C for all testing sessions and volunteers were tested at the same time of day as the strength assessment. Participants were required to complete an exercise-session checklist before participation to confirm adherence to pretesting instructions and absence of URTI symptoms. The resistance-exercise timeline (with differential gene expression data) used in this study is shown in Figure 1. Briefly, blood was collected at three time points, pre-, post-, and 2 hours following exercise. Prior to the first blood draw, each participant was required to rest quietly in a seated position for 10 min before sampling from an antecubital vein.
Figure 1.
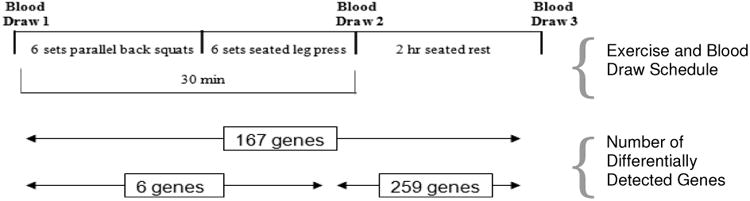
Resistance exercise protocol timeline with corresponding gene response. Each exercise consisted of one set of 10 repetitions at 45% and one set at 55% of 1-RM and four sets of 10 repetitions at 65% of 1-RM. Repetitions were performed with a 2:2 cadence with 2 min rest between sets. Blood was immediately drawn from each participant and extracted for total RNA. Microarray analysis was performed and differentially expressed genes were calculated.
Exercise Protocol
The resistance exercises employed were selected to recruit and activate a large amount of muscle tissue. Participants performed the parallel back squat and the seated leg press, both, which utilized major muscle groups in the lower extremities. The exercise protocol required each participant to complete six sets of the parallel back squat, followed by six sets of the seated leg press. Each exercise consisted of two warm-up sets of 10 repetitions at 45% and 55% of 1-RM and four sets of 10 repetitions at 65% of 1-RM. All repetitions were paced with the use of a metronome set at a 2:2 cadence, with a 2 min resting period between sets. The total time to complete the exercise protocol was approximately ∼30 min. A second blood sample (post) was immediately collected after the completion of the exercise session. Participants were then instructed to rest quietly for 2 hr. The final blood sample (2hr) was collected after the 2 hr recovery period.
Blood Collection, RNA Extraction, and Bioanalyzer Analysis
Blood was sampled from the antecubital vein of each subject while seated at baseline, immediately post-exercise, and after 2 hr of recovery. Vacutainers containing the anticoagulant (EDTA) were used for collection. Due to the delicacy of RNA, the duration from blood sampling to stabilization of RNA never exceeded 3 min. Blood was collected and immediately stored at -80°C at a ratio of 4 parts TriReagent BD to 1 part whole blood. Within 24 hours, total RNA was extracted from the blood by adjusting the ratio of TriReagent to blood volume as described by the protocol, and further extracted using the standard blood protocol of TriReagent BD (Molecular Research Center, Cincinnati, OH). The resulting RNA was quantified on the NanoDrop ND1000 spectrophotometer and evaluated for integrity using Agilent 2100 bioanalyzer. All RNA concentrations were between 13 and 77 ng/uL and had RIN values (RNA Integrity Numbers) greater than 7.0 indicating excellent quality RNA.
Microarray Analysis
Microarray target preparation was performed using the NuGEN Ovation V2 and WB reagents (NuGEN Technologies, San Carlos, CA) as specified by the manufacturer with a total RNA input of 50 ng. The resulting cDNA was purified using the DNA clean and concentrator-25 (Zymo Research, Orange, CA), fragmented, biotin labeled, and prepared as a hybridization mixture as described in the methods of the NuGEN Encore labeling reagents. Microarray hybridizations were performed using the Affymetrix U133a 2.0 GeneChips for 16h at 45°C at 60 rpm. GeneChips were subsequently stained using a double strepavidin phycoerythrin protocol on the FS450 fluidic station, scanned using the GS3000-7G scanner, and processed with the GeneChip Operating Software (Affymetrix Corp, Santa Clara, CA) (3).
Microarray Data Analysis
Affymetrix GeneChip Operating Software (GCOS) was used to generate both image files (DAT and CEL) and probe intensity files (CHP). Data obtained from the GCOS CHP files includes quantitative gene signal information, which can be used in downstream complex data analysis. Quantitative data from each gene is determined by a set of DNA probes that interrogate at least 11 locations across each gene. Collectively, this “probe set” provides a single value summation of each gene response. Probe statistics are background corrected and normalized using the R Language (2) and Environment for Statistical Computing with BioConductor tools (BioConductor) (1). Global expression statistics are calculated for each probe set from each sample using the Robust Multichip Average (RMA) statistic of Speed et al (6, 17).
