Summary
Presenilins play essential roles in memory formation, synaptic function, and neuronal survival. Mutations in the Presenilin-1 (PSEN1) gene are the major cause of familial Alzheimer’s disease (FAD). How PSEN1 mutations cause FAD is unclear, and pathogenic mechanisms based on gain or loss of function have been proposed. Here, we generated Psen1 knockin (KI) mice carrying the FAD mutation L435F or C410Y. Remarkably, KI mice homozygous for either mutation recapitulate the phenotypes of Psen1−/− mice. Neither mutation altered Psen1 mRNA expression, but both abolished γ-secretase activity. Heterozygosity for the KI mutation decreased production of Aβ40 and Aβ42, increased the Aβ42/Aβ40 ratio, and exacerbated Aβ deposition. Furthermore, the L435F mutation impairs hippocampal synaptic plasticity and memory and causes age-dependent neurodegeneration in the aging cerebral cortex. Collectively, our findings reveal that FAD mutations can cause complete loss of Presenilin-1 function in vivo, suggesting that clinical PSEN mutations produce FAD through a loss-of-function mechanism.
INTRODUCTION
Dominant mutations in the PSEN1 and PSEN2 genes encoding Presenilin-1 (PS1) and Presenilin-2 (PS2) are the major cause of familial Alzheimer’s disease, and more than 200 distinct causative mutations distributed throughout the coding sequences have been identified (http://www.alzforum.org/mutations). Presenilin (PS) comprises the catalytic component of γ-secretase (Li et al., 2000), which carries out intramembranous cleavage of type I transmembrane proteins, including the amyloid precursor protein (APP) and Notch receptors (De Strooper et al., 1999; Song et al., 1999; Struhl and Greenwald, 1999). Psen1-null mice display perinatal lethality with developmental defects and impaired neurogenesis attributable to loss of Notch signaling activity (Handler et al., 2000; Shen et al., 1997), whereas Psen2-null mice have no detectable brain phenotypes (Saura et al., 2004; Steiner et al., 1999). In the adult brain, Presenilins play essential roles in synaptic function, learning and memory, and neuronal survival (Saura et al., 2004; Wines-Samuelson et al., 2010; Yu et al., 2001; Zhang et al., 2009; Zhang et al., 2010).
Despite these insights into PS function in the brain, the mechanism by which PSEN mutations lead to neurodegeneration and dementia in FAD remains a key unresolved issue. Both gain-of-function (Hardy and Selkoe, 2002) and loss-of-function (Shen and Kelleher, 2007) pathogenic mechanisms have been proposed. FAD mutations in PSEN have been reported to increase Aβ42 production selectively, suggesting that PSEN mutations may cause FAD via increased Aβ42/Aβ40 ratio (Borchelt et al., 1996; Duff et al., 1996; Scheuner et al., 1996). However, FAD patients carrying PSEN mutations develop the disease at a significantly earlier age of onset, relative to APP mutations (Ryman et al., 2014), even though the increase in the Aβ42/Aβ40 ratio conferred by PSEN mutations is typically rather small. Furthermore, overproduction of Aβ including Aβ42 has failed to produce significant neurodegeneration in mice (Games et al., 1995; Hsiao et al., 1996; Mucke et al., 2000), suggesting that Aβ42 overproduction itself may be insufficient to initiate neurodegeneration. Surprisingly, conditional inactivation of Presenilins (Beglopoulos et al., 2004; Saura et al., 2004; Wines-Samuelson et al., 2010) or another component of the γ-secretase complex Nicastrin (Tabuchi et al., 2009) in the adult mouse brain produced widespread neurodegeneration, inflammation and tau hyperphosphorylation, raising the possibility that PSEN mutations may cause neurodegeneration and dementia in FAD via a loss of essential PS functions.
Using a sensitive cell-based assay system, we found that a series of clinical PSEN1 mutations uniformly impaired γ-secretase activity with the L435F and C410Y mutations causing virtually complete loss of γ-secretase-dependent processing of APP and Notch (Heilig et al., 2013; Heilig et al., 2010). These findings raised the pivotal question of whether FAD mutations cause a loss of PS function in vivo, and whether loss of PS function by clinical mutations can trigger key AD-related phenotypes. To address these questions, we introduced the L435F or C410Y mutation into the genomic Psen1 locus, and analyzed their impact on PS1 function and γ-secretase activity in the developing and adult brain. Strikingly, both the L435F and C410Y mutations yielded null alleles in vivo, as knockin (KI) mice homozygous for either mutation displayed morphological, neurodevelopmental and biochemical phenotypes indistinguishable from those of Psen1-null mice. Moreover, replacement of a wild-type Psen1 allele with the L435F KI allele in adult mice produced a series of neuropathological, synaptic and behavioral changes relevant to FAD, including elevation of the Aβ42/Aβ40 ratio and accelerated Aβ deposition in a human APP transgenic background, impaired hippocampal synaptic plasticity and memory, and cerebral cortical neurodegeneration. Collectively, these findings support the hypothesis that loss of PS essential functions plays an important role in FAD pathogenesis.
