Abstract
Oncogene-induced senescence (OIS) is a tumor-suppressing response that must be disrupted for cancer to develop. Mechanistic insights into OIS have begun to emerge. Activation of the p53/p21WAF1 and/or p16INK4A tumor-suppressor pathways is essential for OIS. Moreover, the DNA damage response, chromatin remodeling and senescence-associated secretory phenotype (SASP) are important for the initiation and maintenance of OIS. This review discusses recent advances in elucidating the mechanisms of OIS, focusing on the roles of the p38 MAPK and PI3K/AKT/mTOR pathways. These studies indicate that OIS is mediated by an intricate signaling network. Further delineation of this network may lead to development of new cancer therapies targeting OIS.
Keywords: oncogene-induced senescence, p38, PI3K, AKT
Introduction: oncogene-induced senescence
Replicative senescence is a form of irreversible growth arrest associated with the exhaustion of replicative potential of in vitro cultured cells [1]. In the case of human cells, replicative senescence occurs as a result of telomere erosion during cell division [2]. Replication-independent senescence can also be induced prematurely in young cells by activation of oncogenes. It was discovered in 1997 that in early-passaged normal human and murine fibroblasts, oncogenic ras induces an initial phase of hyperproliferation, followed by an irreversible growth arrest that is phenotypically indistinguishable from replicative senescence [3]. This form of premature senescence is termed oncogene- induced senescence, or OIS. The induction of OIS was initially reported to be independent of telomere length and telomerase activity [4], but a recent study indicates that oncogenes such as ras induce telomere dysfunction, including telomere attrition in primary fibroblasts, and that OIS is not stable in cells with high telomerase activity [5]. Like replicative senescence, OIS is identified by senescence biomarkers such as senescence-associated β-galactosidase (SA-β-gal). In addition to oncogenic ras, OIS can also be induced by other oncogenes, such as BRAFV600E, AKT, E2F1, cyclin E, mos, and Cdc6, and by inactivation of tumor suppressor genes including PTEN and NF1 [6].
Like apoptosis, oncogene-induced senescence (OIS) is a tumor suppressing defense mechanism that must be compromised by additional mutations during tumorigenesis. It has long been recognized that OIS inhibits oncogenic transformation in cell culture [7]. Later studies demonstrate that OIS indeed occurs in multiple human tumor types and mouse cancer models, and serves as an initial barrier to cancer development in vivo [6].
The molecular mechanisms and signaling pathways that mediate OIS have begun to emerge [6]. Almost all the OIS inducers trigger activation of p53, which induces the expression of its transcriptional target p21WAF1, and/or increase the expression of p16INK4A [3]. p21WAF1 and/or p16INK4A both inhibit the activity of cyclin-dependent protein kinases (CDKs) that phosphorylate and inactivate the Retinoblastoma protein (Rb), leading to accumulation of the hypo-phosphorylated, active form of Rb that mediates cell-cycle arrest and other phenotypes of senescence. Some oncogenes induce OIS through DNA damage responses, which can be generated by reactive oxygen species (ROS) that accumulate as a result of oncogene activation [8] or by hyper-replication of DNA caused by sustained oncogenic signals [9,10]. OIS induction is also accompanied by accumulation of senescence-associated heterochromatic foci (SAHFs), which recruit Rb and heterochromatin proteins to stably silence the expression of E2F target genes that are necessary for cell proliferation [11]. These changes in chromatin brought about by SAHF formation are believed to mediate the irreversibility of OIS. Moreover, like replicative senescence, OIS is characterized by the senescence-associated secretory phenotype (SASP), referring to increased expression and secretion of inflammatory cytokines, chemokines, growth factors, proteases and other proteins in senescent cells [12]. The SASP factors are critical for the initiation and maintenance of senescence in a cell autonomous fashion [13–16], and some of them signal the immune system to clear senescent cells in vivo [17,18]. Some of the SASP factors are upregulated at the mRNA level by the transcription factors nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and CCAAT-enhancer-binding protein β (C/EBPβ) [13,15,19].
In this review, we discuss the molecular mechanisms and signal transduction pathways for OIS that have emerged from recent studies, focusing on the roles of the p38 mitogen-activated protein kinase (MAPK) and the phosphoinositide 3-kinase (PI3K)/cellular homolog of murine thymoma virus Akt8 oncoprotein (AKT)/mammalian target of rapamycin (mTOR) pathways.
OIS and the p38 MAPK pathway
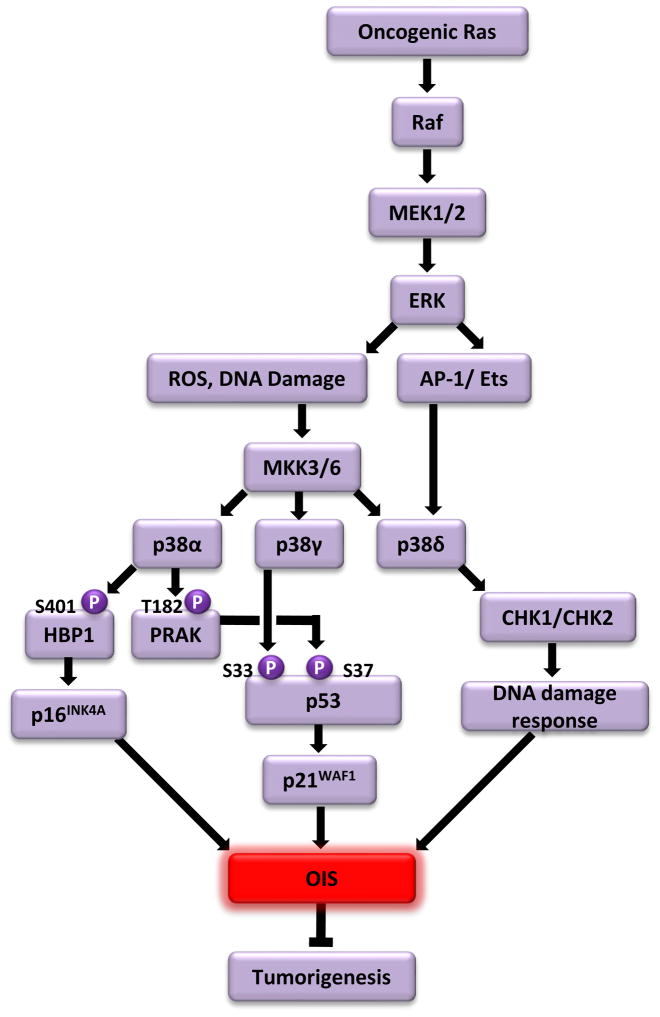
The p38 MAPK pathway was initially identified as a mediator of inflammation and stress responses (Box 1). Recent studies indicate that the p38 pathway also mediates OIS and tumor suppression (Fig. 1).