Immune Cells and Lactate
Whole blood containing EDTA was analyzed for complete blood counts and leukocyte subsets (Beckman Coulter AcT Diff2, Brea, CA). Total leukocytes and leukocyte subsets were corrected for changes in plasma volume via hematocrit and hemoglobin changes using the Dill and Costill (1974) method (12). Lactate determination at all three time periods was accomplished using fingersticks and analyzed using the Accutrend Lactate meter (Roche Diagnostics, Germany).
Statistical Analyses
One-way analysis of variance (ANOVA) was used to determine whether there were significant changes in the dependent variables. Tukey post hoc analysis was utilized to isolate differences. The statistical significance level was set a 0.05. Linear modeling was performed on the microarray data using the Bioconductor limma package, which implements the method of Smythe (2005) (36). Smythe's (2005) method borrows information across genes to improve inference based on small sample sizes. It provides, for each contrast, a moderated t-statistic, p-value, and p-value adjusted (19) for the purpose of controlling the false discovery rate. Differentially expressed genes were loaded into the Ingenuity Pathways Analysis (Ingenuity® Systems, www.ingenuity.com) database for biological pathway analysis.
Results
Lactate
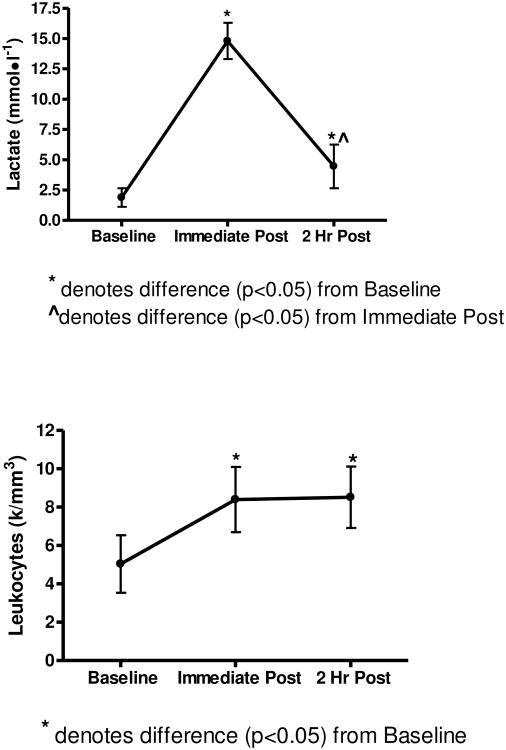
A significant main effect (p<0.05) for time was observed for lactate. The resistance exercise induced a ∼7 fold increase (p<0.05) from baseline to post-exercise while after 2hr post-exercise, the lactate levels subsided to approximately 2 fold over baseline (see Figure 2).
Figure 2.
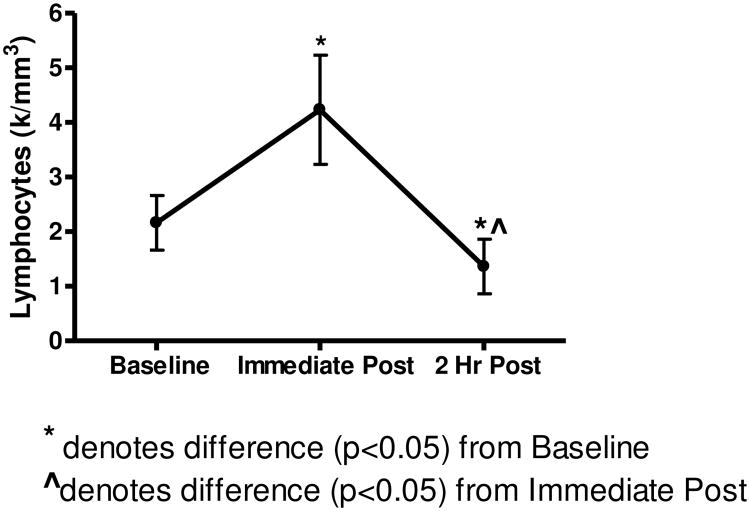
Lactate, Leukocyte, and Lymphocyte data show changes in response to exercise time points.
Immune Responses
A significant main effect (p<0.05) for time was observed for leukocytes, monocytes, granulocytes, and lymphocytes. Tukey post hoc analysis revealed that leukocytes, monocytes, and lymphocytes were elevated immediately post-exercise (p<0.05) over baseline values. At 2 hr post-exercise, leukocytes and granulocytes remained elevated (p<0.05), whereas lymphocytes were lower than (p<0.05) baseline values (Figure 2).