RESULTS
FAD mutations L435F and C410Y preserve Psen1 mRNA expression but reproduce Psen1-null phenotypes in vivo
To determine the impact of FAD mutations in vivo, we generated two independent KI mice in which the FAD-linked L435F or C410Y mutation was introduced into the genomic Psen1 locus. The L435F mutation was identified in early-onset FAD with cerebral cotton wool plaque neuropathology (Heilig et al., 2010; Rogaeva et al., 2001). The C410Y substitution was one of the first five FAD mutations reported in PSEN1 (Sherrington et al., 1995), and has been extensively characterized including its association with cerebral cotton wool plaques (Haleem et al., 2007; Klunk et al., 2007; Moonis et al., 2005). The L435F or C410Y missense mutation was introduced into Psen1 exon 12 or 11, respectively, by homologous recombination (Figure 1A), and further verified by Southern analysis and sequencing (Figure S1A–C).
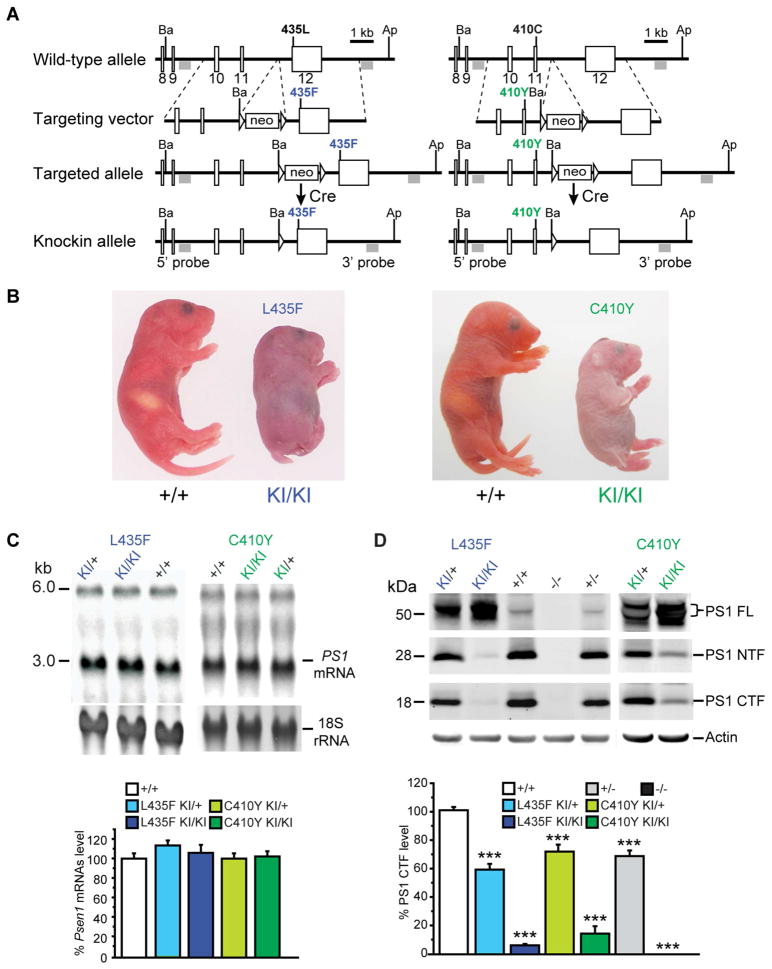
Figure 1. L435F and C410Y KI/KI mice exhibit phenotypes indistinguishable from Psen1−/− mice.
(A) Targeting strategy for the generation of L435F and C410Y KI mice. Boxes represent Psen1 exons 8–12, and the locations of the 5′ and 3′ external probes are shown. The L435F mutation is located in exon 12 and C410Y mutation is located in exon 11. Ba and Ap denote BamHI and ApaI restriction sites, respectively.