Box 1. The p38 mitogen-activated protein kinase (MAPK) pathway.
The p38 pathway is one of the major mitogen-activated protein kinase (MAPK) pathways, and was initially identified as a mediator of inflammation and stress responses [65]. The essential role of this pathway in cellular responses to components of microorganisms, inflammatory cytokines, and environmental stresses has been well established. Four isoforms of p38 (p38α, p38β, p38γ and p38δ; also known as SAPK2a, 2b, 3 and 4, respectively) exist in the mammalian genome, each encoded by a different gene. These isoforms differ in their tissue distributions, regulation by upstream stimuli, and selectivity for upstream regulatory kinase and phosphatases as well as downstream targets. Like the other MAPK pathways, the p38 signaling cascade involves sequential activation of MAP kinase kinase kinases (MAP3Ks), such as MTK, MLK2, MLK3, DLK, ASK and TAK1, and MAP kinase kinases (MKKs) including MKK3, MKK6, and MKK4, which directly activate p38 through phosphorylation in a cell type- and stimulus-dependent manner. A MKK-independent mechanism for p38 activation was also observed, in which interaction between p38 and a chaperone protein called transforming growth factor-beta-activated protein kinase 1 (TAK1)-binding protein 1 (TAB1) leads to autophosphorylation and activation of p38 [66]. After induction, p38 activity can be dampened through downregulation by dual-specific MAP kinase phosphatases (MKP)-1, -4 and –5, or other phosphatases such as PP2C [65]. The functions of p38 are mediated by downstream substrates, including transcription factors and cell-cycle regulators, and a family of Ser/Thr protein kinases consisting of MAPK-Activated Protein Kinases 2 and 3 (MK2 and MK3), p38-Regulated/Activated Kinase (PRAK/MK5), MAPK-Interacting Protein Kinase 1 (MNK1), Mitogen- and Stress-Activated Protein Kinases-1 and -2 (MSK1 and MSK2), and Casein Kinase 2 (CK2) [67]. In addition to inflammation and stress responses, the p38 pathway is involved in the control of cell cycle and proliferation [68], and has been shown to mediate both tumor suppression and tumor promotion in a context-dependent manner [69,70].
Fig. 1.
Oncogenic ras-induced senescence is mediated by sequential activation of the Raf- MAPK/extracellular signal-regulated kinase kinase (MEK)-extracellular signal-regulated kinases (ERK) and mitogen-activated protein kinase kinase (MKK)3/6-p38 pathways. Oncogenic ras initially activates the Raf-MEK-ERK pathway. The persistent activation of the Raf-MEK-ERK pathway leads to accumulation of ROS and DNA damage, which activate the stress-induced MKK3/6-p38 pathway. Unique among the p38 isoforms, the expression level of p38δ is stimulated by the AP-1 and Ets transcription factors, which are activated by the Raf-MEK-ERK pathway in response to oncogenic ras. Three p38 isoforms, p38α, p38γ and p38δ, mediate OIS through differential mechanisms. p38α induces the transcription of p16INK4A by directly phosphorylating and activating the HBP1 transcription factor, and activates a downstream substrate kinase PRAK, which in turn activates p53 by phosphorylating p53 at Ser37. p38γ stimulates the activity of p53 by directly phosphorylating p53 at Ser33. Once activated, p53 induces the expression of its transcriptional target p21WAF1, which, together with p16INK4A, triggers senescence. p38δ mediates OIS through a p53- and p16INK4A-indepdent mechanism, possibly by regulating the activity of two DNA damage checkpoint kinases CHK1 and CHK2 and participating in ras-induced DNA damage responses.
Sequential activation of the ERK and p38 MAPK pathways in OIS
Multiple studies have revealed a major role of the p38 pathway in OIS induced by oncogenic ras or its downstream effector raf-1[20–23]. The protein kinase activity of p38 is stimulated by oncogenic Ha-rasV12 or active raf-1 through its upstream kinases mitogen-activated protein kinase kinase (MKK)3 and MKK6 in human and murine fibroblasts, coinciding with induction of senescence [20–23]. Constitutive activation of p38 by ectopically expressed active mutants of MKK3 or MKK6 causes senescence, whereas inhibition of p38 activity by the pharmacological p38 inhibitor SB203580, a stably expressed dominant-negative mutant of MKK3 or MKK6, or shRNA-mediated knockdown of p38 expression overrides senescence induction [20,21,24,25]. These studies strongly indicate a central role for p38 in OIS.
Several studies indicated that oncogenic ras-induced senescence is also mediated by Raf through the MAPK/extracellular signal-regulated kinase kinase (MEK)-extracellular signal-regulated kinases (ERK) MAPK pathway [7,26,27]. Constitutively active mutants of Raf-1 and MEK1 induce senescence; consistently, inhibition of this pathway by chemical inhibitors of MEK1, dominant negative constructs of MEK1, or shRNA for ERK2 disrupts oncogenic ras-induced senescence. Further investigation revealed that the MKK3/6-p38 pathway acts downstream of the Raf-MEK-ERK cascade to mediate ras-induced OIS [20,21] (Fig. 1). Constitutively active MEK induced MKK3/6-p38 activation and senescence, and induction of p38 activity by ras requires not only active MKK3/6, but also active MEK. More importantly, although the p38 inhibitor SB203580 disrupts OIS induced by oncogenic ras, active MEK1 and active MKK3/6, the MEK inhibitor U0126 only prevented senescence induced by ras and active MEK1, but not by active MKK3/6. These studies place MKK3/6 and p38 downstream of MEK. The mechanism by which MKK3/6-p38 is activated following MEK-ERK activation remains to be determined. A likely intermediate between MEK-ERK and MKK3/6-p38 is reactive oxygen species (ROS), which is induced by ras and mediates both p38 activation and OIS [28–30]. Oncogenic ras-induced accumulation of intracellular ROS is ERK-dependent during senescence induction [31]. Furthermore, shRNA-mediated depletion of ROS-generating oxidases nicotinamide adenine dinucleotide phosphateoxidase (Nox)1 and Nox4 leads to inhibition of oncogenic ras-induced p38 activation and disruption of OIS [32]. These findings suggest that ROS may act downstream of ERK to mediate p38 activation during ras-induced senescence. Another possible candidate is DNA damage. Hyperproliferative signals generated by activated MEK-ERK may cause hyper-replication of DNA, generating DNA damage that is known to activate p38 and induce senescence [9,10,33]. Of note, the involvement of ROS and DNA damage in the sequential activation of the ERK and p38 pathways may not be mutually exclusive, as ROS is a known DNA damaging agent. Specifically, it has been shown that oncogenic ras-induced ROS causes activation of DNA damage responses [34]. Another study demonstrated a positive feedback loop between ROS production and DNA damage responses: DNA damage responses trigger mitochondrial dysfunction leading to enhanced ROS production through a signaling pathway involving p53, p21WAF1, Gadd45a, p38 and TGFβ, and ROS contributes to the long-term maintenance of DNA damage responses, which are essential for induction of senescence [35].