Gene Expression
Using a conservative false discovery rate (FDR) of p<0.05 and a two-fold change of or higher threshold, 167 genes were differentially detected between baseline and 2 hr post-exercise, 259 genes between 2 hr post-exercise and immediately post-exercise, and six genes between immediately post-exercise and baseline indicating the greatest gene response was seen at the 2 hour post-exercise (Figure 1). Farther analysis of these differentially detected genes by plotting using similarity overlapping in a Venn diagram, indicate that although cellular populations and lactate levels are changing, that the majority of differentially expressed genes remain the same as indicated by the 139 genes in the overlap region. Conversely, the non-overlapping genes may be related to cellular population, lactate, or other changes (see Figure 3). The Venn diagram also displays 10 of the highest and lowest differentially expressed genes (by gene symbol) for the corresponding region. Complete data sets are available in the NCBI's Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/).
Figure 3.
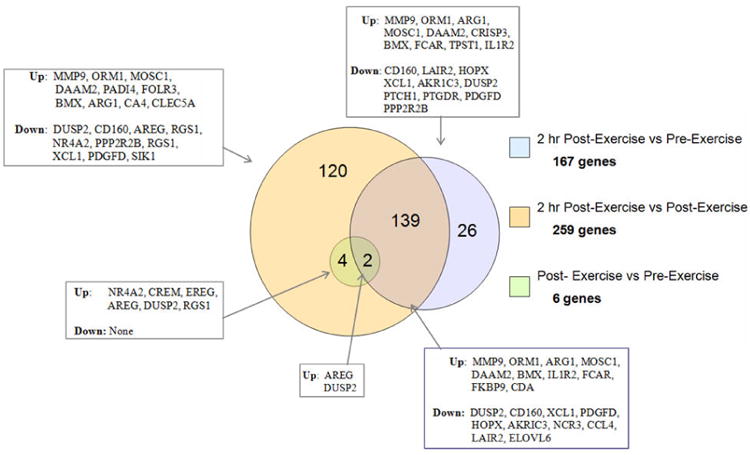
Venn Diagram showing the number and similarity of differentially detected genes from each comparison. These data indicate that 139 of the167 genes differentially detected between the 2 hr after exercise and pre-exercise were the same genes as those detected between 2hr after exercise vs immediate post exercise. All 6 genes detected for the immediate post exercise data vs pre exercise were also seen in the 2hr after exercise vs immediate post exercise.
Principal component analysis (PCA, (32)) as well as sample clustering (heatmap and sample dendrogram, not shown) indicated clear separation between the 2-hour post-exercise samples and all other samples as well as the expected inherent variation between participants reflected on the axis of PC 2 (see Figure 4).
Figure 4.
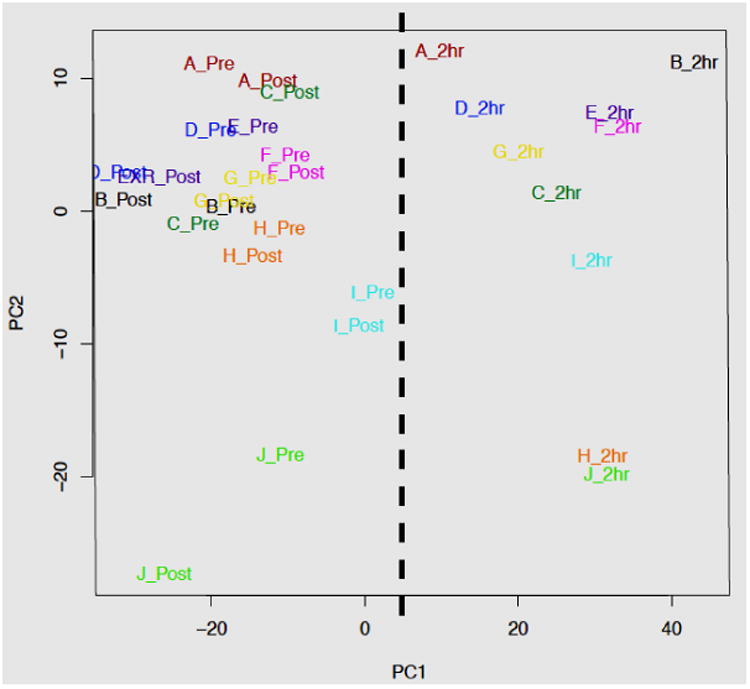
The Principle Component Analysis (PCA) plot of the microarray data indicates that the primary source of variation among samples correlates with the sample time points, distinguishing the 2hr post-exercise samples from the pre- and Immediate post-exercise samples (PC1, dashed line). The second principal component (PC2) largely captures differences in expression between participants. Samples are labeled by participant (A-J) and sample time point (pre = pre-RE, ipost = immediate post-exercise, and 2hr = 2 hr post-exercise), and color-coded by participant.
The top canonical pathways identified by Ingenuity Pathways Analysis (Ingenuity® Systems, www.ingenuity.com) from the genes differentially expressed between the 2 hr post-exercise and the pre-exercise blood samples are presented in Table 2. The percentage of these genes that are either differentially regulated in the 2 hr post-exercise samples is indicated. All of the pathways were statistically significant at p < 0.05, suggesting that the differentially expressed genes identified between the samples are most likely involved in these particular pathways due to the biology of the system.
