INTRODUCTION
We have previously indicated that the ideal animal tumor model should mimic the human disease (107). This means that the investigator should be able to ascertain the influence of host factors on the initiation of tumorigenesis, mimic the susceptibility of tumor response based on age and reproductive history, and determine the response of the tumors induced to chemotherapy. The utilization of experimental models of mammary carcinogenesis in risk assessment requires that the influence of ovarian, pituitary and placental hormones, among others, as well as overall reproductive events are taken into consideration, since they are important modifiers of the susceptibility of the organ to neoplastic development. Several species, such as rodents, dogs, cats, and monkeys, have been evaluated for these purposes; however, none of them fulfill all the criteria specified above. Rodents, however, are the most widely used models; therefore, this work will concentrate on discussing the rat rodent model of mammary carcinogenesis.
GENERAL CONCEPTS
Spontaneous mammary tumors are frequently observed in long-term rodent studies. In mice the development of “spontaneous” mammary tumors is linked to the infection of female mice with either an exogenous mouse mammary tumor virus (MMTV), or a less virulent endogenous provirus and has been discussed in a previous publication (107). In the rat, the majority of spontaneously developed tumors, with the exception of leukemia, are neoplasms of endocrine organs or of organs under endocrine control. Spontaneous mammary tumors develop in females of various strains of rats, such as August, Albany-Hooded, Copenhagen, Fisher, Lewis, Osborne-Mendel, Sprague-Dawley, Wistar and Wistar/Furth (107). Spontaneous mammary tumors are third in incidence among spontaneous tumors found in the Fisher 344 rat used in the National Cancer Institute/National Toxicology Program (NCI/NTP) carcinogenicity bioassays (107). They are predominantly benign tumors, i.e., fibroadenomas, fibromas and more rarely adenomas. Malignant tumors such as adenocarcinomas are rare, although they are the most frequent tumors induced by chemical carcinogens (107). The development of spontaneous tumors varies as a function of strain, age and endocrine influences. Hormone withdrawal inhibits tumor development, and hormone supplementation, such as chronic administration of estrogens, increases the incidence of adenocarcinomas, whereas chronic administration of prolactin or of growth hormone stimulates benign tumor growth (107). The long latency period for spontaneous tumor development, up to two years in susceptible strains to develop a 50–70% tumor incidence, limits the usefulness of this model for experimental studies.
COMPARATIVE ASPECTS OF MAMMARY GLAND DEVELOPMENT IN THE HUMAN AND IN THE RAT
The mammary gland differs from almost all other organs and glandular elements. During the process of development they are composed of an immature mammary parenchyma surrounded by mature stromal tissue. In other glands, on the other hand, both parenchyma and stroma develop harmoniously at the same time and in definitive space. In the mammary gland, the glandular parenchyma penetration into the surrounding tissue occurs during the prepubertal period rather slowly. By this time, the tissue surrounding the epithelium is fat, connective tissue and blood vessels which have become completely mature, whereas the gland itself remains in an embryonal state. It is during pubertal growth in both the rat and the human that the epithelial component begins to be stimulated by hormonal impulses to penetrate into the mature surrounding tissue. The postnatal development of the rat mammary gland has been thoroughly studied in the S-D strain of rats (107). However, it is not known whether differences in susceptibility to carcinogenesis exhibited by other strains of rats are related to variations in the pattern of gland development and branching or in relation with variations in the physiology of the animals. In the human female, the developmental pattern of the mammary gland is fragmentary known, and no comparative studies among women of different races, genetic backgrounds or environments are available (114, 119).
There are basic differences between the developmental pattern of the human and rat mammary glands. For example, in the human, the ductal structures grow along connective tissue septa, and rarely the lobular structures grow into fat tissue. In the rat, instead, there is a constant growth of ductal and lobular structures into the adjacent fat tissue. This difference must be considered when the influence of the parenchyma on the stroma is studied in one species or the other. Another difference that might be of importance is that in the rat there is a gradient in development from the nipple to the distal portion, which is the last to differentiate; in the human, the growth or the development is more laberyinthic and a different degree of development in different portions of the breast is observed (114, 119).
PATHOGENESIS
Pathogenesis of rat mammary tumors
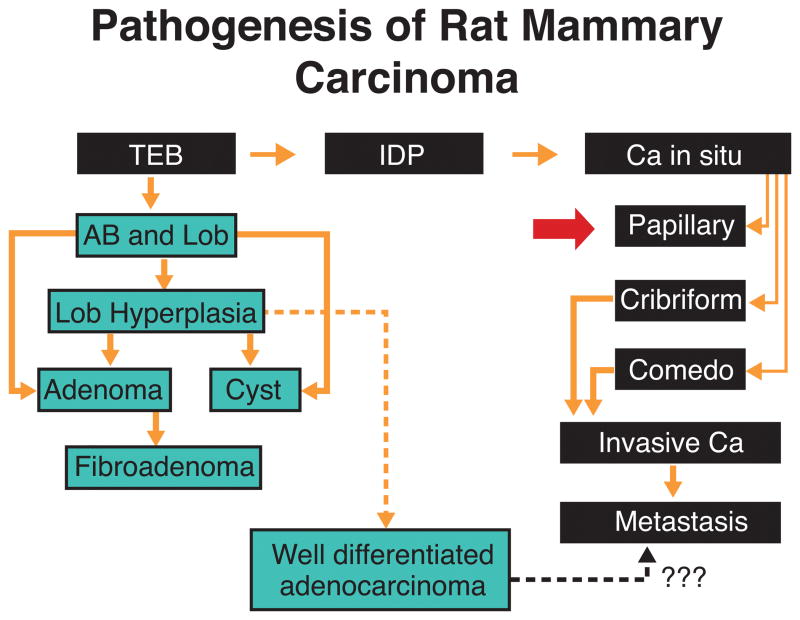
Mammary gland tumors induced in rats by a single dose of DMBA, NMU or irradiation comprise a spectrum of morphology from benign, typical fibroadenomas and adenomas to papillomas with hyperplastic, atypical and dysplastic epithelium and significant stromal and myoepithelial components, to tumors that are architecturally and cytologically malignant and invade adjacent normal tissue. Metastases from even the most anaplastic tumors are low in frequency less than 10% (145). Carcinogenic initiation occurs primarily in the epithelium of terminal end buds (TEBs) (Figure 1) while they are developing into alveolar buds (ABs) and terminal ducts (TDs); these structures are considered to be equivalent to the terminal ductal lobular unit (TDLU) described in the human breast (107, 149).
Figure 1.
Pathogenesis of chemically-induced rat mammary tumors. The undifferentiated terminal end bud (TEB) affected by the carcinogen progresses to intraductal proliferation (IDP), and in situ ductal carcinoma (Ca In Situ) that exhibit various histopathological types. Further tumor growth and coalescence of neighboring lesions originate invasive carcinomas (Invasive Ca), which might become metastatic. When the carcinogen affects more differentiated structures, such as alveolar buds (AB) and lobules (Lob) the lesions developed are more benign in nature and appear later than the ductal carcinomas. Alveolar bud hyperplasia (A.B. Hyperplasia). (Reprinted from Russo J and Russo IH, Cancer Epidemiol Biomarkers & Prev 3, 353–364, 1994, with permission).
Excessive epithelial proliferation and development of progressive cytologic abnormalities result from carcinogen exposure and ultimately produce tumors (117, 114). This process can be initiated with the administration of DMBA to virgin rats (117). The administration of DMBA when the animals are between the ages of 45 and 55 days induces the largest number of transformed TEBs. Affected TEBs, instead of differentiating into ABs, become larger, and they are called intraductal proliferations. Intraductal proliferations become progressively larger and their confluence leads to the formation of carcinomas (114, 117).
Tumor development does not occur as a random event in all six pairs of mammary glands. Virgin animals treated with the carcinogen develop a greater number of tumors in those glands located in the thoracic region than in glands located in the abdomino-inguinal area. These topographic differences in tumor incidence appear to be due to the asynchronous development of the thoracic mammary glands, which retain the undifferentiated TEBs for a longer period of time than mammary glands located in different topographic areas (107, 114)
However, a trend in a different direction has been observed in irradiated animals (145). Those TEBs that were already differentiated into ABs before DMBA administration do not develop carcinomas but either remain unmodified, undergo dilation, giving rise to hyperplastic lobules (Figure 1), exhibit epithelial proliferation forming tubular adenomas, or give rise to cystic dilations. Hyperplastic lobules and cysts appear later than IDPs, the first ones being observed at 5 to 6 weeks after DMBA administration (114, 117, 118, 119).
The observation that mammary carcinomas arise from undifferentiated structures of the gland, namely TEBs, whereas benign lesions, such as adenomas, cysts, and fibroadenomas arise from structures that were more differentiated at the time of carcinogen administration, indicates that the carcinogen requires an adequate structural target and the type of lesion induced is dependent upon the area of the mammary gland that the carcinogen affects. Thus, the more differentiated the structure at the time of carcinogen administration, the more benign and organized is the lesion which develops (107).
The high susceptibility of the TEB to neoplastic transformation is attributed to the presence of stem cells and the cell kinetic properties of its lining epithelium, whose rate of cell proliferation and of DNA synthetic activity (DNA-LI) are maximal at the tip, and decrease toward the ductal or proximal portion of the gland. The TEB is also characterized by having the highest growth fraction, which progressively diminishes in the more differentiated ABs and lobules (113). The high rate of cell proliferation is associated with the short length of the cell cycle (Tc), which in TEBs of young virgin rats has an average length of 11 hours, lengthening to 21 and 28 hours in TDs and ABs, respectively. Using these cell kinetic parameters we have calculated the rate of cell loss in each one of the compartments of the mammary tree. Interestingly, the TEB is the structure with the highest proliferative ratio and the lowest percentage of cell loss. DMBA is metabolized by mammary epithelial cells to polar metabolites, including epoxides (118, 137, 138), that may be responsible for causing DNA damage.
When dissociated breast epithelial cells of TEBs obtained from virgin animals and lobular cells obtained from parous animals are grown in vitro, they exhibit different rates of formation of polar metabolites. Cells of TEBs produce more polar and less phenolic metabolites than do lobular cells, indicating that the former, in addition to their higher proliferative activity, produce more epoxides, with a greater binding of DMBA to DNA (114, 119, 137, 138). Autoradiographic studies show that the greatest uptake of [3H]DMBA occurs in the nucleus of the epithelial cells of TEBs, and the lowest uptake is observed in ABs and lobules, indicating that the highest DMBA-DNA binding is associated with the structure of the gland with the highest replicative properties (119). The ability of the cells to remove DMBA adducts from the DNA, an indication of their capability to repair the damage, is less in TEB cells than in lobular cells. This is attributed to the shorter Go and Tc, and not to lack of reparative enzymes (114, 137, 138).
Pathogenesis Human Breast Tumors
The nature and site of origin of neoplastic growth in human breast has been the subject of numerous studies based on examination of surgical and autopsy tissues (10, 23, 24, 31,32, 47, 48, 68, 69, 70, 74, 93, 115, 121, 123, 146).
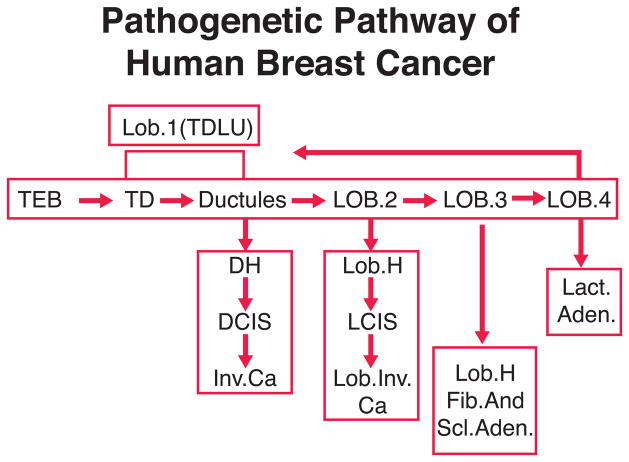
The TD or terminal duct is best regarded as having two parts. The first part is closest to the nipple and is called extra lobular or entering TD. The second part of the TD is referred to as the intralobular or axial TD. The ductules, together with the intralobular or axial TD, and the distinctive loose intralobular connective tissue, are the three recognized constituents of the lobule. The lobule type 1 (Figure 2), essentially synonymous with the terminal ductal lobular unit or TDLU, is the basic morphologic and functional microstructure of the mammary gland (2, 107, 148, 149). The preponderance of evidence indicates that most mammary carcinomas arise from lobules type 1 (TDLU) or their precursors (Figure 2). Lesions originated in lobules type 1 include at least: carcinoma in situ, the common infiltrating duct carcinomas, and perhaps some other kinds of infiltrating carcinomas. In contrast, it is thought that classical lobular carcinoma in situ may arise from the more differentiated lobules type 2 (Figure 2).
Figure 2.
Chart representing the pathogenetic pathway of human breast cancer. lesions, (Reprinted from Russo, J and Russo, IH, Cancer Epidemiol Biomarkers & Prev 3, 353–364, 1994, with permission).
Comparative aspects of pathogenesis
The TEB in the human female is a prepubertal structure and the biology and the differentiation of the structure from the prepubertal to the pubertal age needs to be studied. Most of the data collected for the study of mammary carcinogenesis are derived from observations of postpubertal breasts. In the postpubertal breast, most of the structures of the gland are mainly composed of TDs and ductules forming lobules type 1, which are the basic TDLU. The terminal ductal structures of the terminal ductal lobular unit originate preneoplastic lesions which evolve to ductal carcinoma in situ, progressing to invasive carcinoma and finally metastasize. The differentiation of lobules type 1 to type 2 is also important since morphologic observations suggest that lobules type 2 could be involved in the development of atypical lobules which originate lobular carcinoma in situ (Figure 2) and its invasive form.
Lobules type 3 present in the human breast seem to give origin to hyperplastic or hypersecretory lobules, fibroadenomas, sclerosing adenosis (Figure 2) and apocrine cysts, whereas lactating adenomas seem to originate from lobules type 4 (Figure 2). In the rat, these more benign lesions originate from virginal and mature lobules.
An important difference between the pathogenetic pathway in the rat and human is at the level of the TDLU. The TEB in the rat would be equivalent to the intralobular TD in the human, the area which is most susceptible to neoplastic growth. However, an important gap in our knowledge is the lack of observations of early carcinomas in the human breast, in order to determine whether they originate from more undifferentiated TEBs before the lobular elements have formed. Another possibility is that in humans, carcinogenesis takes place in more differentiated structures, or TDLU. The presence of lobules type 1 in the postmenopausal woman may explain the rising incidence of neoplasia in older women, however, this does not rule out the possibility that malignant transformation had occurred much earlier in life. Chemically induced mammary carcinogenesis requires active cell replication which is maximal in the TEB. In the human breast, the highest peak of cell replication occurs in the TD during early adulthood, decreasing considerably with age (107, 119). This observation indicates that during early adulthood, when cell turnover is more rapid, more chances exist for a neoplastic process to be initiated. Epidemiologic findings reveal that the young woman’s breast is more susceptible to ionizing radiation. This could be explained as a consequence of incomplete differentiation of the gland, meaning one having TDs with high replicative properties (17, 18, 106). These observations are supported by experimental data showing that the TDLU or lobule type 1 of the human female has the highest in vitro binding of carcinogen to DNA. When these structures are cultured and treated in vitro with carcinogens, they express phenotypical changes of transformation that are not expressed by lobules type 2 and 3 (111).