Differential roles of p38 isoforms in OIS
The mammalian genome encodes four isoforms of p38: p38α, p38β, p38γ and p38δ (Box 1). Recent studies demonstrate that these p38 isoforms play different roles in OIS [24,25]. Senescence induction by ras is mediated by p38α, p38γ and p38δ, but not p38β, although all 4 isoforms are activated in senescent cells. shRNA-mediated silencing of p38α, p38γ or p38δ disrupts ras-induced senescence, whereas shRNAs for p38β have no effect. In addition, the constitutively active mutant of p38α, p38γ or p38δ, but not that of p38β, induces senescence in primary human fibroblasts.
p38α, p38γ and p38δ seem to mediate OIS through different mechanisms (Fig. 1). It was shown in an early study that Ser33 of p53 is a substrate of p38α in vitro, and that phosphorylation of Ser33 contributes to p53 activation upon DNA damage [36]. Consistent with this report, recombinant p38α, p38β, p38γ and p38δ all can phosphorylate p53-Ser33 in vitro with similar efficiency [24,25]. However, in cells oncogenic ras-induced phosphorylation of p53-Ser33 appears to be mediated by p38γ, but not the other isoforms [24,25]. When immunoprecipitated from senescent cells only p38γ, but not p38α or p38δ, phosphorylates p53-Ser33. In addition, oncogenic ras-induced phosphorylation of p53-Ser33 and the subsequent increase in transcriptional activity of p53 (as measured by p21WAF1 expression and activity of a p53-responsive luciferase reporter) in senescent cells is greatly reduced by p38γ shRNA, but not by shRNA for p38α or p38δ. Moreover, the constitutively active form of p38γ, but not that of p38α, p38β or p38δ, induces p53-Ser33 phosphorylation and p21WAF1 expression in cells. These findings indicate that during oncogenic ras-induced senescence in cells, phosphorylation of p53-Ser33 and activation of p53 is mainly mediated by p38γ, but not the other isoforms. The mechanism underlying this discrepancy between the in vitro and in vivo activities of the p38 isoforms toward p53 is currently unknown. It is possible that during senescence induction in cells, the kinase activity of p38γ toward p53 is enhanced as a result of posttranslational modification or binding to a positive regulator. Alternatively, the p53 kinase activity of the other p38 isoforms may be repressed by posttranslational modification or an associated inhibitory protein in cells.
Although p38α does not contribute to p53 activation in ras-induced senescence, it is essential for oncogenic ras-induced expression of p16INK4A, another key senescence effector. p38α shRNA, but not p38γ shRNA, abolishes induction of p16INK4A by ras [24]. The regulation of p16INK4A by p38α is likely to be mediated by the transcription factor high mobility group (HMG) box-containing protein 1 (HBP1). Without distinguishing the effects of different p38 isoforms, one study showed that HBP1 is a direct substrate of p38, and phosphorylation of HBP1 at Ser401 by p38 leads to stabilization of the HBP1 protein [37]. The HBP1 protein level indeed increases in cells undergoing ras- or active MKK6 (MKK6E)-induced senescence, where p38 is activated [38,39]. HBP1 mediates senescence induction by the ras-p38 pathway through upregulation of p16INK4A, as HBP1 shRNAs abrogate senescence and p16INK4A induction by oncogenic ras or activated p38, whereas HBP1 overexpression induces senescence and p16INK4A expression [38,39]. Furthermore, HBP1 directly binds to the p16INK4A promoter and upregulates p16INK4A transcription during oncogenic ras-induced senescence, and oncogenic ras and MKK6E enhances the promoter activity of p16INK4A in a HBP1-dependent fashion [39]. These results indicate that p38 induces the transcription of p16INK4A by phosphorylating and activating the p16INK4A transcription factor HBP1 in ras-induced senescence. It will be interesting to investigate the possibility that HBP1 is specifically phosphorylated and activated by p38α to mediate p16INK4A induction.
In contrast to p38α and p38γ, p38δ mediates oncogenic ras-induced senescence in a p53- and p16INK4A-independent manner [25]. Although p38δ shRNA disrupts OIS and the constitutively active mutant of p38δ triggers senescence, neither has any effect on p16INK4A expression, p53-Ser33 phosphorylation or p53 transcriptional activity. Interestingly, p38δ shRNA reduces ras-induced activating phosphorylation of checkpoint kinase (CHK)1 and CHK2, two DNA damage checkpoint kinases, suggesting that p38δ may participate in the DNA damage responses to oncogenic ras. However, how p38δ regulates DNA damage responses in a p53- and p16INK4A-indendent fashion remains unclear. p38δ is unique among the p38 isoforms in that its expression is upregulated during ras-induced senescence [25]. Oncogenic ras induces the Raf-1-MEK-ERK pathway, which, in turn, activates the activator protein (AP)-1 and v-Ets avian erythroblastosis virus E26 oncogene homolog (Ets) transcription factors that are bound to the p38δ promoter, leading to increased transcription of p38δ. Therefore, induction of the pro-senescent function of p38δ by oncogenic ras is achieved through two mechanisms: transcriptional activation by the Raf-1-MEK-ERK-AP-1/Ets pathway, which increases the cellular concentration of the p38δ protein, and posttranslational modification by MKK3/6, which stimulates the enzymatic activity of p38δ.