Table 2.
Top pathways, functions, and networks identified Ingenuity Pathways Analysis of the genes differentially expressed (DE) between 2-hour post-exercise and immediate post-exercise blood samples. Canonical pathways are known pathways for which there is a significant association with the molecules in the data set. Biological functions are composed of the molecules in the data set that are know to be involved in various diseases and functions, and are further divided statistical significance subcategories.
| Canonical pathways | P-value | Number of differentially expressed molecules to total in pathway |
|---|---|---|
| Natural Killer Cell Signaling | 1.6 × 10-7 | 20/115 (17.4%) |
| NF-kB Signaling | 8.9 × 10-5 | 17/149 (11.4%) |
| Cytotoxic T Lymphocyte-mediated Apoptosis of Target Cells | 3.7 × 10-4 | 7/31 (22.6%) |
| T Cell Receptor Signaling | 9.9 × 10-4 | 13/110 (11.8%) |
| Communication between Innate and Adaptive Immune Cells | 1.6 × 10-3 | 10/90 (11.1%) |
|
| ||
| Biological functions | P-value | Number of differentially expressed molecules |
|
| ||
| Inflammatory Response | ≤ 5.8 × 10-3 | 132 |
| Infectious Disease | ≤ 8.0 × 10-3 | 100 |
| Respiratory Disease | ≤ 8.0 × 10-3 | 74 |
| Inflammatory Disease | ≤ 7.3 × 10-3 | 193 |
| Immunological Disease | ≤ 8.0 × 10-3 | 187 |
| Antigen Presentation | ≤ 6.6 × 10-3 | 125 |
| Cell-To-Cell Signaling and Interaction | ≤ 8.0 × 10-3 | 140 |
| Cell-mediated Immune Response | ≤ 8.0 × 10-3 | 139 |
| Humoral Immune Response | ≤ 5.4 × 10-3 | 122 |
| Hematological System Development and Function | ≤ 5.4 × 10-3 | 134 |
| Immune Cell Trafficking | .≤ 7.4 × 10-3 | 88 |
Discussion
This study focused on identifying significant transcriptional and immunological changes in human PBMCs, in response to resistance exercise. This is one of the first studies to examine the transcriptional changes in PBMCs in response to RET and the data presented here clearly demonstrated that a brief bout of RET stimulated both leukocyte and leukocyte-subset mobilization and a unique set of genomic changes. While increases in immune cell population in response to both endurance and resistance exercises have been well documented, the transcriptional changes on the genes involved are not well understood. Using a microarray approach, this study identified differential gene expression in PBMCs (167 genes at p < 0.01) between pre- and 2 hr post-exercise and grouped genomic changes into relevant key signaling pathways. Although genomic changes in skeletal muscle have been identified as a result of resistance exercise (7, 8, 11, 31) we identified several specific genes from resistance exercise post-exercise, as having potential roles into the mechanism of recovery. Although some genes related to immunological responses were shown to be increased in response to both aerobic and resistance exercise, we identified several genes that were uniquely altered in response to RET which may contribute to a better understanding of the processes involved in acute muscle damage and repair.
Cellular Immune Response During Resistance Exercise
As hypothesized, these data show increases in total leukocyte, lymphocytes, and monocytes immediately after resistive exercise (Figure 2). Our findings are in agreement with previous studies that have described a mobilization of total leukocytes and their subsets immediately post-exercise, stimulated by an acute bout of resistance exercise (9, 15, 21, 34). Based on the exercise protocol utilized in this study, the 2:2 cadence not only kept all subjects on the same pace, but also may have induced a small degree of muscle damage. The high intensity of the RET bout was evidenced by the ∼7 fold increase in blood lactate levels immediately following exercise. Resistance exercise of this nature with pronounced eccentric muscle contraction has been reported to increase inflammation (34) in the surrounding tissue, leading to the concomitant inflammatory response as noted by the temporary leukocytosis post-exercise (23, 30). Typically, neutrophils are the first inflammatory cells to infiltrate the affected muscles and are purported to participate in phagocytosis (23, 30, 35). Additionally, inflammatory signaling molecules and other leukocytes, such as monocytes are further activated. Studies examining immune responses to resistance exercise have consistently reported a significant leukocytosis (9, 20, 21, 25), (28, 33). Nieman, Henson, et al. (1995) reported that a single session of resistance training induced leukocytosis and lymphocytosis immediately post-exercise and lymphocytopenia 2 hr later. The degree of these immune responses was similar to those reported during and after high-intensity prolonged endurance exercise (26). Further, after the 2 hr recovery, total leukocytes remain elevated, while lymphocyte counts decline below baseline values following the recovery period. This decline in lymphocyte population has been previously shown after either prolonged endurance or exhaustive resistance exercises (26).