It has been shown that in human the usual ductal hyperplasia has few similarities to ADH, DCIS, or invasive cancer. ADH have many similarities to low-grade DCIS (16, 125, 129). In contrast, low-grade DCIS appears to be genetically distinct from high-grade DCIS (16,129). It seems that different forms of invasive breast cancer that develop mostly from different types of DCIS lesions, with low-grade DCIS lesions giving rise to low-grade invasive breast carcinomas, and high-grade DCIS lesions giving rise to high-grade invasive breast carcinomas (16, 77, 125, 129).
Comparative Histopathological Classification of Mammary Tumors between Human and Rat
The validity of rat mammary carcinogenesis for the study and understanding of the biology of human mammary carcinogenesis and for validating the development of strategies for breast cancer prevention and cure requires that in these two models, in addition to similarities in the basic pathogenesis and developmental concepts that: (a) the histotype of the tumors is similar; (b) that the cell types present in the rat react with similar immunocytochemical markers as do the human ones, and (c) that the biologic behavior of tumors is comparable in the two species. Rat mammary tumors have been classified by several authors (53, 107, 110, 116, 145). There is agreement that tumors appearing histologically malignant in the rat have features in common with the intraductal and infiltrating ductal carcinomas in humans, but few spontaneously metastasize. Similarly, benign fibroadenomas and adenomas that occur in rats closely resemble the human benign tumors.
The majority of induced tumors are papillomatous with hyperplastic epithelium containing both epithelial and myoepithelial cells, and appear cytologically benign, or they may be composed of epithelial cells with varying degrees of cytologic atypia, growing in solid, papillary adenomatous patterns. In most tumors, the abnormal epithelium tends to remain rigidly confined by the adjacent stroma, and shows no clear evidence of invasion. All of these features contribute to the difficulty of making clear distinctions between benign and malignant lesions. In Table 1 are shown the main histological types of mammary tumors found in the rat. It is clear that most of the lesions found in the rat mammary glands have their counterpart in human pathology. However, there are specific lesions in humans, such as Paget’s disease of the nipple and infiltrating ductal carcinoma, scirrhous, and medullary type that have not been reported in the rat mammary gland. Lobular carcinoma, in situ or invasive, has not been reported in the rat (116).
Table 1.
Classification of rat mammary gland tumors
|
THE ROLE OF AGING AND PARITY IN THE RAT AS DETERMINANTS OF A LOWER SUSCEPTIBILITY TO CANCER AND BENIGN LESIONS
The susceptibility of the mammary gland to carcinogenesis decreases significantly with age. This has been explained as a consequence of a decrease in the number of undifferentiated structures. It has been reported that mammary tumorigenesis by a carcinogen is inhibited in rats in which mammary growth has been pre-stimulated by hypothalamic lesions (27) or pituitary grafting (150), or when carcinogen is administered to lactating rats (30, 62). Decreased tumor incidence has also been observed when mice and rats (106, 109, 112) are inoculated with chemical carcinogens after pregnancy and lactation. In the rat (106) it has been demonstrated that the protective effect seen during pregnancy and lactation extends to the post-weaning period. Thus, administration of a single dose of DMBA to parous rats when the glands have regressed to a resting stage induces a much lower incidence of mammary carcinomas. This indicates that it is not the hormonal status of pregnancy and lactation by itself that protects the gland, but the permanent changes induced in the gland structure and in the biological properties of the gland epithelium which are independent of the hormonal status of the host (26). The degree of differentiation of the mammary gland can be described at a given time in the life span of the rat according to the number of TEB, TD, AB, and lobules, and their level of DNA synthesis and proliferative activity (107). A decrease in the density of TEB is observed with aging, and in the glands of multiparous rats, TEB are undetectable. Terminal ducts (TD) are the last generation of ducts which result from the lack of differentiation of TEB -> AB -> lobules. These TD share some features of the TEB, such as a high DNA labeling index. Pregnancy and lactation induce further differentiation of TD to AB and lobules, and as a consequence, TDs are rarely observed in the mammary gland of resting multiparous rats (107, 108, 114).
The highest incidence of tumors is observed in YV rats, and the lowest in multiparous rats. Histological evaluation of the tumors reveals that 100% of the tumors developed in YV rats are adenocarcinomas. In multiparous rats, only 21 % of tumors are carcinomas. In OV rats, 63% of the tumors are carcinomas. Numerous lesions induced by DMBA are of a benign nature. Thus, fibroadenomas are twice as frequent in multiparous rats as in age-matched OV rats (107, 114). However, YV and OV rats develop a higher incidence of HAN than multiparous rats. In addition, the incidence of adenomas and cysts is similar among YV and OV rats, but the incidence is much lower in the mammary gland of multiparous rats. In conclusion, the lower incidence of carcinomas in the gland of multiparous rats is related to the absence of TEB, and to the lower number of TDs with low DNA-LI.
The refractoriness of parous rats to carcinogenesis can, therefore, be explained as a consequence of a higher degree of differentiation of the gland after pregnancy and lactation. On the other hand, the higher susceptibility of the mammary gland of virgin rats is explained by the presence of TEB and TD with very high DNA-LI. However, OV rats have more tumors than multiparous rats, demonstrating that the lower susceptibility observed in multiparous rats is not due to age alone but to the higher degree of differentiation that pregnancy and lactation induces in the mammary gland (107, 114).
DIFFERENTIAL RESPONSE OF THE RAT AND HUMAN MAMMARY GLAND TO PROLACTIN
Prolactin effect on the stem cells of the rat mammary gland
Transgenic and gene disruption techniques have shown that complete prolactin (PRL) deficiency results in the arrest of mammary organogenesis at an immature pubertal state. In this arrested developmental state, the epithelial component of the gland consists of a basic ductal system and terminal end buds, but none of the lobuloalveolar system. PRL induces the differentiation and growth of alveolar progenitor cells from the ductal epithelium (25, 131). This development of alveoli from precursor cells in the ductal epithelium may involve both clonal growth from committed precursors and induction of phenotypic changes in cells that are near specialized “organizer” cells. In addition PRL is also an essential survival factor for lobuloalveolar cells during both pregnancy and lactation (6, 19, 63,140). Therefore local or systemic increase of PRL will maintain the lobular structures. The mammary gland is an important site of PRL synthesis and secretion. PRL is present in significant concentrations in milk (8, 67). It has been suggested that locally synthesized PRL in the mammary gland might act as a growth factor for both normal breast epithelium and breast cancer cells (28) and as a consequence the maintenance of the epithelial cell proliferation.
Prolactin effects in human mammary gland are restricted to the Lobule 4 and development towards lactation
During pregnancy, the lobuloalveolar epithelium undergoes extensive proliferation under the influence of PRL, Placenta lactogens (PLs), progesterone, and local growth factors such as RANK-ligand and IGF-2 (13, 61,135). Placental lactogens (PLs) are synthesized during pregnancy in most mammals. The major stimulus to mammary gland development during pregnancy is presumably PLs, rather than pituitary PRL (56). Loss of PLs and placental steroids at parturition is accompanied by elevation of pituitary PRL secretion, and a corresponding shift to pituitary-dominated regulation of mammary gland function during lactation. The importance of this shift is that pituitary PRL is strongly regulated by a suckling-induced neuroendocrine reflex, which allows nursing activity to determine directly the lactational stimulus to the mammary glands. During and after parturition, progesterone, estrogen, and placental lactogen fall precipitously, and PRL rises. The lobuloalveolar epithelium in the lobules type 3 and 4 are converted to a secretory phenotype. At the end of lactation, involution of the lobuloalveolar system occurs in response to milk stasis and falling systemic lactogens (124).
Therefore PRL and PLs, each of which binds to the PRL-R, act during three stages of mammary gland development: lobule budding during organogenesis, lobuloalveolar expansion during pregnancy, and lactational differentiation after parturition.
FIBROADENOMAS IN THE HUMAN BREAST AND IN THE RAT MAMMARY GLAND
Fibroadenomas in the human breast
Fibroadenoma is the most common benign tumor of the breast in young women in the childbearing period. Gradual enlargement over a period of months or years is the rule in early adolescence and in pregnancy. Married and unmarried women were affected in approximately equal numbers. Among the married women, those with children predominated in a ratio of two to one.
Fibroadenoma is a benign, confined tumor of the breast that has a mixture of glandular and mesenchymal elements. Fibroadenomas that are allowed to grow after initial detection usually cease to grow when they reach 2 to 3cm in diameter (55). Blacks more commonly develop fibroadenomas compared with whites and at a younger age as well. Fibroadenomas in blacks are also more likely to recur. Infarcts of the breast may occur during pregnancy or lactation with a resultant discrete mass. Fibroadenoma is the most common benign tumor of the breast in young women in the childbearing period; however, more rapid growth may occur during menopause. The gross appearance of fibroadenomas (FA) is usually characteristic and often diagnostic by its sharp circumscription and smooth boundary with surrounding breast tissue. The cut surface is white, and the epithelial elements are light brown areas. The fibro-adenoma is usually a solitary lesion, but in few cases multiple tumors are present and the interval between the excision of a previous tumor and the second mass vary from two to 25 years. The tumor occurs most often in the outer and upper quadrant. The upper half of the breast is more frequently affected than the lower and the outer more often than the inner portion. The sizes vary but more often is between 2 and 5 cm. in diameter (55).
Cysts from 1 to several mm. in diameter may be found in older growths containing also hard, hyalinized fibrous tissue with occasional areas of calcification. In the majority of fibroadenomas, the number and the size of the ducts are increased. Approximately 1 of 200 fibroadenomas show infarction (152).
Microscopically, fibrous tissue composes most of the fibroadenoma (FA); either the stroma may surround and easily definable duct like epithelial structures pattern known as pericanalicular, or the epithelium may be stretched into curvilinear arrangements known as intracanalicular. Smooth muscle is an extremely rare component of fibroadenomas (52). The epithelium within an FA may have the same appearance as elsewhere in the breast, including apocrine metaplasia (5). Rarely, squamous metaplasia is present (122).
The epithelia forms tubules that are composed of cuboidal or low columnar cells with round uniform nuclei resting on a myoepithelial cell layer. The stroma is made up of loose connective tissue rich in acid mucopolysaccharides, but it may be partially or totally composed of a dense fibrous type. The spindle cells are predominantly CD34-positive fibroblasts, admixed with scattered Factor XIIIa-positive cells (89, 128). The cellularity of the stroma varies from case to case and the alternative diagnosis of phylloides tumor should be considered.
Fibroadenomas often resemble the normal mammary tissue of adolescence. The hypertrophy of duct epithelium, scarcity of mammary lobules and the increase in pale-staining periductal connective tissue found in adolescence are also characteristic of these new growths. The ordinary FA may be looked upon as an exaggerated form of puberty hypertrophy affecting an isolated portion of the breast. The accuracy of this interpretation can be proved experimentally. Typical benign FA occurs in the breast of rats and rabbits that have been continuously stimulated with increased amounts of estrogenic hormone.
Ultrastructurally, the most interesting feature of FAs is the constant presence of a multilayered basal lamina around the epithelial and endothelial cells (21). The stromal cells have features of fibroblasts (102). Fibroadenomas contain progesterone receptors almost universally, and estrogen receptors in approximately one fourth of the cases (143). Approximately 20% of fibroadenomas have been found to have clonal chromosome aberrations suggesting that fibroadenoma is a benign neoplasm of the specialized stroma of the breast with an accompanying epithelial component (43).
According to Rosai (103) the morphologic variations in FA are plentiful, and among them are hyalinization, calcification, and/or ossification of the stroma. Presence in the stroma of multmucleated giant cells of reactive nature can also be found (9). Presence in the stroma of mature adipose tissue, smooth muscle, or metaplastic cartilage has been reported, (4, 52, 86, 90, 96, 127) as well as prominent myxoid changes (20). Sclerosing adenosis occurs in less than 10% of cases (5). Lactational changes are manifested by an increase in the amount of cytoplasm in the epithelial cells, which appear vacuolated, and by dilatation of the glandular lumina by secretion.
There is a distinct type of FA that tends to occur in adolescents (often in blacks and sometimes involving both breasts), reach a large size (over 10 cm), and show hypercellularity of glands and/or stroma (33, 38, 40, 87, 98). No differences have been found in fibroadenomas removed from patients taking oral contraceptives and those in control cases, except for the occasional formation of acini in the former (39).
A variant of fibroadenoma is the tubular adenoma (59). Grossly tubular adenomas have a fine nodularity and the tubular structures are seen but there is not lobular configuration is evident. Tubular adenomas may have evidence of secretory activity, but when not occurring in association with pregnancy or lactation should not be termed “lactating adenomas.” Lactating adenomas are certainly analogous in some ways to tubular adenomas, and may represent a physiologic response of the tubular adenoma to pregnancy. In addition to showing lactational changes, the adenomas presenting in pregnancy have a more evident lobular anatomy than that seen in most tubular adenomas.
Traditionally, the risk for subsequent carcinoma in patients with typical fibroadenoma has not been considered to be higher than for the general population (44, 97). Malignant changes in fibroadenomas are found in only 0.1 % of cases (51, 85, 97). They usually involve the epithelial component, and the large majority are in situ lesions (35,44, 97). As well described by Rosai (103) “in some cases the malignant tumor is entirely within the confines of the FA, but in others it involves the surrounding breast as well”. The latter may simply represent extension into the FA by a carcinoma originating elsewhere in the breast. In a series of 105 fibroadenomas containing carcinoma, 95% of the cases were in situ lesions, and lobular and ductal types occurred with equal frequency. Nine of ten fibroadenomas harboring an invasive carcinoma also contained carcinoma in situ (CIS), supporting the origin of the invasive component in the fibroadenoma. CIS within the fibroadenoma was associated with CIS in the surrounding breast in 21% of the cases (44). A large-scale epidemiologic study reported by Dupont and Page (36) concluded that FA represents a low long-term risk for breast carcinomas and that this risk is increased in women with complex fibroadenomas, ductal hyperplasias, or a family history of breast carcinoma. This risk is not further increased if the fibroadenoma contains foci of atypical epithelial hyperplasia (22). Sarcomatous transformation of the stroma of a fibroadenoma is an even rarer phenomenon (29).