(B) Newborn L435F and C410Y KI/KI pups resemble Psen1−/− pups with shortened tail and body axis.
(C) Northern analysis of brain total RNA at P0 shows normal Psen1 mRNA size and level in L435F and C410Y KI/KI and KI/+ mice (n≥5).
(D) Western analysis of brain lysates at P0 shows residual amounts of PS1 NTFs and CTFs in L435F and C410Y KI/KI brains and drastic accumulation of PS1 holoprotein in L435F and C410Y KI/KI and KI/+ brains in a KI gene dosage-dependent manner, whereas these PS1 species are undetectable in Psen1−/− brains (n≥4). Data are represented as mean ± SEM. ***p < 0.001.
Strikingly, homozygous Psen1L435F/L435F (L435F KI/KI) and Psen1C410Y/C410Y (C410Y KI/KI) mice display developmental abnormalities indistinguishable from the phenotypes observed in Psen1−/− mice (Shen et al., 1997), including perinatal lethality, shortened rostro-caudal body axis and shortened, kinked tails (Figure 1B; Table S1). To determine whether introduction of the FAD mutation disrupts Psen1 expression, we examined Psen1 mRNA levels in L435F and C410Y KI/KI, KI/+ and Psen1+/+ brains at postnatal day 0 (P0). In contrast to the absence of Psen1 mRNA in Psen1−/− mice (Shen et al., 1997), Northern analysis showed normal levels of full-length Psen1 mRNA in KI/KI brains (Figure 1C). Western analysis revealed drastically reduced levels of endoproteolytically processed PS1 N- and C-terminal fragments (NTFs and CTFs) in KI/KI brains, and corresponding accumulation of PS1 holoprotein in a KI allele dosage-dependent manner (Figure 1D). Thus, the L435F and C410Y mutations do not affect Psen1 mRNA expression but abolish the normal presenilinase activity of PS1. To assess whether the L435F mutation affects the expression of γ-secretase complex components, we measured levels of Nicastrin and Pen-2, and found that they are similar in Psen1+/+, L435F KI/KI, and KI/+ brains but significantly reduced in Psen1−/− brains (Figure S1D), suggesting that PS1 holoprotein with the L435F mutation is sufficient to maintain normal levels of other γ-secretase components, despite the drastic reduction of PS1 NTFs and CTFs.
Impaired Notch signaling and neurogenesis in L435F and C410Y KI/KI mice
Presenilin is required for the proteolytic cleavage of Notch to release its intracellular effector domain (NICD) (Struhl and Greenwald, 1999). We assessed Notch signaling activity using an in vitro assay for de novo NICD production, and found that NICD production is markedly reduced in L435F and C410Y KI/+ brains and largely eliminated in KI/KI brains (Figure 2A). Northern analysis revealed that levels of the Notch effector gene Hes5 are decreased in L435F and C410Y KI/KI brains to a similar extent as in Psen1−/− brains (Figure 2B). These data provide further confirmation that the L435F and C410Y KI mutations impair Notch signaling.
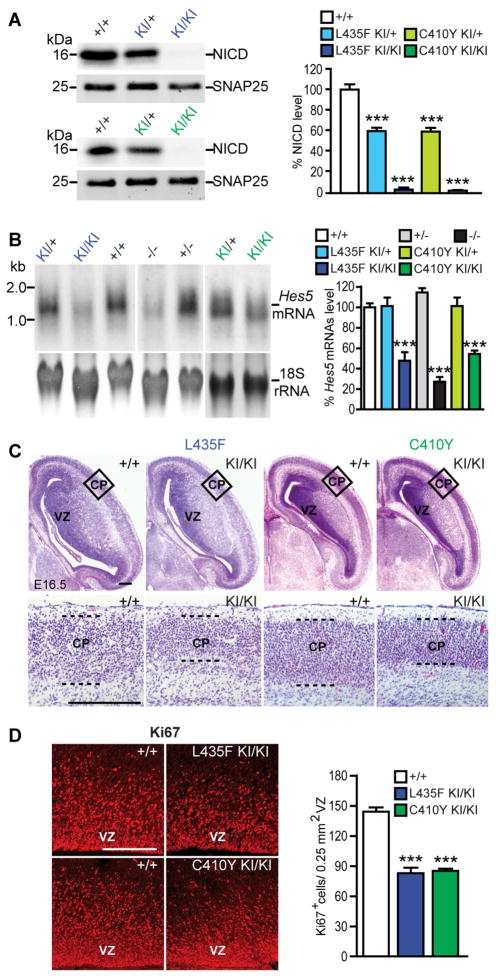
Figure 2. Impaired neurogenesis and Notch signaling in L435F and C410Y KI/KI mice.