PBMC Genomic Response
Microarray data suggest that while there is a small genetic response to the resistance exercise 30 minutes following the exercise, the genetic response two hours post-exercise is large and significant, and presents a strong signature of immunological stress (Table 2). Analysis of transcriptional principal components (PCA) is a method to graphically reveal total sample variation from microarray data into components that capture most of that variation (12). The plot of those components indicates that the primary source of variation among samples correlates with the sample time points, distinguishing the 2hr post-exercise samples from the pre- and immediate post-exercise samples (Figure 4, PC1, dashed line), while the pre-exercise and immediate post-exercise samples are inseparable along the x-axis. The distinction of the 2hr post-exercise samples and the lack of separation between the pre- and immediate post-exercise samples are reflective of the number of differentially expressed gene identified by linear modeling between the time points (Figure 4). The second principal component (PC2), which represents the second greatest source of variation, appears to largely capture differences in expression between participants. This PCA plot presents convincing evidence that the experimental variables (gene response time and participant variation) clusted appropriately and could easily be modeled, indicating good experimental controls with high signal to noise.
Ingenuity IPA consistently identified immunological and inflammatory-related response among the biological processes most represented by the differentially expressed genes identified in the 2 hr post-exercise minus pre-exercise samples (p<0.01) (Table 2). Statistically significant changes in gene expression in canonical pathways such as Natural Killer Cell signaling, NF-κB, Cytotoxic T lymphocyte-mediated Apoptosis and T cell receptors, suggesting that genes involved in these pathways are critical to mediating the host response to resistance exercise and may be critical targets for future studies.
Differential expression of Genes related to Canonical Pathways
Genes related to inflammatory disease, immunological disease and cell-to-cell signaling showed the greatest changes (as shown by number of genes differentially expressed) from pre-exercise to 2 hr post-exercise. These data concur with a previous study examining changes in aerobic exercise and showed that the greatest changes in genes expression involved stress response and inflammatory response (11). It is important to note that all gene expression studies on PBMC have an “apparent” transcriptional response solely based on cell population dynamics, but this bias can be reduced if one looks at consistent gene changes over time as cellular populations fluctuate and revert back to baseline. These data can be obtained from our data set by examining the intersect population (139 genes) in the venn diagram (Figure 3) which somewhat reduces the apparent cell population effect.
From data shown in Figure 5, the most differentially expressed at the 2 hr post-exercise recovery time point were matrix metalloproteinase 9 (MMP 9), orosomucoid 1 (ORM 1) and arginase 1 (ARG 1), which showed significant up regulation, and CD160 which was significantly down-regulated. As previously shown, RET induces skeletal muscle damage and this is reflected in the genetic output of PBMCs. As indicated, both MMP9 and ARG1 were significantly increased in response to RET suggesting that there is significant skeletal muscle damage and associated repair occurring. MMP9, also referred to as gelatinase B, has been classified by its protein and domain structure as the largest and most complex of the MMP family (13). When muscle damage occurs, MMP9 and ARG1 antagonize one another in response to inflammatory cytokines. Functionally, MMPs are enzymes responsible for the destruction of extracellular matrix, and are linked to inflammation and tissue remodeling (18). Both MMP9 and ARG1 are associated with the NF- κB signaling cascade and this is in accordance with an increase in NF- κB signaling also displayed in PBMC transcriptome following RET. MMP9 is quite complex and elicits a widespread effect, including activation of other MMPs, gene transcription and protein secretion by both cytokines and chemokines, proenzyme activation by components of the plasminogen activation system and action of tissue inhibitors of matrix metalloproteinases. Thus, these responses may indicate that following an acute bout of RET and likely induction of muscle damage, there is a rapid and robust intracellular repair and remodeling response.
Figure 5.
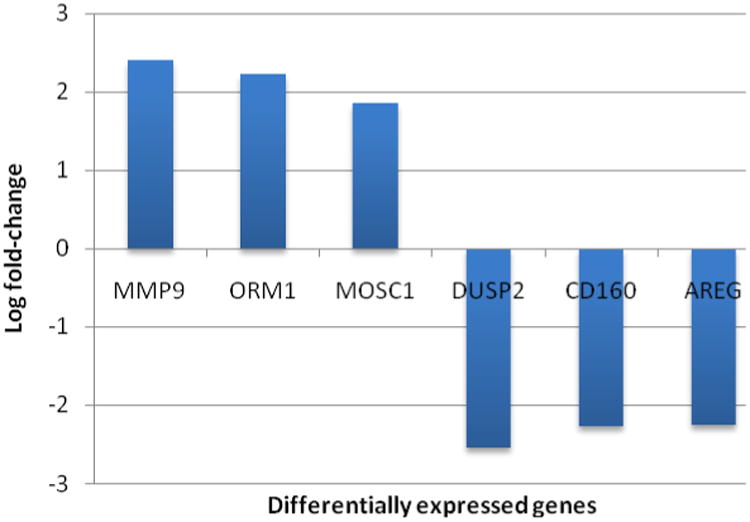
The three most up- or down-regulated genes between the 2-hour post-exercise and the pre-exercise whole blood samples based on microarray analysis. The data were sorted using a binary filter of p-value <0.05 and at least a 2-fold difference in expression, and pre-exercise samples were used as the reference (i.e. 2-hour post-exercise minus pre-exercise) and thus positive fold change indicates up-regulation two hours post-exercise, while negative indicates down-regulation.