The role of a p38 downstream substrate kinase PRAK in OIS
Besides regulating the activity of p53 through direct phosphorylation, p38 also mediates ras-induced senescence through a downstream Ser/Thr protein kinase called p38 regulated/activated protein kinase (PRAK), or MAP kinase activated protein kinase 5 (MAPKAPK5 or MK5)[40] (Fig. 1). PRAK, which is activated by ras in a p38-dependent manner, is essential for senescence induction by ras in primary human and murine fibroblasts. In mice, senescence induction is also compromised by PRAK deficiency in skin papillomas induced by DMBA, an environmental carcinogen that prompts oncogenic ras mutations. The function of PRAK in OIS is at least in part mediated by p53. PRAK is essential for ras-induced transcriptional activity of p53. Upon activation by the Ras-p38 cascade, PRAK directly phosphorylates p53 at Ser37, a residue required for p53 to mediate ras-induced senescence. This report also demonstrates that in addition to the PRAK phosphorylation site Ser37, other p53 residues that are phosphorylated during oncogenic ras-induced senescence, including the p38 phosphorylation site Ser33, are important for the ability of p53 to mediate OIS [40]. Therefore, activation of p53 during OIS requires the coordinated phosphorylation of different residues by multiple components of the p38 pathway.
The identity of the p38 isoform responsible for PRAK phosphorylation in OIS is unclear; although in vitro PRAK is mainly activated by p38α and p38β, but not by p38γ and p38δ [41]. This raises a possibility that PRAK is activated primarily by p38α in OIS, as p38β is dispensable for OIS.
A posttranslational modification cascade involving p38, Tip60 and PRAK in OIS
In an attempt to delineate the pathway mediating the function of PRAK in OIS, a yeast-2-hybrid screen was performed to search for PRAK-interacting proteins [42]. This screen led to the identification of Tip60, a member of the MOZ, YBF2, SAS2 and Tip60 (MYST) family of acetyltransferases. Tip60 has been previously implicated in multiple cellular processes, including DNA damage responses, apoptosis, and cell proliferation [43,44], and has been shown to function as a tumor suppressor [45]. This study demonstrates that Tip60 is essential for ras-induced senescence, thus providing a mechanism underlying the tumor suppressing activity of Tip60[42]. Further investigation reveals a posttranslational modification cascade involving p38, Tip60 and PRAK, three proteins essential for OIS (Fig. 2). Upon activation by ras, p38 (likely p38α and p38δ) induces the acetyltransferase activity of Tip60 through phosphorylation of Thr158; activated Tip60, which directly interacts with PRAK, in turn induces the protein kinase activity of PRAK through acetylation of Lys364 in a manner that depends on phosphorylation of both Tip60 and PRAK by p38. These posttranslational modifications are critical for the pro-senescent function of Tip60 and PRAK, as substitutions that disrupt these modifications (T158A in Tip60 and K364R in PRAK) render Tip60 and PRAK incapable of restoring ras-induced senescence in cells expressing shRNAs for Tip60 and PRAK, respectively. These findings have thus defined a novel signaling circuit within the p38 pathway, which mediates OIS. The impact of this circuit on OIS induction is at least partly achieved through regulation of p53 activity, because PRAK-mediated p53-Ser37 phosphorylation in response to oncogenic ras is greatly reduced in cells with Tip60 knockdown. Of note, it was shown previously that Tip60 regulates the activity of p53 by directly acetylating p53 at Lys120[46,47]. Oncogenic ras indeed induces acetylation of p53-Lys120; however, ras-induced p53-Lys120 acetylation is not reduced by Tip60 shRNA [42], suggesting that p53-Lys120 acetylation in OIS may be mediated by a different acetyltransferase.
Fig. 2.
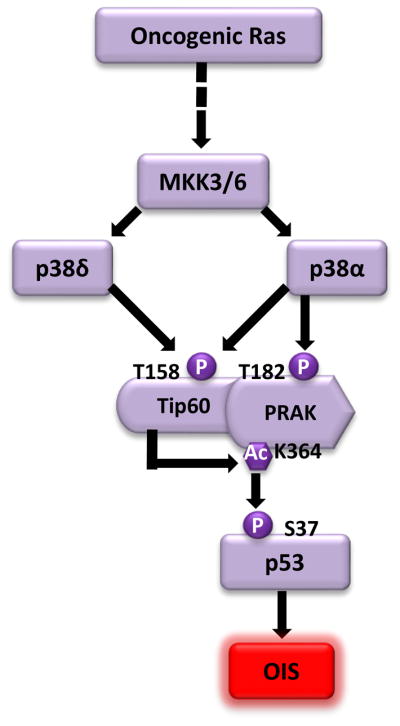
A posttranslational modification cascade that regulates OIS. Upon activation by oncogenic ras, p38α and p38δ induce the acetyltransferase activity of Tip60 through phosphorylation of the Thr158 residue. Activated Tip60, which directly interacts with PRAK, induces the protein kinase activity of PRAK through acetylation of the Lys364 residue. Phosphorylation of PRAK at Thr182 by p38α is required for the optimal acetylation of PRAK by Tip60. Activated PRAK in turn phosphorylates p53 at Ser37, thereby stimulating the activity of p53 in OIS induction.
A tumor suppressing function of the p38 pathway
The role of p38 MAPK in OIS suggests that this pathway may be involved in the suppression of tumor development. Indeed, the tumor-suppressing function of several p38 pathway components has been demonstrated in mouse cancer models in vivo.
Conditional deletion of p38α in adult mice enhances both initiation and progression of K-RasG12V-induced lung cancer due to hyperproliferation of lung epithelium and reduced differentiation of lung progenitor cells [48]. An independent study demonstrates that, compared to wild type animals, mice with liver-specific deletion of p38α show accelerated diethylnitrosamine (DEN)-phenobarbital (Pb)-induced liver tumor development and increased DEN-induced hepatocyte proliferation, which correlates with upregulation of c-Jun N-terminal kinase (JNK)-c-Jun activity [49]. Notably, simultaneous deletion of c-Jun in liver abolishes the enhancement of liver tumor induction and tumor cell proliferation observed in p38α-deficient mice, indicating that p38α inhibits the JNK-c-Jun pathway to control liver cell proliferation and suppress cancer development. Taken together, these results have demonstrated a tumor-suppressing function of p38α in vivo. However, it is unclear whether p38α deficiency promotes tumorigenesis by disrupting OIS in these studies.