Fibroadenomas in the rat mammary gland
The incidence of spontaneous mammary tumors in several different strains and stocks of female rats have been published (145) ranging in duration from 18 months to the natural life span of the rats (Tables 2 and 3). In general, the mean incidence of fibroadenoma in females of most rat strains varies from approximately 20% to 40%. On average, fibroadenomas occur more commonly in Sprague-Dawley rats than in other strains with values as high as 68% (145) an instead in the ACI/N rats with the lowest incidence (4.8%) (79).
Table 2.
Differential diagnosis of terminal end bud (TEB), intraductal proliferation (IDP), and carcinoma in situ (CIS)
| Tissue Components | TEB | IDP | CIS |
|---|---|---|---|
| Basement membrane | Present | Present | Present |
| Periductal stroma | Normal | Moderate desmoplastic reaction | Marked desmoplastic reaction |
| Inflammatory reaction | Absent | Moderate | Marked |
| Luminal border | Smooth | Serrated | Irregular |
| Secondary luminal | Absent | Absent | Present in cribriform pattern |
| Micropapillae | Absent | Some | May be prominent |
| Epithelium | Heterogeneous three cell types | Predominance of one cell type | Predominance of one cell type |
| Mitoses | Numerous | Numerous | Numerous |
Table 3.
Incidence of Invasive Rat Mammary Cancer Induced by DMBA
| Histological type | Number of cases | Incidence |
|---|---|---|
| Invasive Papillary Adeno carcinoma Type I | 35 | 5.99 |
| Invasive Papillary Adeno carcinoma Type II | 152 | 26.02 |
| Invasive Papillary Adeno carcinoma Type I + II | 136 | 23.10 |
| Invasive Cribriforme carcinoma | 158 | 27.05 |
| Invasive Comedo carcinoma | 5 | 0.95 |
| Invasive Tubular Carcinoma | 39 | 6.67 |
| Adenoidcystic carcinoma | 59 | 10.10 |
| Total number of cancer studied | 584 | 100.00 |
As in humans, spontaneous mammary tumors in rats occur far more commonly in females than in males. Mammary tumors have been observed in virtually every strain, although none were observed in males of some strains of rats (15). Fibroadenomas are found in approximately 1–2%. of male rats, regardless of strain, although incidences as high as 11% have been reported in Sprague-Dawley (3) and Wistar (72) rats. In a full life-span study in F344 rats, 13.4% of the males had either a fibroadenoma or an adenoma (133).
Mammary tumors, like most other neoplasms in the rat, occur with increasing frequency as the animal ages (15, 133). The prevalence of mammary tumors, regardless of type, is essentially 100% in female F344 rats older than 137 weeks (133) and in female Sprague-Dawley rats between 118 and 130 weeks of age (145). Although the age-specific prevalence rates of fibroadenomas and adenocarcinomas in these two strains continued to increase with increasing age, Burek (15) found that in WAG/Rij and BN/BiRij rats only adenocarcinomas continued to increase in each age group. Fibroadenomas, however, had a peak risk period between 31 and 36 months of age, and their incidence decreased in older rats. The age-specific prevalence rate of both fibroadenomas and adenocarcinomas decreased slightly during an earlier age period in both F344 (85–97 weeks) and Sprague Dawley (53–65 weeks) rats (133).
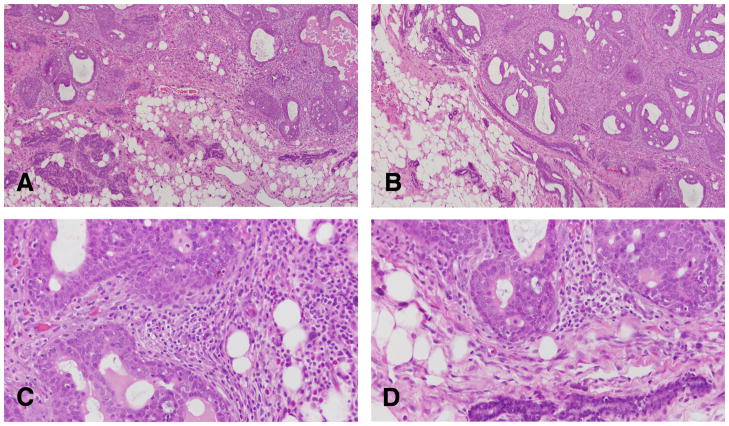
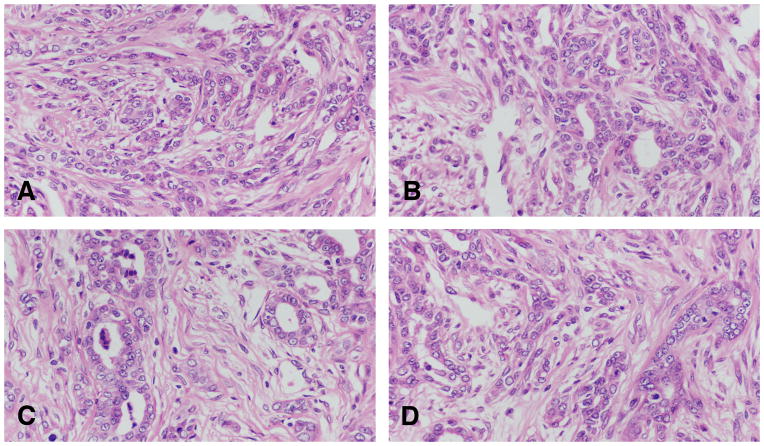
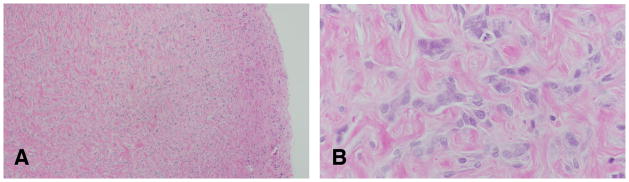
As in the human, the fibroadenoma in the rat mammary gland is a benign tumor composed of both well-differentiated epithelial and fibrous connective tissue (Figure 3). Fibroadenomas may range in size from a few millimeters to 8 cm or more in diameter. As described by van Zwieten (145) these tumors are spherical or discoid, smooth or slightly bosselated masses that are freely movable in the subcutis. Their consistency can be firm and tough or soft and rubbery, and the cut surface is generally white or pink and has a distinctive lobulated pattern. The histological appearance of fibroadenomas can vary depending on the relative proportions of epithelium and fibrous connective tissue (Compare Figures 3A and B with Figures 4A,B,C and D). Thus, fibroadenomas can range from those composed predominantly of epithelial tissue to those composed predominantly of connective tissue. The alveolar structures in some fibroadenomas may be widely distended with secretion (Figures 4A,B,C and D). Some fibroadenomas contain multiple foci of epithelium, showing unusual growth patterns with cellular pleomorphism and atypia. These foci can range from small papillary projections within the ductules in some tumors to solid, cribriform, or papillary epithelial formations within clusters or distended ductules in others. Occasionally, adenocarcinomas arise within fibroadenomas, suggesting that the smaller foci of cellular atypia may represent an early premalignant change (145).
Figure 3.
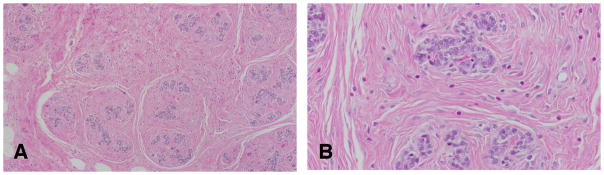
A: The fibroadenoma is a benign tumor composed of both well-differentiated epithelial and fibrous connective tissue. B: Glandular epithelial cells surrounded by dense connective tissue. Stained with H&E, ×4 and ×40 respectively.
Figure 4.
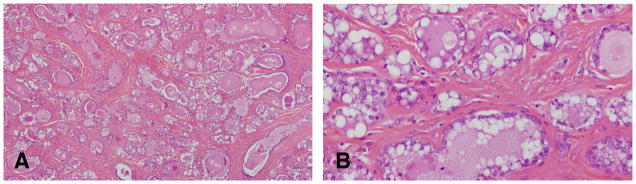
A: Fibroadenoma composed predominantly of alveolar structures distended with secretion. B: the epithelial cells are secreting lipids and proteinaceous material. Stained with H&E, ×4 and ×40 respectively.
Experimental induction of Fibroadenomas using hormones
The rapid growth of a preexisting fibroadenoma during pregnancy indicates a special responsiveness to estrogen. The increase in the number of ducts, in their lining cells and in the amount of periductal fibrous tissue found in growing fibro-adenomas duplicates the histologic pattern of rapid mammary growth in normal adolescence and in early pregnancy. This pattern is repeated when the mammary gland of the rat or monkey or human is stimulated with high doses of estrogen (50). Estrogen in combination with gonadotropic hormone increased the number of successfully transplanted fibroadenomas in rats and stimulated hyperplasia in these growths (58). Geschickter (49) produced fibroadenomas in the mammary glands of rats by implanting pellets of estrone under the skin to provide a constant, intense estrogenic stimulation. He also found that a constant absorption for many months of excessive amounts of estrogen leads to the formation of fibroadenomas, however, fluctuations of the concentration of estrogen induced large cysts but no fibroadenomas. This corroborates the clinical observation that fibroadenomas have a tendency to form during adolescence, pregnancy and the menopause when a relatively constant estrogenic stimulation is maintained, rather than in cyclic women where there is a repeated rise and fall in the level of the hormone (49).
Experimental induction of fibroadenomas using chemical carcinogens
The two most widely used experimental systems for the study of mammary tumorigenesis are the models in which tumors are induced in the Sprague-Dawley (S-D) rat by 7,12-dimethylbenz (a) anthracene (DMBA) or in the S-D or Fischer 344 rat by N-methylnitrosourea (NMU). DMBA, given by gavage in a single dose of 2.5 to 20 mg induces tumors with latencies that generally range between 8 and 21 weeks with and final tumor incidences close to 100% if sufficient time elapses before necropsy (110). NMU, given by intravenous or subcutaneous injection in a single dose of 25 or 50 mg/kg body weight yields tumors with similar latency and incidence (110).
Tumor latency is, in general, inversely related to carcinogen dose, whereas both tumor incidence and number are directly related, if relatively early end points are used. For the study of modulating factors, tumor latency is often the most sensitive end point. Tumor histology is influenced by carcinogen dose. In S-D rats given a single NMU dose of 10 mg/kg body weight, 42% of tumors were malignant, whereas 86 to 94% were malignant at doses from 35 to 50 mg/kg (83). Tumor incidence, number of malignant tumors/rat and latency all showed a dose response, but number of benign tumors/rat did not (83).
The susceptibility of the mammary gland to DMBA or NMU-induced carcinogenesis is strongly age-dependent and is maximal when the carcinogens are administered to animals between the ages of approximately 45 and 60 days, that is the age of sexual maturity. Active organogenesis and high rate of proliferation of the glandular epithelium are characteristics of that period (54, 84, 104, 112, 113). DMBA activation in the gland is also high, but it may not be a significant factor, since NMU, which is similarly most effective at that age, does not require activation. The age-related changes in susceptibility are independent of dietary fat content; increased tumorigenesis occurs in rats fed high-fat diet at all ages tested. In virgin rats treated with DMBA, tumors that develop are largely carcinomas, although the proportion can be altered by carcinogen dose and dietary fat. The administration of DMBA to virgin rats of different ages induces tumors with an incidence which is directly proportional to the density of highly proliferating TEBs (112). A 100% incidence of carcinomas is obtained when DMBA is administered to rats aged 30 to 55 days, but the highest number of tumors/animal is observed when the carcinogen is given to animals when they are 40 to 46 days of age, a period when TEBs are most actively differentiating into ABs. The sharp decrease in the number of TEBs observed in animals older than 55 days is also accompanied by a lower incidence of tumors as well as a lower number of tumors/animal (112, 113,114, 119).
GENETIC INFLUENCES IN RAT MAMMARY TUMORIGENESIS
Genetic factors control the susceptibility of different rat strains to DMBA or NMU mammary tumorigenesis. Of the commonly used strains, SD and Wistar-Furth are the most susceptible; Fischer 344 and ACI rats show intermediate susceptibility, and Copenhagen rats are essentially completely resistant even to direct application of DMBA to the gland (64). Copenhagen rats did develop fibrosarcomas in response to parenteral DMBA. In contrast, tumor induction by diethylstilbestrol (DES) is demonstrable in the ACI but not in the SD strain of rats, although a co-carcinogenic effect of DES with DMBA can be shown in SD rats (11, 105). Both malignant and benign tumors are increased by the combined treatment, but there is a relatively greater increase in benign tumors. In extensive analyses comparing DMBA tumorigenesis, mammary gland growth rate, serum hormone levels, and DMBA:toxicokinetics in female rats of several strains and F1 hybrids between the strains, Isaacs (64, 65, 145) found no major difference that correlated with susceptibility to tumorigenesis. Transplantation studies demonstrated that inherent characteristics of the gland and not of the host animal determine response to DMBA. Glands from rats from a resistant or a susceptible strain were transplanted into F1 hybrids between the 2 strains and directly exposed to DMBA. While similar percentages of glands from the two strains developed malignant changes (60% in resistant, 80% in susceptible), macroscopically detectable tumors developed in 70% of susceptible and only 10% of resistant glands. The result clearly suggests that genetic factors govern the progression from microscopic to macroscopic tumor rather than from normal to histologically malignant epithelium (65).
RELEVANCE OF DUCTAL CARCINOMA IN SITU IN THE RAT TO HUMAN DUCTAL CARCINOMA IN SITU
Morphological features of DCIS in the rat
The earliest change observed in the mammary parenchyma after carcinogen treatment of virgin rats is the dilation of terminal ductal structures, namely the TEBs. They exhibit thickening of the epithelial lining, which may be up to six layers thick. These cells have a large, round nucleus, prominent nucleolus and coarse chromatin along the inner leaflet of the nuclear membrane. These early lesions called intraductal proliferations (IDPs) (107, 117) (Figures 5 and 6) represent the transition between the normal TEB (108, 110, 117, 119, 120) and carcinoma in situ; the criteria for identifying these three types of structures are outlined in Table 2 (108, 110 117, 119, 120). IDPs appear to evolve into carcinoma in situ through the development of: (a) micropapillae, which might be the only pattern present or combined with cribriform pattern; (b) pseudolumina, forming a cribriform pattern, or (c) a comedo pattern. A progressively increasing desmoplastic reaction in the stroma surrounding the transformed ductal structures is a hallmark of neoplastic progression.