In contrast, Arginase 1 (ARG 1) is a hydrolytic enzyme shown to reduce activity of MMP9 (37). The inhibitory effects of ARG1 on MMP2 and MMP9 activation have been shown to reduce reactive oxygen species, and muscle destruction suggesting that an increase in ARG1 in this study is a direct result on increases in MMP9 activity. Interestingly, ARG 1 is also able to elicit it function on the body through regulation of NO because it competes with nitric oxide synthase (NOS) for arganine (37). According, to Bansal and Ochoa (2003) this competition for arginine between ARG1 and NOS may lead to macrophage and T-lymphocyte activation (5). It is for this reason that arginase expression is implicated in both the immune response and inflammation associated with infectious disease (37). It is possible that the induction of MMP9 and ARG1 is biphasic, where MMP9 is induced earlier in response to RET and ARG1 is induced as a protective mechanism during muscle repair. A closer look at the transcriptional time course in PBMCs following RET would be useful for examining the relationship of these two proteins.
Orosomucoid 1 (ORM 1) or α 1- acid glycoprotein (αAGP) was also significantly increased in response to RET. ORM is a plasma protein expressed in hepatocytes and secreted into the plasma under stressful conditions, injury, infection, inflammation, and has been implicated is suggested to have roles in immunomodulatory, barrier and carrier functions (22). ORM has been shown to inhibit mitogen-induced proliferation of lymphocytes and this may be partially responsible for the reduction in lymphocytes observed in this study. In our data, 2 hr following RET, there was a marked increase in leukocyte populations; however, lymphocyte populations were decreased. The resulting decrease in lymphocytes could be a direct result of an increase in circulating ORM from the PBMCs and continued studies would be of interest to demonstrate that ORM is or is not responsible for halting proliferation of resident lymphocyte populations in the case of RET. Interestingly, other studies in both aerobic exercise and tumor progression experiments that an increase of lactate in the blood stream may cause a regression or inhibition of TCR, lymphocytes, and associated pathways(24, 14). However, this has not yet been show in RET, but may not be surprising as the levels of lactate in this study were significantly elevated (Figure 2)
CD160 was the only molecule to show significant down regulation at the 2 hr post-exercise recovery point. CD160 is a cell membrane receptor, also referred to as BY55 (29). CD160 is a 27 kDa glycoprotein whose expression is linked to peripheral blood natural killer cells, as well as CD8 T lymphocytes. Much like the other genes implicated in our study, CD160 is linked to immunity, which can be seen through its expression, and the resulting decrease in expression may be correlated to the displayed decrease in circulating lymphocytes 2 hr post exercise. According to Nikolova et al. (2002), CD160 is expressed on intestinal intraepithelial T lymphocytes, circulating natural killer cells (CD56DIM+ and CD16+), and small amount of other circulating lymphocytes such as CD8bright+, responsible for mediating cytotoxic activity. The displayed decrease in CD160 and in the increase of ORM shed light on the regulatory mechanisms of lymphocyte populations in the blood following exercise. A more extensive examination of the time course of PBMCs transcriptional response to RET would be useful in order to determine the relationship between these two genes and whether they are influenced by high lactate or other regulatory events.
In summary, an acute bout of resistance exercise disrupts cellular homeostasis, and induced a transient redistribution of certain leukocytes. The exercise bout also changed the gene expression in the peripheral blood, which is different from previous reports suggesting that genetic response differs as a result of different exercise. Future studies should validate the differentially expressed genes with RT-qPCR and confirm their role in the post-exercise response. Detailed flow cytometric analysis and sorting on specific cell populations could provide an understanding on the exact transcriptional responses of each cell type and how they potentially behave as a population.
Acknowledgments
The views, opinions, and findings in this report are those of the authors and should not be construed as official Department of the Army position, policy, or decision unless so designated by other official designation. All experiments were carried out in accordance to state and federal guidelines. This publication was made possible by the Vermont Genetics Network through Grant Number P20 RR16462 from the INBRE Program of the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. The authors would like to thank all the men who participated in this e exercise study, Dr. Jeff Bond for bioinformatics support at the University of Vermont (UVM), and Tim Hunter in the VGN microarray core lab (UVM) for assistance in the experimental design.