Another study shows that the p38 downstream kinase PRAK also suppresses tumorigenesis in vivo. PRAK deletion renders mice prone to skin papilloma induction by DMBA, which induces activating ras mutations, and accelerates lymphomagenesis in Eμ-N-RasG12D transgenic mice, which specifically express an activated N-ras transgene in hematopoietic cells [40,50]. In these models, enhanced tumorigenesis is observed in both PRAK−/− and PRAK+/− mice. Thus, the tumor-suppressing function of PRAK is haploinsufficient. Acceleration of DMBA-induced skin carcinogenesis in PRAK-deficient mice is accompanied by compromised senescence induction, suggesting that the tumor-suppressing effect of PRAK is achieved via its ability to mediate OIS.
Modulation of p38 activity by manipulating its upstream regulators can also impact tumor development in vivo. Deletion of the p38 phosphatase Wip1, which results in p38 activation, inhibits mammary tumorigenesis in mice with mammary gland-specific expression of oncogene erbB2 or v-Ha-ras, both driven by the mouse mammary tumor virus (MMTV) promoter [51]. Inactivation of p38 by SB203580 treatment restores MMTV-erbB2-induced mammary tumor formation in Wip1−/− mice. In a reciprocal experiment, mice with targeted expression of Wip1 in the breast epithelium are prone to mammary tumor induction by the MMTV-erbB2 transgene, and the enhancement of tumorigenesis by Wip1 is eliminated when a constitutively active MKK6 is introduced into these mice [52]. These results indicate that p38 is the target for the tumor-promoting function of Wip1 during breast tumorigenesis in MMTV-erbB2 mice. In addition, deletion of Gadd45a, a p38 binding partner and activator, accelerates MMTV-ras induced mammary tumorigenesis [53], partly due to decreased OIS as a result of impaired p38 activation.
Consistent with the tumor-suppressing function of p38, this pathway is downregulated in human cancers. In a study surveying 20 hepatocellular carcinoma patients, the p38 and MKK6 kinase activities are significantly lower in tumorous lesions as compared to the paired nontumorous tissues, and larger tumors exhibit lower levels of p38 and MKK6 activity than the smaller tumors [54]. p38α expression is also lower in human lung tumors as compared with normal lung tissues [48]. The p38 phosphatase Wip1 is frequently activated through amplification in human breast cancer, resulting in impaired p38 activation in these cells [22,55]. Furthermore, Gstm1, a member of the glutathione S-transferase family that inhibit p38 activation by oxidative stress, is overexpressed in multiple types of cancers [31].
In summary, recent studies have demonstrated a novel function of the p38 pathway in tumor suppression, which is at least partly attributed to the ability of this pathway to mediate OIS.
OIS and PI3K/AKT/mTOR pathway
The PI3K/AKT/mTOR pathway is frequently activated in human cancer (Box 2). Alterations that lead to constitutive activation of this pathway in cancer include genetic and epigenetic inactivation of PTEN, overexpression or activating mutation of PI3K (PIK3CA) and overexpression of AKT [56]. Recent studies indicate that activation of this pathway through multiple components lead to induction of OIS, and have begun to reveal the downstream effectors (Fig. 3A). It was reported initially that a constitutively active, myristoylated form of AKT induces OIS in primary murine fibroblasts and multiple lines of primary cultured human endothelial cells via a p53/p21WAF1 dependent pathway [57]. This is mediated by downregulation of the forkhead transcription factor FOXO3a, which is an AKT downstream substrate. FOXO3a upregulates the transcription of radical scavenger genes, such as manganese superoxide dismutase (MnSOD), which protect cells from oxidative damage. Active AKT inhibits the transcriptional activity of FOXO3a and thereby downregulates MnSOD, leading to an increased level of ROS that induces activation of the p53/p21WAF1 pathway and senescence.
Box 2. The PI3K/AKT/mTOR signaling pathway.
The phosphoinositide 3-kinase (PI3K)/cellular homolog of murine thymoma virus Akt8 oncoprotein (AKT)/mammalian target of rapamycin (mTOR) pathway is one of the most important intracellular pathways, and is frequently activated in human cancers. Under normal physiological conditions, the activity of the PI3K/AKT/mTOR pathway is tightly controlled [56,71,72]. Upon activation by receptor tyrosine kinases or G-protein coupled receptors, PI3K is translocated to the plasma membrane, leading to phosphorylation of phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3). This step is negatively regulated by the phosphatase and tensin homolog (PTEN) phosphatase, which dephosphorylates PIP3 to PIP2. PIP3 recruits the serine-threonine kinase AKT and its activating kinase PDK1 (3-phosphoinositide-dependent kinase 1) to the plasma membrane, where PDK1 activates AKT by phosphorylating threonine 308. Activated AKT phosphorylates tuberous sclerosis complex 2 (TSC2) and thereby inhibits the GTPase-activating protein (GAP) activity of the TSC1/TSC2 complex towards Ras homolog enriched in brain (Rheb). This allows the GTP-bound, active form of Rheb to accumulate and activate mTOR. mTOR forms either mTOR complex 1 (mTORC1) with the regulatory-associated protein of mTOR (Raptor) protein, or mTORC2 with the rapamycin-insensitive companion of TOR (Rictor) protein. Activation of mTORC1 results in increased protein translation through inhibitory phosphorylation of eukaryotic translational initiation factor eIF4E-binding protein 1 (4EBP1), which in turn inhibits eIF4E, and activating phosphorylation of p70S6 kinase (S6K), which in turn phosphorylates ribosomal S6 protein. Activated mTORC2 serves as a positive feedback loop leading to additional activating phosphorylation of AKT. AKT also regulates transcription by inducing the phosphorylation-dependent degradation of forkhead box O (FOXO) transcription factor and inhibitory phosphorylation of glycogen synthase kinase 3β (GSK3β), which negatively regulates the function of c-Jun and Myc transcription factors.