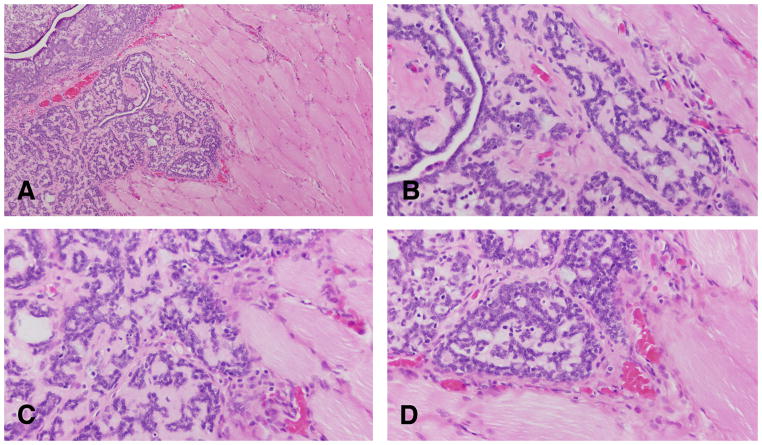
Figure 5.
The earliest change observed in the mammary parenchyma after carcinogen treatment of virgin rats is the dilation of terminal ductal structures, namely the TEBs forming the intraductal proliferation or IDP (A, B, C and D). They exhibit thickening of the epithelial lining, which may be up to six layers thick. These cells have a large, round nucleus, prominent nucleolus and coarse chromatin along the inner leaflet of the nuclear membrane (B,C and D). Stained with H&E, ×20.
Figure 6.
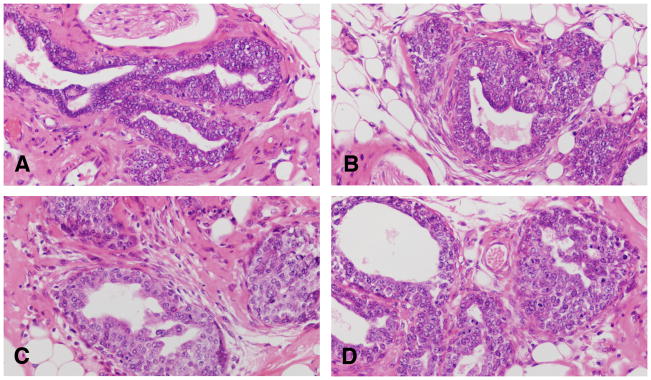
A and B: The IDPs are early lesions that represent the transition between the normal TEB and carcinoma in situ. C and D: IDPs appear to evolve into carcinoma in situ through the development of: (a) micropapillae, which might be the only pattern present or combined with cribriform pattern; (b) pseudolumina, forming a cribriform pattern, or (c) a comedo pattern. A progressively increasing desmoplastic reaction in the stroma surrounding the transformed ductal structures is a hallmark of neoplastic progression. Stained with H&E, ×20.
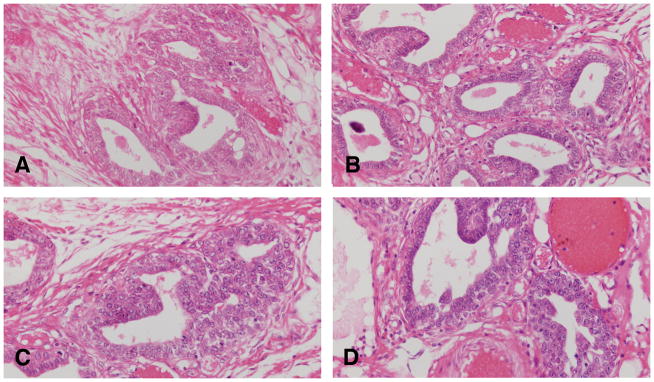
In the intraductal papillary carcinoma (Figure 7A and B), the ductal structures are dilated, and the lining epithelium grows inward, forming epithelial papillae devoid of fibrovascular core. Most of the epithelial cell population is uniform in size and shape. Mitotic figures are often found. The stroma, which is separated from the epithelium by a well-defined basement membrane, exhibits a slight to marked desmoplastic reaction, with replacement of fat by fibroblasts, and infiltration by lymphocytes and mast cells.
Figure 7.
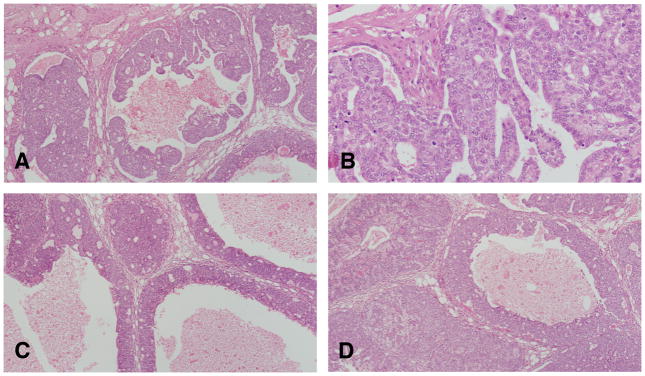
A and B: In the intraductal papillary carcinoma the ductal structures are dilated, and the lining epithelium grows inward, forming epithelial papillae devoid of fibrovascular core. Most of the epithelial cell population is uniform in size and shape. Mitotic figures are often found. The stroma, which is separated from the epithelium by a well-defined basement membrane, exhibits a slight to marked desmoplastic reaction, with replacement of fat by fibroblasts, and infiltration by lymphocytes and mast cells. C and D: The intraductal cribriform carcinoma is the result of epithelial cell proliferation in a solid pattern with formation of’ secondary lumina. The tumors are cytologically similar to papillary carcinomas and like them; they elicit a stromal reaction and lymphocytic infiltration. Stained with H&E, ×20.
The intraductal cribriform carcinoma is the result of epithelial cell proliferation in a solid pattern with formation of’ secondary lumina (Figure 7C and D). The tumors are cytologically similar to papillary carcinomas and like them; they elicit a stromal reaction and lymphocytic infiltration.
The intraductal ductal comedocarcinoma is characterized by intraductal growth of epithelium and accumulation of necrotic cellular debris in the lumen). This necrotic material is similar to the one observed in the invasive comedocarcinoma (Figure 8). The surrounding stroma may exhibit a marked desmoplastic reaction. Comedo and cribriform patterns may be present simultaneously in the same tumor. Less frequently a papillary component is present as well.
Figure 8.
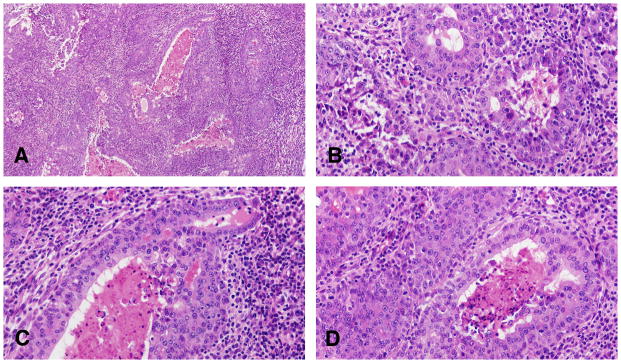
A: The invasive comedocarcinoma is characterized by intraductal growth of epithelium and accumulation of necrotic cellular debris in the lumen Stained with H&E, ×4, B, C and D: invasive comedo carcinoma containing the necrotic material similar to the one observed in the non invasive comedocarcinoma. The surrounding stroma may exhibit a marked desmoplastic reaction as seem in B, C and D. The invasive comedo carcinomas appear as distended ductal structures lined by a multilayered epithelium surrounding necrotic debris. Invasion occurs as an extension of duct-like structures or sheets of epithelial cells arranged in a serpiginous pattern into a stroma in which desmoplastic reaction and inflammatory cell infiltration may occur. Individual neoplastic cells are pleomorphic. Some of these tumors resemble the comedo carcinoma of the human breast. Stained with H&E, ×40.
Human ductal carcinoma in situ or DCIS
DCIS constitutes 30% to 40% of the breast cancer cases diagnosed mammographically, with one case of DCIS detected in every 1300 screening mammograms (130). The architectural subtypes of DCIS are very similar to those described for the rat. However, for clinical purposes they have been divided into noncomedo and comedo subtypes; noncomedo subtypes were further subdivided into cribriform, micropapillary, solid and papillary, while the comedo subtype was defined by high-grade cells, prominent central necrosis, and associated pleomorphic microcalcifications (42, 139). Additional classification systems have been proposed (37, 60, 126) accounting the degree of atypia of the nuclei that correlates with clinical outcomes (37, 60,). In this system, the nuclear grade of the DCIS lesions is defined as low grade (grade 1), intermediate grade (grade 2), and high grade (grade 3) (73). In the rat most of the nuclei are grade 1 or 2 and rarely grade 3 are observed. Other features like the size (73) and the margins (57, 125, 136) that are important prognostic parameters in human are not relevant in the rat mammary pathology.
Differential diagnosis
In the human as well as in the rat the lesions that needs to be differentiated with DCIS is the atypical ductal hyperplasia (ADH) (91, 92, 125) and occasionally must be differentiated from invasive carcinomas; a frequent such mimicker is invasive cribriform carcinoma that needs to be distinguished mostly from cribriform DCIS (41, 81, 154). The criteria listed in Table 6 can be use in the rat and to certain extend in the human lesions. An elegant review of DCIS in human has been published (130) indicating the most important concerns in the differential diagnosis of DCIS in the human breast are microinvasion around DCIS lesions, extension of a DCIS lesion into adjacent benign structures or foci of sclerosing adenosis, the presence of foci of lymphatic/vascular invasion mimic DCIS lesions and the comedo-type DCIS lesions often need to be distinguished from the pleomorphic subtype of lobular carcinoma in situ (LCIS) lesions (46, 66, 132).
Table 6.
Differential diagnosis of tumoral masses developing in mammary regions
| Structure or lesion | Main features |
|---|---|
| Lactating gland | Enlargement of the gland, uniformity affects all the pairs of glands |
| Salivary gland hyperplasia | Submandibular location; acini with serous or mucinous type epithelium |
| Clitoridal gland hyperplasia | Prepubic location; uniformly large cells with round, leptochromatic nuclei arranged in acini |
| Lymph nodes | Normal or reactive, specific locations, characteristic architecture |
| Abscesses | Encapsulated, tense, or fluctuant. Numerous polymorphonuclear leukocytes |
| Skin and adnexal tumors | Basal and squamous cell carcinomas, trichoepithelioma, trichilemmoma, sebaceous adenoma and carcinoma, fibroma, fibrosarcoma |
| Hibernomas | Typical architecture with numerous fat droplets of varying sizes |
INVASIVE CARCINOMA IN THE RAT VERSUS THE HUMAN
Histopathology of invasive carcinoma in the rat mammary gland
The diagnosis of invasive carcinomas is based upon the presence of unequivocal growth of malignant epithelial cells into the adjacent stroma. The presence of invasion can be difficult to judge because under normal conditions the mammary gland ducts grow diffusely into the fat pad, adjacent muscle, and subcutaneous tissue of the skin. In table 1 are depicted the subtypes of invasive lesions found in the rat mammary gland. In Table 3 is the histological type more frequently found in a large series of 584 tumors induced by DMBA in the rat mammary gland in our laboratory at the FCCC.
The invasive papillary carcinomas are the most typical and frequent of the 7,12-dimethylbenz[a]anthracene (DMBA)- and N-methyl-N-nitrosourea (NMU)-induced tumors in the rat (107) (Table 3). Most of the tumors are detectable by palpation; they efface the normal architecture of the gland, invading surrounding structures. When they invade the skin they ulcerate and undergo local necrosis. Papillary carcinomas contain delicate fibrovascular cores, often heavily infiltrated by lymphocytes and mast cells. The fibrovascular cores are considerably thinner than those seen in intraductal papillomas. On top of the fibrovascular core grows the epithelium, which depending upon its thickness and cytologic characteristics allows one to classify these lesions into grade 1 or grade 2 (Table 4). Papillary carcinomas grade 1 are composed of 1–2 layers of epithelial cells, which in turn emit short epithelial papillae devoid of fibrovascular cores (Figures 9 and 10). This type of tumors comprises 5.91% of all the invasive cancers (Table 3). The papillary carcinoma grade 2 is also formed by papillary projections; however, the cores of connective tissue are sparser than those observed in the papillary carcinoma grade 1, and the secondary projections (papillae) of epithelium are solid clusters of cells (Figure 11). The luminal borders of these cell projections are in some occasion serrated, whereas those present in papillary carcinomas grade 1 are smooth (Table 4). The epithelial cells in this tumor type are slightly more pleomorphic than those of the papillary carcinoma grade 1. In Table 4 are depicted the basic histological and cytological differences between these tumor subtypes. Occasionally, in papillary carcinomas grade 2, the luminal spaces may become dilated or cystic; these lesions are called cystic papillary carcinomas. They may well represent a further evolution of a papillary or cribriform type. The papillary Carcinoma type 2 represents 26.05% of the invasive carcinoma and 23.10% are mixed with papillary carcinoma type 1 (Table 3).
Table 4.
Differential diagnosis between papillary carcinoma grade 1 and grade 2
| Components | Papillary Carcinoma | |
|---|---|---|
| Grade 1 | Grade 2 | |
| Fibrovascular core | prominent | sparse |
| Epithelium | 1–3 layer thick | 5–10 layer thick |
| Micropapillae | present | present |
| Luminal border | smooth | serrated |
| Cytological characteristics | moderate pleomorphism | marked pleomorphism |
| Nucleolus | inconspicuous | prominent |
| Mitoses | scarce | numerous |
Figure 9.
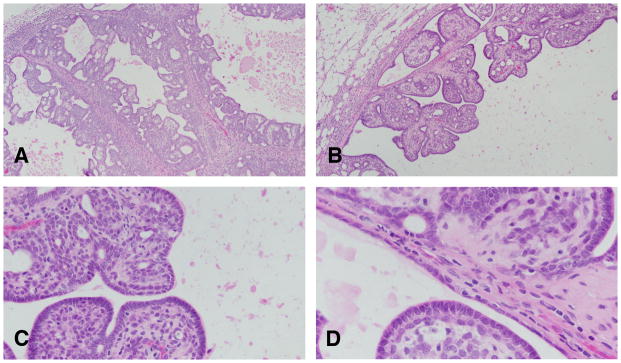
A: Invasive papillary carcinomas type 1 contain delicate fibrovascular cores, often heavily infiltrated by lymphocytes and mast cells. Stained with H&E, ×4. B: The fibrovascular cores are considerably thinner than those seen in intraductal papillomas. Stained with H&E, ×10. C and D: On top of the fibrovascular core grows the epithelium, which are composed of 1–2 layers of epithelial cells, which in turn emit short epithelial papillae devoid of fibrovascular cores. Stained with H&E, ×40.
Figure 10.