Contributor Information
L. A. Carlson, University of New England, 11 Hills Beach Road, Biddeford, ME 04005
R. J. LeClair, University of New England, 11 Hills Beach Road, Biddeford, ME 04005; Castleton State College, Castleton, VT
S. W. Tighe, University of Vermont, Vermont Genetics Network, 109 Carrigan Drive, Burlington, VT 05495
J. Dragon, University of Vermont, Vermont Genetics Network, 109 Carrigan Drive, Burlington, VT 05495
R. W. Kenefick, US Army Research Institute of Environmental Medicine, Natick, MA
N. W. Westcott, Castleton State College, Castleton, VT
References
- 1.BioConductor: Open Source Software for Bioinformatics.
- 2.R: A Language and Environment for Statistical Computing.
- 3.Affymetrix. Genechip Expression Analysis Technical Manual. Affymetrix; Sanat Clara: 2003. [Google Scholar]
- 4.Bachle LA, Smith DD, Petzel D. Isolation and characterization of insulin from the Brockmann body of Dissostichus mawsoni, an Antarctic teleost fish. J Pept Res. 2000;56:47–54. doi: 10.1034/j.1399-3011.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 5.Bansal V, Ochoa JB. Arginine availability, arginase, and the immune response. Curr Opin Clin Nutr Metab Care. 2003;6:223–228. doi: 10.1097/00075197-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 7.Buford TW, Cooke MB, Shelmadine BD, Hudson GM, Redd L, Willoughby DS. Effects of eccentric treadmill exercise on inflammatory gene expression in human skeletal muscle. Appl Physiol Nutr Metab. 2009;34:745–753. doi: 10.1139/H09-067. [DOI] [PubMed] [Google Scholar]
- 8.Buttner P, Mosig S, Lechtermann A, Funke H, Mooren FC. Exercise affects the gene expression profiles of human white blood cells. J Appl Physiol. 2007;102:26–36. doi: 10.1152/japplphysiol.00066.2006. [DOI] [PubMed] [Google Scholar]
- 9.Carlson LA, Headley S, DeBruin J, Tuckow AT, Koch AJ, Kenefick RW. Carbohydrate supplementation and immune responses after acute exhaustive resistance exercise. Int J Sport Nutr Exerc Metab. 2008;18:247–259. doi: 10.1123/ijsnem.18.3.247. [DOI] [PubMed] [Google Scholar]
- 10.Clarkson PM, Tremblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol. 1988;65:1–6. doi: 10.1152/jappl.1988.65.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Connolly PH, Caiozzo VJ, Zaldivar F, Nemet D, Larson J, Hung SP, Heck JD, Hatfield GW, Cooper DM. Effects of exercise on gene expression in human peripheral blood mononuclear cells. J Appl Physiol. 2004;97:1461–1469. doi: 10.1152/japplphysiol.00316.2004. [DOI] [PubMed] [Google Scholar]
- 12.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 13.Dubois B, Opdenakker G, Carton H. Gelatinase B in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neurol Belg. 1999;99:53–56. [PubMed] [Google Scholar]
- 14.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 15.Henson DA, Nieman DC, Blodgett AD, Butterworth DE, Utter A, Davis JM, Sonnenfeld G, Morton DS, Fagoaga OR, Nehlsen-Cannarella SL. Influence of exercise mode and carbohydrate on the immune response to prolonged exercise. Int J Sport Nutr. 1999;9:213–228. doi: 10.1123/ijsn.9.2.213. [DOI] [PubMed] [Google Scholar]
- 16.Henson DA, Nieman DC, Parker JC, Rainwater MK, Butterworth DE, Warren BJ, Utter A, Davis JM, Fagoaga OR, Nehlsen-Cannarella SL. Carbohydrate supplementation and the lymphocyte proliferative response to long endurance running. Int J Sports Med. 1998;19:574–580. doi: 10.1055/s-2007-971962. [DOI] [PubMed] [Google Scholar]
- 17.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanbe N, Tanaka A, Kanbe M, Itakura A, Kurosawa M, Matsuda H. Human mast cells produce matrix metalloproteinase 9. Eur J Immunol. 1999;29:2645–2649. doi: 10.1002/(SICI)1521-4141(199908)29:08<2645::AID-IMMU2645>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Klipper-Aurbach Y, Wasserman M, Braunspiegel-Weintrob N, Borstein D, Peleg S, Assa S, Karp M, Benjamini Y, Hochberg Y, Laron Z. Mathematical formulae for the prediction of the residual beta cell function during the first two years of disease in children and adolescents with insulin-dependent diabetes mellitus. Med Hypotheses. 1995;45:486–490. doi: 10.1016/0306-9877(95)90228-7. [DOI] [PubMed] [Google Scholar]