(A) In vitro γ-secretase assay using CHAPSO-solubilized E18.5 brain fractions and recombinant Notch substrate N102-FmH followed by Western analysis reveals that NICD production is reduced in L435F KI/+ (n=3) and C410Y KI/+ (n=4) mice, and abolished in L435F KI/KI (n=3) and C410Y KI/KI (n=3) mice, compared to Psen1+/+ controls (n=6). SNAP25 is used as internal loading control for membrane fractions.
(B) Northern analysis of Hes5 expression. Levels of Hes5 mRNA are markedly reduced in L435F KI/KI (n=9), C410Y KI/KI (n=3), Psen1−/− (n=3) brains relative to Psen1+/+ controls (n=7).
(C) Hematoxylin/eosin staining of comparable brain sections from L435F KI/KI, C410Y KI/KI and littermate controls at E16.5 shows that the ventricular zone (VZ) and the developing cortical plate (CP) are thinner in L435F KI/KI and C410Y KI/KI mice. The bottom panels show higher power views of the boxed areas in the developing CP. Dashed lines show the boundaries of the CP. Scale bar: 0.25mm.
(D) The number of proliferating neural progenitor cells in the VZ labeled by Ki67 immunoreactivity is reduced in L435F KI/KI and C410Y KI/KI brains at E16.5 (n=4 for each genotype). Scale bar: 0.25mm. Data are represented as mean ± SEM. ***p < 0.001.
Inactivation of PS1 function causes neural developmental defects, including depletion of neural progenitor cells and impaired neurogenesis (Handler et al., 2000; Kim and Shen, 2008; Shen et al., 1997). We therefore evaluated the impact of the L435F and C410Y mutations on brain development. Similar to Psen1−/− mice (Handler et al., 2000; Shen et al., 1997), L435F and C410Y KI/KI mice display pronounced thinning of the ventricular zones within the lateral ganglionic eminence and developing telencephalon at E16.5, compared to littermate Psen1+/+ mice (Figure 2C). Moreover, the number of proliferating cells detected by Ki67 immunoreactivity is significantly reduced in the ventricular zone of L435F and C410Y KI/KI brains (Figure 2D). These phenotypes bear striking resemblance to those in Psen1−/− mice (Shen et al., 1997), further indicating that the effects of the L435F and C410Y mutations on neurodevelopment and γ-secretase-mediated cleavage of Notch are comparable to the null mutation.
Inactivation of γ–secretase by the L435F and C410Y mutations
To investigate further the impact of the L435F and C410Y mutations on γ-secretase activity, we examined proteolytic processing of APP. Western analysis revealed that APP CTFs, the direct substrate of γ-secretase, accumulate to a similar extent in L435F and C410Y KI/KI mice and Psen1−/− mice, indicating comparable deficiencies in γ-secretase activity (Figure 3A; Figure S2A). We next analyzed γ-secretase activity using an in vitro assay for de novo Aβ production (Takahashi et al., 2003; Watanabe et al., 2012), and found that production of Aβ40 and Aβ42 is absent in KI/KI brains and markedly reduced in KI/+ brains for each mutation (Figure 3B; Figure S2B). We also measured the steady-state levels of endogenous mouse Aβ directly from brain homogenates. Strikingly, endogenous Aβ40 and Aβ42 are undetectable in KI/KI brains, whereas Aβ40 and Aβ42 levels in KI/+ embryonic brains are similar to those in Psen1+/+ brains for each mutation (Figure 3C; Figure S2C). N-Cadherin CTF1, another γ-secretase substrate (Marambaud et al., 2003), also displayed significant accumulation in L435F and C410Y KI/KI as well as Psen1−/− brains (Figure S2D). These results demonstrate that the L435F and C410Y mutations disrupt γ-secretase activity to an extent comparable to complete loss of PS1 function.