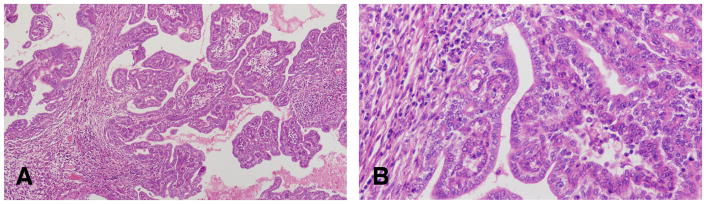
A and B: The luminal borders of these cell present in papillary carcinomas grade 1 are smooth. The epithelial cells in this tumor type are less pleomorphic than those of the papillary carcinoma grade 2. Stained with H&E, ×4 and ×40 respectively.
Figure 11.
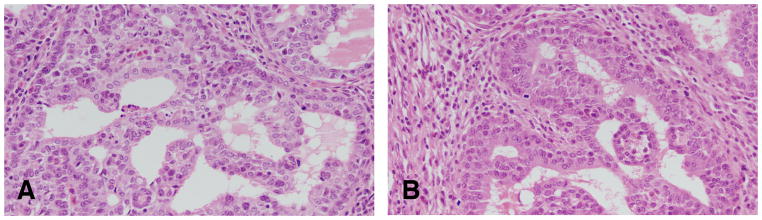
A and B: Papillary carcinoma grade 2 is formed by papillary projections; however, the cores of connective tissue are more sparse than those observed in the papillary carcinoma grade 1, and the secondary projections (papillae) of epithelium are solid clusters of cells. The luminal borders of these cell projections are in some occasion serrated,. The epithelial cells in this tumor type are pleomorphic, Stained with H&E, ×40.
The invasive cribriform carcinoma (Figures 12–16) exhibits the same cellular arrangement as the in situ lesions in which the solid sheets of neoplastic epithelial cells are interrupted by round or irregularly shaped secondary lumina of variable size. Invasion is characterized by penetration of haphazardly arranged, finger-like projections of epithelium into the surrounding stroma (Figures 15 and 16). The cribriform pattern may be maintained even in small clusters of cells infiltrating the dermis, skeletal muscle, and connective tissue, as well as in metastatic lesions (Figures 16A and B). Individual neoplastic cells are moderately to markedly pleomorphic. The degree of pleomorphism varies from tumor to tumor and even in different areas of the same tumor. Interestingly enough, even in the most pleomorphic tumors the glandular pattern is still present, with secretory material within the newly formed lumina (Figure 14). The infiltrating neoplastic cells are in general surrounded by a connective tissue that exhibits a marked desmoplastic reaction, with heavy lymphocytic and mast cell infiltrations (Figures 12 and 13). The cribriform type of tumor appears in general as a uniform pattern, but it may be associated with papillary or comedo patterns in the same tumor. The invasive cribriform carcinomas represent 27.05% of the mammary cancers in the rat (Table 3).
Figure 12.
A and B: The invasive cribriform carcinoma exhibits the same cellular arrangement as the in situ lesions in which the solid sheets of neoplastic epithelial cells are interrupted by round or irregularly shaped secondary lumina of variable size. Stained with H&E, ×4. C and D: the cluster of neoplastic cells invading the stroma are surrounded by a profuse inflammatory response. Stained with H&E, ×20.
Figure 16.
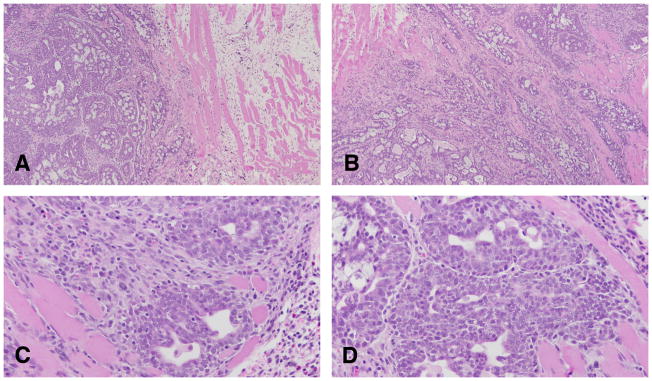
A and B: The infiltrating neoplastic cells are, in general, surrounded by a connective tissue that exhibits a marked desmoplastic reaction, with heavy lymphocytic and mast cell infiltrations. The cribriform type of tumor appears, in general, as a uniform pattern, but it may be associated with papillary or comedo patterns in the same tumor. Stained with H&E, ×40.×4. C and D: The tumor cells are surrounded by lymphocytes in the invasive areas to the muscle wall. Stained with H&E, ×40.
Figure 15.
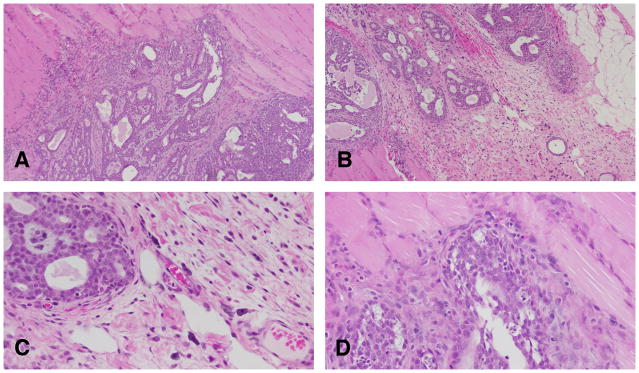
A and B: Cribriform carcinoma invading the muscle wall. Stained with H&E, ×4. C and D: Neoplastic cells are moderately to markedly pleomorphic. The degree of pleomorphism varies from tumor to tumor and even in different areas of the same tumor. Stained with H&E, ×40.
Figure 14.
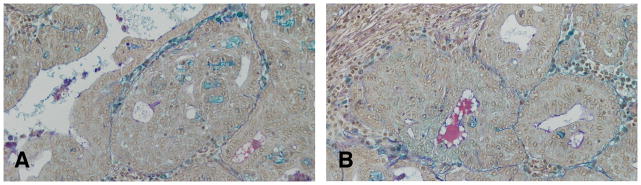
A and B: In the invasive cribriform carcinoma even in the most pleomorphic tumors, the glandular pattern is still present, with secretory material within the newly formed lumina. Formation of intercellular spaces shows Alcian blue and PAS positive reaction identified as proteoglycans. Deposition of proteoglycans around a carcinoma in situ, and around mast cellsis also observed. Stained with Alcian blue and PAS, ×10.
Figure 13.
A and B: In the invasive cribriform the Invasion is characterized by penetration of haphazardly arranged, finger-like projections of epithelium into the surrounding stroma. B: The cribriform pattern may be maintained even in small clusters of cells infiltrating the dermis, skeletal muscle, and connective tissue, as well as in metastatic lesions. Stained with H&E, ×4 and ×40 respectively..
The invasive comedo carcinomas (Figure 8) represents only 0.95% of the invasive cancer and are more frequently found in animals that have been treated with chemical carcinogens at a young age. The lesions appear as distended ductal structures lined by a multilayered epithelium surrounding necrotic debris. Invasion occurs as an extension of duct-like structures or sheets of epithelial cells arranged in a serpiginous pattern into a stroma in which desmoplastic reaction and inflammatory cell infiltration may occur. Individual neoplastic cells are pleomorphic. Some of these tumors resemble the comedo carcinoma of the human breast (Figures 8B, C and D) (41).
The invasive tubular carcinomas are composed of tubular or alveolar structures but are more irregular in the invasive areas forming distorted glandular structures in the areas of invasion surrounded by desmoplastic reaction (Figure 17). Some of them present scanty secretory material. These lesions could be derived from adenomas,. Several transitional steps between adenomas and fully manifest carcinomas are found, which in some cases makes it difficult to differentiate one from the other. Tubular carcinomas, however, differ cytologically enough from tubular adenomas to allow their identification (Table 5). The epithelial cells composing the tubular carcinoma have increased nuclear size, and the nuclei contain prominent nucleoli; tubular adenomas (Figure 17), on the other hand, have cells with smaller nuclei, and nucleoli are absent or inconspicuous. Tubular carcinomas represent 6.67% of he invasive lesions in the rat mammary gland (Table 3).
Figure 17.
A,B,C and D: The invasive tubular carcinomas are composed of tubular or alveolar structures but are more irregular in the invasive areas forming distorted glandular structures in the areas of invasion surrounded by desmoplastic reaction. The epithelial cells composing the tubular carcinoma have increased nuclear size, and the nuclei contain prominent nucleoli. Stained with H&E, ×40.
Table 5.
Differential diagnosis between tubular adenomas and tubular adenocarcinomas
| Components | Tubular adenoma | Tubular carcinoma |
|---|---|---|
| Tubular structure | Present | Present |
| Lumen | Prominent | Present or absent |
| Secretion in lumen | Present | Present, in some cases prominent |
| Epithelium | Three cell types | Almost always one cell type |
| Cell morphology | Cuboidal, polarity preserved | Pleomorphic, loss of polarity |
| Nuclear features | Round shape, small nucleus | Oval, enlarged, prominent nucleus |
| Stroma | Scanty, scarce | Scanty or absent |
Ten percent of the invasive cancers in the rat mammary glands are the adenoid cystic type that (Figure 18) is characterized by a biphasic cellular pattern of myoepithelial and epithelial cells surrounded by a lightly eosinophilic stroma. In the human is a rare basal- like breast cancer (71, 82, 147). In the rat this tumor has the same markers as the luminal type
Figure 18.
A: Invasive adenoid cystic type stained with H&E, ×4. B,C and D: The tumor is characterized by a biphasic cellular pattern of myoepithelial and epithelial cells surrounded by a lightly eosinophilic stroma. Stained with H&E, ×40.
STROMAL NEOPLASMS
The stromal tumors in the rat are very similar to those described in human (Table 1) among them are fibromas well-circumscribed and non-encapsulated tumors composed of proliferating fibroblasts arranged in interlacing bundles and embedded in variable amounts of collagen fibers. In some tumors there are isolated remnants of glandular epithelium, which suggests that they originate from fibroadenomas (Figure 19). The malignant counterpart is the fibrosarcoma composed of malignant fibroblasts exhibiting the expected anaplastic characteristics and increased number of mitoses are the essential components of fibrosarcomas.
Figure 19.
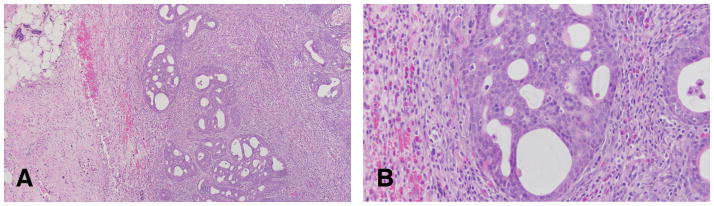
A: Fibromas well-circumscribed and non-encapsulated tumors composed of proliferating fibroblasts arranged in interlacing bundles and embedded in variable amounts of collagen fibers. B: In some tumors there are isolated remnants of glandular epithelium, which suggests that they originate from fibroadenomas. Stained with H&E, ×4 and ×40, respectively.
A combination of epithelial and stromal components are often found in the rat mainly fibroadenomas that were described more in details in a previous section due to their predominance in the rat pathology. The carcinosarcomas is a rare entity, with malignant characteristics in both the epithelium and the stroma. The epithelial component varies from well-differentiated tubular structures to poorly demarcated and elongated cells, which are difficult to differentiate from neoplastic stromal cells. The specific markers keratin, myoglobin, desmin, and vimentin are useful for separating spindle-shaped epithelial cells from the stromal component. Nuclei vary in size and shape, and giant, multinucleated cells may be found. Mitoses are common in both the epithelium and the stroma.
NON-NEOPLASTIC LESIONS
Among the non-neoplastic lesions in the mammary gland, cystic changes are the ones most frequently found. Cysts can originate from either ductal or lobular elements. Those derived from ducts exhibit a 10 to 100-fold increase in the normal diameter of the duct. They are lined by flat, cuboidal epithelial cells, and have myoepithelial cells which are compressed against the basement membrane. Duct ectasia or galactocele, are characterized by the accumulation within the lumen of eosinophilic granular material composed of lipids and protein secretion. Crystals similar to cholesterol and focal calcifications are common.
Lobular cysts are characterized by a grape-like configuration. The small cystic dilatations can be confluent, forming one large cyst lined by low cuboidal epithelial cells, although some may contain cells with large vacuoles and decapitation of the apical portion of the cytoplasm. The nuclei are, in general, round or oval and are compressed against the basement membrane.
DIFFERENTIAL DIAGNOSIS OF MAMMARY TUMORS
Although a common practice for evaluating the tumorigenic response of the rat mammary gland to carcinogens is the quantization of palpable tumors, it is important to keep in mind that palpable lesions are lumps or swellings whose nature can be determined only through histologic examination. In Table 6 are listed normal organs, non-neoplastic lesions, and tumors of non-mammary origin that under gross examination can be confused with mammary tumors. Whether spontaneous or carcinogen-induced rat mammary tumors are benign or malignant can be determined by criteria derived from: gross examination (macroscopic criteria), histopathologic examination, and analysis of the biological behavior of the tumor (Table 7).
Table 7.
Criteria of malignancy in mammary tumors
| I. Macroscopic criteria |
|
| II. Histopathologic criteria |
|
| III. Biologic criteria |
|
The two major criteria to be taken into consideration upon gross examination of a tumor are its rate of growth and macroscopic appearance (Table 7). Generally, malignant tumors tend to grow faster; however, some exceptions to this rule are observed. We have also found that tumors in the mammary glands located in the thoracic region grow faster than those arising in glands located in the abdominal region (107). The gross appearance of carcinomas is generally soft and fleshy. They are well vascularized and contain areas of necrosis and hemorrhage. Some tumors have cysts containing blood and necrotic material. Fibroadenomas, on the other hand, are white, with a rubbery and firm consistency, and they shell out from their capsule when they are sectioned. Carcinomas can be firm if they have elicited an intense desmoplastic response.
Among the criteria of malignancy, the most important one is the loss of the tubular-alveolar pattern of the normal mammary gland, a pattern maintained in the adenomas and fibroadenomas (Table 7). Cytologically, malignant cells are larger than their normal counterparts and have an increased nucleocytoplasmic ratio. The enlarged nuclei contain coarse chromatin and more prominent nucleoli. The epithelial heterogeneity expressed by the normal gland, or even by benign lesions, in which at least myoepithelial, dark, and intermediate or clear cell types are identified, is rarely observed in malignant lesions (107). The predominant cell in malignancy is the intermediate type, dark cells are rarely observed, and very few myoepitlielial cells remain, especially in the invasive lesions. The number or mitoses is generally higher in malignant lesions than in benign ones. Finally, the invasion of the stroma and neighboring tissues, such as muscle and dermis, is a hallmark of malignant tumors. The stromal response to invasion, as demonstrated by fibrosis and inflammatory infiltration, is generally more prominent in the malignant lesions than in the noninvasive or benign ones.