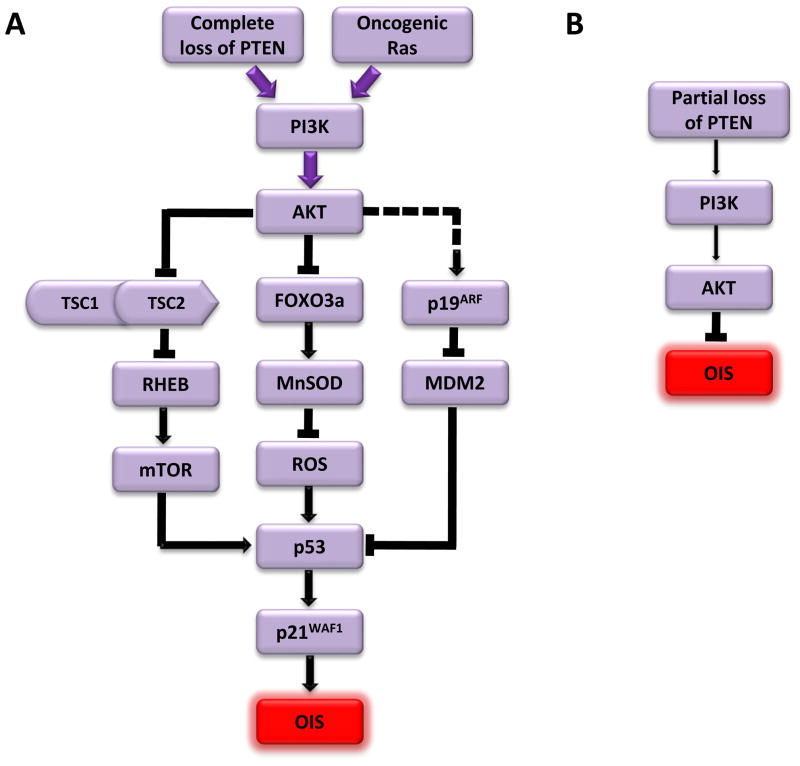
Fig. 3. The role of the PI3K/AKT/mTOR pathway in OIS.
(A) Complete loss of PTEN or ectopic expression oncogenic ras or constitutively active forms of PI3K or AKT leads to strong activation of the PI3K/AKT/mTOR pathway (purple, thick arrows). Activated mTOR stimulates the translation of p53, which in turn stimulates p21WAF1 expression and triggers OIS. Strong activation of AKT also inhibits the FOXO3a transcription factor that upregulates the transcription of a radical scavenger gene MnSOD, leading to decreased MnSOD expression, accumulation of ROS, and activation of the p51/p21WAF1 cascade that mediates OIS. In addition, complete loss of PTEN induces the expression of p19ARF, an inhibitor of MDM2 that serves as a negative regulator of p53, resulting in increased expression and activity of p53.
(B) Partial loss of PTEN leads to moderate activation of the PI3K/AKT pathways (thin arrows), which disrupts OIS.
In addition to AKT activation, loss of PTEN also triggers p53-dependent OIS in primary murine fibroblasts [58]. Complete loss of PTEN induces the expression of p19ARF, an inhibitor of the p53-specific E3 ubiquitin-protein ligase MDM2. This leads to stabilization of the p53 protein and increased expression of p21WAF1, resulting in senescence. More importantly, homozygous deletion of PTEN in prostatic epithelium in mice induced premalignant lesions containing large numbers of senescent cells, which was accompanied by AKT activation and upregulation of p19ARF, p53 and p21WAF1 proteins. Loss of p53 disrupts senescence in the prostate and enhances the development of invasive prostate cancer in PTEN-null mice. These results indicate that loss of PTEN, through the resulting activation of the PI3K/AKT pathway, triggers a p19ARF/p53/p21WAF1-dependent senescence program that suppresses cancer development in vivo. Moreover, a cancer-derived, constitutively active mutant of the catalytic subunit of PI3K (PIK3CA)-E545K, also induces senescence in primary human fibroblasts [59]. These findings indicate that activation of the PI3K/AKT/mTOR pathway through multiple components can trigger OIS (Fig. 3A).
Further supporting the importance of the PI3K/AKT/mTOR pathway in OIS, mTOR is required for oncogenic ras-induced senescence in primary human fibroblasts [60]. Inhibition of the mTORC1 complex by the mTORC1-specific inhibitor rapamycin or shRNAs against mTOR or Raptor delays both replicative and oncogenic ras-induced senescence, whereas inhibition of the mTORC2 complex by Rictor shRNA has no effect. In addition, rapamycin treatment disrupts senescence and p53 accumulation induced by loss of PTEN or constitutively active AKT in primary human and murine cells [59,61]. Treatment with a rapamycin analogue RAD001 also reduces induction of senescence and p53 expression in PTEN-null prostate epithelium in mice [61]. Moreover, PTEN loss induces the translation of the p53 gene in primary murine fibroblasts, presumably as a result of the upregulation of overall gene translation by activated mTOR [61]. These findings indicate that mTORC1, but not mTORC2, is an essential component in the OIS pathway.
Unlike classical OIS, such as that induced by Ha-rasV12, Mos and CDK6, senescence induced by PTEN loss or activated AKT is not accompanied by DNA damage responses [59,61]. Defects in the DNA damage response pathway abrogate Ha-rasV12-induced senescence, but have no effect on senescence induced by PTEN loss [61]. In addition, whereas Ha-rasV12-induced senescence is preceded by an initial period of enhanced cell proliferation [3,7], PTEN loss- and AKT-induced senescence occurs rapidly without an early phase of hyperproliferation and does not rely on DNA replication [59,61]. These results suggest that different oncogenes induce senescence through overlapping, but distinct pathways. Interestingly, a recent study indicates that the nuclear function of PTEN is required for homologous recombination repair of DNA double-strand breaks induced by DNA-damaging agents, and that cells lacking PTEN display persistent DNA damage responses due to defects in DNA repair [62]. It is possible that the DNA repair function of PTEN only becomes essential in the face of genotoxic stress, and thus loss of PTEN in the absence of DNA-damaging agents is not sufficient to trigger DNA damage responses.
The role of the PI3K/AKT/mTOR pathway in OIS is challenged by a recent report that instead of mediating senescence, activation of this pathway abrogates senescence induction by BRAFV600E [63]. shRNA-mediated depletion of PTEN, which induces AKT activation, disrupts BRAFV600E-induced senescence in both primary human fibroblasts and primary human melanocytes. Using a BRAFV600E knock-in mouse model with melanocyte-specific expression of BRAFV600E that develops nevus-like lesions characterized by melanocytic hyperplasia, the authors found that injection of a lentivirus carrying PTEN shRNA into the nevi leads to tumor formation, suggesting that PTEN depletion allows BRAFV600E-expressing nevus cells to resume proliferation. However, whether the tumor formation was indeed due to disruption of senescence in nevi was not shown.