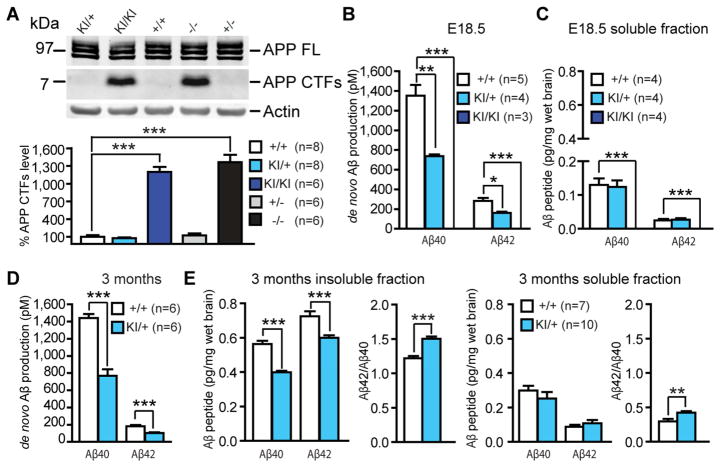
Figure 3. Abolished Aβ production in L435F KI/KI mice and reduced Aβ40 and Aβ42 production in L435F KI/+ mice.
(A) APP CTFs accumulate (>10-fold) in L435F KI/KI and Psen1−/− brains relative to littermate controls at E18.5 (n≥6).
(B) ELISA measurements of Aβ40 and Aβ42 following in vitro γ-secretase assay. In vitro γ-secretase assay reveals that de novo generation of Aβ40 and Aβ42 in L435F KI/+ brains at E18.5 (n=4) is reduced by 46% and 43%, respectively, compared to Psen1+/+ brains (n=5), and they are undetectable in L435F KI/KI brains (n=3).
(C) ELISA measurements of endogenous Aβ40 and Aβ42 from brain homogenates at E18.5. Levels of stead-state endogenous Aβ40 and Aβ42 are similar between L435F KI/+ and Psen1+/+ brains, and they are undetectable in L435F KI/KI brains (n=4 for each genotype).
(D) ELISA measurements of Aβ40 and Aβ42 following in vitro γ-secretase assay in 3-month old mice. De novo generation of Aβ40 and Aβ42 in L435F KI/+ cortical homogenates is reduced by 47% and 43%, respectively, compared to Psen1+/+ cortices (n=6 for each genotype).
(E) ELISA measurements of stead-state endogenous Aβ40 and Aβ42 in both insoluble and soluble fractions from cortical samples at 3 months of age. In insoluble fractions, levels of endogenous Aβ40 and Aβ42 are reduced in L435F KI/+ cortices (n=10) relative to controls (n=7), and the Aβ42/Aβ40 ratio is increased. In soluble fractions, levels of endogenous Aβ40 and Aβ42 are not significantly different between Psen1L435F/+ and Psen1+/+ cortices, but the Aβ42/Aβ40 ratio is significantly increased in Psen1L435F/+ cortices. Data are represented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
Reduction of Aβ40 and Aβ42 production and elevation of the Aβ42/Aβ40 ratio in adult KI/+ brains
Although L435F KI/KI mice exhibit perinatal lethality, L435F KI/+ mice are viable and fertile. Notably, L435F KI/+ mice provide a precise genetic animal model of the PSEN1 mutation in FAD. To determine how the L435F mutation affects Aβ production in the adult brain, we performed in vitro assay using L435F KI/+ and littermate Psen1+/+ mice at 3 months of age. Levels of de novo Aβ40 and Aβ42 production in L435F KI/+ mice are reduced to approximately half of those in Psen1+/+ mice (Figure 3D). We next measured steady-state levels of endogenous Aβ40 and Aβ42 in both insoluble and soluble fractions from the cerebral cortex at 3 months of age. In insoluble fractions, levels of Aβ40 and Aβ42 are significantly reduced (by 29% and 17%, respectively) and the Aβ42/Aβ40 ratio is correspondingly increased (~15%) in L435F KI/+ mice (Figure 3E). In soluble fractions, levels of Aβ40 and Aβ42 are lower than that in insoluble fractions and not significantly altered but the ratio of Aβ42/Aβ40 is elevated in L435F KI/+ mice compared to Psen1+/+ mice (Figure 3E). These results show that heterozygosity for the L435F mutation elevates the Aβ42/Aβ40 ratio due to a greater reduction in accumulation of Aβ40 relative to Aβ42.