The most reliable criterion of malignancy is the ability of a tumor to metastasize to distant organs, such as lymph nodes or lungs. Very few authors report the finding of metastases from either spontaneous or experimentally induced rat mammary tumors. This lack of metastasizing ability of rat tumors could be attributed to the short period of time that treated animals have been followed in most studies, since only when the study is prolonged for 2 or 3 years, essentially the whole life span of the animal, do metastases become evident. The histopathologic type of a tumor does not seem to affect its metastasizing ability, since it has been found that cribriform, comedo, or papillary carcinomas produce metastases with similar frequency (107). Transplantability is considered another reliable criterion of malignancy in the mammary gland tumors of the rat. The ability of neoplastic lesions to elicit angiogenesis has been postulated to be a biological marker of malignancy, but it has not been extensively used (12, 80, 110).
BIOLOGICAL IMPORTANCE OF THE CHEMICAL CARCINOGEN-INDUCED RAT MAMMARY TUMOR MODEL
The DMBA rat mammary model has been able to demonstrate that the carcinogen acts on the intermediate cell of the TEB, and that this structure is the one that evolves to IDP and carcinoma in situ. There are several factors that regulate the susceptibility of the TEB; some of them are: a) topographic location of the mammary gland, b) age of the animal, and c) reproductive history. The high proliferative activity of the TEB is associated with higher binding of the carcinogen, and its short cell cycle makes them less likely to repair the DNA damaged by the carcinogen. Even though most of the TEBs are transformed to IDPS, not all of them evolve to carcinomas. The regulatory mechanism of this process is more complex due to intrinsic properties of the TEB. We have observed that IDPs progressing to carcinomas, secrete proteoglycans and attract lymphocytes and mast cells, emphasizing the importance of the interaction of the initiated cells with the host as a mechanism in the progression of the disease. It is clear that the understanding of the mechanisms that modulate the progression of an IDP to a carcinoma will not only further our knowledge and understanding of carcinogenesis, but will also provide the tools for the prevention of the disease, as a result of the development of strategies for stopping the progression of initiated cells to fully manifested malignancy (107).
MOLECULAR CLASSIFICATION OF HUMAN BREAST CANCER AND ITS COUNTERPART IN THE RAT
The use of gene expression microarray technology has introduced a new classification based on molecular signatures (95, 134, 144, 151) and allowed for the identification of 4 groups with distinct molecular features of mammary cells (estrogen receptor positive/luminal like, basal-like, HER2/neu positive, and normal breast) (Table 8). The tumors were classified into basal-like, HER2/neu over expressing, luminal-like types, and normal breast tissue–like subgroup. The normal breast-like subtype originally proposed has since been recognized as probably arising from normal-tissue contamination (95, 134, 144, 151). The most well-characterized and widely accepted molecular subtypes are the luminal A, luminal B, HER2/neu, and basal like types. The basal like subtype commonly displays a triple-negative phenotype often defined by a lack of estrogen receptor and progesterone receptor protein expression, with an absence of HER2/neu overexpression (34, 45, 75, 78, 88, 94, 99, 100, 101, 141, 142). Similarly, luminal A, luminal B, basal-like and HER2/neu subtypes of DCIS were described (14, 76).
Table 8.
Molecular subtypes of breast cancer
| Molecular Subtype | Representative Gene | Immunophenotype |
|---|---|---|
| Luminal A | ESR1(estrogen receptor 1) | ER+ and/or PR+, HER2/Neu2−, low Ki-67 |
| Luminal B | ESR1 | ER+ and/or PR+, HER2/neu + |
| Basal-like | KRT5 (keratin 5) KRT17 (keratin 17 LAMC2 (laminin, γ 2) |
ER−, PR−, HER2/neu2, CK 5/6+, and/or EGFR+ |
| HER2/neu positive | ERB2(her 2 neu) | ER− PR−, HER2/neu+ |
Adapted from: AM Gruver, BP Portier, RR Tubbs. Molecular Pathology of Breast Cancer. The Journey from Traditional Practice toward Embracing the Complexity of a Molecular Classification. Arch Pathol Lab Med. 2011;135:544–557
Using three markers, ER, PgR, and HER2/neu (Table 9) (Figures 20 and 21) we have reclassified the mammary tumors in the rat. The ER and PgR was evaluated according to the Allred’s criteria (1). The expression of HER2 was evaluated by manual tracing of the tumor areas and run by Aperio Image Membrane Algorithm. They were evaluated as negative(−) weakly positive (+), moderate positive(++) when the some spots were reacted in the cell membrane and 3 + was defined as a circumferential membrane staining that is complete, intense, and within > 10% of tumor cells. These kinds of tumors were defined as HER2 positive (153).
Table 9.
Immunocytochemical evaluation of rat mammary tumors
| Antibody | Provider | Host species | Dilution | Primary antibody Incubation time |
|---|---|---|---|---|
| ER-alpha (MC-20) | Santa Cruz | Rabbit polyclonal | 1:400 | 30 min |
| Anti-PR (C-19) | Santa Cruz | Mouse monoclonal | 1:800 | 30 min |
| c-erbB-2/Her2/neu Ab-17 | Thermo Scientific | Mouse IgG | 1:1000 | 30 min |
Figure 20.
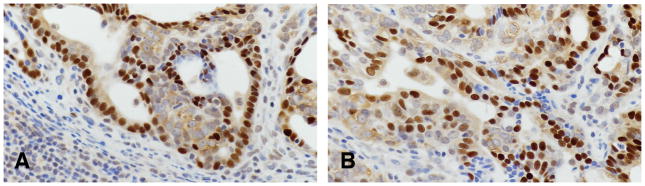
Invasive carcinoma of Luminal type A containing positive cells for ER(A) and Pg R(B)x40.
Figure 21.
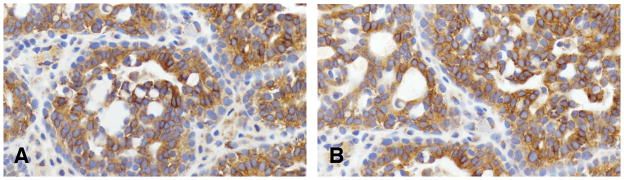
Invasive carcinoma of Luminal type A containing positive cells for HER2/neu (A) and (B)x40.
In 59 rat mammary tumors listed in Table 9 and histologically classified as invasive papillary, cribriform, comedo, tubular type and adenoid cystic were reacted with antibodies detecting ER alpha, PgR and HER2/neu positive. The proportion of ER alpha positive cells vary from 15.1% to 57.5%. The proportion of PgR positive cells varied from 11.1% to 61.1%. The HER2/neu was found only positive in a fraction of the tumors that were ER and PgR positive and classified as Luminal A, comprising 76.2%, whereas the other fraction of the tumors were HER2/neu negative and considered Luminal type B, comprising only 23.8% of the tumors.
At the time of the publication of this work the authoritative work of Santagata [155] was published showing that the use of other immuno-cytochemical markers like Vitamin D and androgen receptors in addition to Her2/neu and estrogen receptor may allow to better differentiate cancer subtypes in the human. Therefore the study in the rat model using new immuno-cytochemical markers as well as the comparison with the same makers in preneoplastic lesions and the final comparison with the human lesions warrants a new series of studies besides the one described in the present work.
Final considerations
The rat mammary tumor model is well suited for studying in situ and invasive lesions. However, unless the animals are kept for longer periods of time and the methodology for detecting early metastatic events improves, the rat model is not a good model for studying the metastatic process. The classification of the tumors matches well with the criteria used in the human pathology, and provides an adequate model for understanding these phases of the human disease. The role of immunosurveillace is an area that should be better studied and could potentially provide new insight into the inflammatory processes involved in the initiation and progression of mammary neoplasia. In turn it could provide an adequate model for testing vaccines and other tools using cytokines inhibitors.
The use of immunocytochemical markers allows us to differentiate two cell subtypes, Luminal A and B. We have not detected any of the tumors studied as positive only for HER2/neu or as triple negative. An important consideration is that the presence of histochemical markers does not indicate that the tumors in the rat will behave exactly as they do in the human disease, but will provide an initial point of comparison and more studies on this subject could be extremely beneficial.
Table 10.
Invasive Rat Mammary Cancer Induced by DMBA classified using the histological criteria and immunophenotype
| Histological type | Luminal | ||
|---|---|---|---|
| Number of cases | A (%) | B (%) | |
| Invasive Papillary Adenocarcinoma Type I, II and mix if I and II; Invasive Cribriform carcinoma, Invasive Comedo carcinoma and Invasive Tubular Carcinoma Adenoid cystic carcinoma |
59 | 76.29% | 23.8% |
Acknowledgments
A special acknowledgment goes out to Ms. Rose Sonlin for verifying the accuracy of the references and for her help with preparation of the manuscript; and to Ms. Patricia A. Russo for her editorial suggestions. I also acknowledge the work of Dr Yanrong Su in performing the immunocytochemical studies in the rat mammary tumors. This work was supported by NIH core grant CA06927 to Fox Chase Cancer Center and by an appropriation from the Commonwealth of Pennsylvania.
Footnotes
Dedication
We dedicate this work to the memory of Irma H Russo, MD who passed away on June 25, 2013. For the past 22 years Irma has worked at Fox Chase; first as director of surgical and clinical pathology and then as director of the molecular endocrinology section of the Breast Cancer Research Laboratory, which she founded in 1973 with her husband, Jose. Irma was a pioneer in classifying the rat mammary tumors and their correlation with the human pathology in addition to identifying the mechanisms of breast cancer prevention as mediated by pregnancy. The collaboration between Jose and Irma was so deep it was, at times, impossible to determine where one ended and the other began. Thanks to her innate sense of scientific collaboration and creativity, together they published six books and over 200 research. Irma had a very distinguished career in research and remained devoted till the end to the mission of understanding the cellular and molecular basis of breast cancer. She will be remembered not only by her many friends, colleagues, and the more than 50 MDs and PhDs she trained and mentored over the years, but also by every human being who was touched by her good nature.
References
- 1.Allred D, Bryant J, Land S, Paik S, Fisher E, Julian T, Margolese R, Smith R, Mamounas T, Osborne C, Fisher B, Wolmark N. Estrogen receptor expression as a predictive marker of the effectiveness of tamoxifen in the treatment of DCIS: Findings from NSABP Protocol B-24. Breast Cancer Res Treat. 2002;76 (suppl1):S36. [Google Scholar]
- 2.Alpers CE, Wellings SR. The prevalence of carcinoma in situ in normal and cancer-associated breasts. Hum Pathol. 1985;16:796–807. doi: 10.1016/s0046-8177(85)80251-3. [DOI] [PubMed] [Google Scholar]
- 3.Anver MR, Cohen BJ, Lattuada CP, Foster SJ. Age-associated lesions in barrier-reared male Sprague-Dawley rats: a comparison between Hap: (SD) and Crl:COBS[R]CD[R](SD) stocks. Exp Aging Res. 1982;8:3–24. doi: 10.1080/03610738208258390. [DOI] [PubMed] [Google Scholar]
- 4.Arrigoni MG, Dockerty MB, Judd ES. The identification and treatment of mammary hamartoma. Surg Gynecol Obstet. 1971;133:577–582. [PubMed] [Google Scholar]
- 5.Azzopardi J. Problems in Breast Pathology. WE Saunders; Philadelphia: 1979. [Google Scholar]
- 6.Bailey JP, Nieport KM, Herbst MP, Srivastava S, Serra RA, Horseman ND. Prolactin and transforming growth factor-beta signaling exert opposing effects on mammary gland morphogenesis, involution, and the Akt-forkhead pathway. Mol Endocrinol. 2004;18:1171–1184. doi: 10.1210/me.2003-0345. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee S, Reis-Filho JS, Ashley S, Steele D, Ashworth A, Lakhani SR, Smith IE. Basal-like breast carcinomas: clinical outcome and response to chemotherapy. J Clin Pathol. 2006;59:729–735. doi: 10.1136/jcp.2005.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996;17:639–669. doi: 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- 9.Berean K, Tron VA, Churg A, Clement PB. Mammary fibroadenoma with multinucleated stromal giant cells. Am J Surg Pathol. 1986;10:823–827. doi: 10.1097/00000478-198611000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Black M. In situ carcinoma of the breast. In: Sommers S, editor. Pathol Annu. Appleton; Century, Crofts, New York: 1969. p. 185. [Google Scholar]
- 11.Boylan ES, Calhoon RE. Transplacental action of diethylstilbestrol on mammary carcinogenesis in female rats given one or two doses of 7,12-dimethylbenz(a)anthracene. Cancer Res. 1983;43:4879–4884. [PubMed] [Google Scholar]
- 12.Brem SS, Jensen HM, Gullino PM. Angiogenesis as a marker of preneoplastic lesions of the human breast. Cancer. 1978;41:239–244. doi: 10.1002/1097-0142(197801)41:1<239::aid-cncr2820410133>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Tan J, Dey SK, Dotto GP, Weinberg RA. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell. 2002;3:877–887. doi: 10.1016/s1534-5807(02)00365-9. [DOI] [PubMed] [Google Scholar]
- 14.Bryan BB, Schnitt SJ, Collins LC. Ductal carcinoma in situ with basal-like phenotype: a possible precursor to invasive basal-like breast cancer. Mod Pathol. 2006;19:617–621. doi: 10.1038/modpathol.3800570. [DOI] [PubMed] [Google Scholar]
- 15.Burek JD. Pathology of Aging Rats. CRC Press; Boca Raton: 1978. [Google Scholar]
- 16.Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430–1441. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 17.Calaf G, Martinez F, Russo I, Roi L, Russo J. The influence of age on DNA· Labeling Index of human breast epithelium. Int Res Comm Syst Med Sci. 1982;10:655. [Google Scholar]