Although one cannot rule out the contribution of tissue-specific effects and differences in the specific oncogenes used to induce senescence, one major difference between these studies demonstrating opposite roles of PTEN loss in senescence is the degree of PTEN inactivation. In the prostate cancer studies [58,61], senescence is induced by homozygous, but not heterozygous, deletion of PTEN both in primary fibroblasts in vitro and in prostate epithelium in vivo. Treatment with a PTEN inhibitor VO-OHpic induces senescence in PTEN+/− cells, while the wild type cells are unaffected [61], suggesting that PTEN activity has to be significantly reduced by more than 50% in order for senescence to occur. By contrast, in the study demonstrating the anti-senescence function of PTEN loss [63], PTEN was knocked down by shRNAs, which only partially inhibit PTEN expression. Indeed, the authors failed to overexpress activated PI3K or AKT family members in melanocytes, raising a possibility that strong PI3K/AKT signaling might induce senescence. Thus, it is highly likely that the outcome of PI3K/AKT/mTOR pathway activation with regard to OIS depends on the dosage of the PTEN gene, and thus the signaling strength of the PI3K/AKT/mTOR pathway (Fig. 3). Although strong activation of this pathway is pro-senescent and tumor suppressing (Fig. 3A), moderate activation abrogates senescence induction by other oncogenes leading to enhanced tumorigenesis (Fig. 3B).
Concluding remarks
Mounting evidence has indicated that OIS is an important tumor suppressing defense response that restricts the progression of benign lesions to malignancy in vivo. The molecular mechanisms underlying OIS have begun to be unveiled. There is solid evidence that OIS is almost invariably enforced by the p53/p21WAF1 and/or p16INK4A tumor suppressor pathways. However, the signaling pathways mediating the activation of p53 and p16INK4A can vary depending on the cell type and the OIS inducer. It should also be noted that although the p53/p21WAF1 and p16INK4A pathways are major regulators of OIS, most of the data were obtained using fibroblasts, and the situation might be more complex in other cell types [64]. In addition, activation of p53 and p16INK4A alone is not sufficient to confer the irreversibility of OIS. Additional mechanisms, such as DNA damage responses, chromatin remodeling through SAHF formation, and the senescence-associated secretory phenotype (SASP) are required for the initiation and maintenance of the irreversible growth arrest and other phenotypes of OIS. Recent studies have demonstrated a role of the p38 MAPK and PI3K/AKT/mTOR pathways in these processes leading to OIS. These findings indicate that OIS is not mediated by a simple, linear pathway, but by an intricate signaling network that is often context-dependent. Further investigations are needed to identify novel players in OIS, and to define the crosstalk and cooperation among these different signaling pathways involved in OIS. A better understanding of the mechanisms that mediate OIS may lead to identification of new targets for cancer therapies based on restoration of OIS in cancer cells.
Recent advances in OIS mechanisms are discussed.
The role of the p38 MAPK pathway in OIS is highlighted.
The role of the PI3K/AKT/mTOR pathway in OIS is explored.
Acknowledgments
We thank the Sun laboratory for stimulating discussions. The Sun laboratory is supported by the National Institutes of Health (CA106768, CA131231 and CA172115).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hayflick L. Living forever and dying in the attempt. Exp Gerontol. 2003;38:1231–1241. doi: 10.1016/j.exger.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26:867–874. doi: 10.1093/carcin/bgh296. [DOI] [PubMed] [Google Scholar]
- 3.Serrano M, et al. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 4.Wei S, et al. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 1999;59:1539–1543. [PubMed] [Google Scholar]
- 5.Suram A, et al. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. EMBO J. 2012;31:2839–2851. doi: 10.1038/emboj.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtois-Cox S, et al. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- 7.Lin AW, et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee AC, et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J Biol Chem. 1999;274:7936–7940. doi: 10.1074/jbc.274.12.7936. [DOI] [PubMed] [Google Scholar]
- 9.Bartkova J, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 10.Di Micco R, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 11.Adams PD. Remodeling of chromatin structure in senescent cells and its potential impact on tumor suppression and aging. Gene. 2007;397:84–93. doi: 10.1016/j.gene.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freund A, et al. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Wajapeyee N, et al. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acosta JC, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Yang G, et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci U S A. 2006;103:16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freund A, et al. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002;22:3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwasa H, et al. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–144. doi: 10.1046/j.1365-2443.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- 22.Bulavin DV, et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet. 2002;31:210–215. doi: 10.1038/ng894. [DOI] [PubMed] [Google Scholar]
- 23.Bulavin DV, et al. Loss of oncogenic H-ras-induced cell cycle arrest and p38 mitogen-activated protein kinase activation by disruption of Gadd45a. Mol Cell Biol. 2003;23:3859–3871. doi: 10.1128/MCB.23.11.3859-3871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwong J, et al. p38alpha and p38gamma mediate oncogenic ras-induced senescence through differential mechanisms. J Biol Chem. 2009;284:11237–11246. doi: 10.1074/jbc.M808327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwong J, et al. Induction of p38delta Expression Plays an Essential Role in Oncogenic ras-Induced Senescence. Mol Cell Biol. 2013;33:3780–3794. doi: 10.1128/MCB.00784-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, et al. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin J, et al. Depletion of ERK2 but not ERK1 abrogates oncogenic Ras-induced senescence. Cell Signal. 2013;25:2540–2547. doi: 10.1016/j.cellsig.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Zdanov S, et al. Identification of p38MAPK-dependent genes with changed transcript abundance in H2O2-induced premature senescence of IMR-90 hTERT human fibroblasts. FEBS Lett. 2006;580:6455–6463. doi: 10.1016/j.febslet.2006.10.064. [DOI] [PubMed] [Google Scholar]
- 29.Colavitti R, Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life. 2005;57:277–281. doi: 10.1080/15216540500091890. [DOI] [PubMed] [Google Scholar]