- 20.Koch AJ, Potteiger JA, Chan MA, Benedict SH, Frey BB. Minimal influence of carbohydrate ingestion on the immune response following acute resistance exercise. Int J Sport Nutr Exerc Metab. 2001;11:149–161. doi: 10.1123/ijsnem.11.2.149. [DOI] [PubMed] [Google Scholar]
- 21.Kraemer WJ, Clemson A, Triplett NT, Bush JA, Newton RU, Lynch JM. The effects of plasma cortisol elevation on total and differential leukocyte counts in response to heavy-resistance exercise. Eur J Appl Physiol Occup Physiol. 1996;73:93–97. doi: 10.1007/BF00262815. [DOI] [PubMed] [Google Scholar]
- 22.Lecchi C, Avallone G, Giurovich M, Roccabianca P, Ceciliani F. Extra hepatic expression of the acute phase protein alpha 1-acid glycoprotein in normal bovine tissues. Vet J. 2009;180:256–258. doi: 10.1016/j.tvjl.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Malm C, Nyberg P, Engstrom M, Sjodin B, Lenkei R, Ekblom B, Lundberg I. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. 2000;529(Pt 1):243–262. doi: 10.1111/j.1469-7793.2000.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mihm S, Droge W. Regulation of cytotoxic T-lymphocyte activation by L-lactate and pyruvate. Cell Immunol. 1985;96:235–240. doi: 10.1016/0008-8749(85)90355-7. [DOI] [PubMed] [Google Scholar]
- 25.Miles MP, Leach SK, Kraemer WJ, Dohi K, Bush JA, Mastro AM. Leukocyte adhesion molecule expression during intense resistance exercise. J Appl Physiol. 1998;84:1604–1609. doi: 10.1152/jappl.1998.84.5.1604. [DOI] [PubMed] [Google Scholar]
- 26.Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, Henson DA, Butterworth DE, Schmitt RL, Bailey EM, Warren BJ, Utter A, Davis JM. Carbohydrate and the cytokine response to 2.5 h of running. J Appl Physiol. 1997;82:1662–1667. doi: 10.1152/jappl.1997.82.5.1662. [DOI] [PubMed] [Google Scholar]
- 27.Nieman DC. Upper respiratory tract infections and exercise. Thorax. 1995;50:1229–1231. doi: 10.1136/thx.50.12.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieman DC, Henson DA, Sampson CS, Herring JL, Suttles J, Conley M, Stone MH, Butterworth DE, Davis JM. The acute immune response to exhaustive resistance exercise. Int J Sports Med. 1995;16:322–328. doi: 10.1055/s-2007-973013. [DOI] [PubMed] [Google Scholar]
- 29.Nikolova M, Marie-Cardine A, Boumsell L, Bensussan A. BY55/CD160 acts as a co-receptor in TCR signal transduction of a human circulating cytotoxic effector T lymphocyte subset lacking CD28 expression. Int Immunol. 2002;14:445–451. doi: 10.1093/intimm/14.5.445. [DOI] [PubMed] [Google Scholar]
- 30.Pizza FX, Baylies H, Mitchell JB. Adaptation to eccentric exercise: neutrophils and E-selectin during early recovery. Can J Appl Physiol. 2001;26:245–253. doi: 10.1139/h01-015. [DOI] [PubMed] [Google Scholar]
- 31.Radom-Aizik S, Zaldivar F, Jr, Leu SY, Cooper DM. Brief bout of exercise alters gene expression in peripheral blood mononuclear cells of early- and late-pubertal males. Pediatr Res. 2009;65:447–452. doi: 10.1203/PDR.0b013e3181993473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ringner M. What is principal component analysis? Nat Biotechnol. 2008;26:303–304. doi: 10.1038/nbt0308-303. [DOI] [PubMed] [Google Scholar]
- 33.Shinkai S, Shore S, Shek PN, Shephard RJ. Acute exercise and immune function. Relationship between lymphocyte activity and changes in subset counts. Int J Sports Med. 1992;13:452–461. doi: 10.1055/s-2007-1021297. [DOI] [PubMed] [Google Scholar]
- 34.Simonson SR, Jackson CG. Leukocytosis occurs in response to resistance exercise in men. J Strength Cond Res. 2004;18:266–271. doi: 10.1519/R-12572.1. [DOI] [PubMed] [Google Scholar]
- 35.Smith RM, Giannoudis PV, Bellamy MC, Perry SL, Dickson RA, Guillou PJ. Interleukin-10 release and monocyte human leukocyte antigen-DR expression during femoral nailing. Clin Orthop Relat Res. 2000:233–240. doi: 10.1097/00003086-200004000-00028. [DOI] [PubMed] [Google Scholar]
- 36.Smyth GK. Limma: Linear models for microarray data. In: al RGe., editor. Bioinformatics and Computational Biology Solutions using R and Bioconductor. Springer; NY: 2005. pp. 397–420. [Google Scholar]
- 37.Xu L, Hilliard B, Carmody RJ, Tsabary G, Shin H, Christianson DW, Chen YH. Arginase and autoimmune inflammation in the central nervous system. Immunology. 2003;110:141–148. doi: 10.1046/j.1365-2567.2003.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]