- 18.Calaf G, Martinez F, Russo IH, Russo J. Age related variations in growth kinetics of primary human breast cell cultures. lnt Res Comm Syst Med Sci. 1982;10:307. [Google Scholar]
- 19.Capuco AV, Li M, Long E, Ren S, Hruska KS, Schorr K, Furth PA. Concurrent pregnancy retards mammary involution: effects on apoptosis and proliferation of the mammary epithelium after forced weaning of mice. Biol Reprod. 2002;66:1471–1476. doi: 10.1095/biolreprod66.5.1471. [DOI] [PubMed] [Google Scholar]
- 20.Carney JA, Toorkey BC. Myxoid fibroadenoma and allied conditions (myxomatosis) of the breast. A heritable disorder with special associations including cardiac and cutaneous myxomas. Am J Surg Pathol. 1991;15:713–721. doi: 10.1097/00000478-199108000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Carstens PH. Ultrastructure of human fibroadenoma. Arch Pathol. 1974;98:23–32. [PubMed] [Google Scholar]
- 22.Carter BA, Page DL, Schuyler P, Parl FF, Simpson JF, Jensen RA, Dupont WD. No elevation in long-term breast carcinoma risk for women with fibroadenomas that contain atypical hyperplasia. Cancer. 2001;92:30–36. doi: 10.1002/1097-0142(20010701)92:1<30::aid-cncr1288>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Cheatle GL. Desquamative and dysgenetic epithelial hyperplasia in breast: their situation and characteristics; their likeness to lesions induced by tar. Br J Surg. 1926;13:509. [Google Scholar]
- 24.Cheatle GL. Tumours of the Breast: Their Pathology, Symptoms, Diagnosis, and Treatment. Arnold; London: 1931. [Google Scholar]
- 25.Chepko G, Smith GH. Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell. 1997;29:239–253. doi: 10.1016/s0040-8166(97)80024-9. [DOI] [PubMed] [Google Scholar]
- 26.Ciocca D, Russo J. Prolactin levels in susceptible and nonsusceptible rats to DMBA carcinogenesis. Proc 3rd Ann Cancer Res Conf Ohio Valley-Lake Erie Assoc Cancer Centers; 1980. p. 27. [Google Scholar]
- 27.Clemens l, Welsch C, Meites L. Effects of hypothalamic lesions on incidence and growth of mammary tumors in carcinogen-treated rats. Proc Soc Exp Bioi Med. 1968;127:465–471. doi: 10.3181/00379727-127-32847. [DOI] [PubMed] [Google Scholar]
- 28.Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curran RC, Dodge OG. Sarcoma of breast, with particular reference to its origin from fibroadenoma. J Clin Pathol. 1962;15:1–16. doi: 10.1136/jcp.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dao TL, Bock FG, Greiner MJ. Mammary carcinogenesis by 3-methylcholanthrene. II. Inhibitory effect of pregnancy and lactation on tumor induction. J Natl Cancer Inst. 1960;25:991–1003. [PubMed] [Google Scholar]
- 31.Dawson E. Premalignant conditions in breast carcinoma. In: Severi L, editor. The Morphological Precursors of Cancer. Perugia Univ Press; Perugia: 1962. p. 383. [Google Scholar]
- 32.Dawson EK. A histological study of the normal mamma in relation to tumour growth. I. Early development to maturity. Edinb Med J. 1934;41:653–682. [PMC free article] [PubMed] [Google Scholar]
- 33.Dehner LP, Hill DA, Deschryver K. Pathology of the breast in children, adolescents, and young adults. Semin Diagn Pathol. 1999;16:235–247. [PubMed] [Google Scholar]
- 34.Diaz LK, Cryns VL, Symmans WF, Sneige N. Triple negative breast carcinoma and the basal phenotype: from expression profiling to clinical practice. Adv Anat Pathol. 2007;14:419–430. doi: 10.1097/PAP.0b013e3181594733. [DOI] [PubMed] [Google Scholar]
- 35.Diaz NM, Palmer JO, McDivitt RW. Carcinoma arising within fibroadenomas of the breast. A clinicopathologic study of 105 patients. Am J Clin Pathol. 1991;95:614–622. doi: 10.1093/ajcp/95.5.614. [DOI] [PubMed] [Google Scholar]
- 36.Dupont WD, Page DL, Parl FF, Vnencak-Jones CL, Plummer WD, Jr, Rados MS, Schuyler PA. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;331:10–15. doi: 10.1056/NEJM199407073310103. [DOI] [PubMed] [Google Scholar]
- 37.Sloane JP, et al. European Commission Working Group on Breast Screening Pathology. Consistency achieved by 23 European pathologists in categorizing ductal carcinoma in situ of the breast using five classifications. Hum Pathol. 1998;29:1056–1062. [PubMed] [Google Scholar]
- 38.Eusebi V, Azzopardi JG. Lobular endocrine neoplasia in fibroadenoma of the breast. Histopathology. 1980;4:413–428. doi: 10.1111/j.1365-2559.1980.tb02936.x. [DOI] [PubMed] [Google Scholar]
- 39.Fechner RE. Fibroadenomas in patients receiving oral contraceptives: a clinical and pathologic study. Am J Clin Pathol. 1970;53:857–864. doi: 10.1093/ajcp/53.6.857. [DOI] [PubMed] [Google Scholar]
- 40.Fekete P, Petrek J, Majmudar B, Someren A, Sandberg W. Fibroadenomas with stromal cellularity. A clinicopathologic study of 21 patients. Arch Pathol Lab Med. 1987;111:427–432. [PubMed] [Google Scholar]
- 41.Fisher ER, Gregorio RM, Fisher B, Redmond C, Vellios F, Sommers SC. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4) Cancer. 1975;36:1–85. doi: 10.1002/1097-0142(197507)36:1<1::aid-cncr2820360102>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgibbons PL, Henson DE, Hutter RV. Benign breast changes and the risk for subsequent breast cancer: an update of the 1985 consensus statement. Cancer Committee of the College of American Pathologists. Arch Pathol Lab Med. 1998;122:1053–1055. [PubMed] [Google Scholar]
- 43.Fletcher JA, Pinkus GS, Weidner N, Morton CC. Lineage-restricted clonality in biphasic solid tumors. Am J Pathol. 1991;138:1199–1207. [PMC free article] [PubMed] [Google Scholar]
- 44.Fondo EY, Rosen PP, Fracchia AA, Urban JA. The problem of carcinoma developing in a fibroadenoma: recent experience at Memorial Hospital. Cancer. 1979;43:563–567. doi: 10.1002/1097-0142(197902)43:2<563::aid-cncr2820430224>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 45.Foulkes WD, Stefansson IM, Chappuis PO, Begin LR, Goffin JR, Wong N, Trudel M, Akslen LA. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 46.Fulford L, Reis-Filho J, Lakhani S. Lobular in situ neoplasia. Curr Diagn Pathol. 2004;10:183–192. [Google Scholar]
- 47.Gallagher HS. Early phases in the development of breast cancer. Cancer. 1969;24:1170–1178. doi: 10.1002/1097-0142(196912)24:6<1170::aid-cncr2820240615>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 48.Geschickter C. Diseases of the Breast. Lippincott; Philadelphia: 1945. Pathology of mammary cancer. [Google Scholar]
- 49.Geschickter CF. The Cyclopedia of Medicine. FA Davis; Philadelphia: 1941. Tumors of the breast related to the oestrin hormone. [Google Scholar]
- 50.Geschickter CF, Lewis D, Hartman CG. Tumors of the breast related to the oestrin hormone. Am J Cancer. 1934;21:828–859. [PubMed] [Google Scholar]
- 51.Goldman RL, Friedman NB. Carcioma of the breast arising in fibroadenomas, with emphasis on lobular carcinoma. A clinicopathologic study. Cancer. 1969;23:544–550. doi: 10.1002/1097-0142(196903)23:3<544::aid-cncr2820230305>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 52.Goodman ZD, Taxy JB. Fibroadenomas of the breast with prominent smooth muscle. Am J Surg Pathol. 1981;5:99–101. doi: 10.1097/00000478-198101000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Greaves P, Faccini JM. Rat Histopathology. Elsevier; Amsterdam: 1984. Tumors of the mammary gland. [Google Scholar]
- 54.Grubbs CJ, Juliana MM, Hill DL, Whitaker LM. Suppression by pregnancy of chemically induced preneoplastic cells of the rat mammary gland. Anticancer Res. 1986;6:1395–1400. [PubMed] [Google Scholar]
- 55.Haagensen CD. Diseases of the Breast. 3. WD Sanders; Philadelphia: 1986. [Google Scholar]
- 56.Handwerger S. Clinical counterpoint: the physiology of placental lactogen in human pregnancy. Endocr Rev. 1991;12:329–336. doi: 10.1210/edrv-12-4-329. [DOI] [PubMed] [Google Scholar]
- 57.Harris J, Lippman ME, Morrow M, Osborne CK. Diseases of the Breast. Lippincott Williams & Wilkins; Philadelphia: 2004. [Google Scholar]
- 58.Heiman J, Krehbiel OF. The influence of hormones on breast hyperplasia and tumor growths in white rats. Am J Cancer. 1936;27:450–473. [Google Scholar]
- 59.Hertel BF, Zaloudek C, Kempson RL. Breast adenomas. Cancer. 1976;37:2891–2905. doi: 10.1002/1097-0142(197606)37:6<2891::aid-cncr2820370647>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 60.Holland R, Peterse JL, Millis RR, Eusebi V, Faverly D, van de Vijver MJ, Zafrani B. Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol. 1994;11:167–180. [PubMed] [Google Scholar]
- 61.Hovey RC, Harris J, Hadsell DL, Lee AV, Ormandy CJ, Vonderhaar BK. Local insulin-like growth factor-II mediates prolactin-induced mammary gland development. Mol Endocrinol. 2003;17:460–471. doi: 10.1210/me.2002-0214. [DOI] [PubMed] [Google Scholar]
- 62.Huggins C, Grand LC, Brillantes FP. Critical significance of breast structure in the induction of mammary cancer in the rat. Proc Natl Acad Sci U S A. 1959;45:1294–1300. doi: 10.1073/pnas.45.8.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Humphreys RC, Hennighausen L. Signal transducer and activator of transcription 5a influences mammary epithelial cell survival and tumorigenesis. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1999;10:685–694. [PubMed] [Google Scholar]
- 64.Isaacs JT. Genetic control of resistance to chemically induced mammary adenocarcinogenesis in the rat. Cancer Res. 1986;46:3958–3963. [PubMed] [Google Scholar]
- 65.Isaacs JT. Inheritance of a genetic factor from the Copenhagen rat and the suppression of chemically induced mammary adenocarcinogenesis. Cancer Res. 1988;48:2204–2213. [PubMed] [Google Scholar]
- 66.Jacobs JW. Recently recognized variants of lobular carcinoma in situ (LCIS) with an emphasis on management of LCIS on core needle biopsy. Pathol Case Rev. 2003;8:211–219. [Google Scholar]
- 67.Kacsoh B, Veress Z, Toth BE, Avery LM, Grosvenor CE. Bioactive and immunoreactive variants of prolactin in milk and serum of lactating rats and their pups. J Endocrinol. 1993;138:243–257. doi: 10.1677/joe.0.1380243. [DOI] [PubMed] [Google Scholar]
- 68.Karpas CM, Leis HP, Oppenheim A, Mersheimer WL. Relationship of Fibrocystic Disease to Carcinoma of the Breast. Ann Surg. 1965;162:1–8. doi: 10.1097/00000658-196507000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kern WH, Brooks RN. Atypical epithelial hyperplasia associated with breast cancer and fibrocystic disease. Cancer. 1969;24:668–675. doi: 10.1002/1097-0142(196910)24:4<668::aid-cncr2820240403>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 70.Kiaer W. Relationship of Fibroadenomatosis (Chronic Mastitis) to Cancer of the Breast. Munksgaard; Copenhagen: 1954. [Google Scholar]
- 71.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kroes R, Garbis-Berkvens JM, de Vries T, van Nesselrooy HJ. Histopathological profile of a Wistar rat stock including a survey of the literature. J Gerontol. 1981;36:259–279. doi: 10.1093/geronj/36.3.259. [DOI] [PubMed] [Google Scholar]
- 73.Lester SC, Bose S, Chen YY, Connolly JL, de Baca ME, Fitzgibbons PL, Hayes DF, Kleer C, O’Malley FP, Page DL, Smith BL, Weaver DL, Winer E. Protocol for the examination of specimens from patients with ductal carcinoma in situ of the breast. Arch Pathol Lab Med. 2009;133:15–25. doi: 10.5858/133.1.15. [DOI] [PubMed] [Google Scholar]
- 74.Levin ML, Sheehe PR, Graham S, Glidewell O. Lactation and menstrual function as related to cancer of the breast. Am J Public Health Nations Health. 1964;54:580–587. doi: 10.2105/ajph.54.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, Perou CM. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–271. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- 76.Livasy CA, Perou CM, Karaca G, Cowan DW, Maia D, Jackson S, Tse CK, Nyante S, Millikan RC. Identification of a basal-like subtype of breast ductal carcinoma in situ. Hum Pathol. 2007;38:197–204. doi: 10.1016/j.humpath.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 77.Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, Marchio C, Reis-Filho JS. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology. 2010;57:171–192. doi: 10.1111/j.1365-2559.2010.03568.x. [DOI] [PubMed] [Google Scholar]
- 78.Luck AA, Evans AJ, James JJ, Rakha EA, Paish EC, Green AR, Ellis IO. Breast carcinoma with basal phenotype: mammographic findings. AJR Am J Roentgenol. 2008;191:346–351. doi: 10.2214/AJR.07.2659. [DOI] [PubMed] [Google Scholar]
- 79.Maekawa A, Odashima S. Spontaneous tumors in ACI/N rats. J Natl Cancer Inst. 1975;55:1437–1445. doi: 10.1093/jnci/55.6.1437. [DOI] [PubMed] [Google Scholar]
- 80.Maiorana A, Gullino PM. Acquisition of angiogenic capacity and neoplastic transformation in the rat mammary gland. Cancer Res. 1978;38:4409–4414. [PubMed] [Google Scholar]
- 81.Maluf HM. Differential diagnosis of solid carcinoma in situ. Semin Diagn Pathol. 2004;21:25–31. doi: 10.1053/j.semdp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 82.Marchio C, Weigelt B, Reis-Filho JS. Adenoid cystic carcinomas of the breast and salivary glands (or ‘The strange case of Dr Jekyll and Mr Hyde’ of exocrine gland carcinomas) J Clin Pathol. 2010;63:220–228. doi: 10.1136/jcp.2009.073908. [DOI] [PubMed] [Google Scholar]
- 83.McCormick DL, Adamowski CB, Fiks A, Moon RC. Lifetime dose-response relationships for mammary tumor induction by a single administration of N-methyl-N-nitrosourea. Cancer Res. 1981;41:1690–1694. [PubMed] [Google Scholar]
- 84.McCormick DL, Mehta RG, Thompson CA, Dinger N, Caldwell JA, Moon RC. Enhanced inhibition of mammary carcinogenesis by combined treatment with N-(4-hydroxyphenyl)retinamide and ovariectomy. Cancer Res. 1982;42:508–512. [PubMed] [Google Scholar]
- 85.McDivitt RW, Farrow JH, Stewart FW. Breast carcinoma arising in solitary fibroadenomas. Surg Gynecol Obstet. 1967;125:572–576. [PubMed] [Google Scholar]