- 30.Nicke B, et al. Involvement of MINK, a Ste20 family kinase, in Ras oncogene-induced growth arrest in human ovarian surface epithelial cells. Mol Cell. 2005;20:673–685. doi: 10.1016/j.molcel.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 31.Dolado I, et al. p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell. 2007;11:191–205. doi: 10.1016/j.ccr.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Kodama R, et al. ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Genes Cells. 2013;18:32–41. doi: 10.1111/gtc.12015. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, et al. Involvement of the MKK6-p38gamma cascade in gamma-radiation-induced cell cycle arrest. Mol Cell Biol. 2000;20:4543–4552. doi: 10.1128/mcb.20.13.4543-4552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogrunc M, et al. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Passos JF, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bulavin DV, et al. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999;18:6845–6854. doi: 10.1093/emboj/18.23.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiu M, et al. The transcriptional repressor HBP1 is a target of the p38 mitogen-activated protein kinase pathway in cell cycle regulation. Mol Cell Biol. 2003;23:8890–8901. doi: 10.1128/MCB.23.23.8890-8901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, et al. The HBP1 transcriptional repressor participates in RAS-induced premature senescence. Mol Cell Biol. 2006;26:8252–8266. doi: 10.1128/MCB.00604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, et al. Transcriptional factor HBP1 targets P16(INK4A), upregulating its expression and consequently is involved in Ras-induced premature senescence. Oncogene. 2010;29:5083–5094. doi: 10.1038/onc.2010.252. [DOI] [PubMed] [Google Scholar]
- 40.Sun P, et al. PRAK Is Essential for ras-Induced Senescence and Tumor Suppression. Cell. 2007;128:295–308. doi: 10.1016/j.cell.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 41.New L, et al. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 1998;17:3372–3384. doi: 10.1093/emboj/17.12.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng H, et al. A Posttranslational Modification Cascade Involving p38, Tip60, and PRAK Mediates Oncogene-Induced Senescence. Mol Cell. 2013;50:699–710. doi: 10.1016/j.molcel.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sapountzi V, et al. Cellular functions of TIP60. Int J Biochem Cell Biol. 2006;38:1496–1509. doi: 10.1016/j.biocel.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Squatrito M, et al. Tip60 in DNA damage response and growth control: many tricks in one HAT. Trends Cell Biol. 2006;16:433–442. doi: 10.1016/j.tcb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Gorrini C, et al. Tip60 is a haplo-insufficient tumour suppressor required for an oncogene-induced DNA damage response. Nature. 2007;448:1063–1067. doi: 10.1038/nature06055. [DOI] [PubMed] [Google Scholar]
- 46.Charvet C, et al. Phosphorylation of Tip60 by GSK-3 determines the induction of PUMA and apoptosis by p53. Mol Cell. 2011;42:584–596. doi: 10.1016/j.molcel.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang Y, et al. Tip60-Dependent Acetylation of p53 Modulates the Decision between Cell-Cycle Arrest and Apoptosis. Mol Cell. 2006;24:827–839. doi: 10.1016/j.molcel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 48.Ventura JJ, et al. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39:750–758. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- 49.Hui L, et al. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet. 2007;39:741–749. doi: 10.1038/ng2033. [DOI] [PubMed] [Google Scholar]
- 50.Yoshizuka N, et al. PRAK suppresses oncogenic ras-induced hematopoietic cancer development by antagonizing the JNK pathway. Mol Cancer Res. 2012;10:810–820. doi: 10.1158/1541-7786.MCR-11-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bulavin DV, et al. Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nat Genet. 2004;36:343–350. doi: 10.1038/ng1317. [DOI] [PubMed] [Google Scholar]
- 52.Demidov ON, et al. The role of the MKK6/p38 MAPK pathway in Wip1-dependent regulation of ErbB2-driven mammary gland tumorigenesis. Oncogene. 2006 doi: 10.1038/sj.onc.1210032. [DOI] [PubMed] [Google Scholar]
- 53.Tront JS, et al. Gadd45a Suppresses Ras-Driven Mammary Tumorigenesis by Activation of c-Jun NH2-Terminal Kinase and p38 Stress Signaling Resulting in Apoptosis and Senescence. Cancer Res. 2006;66:8448–8454. doi: 10.1158/0008-5472.CAN-06-2013. [DOI] [PubMed] [Google Scholar]
- 54.Iyoda K, et al. Involvement of the p38 mitogen-activated protein kinase cascade in hepatocellular carcinoma. Cancer. 2003;97:3017–3026. doi: 10.1002/cncr.11425. [DOI] [PubMed] [Google Scholar]
- 55.Rauta J, et al. The serine-threonine protein phosphatase PPM1D is frequently activated through amplification in aggressive primary breast tumours. Breast Cancer Res Treat. 2006;95:257–263. doi: 10.1007/s10549-005-9017-7. [DOI] [PubMed] [Google Scholar]
- 56.Engelman JA, et al. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 57.Miyauchi H, et al. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23:212–220. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Z, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Astle MV, et al. AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene. 2012;31:1949–1962. doi: 10.1038/onc.2011.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kolesnichenko M, et al. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle. 2012;11:2391–2401. doi: 10.4161/cc.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alimonti A, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest. 2010;120:681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bassi C, et al. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science. 2013;341:395–399. doi: 10.1126/science.1236188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vredeveld LC, et al. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes Dev. 2012;26:1055–1069. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bianchi-Smiraglia A, Nikiforov MA. Controversial aspects of oncogene-induced senescence. Cell Cycle. 2012;11:4147–4151. doi: 10.4161/cc.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 66.Lu G, et al. TAB-1 modulates intracellular localization of p38 MAP kinase and downstream signaling. J Biol Chem. 2006;281:6087–6095. doi: 10.1074/jbc.M507610200. [DOI] [PubMed] [Google Scholar]
- 67.Shi Y, Gaestel M. In the cellular garden of forking paths: how p38 MAPKs signal for downstream assistance. Biol Chem. 2002;383:1519–1536. doi: 10.1515/BC.2002.173. [DOI] [PubMed] [Google Scholar]
- 68.Bulavin DV, Fornace AJ., Jr p38 MAP kinase’s emerging role as a tumor suppressor. Adv Cancer Res. 2004;92:95–118. doi: 10.1016/S0065-230X(04)92005-2. [DOI] [PubMed] [Google Scholar]
- 69.Han J, Sun P. The pathways to tumor suppression via route p38. Trends Biochem Sci. 2007;32:364–371. doi: 10.1016/j.tibs.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 70.Yoshizuka N, et al. A Novel Function of p38-Regulated/Activated Kinase in Endothelial Cell Migration and Tumor Angiogenesis. Mol Cell Biol. 2012;32:606–618. doi: 10.1128/MCB.06301-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Polivka J, Jr, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2013 doi: 10.1016/j.pharmthera.2013.12.004. [DOI] [PubMed] [Google Scholar]