- 86.Metcalf JS, Ellis B. Choristoma of the breast. Hum Pathol. 1985;16:739–740. doi: 10.1016/s0046-8177(85)80161-1. [DOI] [PubMed] [Google Scholar]
- 87.Mies C, Rosen PP. Juvenile fibroadenoma with atypical epithelial hyperplasia. Am J Surg Pathol. 1987;11:184–190. doi: 10.1097/00000478-198703000-00002. [DOI] [PubMed] [Google Scholar]
- 88.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moore T, Lee AH. Expression of CD34 and bcl-2 in phyllodes tumours, fibroadenomas and spindle cell lesions of the breast. Histopathology. 2001;38:62–67. doi: 10.1046/j.1365-2559.2001.01053.x. [DOI] [PubMed] [Google Scholar]
- 90.Oberman HA, Nosanchuk JS, Finger JE. Periductal stromal tumors of breast with adipose metaplasia. Arch Surg. 1969;98:384–387. doi: 10.1001/archsurg.1969.01340090160034. [DOI] [PubMed] [Google Scholar]
- 91.Page DL, Anderson TJ. Diagnostic Histopathology of the Breast. Churchill Livingstone; New York: 1987. [Google Scholar]
- 92.Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55:2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 93.Parks AG. The micro-anatomy of the breast. Ann R Coll Surg Engl. 1959;25:235–251. [PMC free article] [PubMed] [Google Scholar]
- 94.Peppercorn J, Perou CM, Carey LA. Molecular subtypes in breast cancer evaluation and management: divide and conquer. Cancer Invest. 2008;26:1–10. doi: 10.1080/07357900701784238. [DOI] [PubMed] [Google Scholar]
- 95.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 96.Petrik PK. Mammary hamartoma. Am J Surg Pathol. 1987;11:234–235. doi: 10.1097/00000478-198703000-00010. [DOI] [PubMed] [Google Scholar]
- 97.Pick PW, Iossifides IA. Occurrence of breast carcinoma within a fibroadenoma. A review. Arch Pathol Lab Med. 1984;108:590–594. [PubMed] [Google Scholar]
- 98.Pike AM, Oberman HA. Juvenile (cellular) adenofibromas. A clinicopathologic study. Am J Surg Pathol. 1985;9:730–736. doi: 10.1097/00000478-198510000-00004. [DOI] [PubMed] [Google Scholar]
- 99.Rakha E, Reis-Filho JS. Basal-like breast carcinoma: from expression profiling to routine practice. Arch Pathol Lab Med. 2009;133:860–868. doi: 10.5858/133.6.860. [DOI] [PubMed] [Google Scholar]
- 100.Rakha EA, El-Sayed ME, Reis-Filho J, Ellis IO. Patho-biological aspects of basal-like breast cancer. Breast Cancer Res Treat. 2009;113:411–422. doi: 10.1007/s10549-008-9952-1. [DOI] [PubMed] [Google Scholar]
- 101.Rakha EA, Reis-Filho JS, Ellis IO. Impact of basal-like breast carcinoma determination for a more specific therapy. Pathobiology. 2008;75:95–103. doi: 10.1159/000123847. [DOI] [PubMed] [Google Scholar]
- 102.Reddick RL, Shin TK, Sawhney D, Siegal GP. Stromal proliferations of the breast: an ultrastructural and immunohistochemical evaluation of cystosarcoma phyllodes, juvenile fibroadenoma, and fibroadenoma. Hum Pathol. 1987;18:45–49. doi: 10.1016/s0046-8177(87)80192-2. [DOI] [PubMed] [Google Scholar]
- 103.Rosai J. Rosai and Ackerman’s Surgical Pathology. 9. Mosby; New York: 2004. [Google Scholar]
- 104.Rose DP, Pruitt B, Stauber P, Erturk E, Bryan GT. Influence of dosage schedule on the biological characteristics of N-nitrosomethylurea-induced rat mammary tumors. Cancer Res. 1980;40:235–239. [PubMed] [Google Scholar]
- 105.Rothschild TC, Boylan ES, Calhoon RE, Vonderhaar BK. Transplacental effects of diethylstilbestrol on mammary development and tumorigenesis in female ACI rats. Cancer Res. 1987;47:4508–4516. [PubMed] [Google Scholar]
- 106.Russo IH, Russo J. Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12-dimethylbenz[a]anthracene. J Natl Cancer Inst. 1978;61:1439–1449. [PubMed] [Google Scholar]
- 107.Russo IH, Russo J. Mammary gland neoplasia in long-term rodent studies. Environ Health Perspect. 1996;104:938–967. doi: 10.1289/ehp.96104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Russo IH, Tewari M, Russo J. Morphology and Development of Rat Mammary Gland. In: Jones TC, Mohr U, Hunt RD, editors. Integument and Mammary Gland of Laboratory Animals. Springer Verlag; Berlin: 1989. pp. 233–252. [Google Scholar]
- 109.Russo J. DNA synthesis and terminal end bud density in mammary gland as determinants of susceptibility to carcinogens. Proc Am Assoc Cancer Res. 1978;19:228. [Google Scholar]
- 110.Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ. Comparative study of human and rat mammary tumorigenesis. Lab Invest. 1990;62:244–278. [PubMed] [Google Scholar]
- 111.Russo J, Reina D, Frederick J, Russo IH. Expression of phenotypical changes by human breast epithelial cells treated with carcinogens in vitro. Cancer Res. 1988;48:2837–2857. [PubMed] [Google Scholar]
- 112.Russo J, Russo IH. DNA labeling index and structure of the rat mammary gland as determinants of its susceptibility to carcinogenesis. J Natl Cancer Inst. 1978;61:1451–1459. [PubMed] [Google Scholar]
- 113.Russo J, Russo IH. Influence of differentiation and cell kinetics on the susceptibility of the rat mammary gland to carcinogenesis. Cancer Res. 1980;40:2677–2687. [PubMed] [Google Scholar]
- 114.Russo J, Russo IH. Biological and molecular bases of mammary carcinogenesis. Lab Invest. 1987;57:112–137. [PubMed] [Google Scholar]
- 115.Russo J, Russo IH. Development of the human mammary gland. In: Neville MC, Daniel C, editors. The Mammary Gland. Plenum; New York: 1987. pp. 67–93. [Google Scholar]
- 116.Russo J, Russo IH, van Zwieten MJ, Rogers AE, Gusterson B, Rogers AE. Classification of neoplastic and non-neoplastic lesions of the rat. In: Jones TC, Mohr U, Hunt RD, editors. Integument and Mammary Glands of Laboratory Animals. Springer Verlag; Berlin: 1989. [Google Scholar]
- 117.Russo J, Saby J, Isenberg WM, Russo IH. Pathogenesis of mammary carcinomas induced in rats by 7, 12-dimethylbenz[a]anthracene. J Natl Cancer Inst. 1977;59:435–445. [Google Scholar]
- 118.Russo J, Tait L, Russo IH. Susceptibility of the mammary gland to carcinogenesis. III. The cell of origin of rat mammary carcinoma. Am J Pathol. 1983;113:50–66. [PMC free article] [PubMed] [Google Scholar]
- 119.Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2:5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- 120.Russo J, Wilgus G, Russo IH. Susceptibility of the mammary gland to carcinogenesis: I Differentiation of the mammary gland as determinant of tumor incidence and type of lesion. Am J Pathol. 1979;96:721–736. [PMC free article] [PubMed] [Google Scholar]
- 121.Ryan JA, Coady CJ. Intraductal epithelial proliferation in the human breast-a comparative study. Canadian journal of surgery Journal canadien de chirurgie. 1962;5:12–19. [PubMed] [Google Scholar]
- 122.Salm R. Epidermoid metaplasia in mammary fibroadenoma with formation of keratin cysts. J Pathol BacterioI. 1957;74:221–222. [Google Scholar]
- 123.Sandison AT. An autopsy study of the adult human breast: with special reference to proliferative epithelial changes of importance in the pathology of the breast. Natl Cancer Inst Monogr. 1962;4:1–145. [PubMed] [Google Scholar]
- 124.Schmitt-Ney M, Happ B, Hofer P, Hynes NE, Groner B. Mammary gland-specific nuclear factor activity is positively regulated by lactogenic hormones and negatively by milk stasis. Mol Endocrinol. 1992;6:1988–1997. doi: 10.1210/mend.6.12.1491685. [DOI] [PubMed] [Google Scholar]
- 125.Schnitt SJ, Collins LC. Biopsy Interpretation of the Breast. Lippincott Williams & Wilkins; Philadelphia: 2009. [Google Scholar]
- 126.Scott MA, Lagios MD, Axelsson K, Rogers LW, Anderson TJ, Page DL. Ductal carcinoma in situ of the breast: reproducibility of histological subtype analysis. Hum Pathol. 1997;28:967–973. doi: 10.1016/s0046-8177(97)90013-7. [DOI] [PubMed] [Google Scholar]
- 127.Shimizu T, Ebihara Y, Serizawa H, Toyoda M, Hirota T. Histopathological study of stromal smooth muscle cells in fibroadenoma of the breast. Pathol Int. 1996;46:442–449. doi: 10.1111/j.1440-1827.1996.tb03635.x. [DOI] [PubMed] [Google Scholar]
- 128.Silverman JS, Tamsen A. Mammary fibroadenoma and some phyllodes tumour stroma are composed of CD34+ fibroblasts and factor XIIIa+ dendrophages. Histopathology. 1996;29:411–419. doi: 10.1046/j.1365-2559.1996.d01-510.x. [DOI] [PubMed] [Google Scholar]
- 129.Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol. 2005;205:248–254. doi: 10.1002/path.1691. [DOI] [PubMed] [Google Scholar]
- 130.Siziopikou KO. Ductal carcinoma in situ of the breast-current concepts and future directions. Arch Pathol Lab Med. 2013;137:462–466. doi: 10.5858/arpa.2012-0078-RA. [DOI] [PubMed] [Google Scholar]
- 131.Smith GH. Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat. 1996;39:21–31. doi: 10.1007/BF01806075. [DOI] [PubMed] [Google Scholar]
- 132.Sneige N, Wang J, Baker BA, Krishnamurthy S, Middleton LP. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductal-lobular) carcinoma in situ of the breast: a report of 24 cases. Mod Pathol. 2002;15:1044–1050. doi: 10.1097/01.MP.0000027624.08159.19. [DOI] [PubMed] [Google Scholar]
- 133.Solleveld HA, Haseman JK, McConnell EE. Natural history of body weight gain, survival, and neoplasia in the F344 rat. J Natl Cancer Inst. 1984;72:929–940. [PubMed] [Google Scholar]
- 134.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Srivastava S, Matsuda M, Hou Z, Bailey JP, Kitazawa R, Herbst MP, Horseman ND. Receptor activator of NF-kappaB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J Biol Chem. 2003;278:46171–46178. doi: 10.1074/jbc.M308545200. [DOI] [PubMed] [Google Scholar]
- 136.Stomper PC, Margolin FR. Ductal carcinoma in situ: the mammographer’s perspective. AJR Am J Roentgenol. 1994;162:585–591. doi: 10.2214/ajr.162.3.8109501. [DOI] [PubMed] [Google Scholar]
- 137.Tay LK, Russo J. 7,12-dimethylbenz[a]anthracene-induced DNA binding and repair synthesis in susceptible and nonsusceptible mammary epithelial cells in culture. J Natl Cancer Inst. 1981;67:155–161. [PubMed] [Google Scholar]
- 138.Tay LK, Russo J. Formation and removal of 7,12-dimethylbenz[a]anthracene--nucleic acid adducts in rat mammary epithelial cells with different susceptibility to carcinogenesis. Carcinogenesis. 1981;2:1327–1333. doi: 10.1093/carcin/2.12.1327. [DOI] [PubMed] [Google Scholar]
- 139.The Consensus Conference Committee. Consensus Conference on the classification of ductal carcinoma in situ. Cancer. 1997;80:1798–1802. doi: 10.1002/(sici)1097-0142(19971101)80:9<1798::aid-cncr15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 140.Travers MT, Barber MC, Tonner E, Quarrie L, Wilde CJ, Flint DJ. The role of prolactin and growth hormone in the regulation of casein gene expression and mammary cell survival: relationships to milk synthesis and secretion. Endocrinology. 1996;137:1530–1539. doi: 10.1210/endo.137.5.8612482. [DOI] [PubMed] [Google Scholar]
- 141.Turner NC, Reis-Filho JS. Basal-like breast cancer and the BRCA1 phenotype. Oncogene. 2006;25:5846–5853. doi: 10.1038/sj.onc.1209876. [DOI] [PubMed] [Google Scholar]
- 142.Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, Savage K, Gillett CE, Schmitt FC, Ashworth A, Tutt AN. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 143.Umekita Y, Yoshida H. Immunohistochemical study of hormone receptor and hormone-regulated protein expression in phyllodes tumour: comparison with fibroadenoma. Virchows Arch. 1998;433:311–314. doi: 10.1007/s004280050254. [DOI] [PubMed] [Google Scholar]
- 144.van ’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Bernards R, Friend SH. Expression profiling predicts outcome in breast cancer. Breast Cancer Res. 2003;5:57–58. doi: 10.1186/bcr562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van Zwieten M. The rat as animal model in breast cancer research. Martinus Nijhoff Publishers; Boston: 1984. [Google Scholar]
- 146.Vorherr H. The Breast. Academic Press; New York: 1974. Development of the female breast. [Google Scholar]
- 147.Weigelt B, Horlings HM, Kreike B, Hayes MM, Hauptmann M, Wessels LF, de Jong D, Van de Vijver MJ, Van’t Veer LJ, Peterse JL. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216:141–150. doi: 10.1002/path.2407. [DOI] [PubMed] [Google Scholar]
- 148.Wellings SR. Development of human breast cancer. Adv Cancer Res. 1980;31:287–314. doi: 10.1016/s0065-230x(08)60660-0. [DOI] [PubMed] [Google Scholar]
- 149.Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975;55:231–273. [PubMed] [Google Scholar]
- 150.Welsch CW, Clemens JA, Meites J. Effects of multiple pituitary homografts or progesterone on 7,12-dimethylbenz[a]anthracene-induced mammary tumors in rats. J Natl Cancer Inst. 1968;41:465–471. [PubMed] [Google Scholar]
- 151.West M, Blanchette C, Dressman H, Huang E, Ishida S, Spang R, Zuzan H, Olson JA, Jr, Marks JR, Nevins JR. Predicting the clinical status of human breast cancer by using gene expression profiles. Proc Natl Acad Sci U S A. 2001;98:11462–11467. doi: 10.1073/pnas.201162998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wilkinson L, Green WO., Jr Infarction of Breast Lesions during Pregnancy and Lactation. Cancer. 1964;17:1567–1572. doi: 10.1002/1097-0142(196412)17:12<1567::aid-cncr2820171208>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 153.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 154.Yaziji H, Gown AM, Sneige N. Detection of stromal invasion in breast cancer: the myoepithelial markers. Adv Anat Pathol. 2000;7:100–109. doi: 10.1097/00125480-200007020-00005. [DOI] [PubMed] [Google Scholar]
- 155.Santagata S, Thakkar A, Ergomul A, Wang B, Woo T, Hu R, Harrell JC, McNamara G, Shcwede M, Culhane AC, Kindelberger D, Rodig S, Richardson A, Tamimi RM, Ince TA. Taxonomy of breast cancer based on normal cell phenotype predicts outcome. J Clin Invest. 2014;124:869–870. doi: 10.1172/JCI70941. [DOI] [PMC free article] [PubMed] [Google Scholar]