The L435F KI mutation exacerbates Aβ plaque deposition in human mutant APP transgenic mice
To investigate how the loss of PS1 function conferred by the L435F mutation affects human Aβ production and plaque formation, we crossed L435F KI/+ mice to transgenic mice overexpressing human wild-type (APPWT, I5 line) or mutant (APPMT, J20 line) APP (Mucke et al., 2000), in which Aβ40 and Aβ42 are dramatically overproduced (Table S2). We first measured levels of human Aβ40 and Aβ42 in insoluble and soluble fractions from the cerebral cortex of Psen1L435F/+; APPWT and Psen1+/+; APPWT mice at 9 months of age (Figure 4A). In Psen1L435F/+; APPWT mice, steady-state levels of human Aβ40 and Aβ42 are significantly reduced in insoluble fractions and the Aβ42/Aβ40 ratio is increased; in soluble fractions, levels of human Aβ40 are markedly reduced and the Aβ42/Aβ40 ratio is increased, although levels of Aβ42 are not significantly changed (Figure 4A). No amyloid plaque deposition was detected in the cerebral cortex of Psen1L435F/+; APPWT mice even at the age of 18 months (Figure S3).
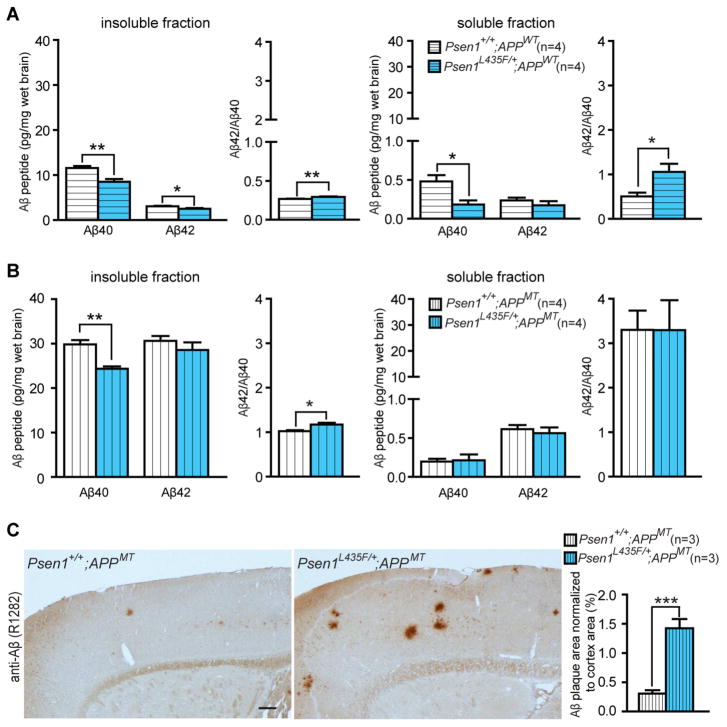
Figure 4. Reduced human Aβ accumulation but accelerated amyloid deposition in L435F KI/+ mice expressing a human mutant APP transgene.
(A) ELISA measurements of steady-state human Aβ40 and Aβ42 in insoluble and soluble fractions from Psen1L435F/+; APPWT (n=4) and controls (n=4) at 9 months of age. In Psen1L435F/+; APPWT mice, human Aβ40 is reduced by ~26% in insoluble fractions and ~62% in soluble fractions, whereas human Aβ42 is reduced by ~19% in insoluble fractions but not significant changed in soluble fractions. Note that levels of Aβ40 and Aβ42 are much higher in insoluble fractions than in soluble fractions (>20-fold for Aβ40, >10-fold for Aβ42). The Aβ42/Aβ40 ratio is significantly enhanced in soluble and insoluble fractions.
(B) ELISA measurements of steady-state human Aβ40 and Aβ42 in insoluble and soluble fractions of cortical homogenates from Psen1+/+; APPMT (n=4) and Psen1L435F/+; APPMT (n=4) at 2 months of age. Levels of human Aβ40 are significantly reduced in insoluble fractions but not in soluble fractions, whereas levels of human Aβ42 are not significantly altered. Note that levels of Aβ40 are drastically higher in insoluble fractions than in soluble fractions (>100-fold). The Aβ42/Aβ40 ratio is significantly increased in insoluble fractions.
(C) Aβ immunostaining with an Aβ antibody (R1282) reveals significantly increased plaque deposition in the cerebral cortex of Psen1L435F/+; APPMT mice compared to Psen1+/+; APPMT mice at 9 months of age. Scale bar: 0.25 mm. Data are represented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
Similarly, we measured levels of human Aβ40 and Aβ42 in insoluble and soluble fractions from the cerebral cortex of Psen1L435F/+; APPMT and Psen1+/+; APPMT mice at 2 months of age (Figure 4B). In Psen1L435F/+; APPMT mice, steady-state levels of human Aβ40 are significantly reduced in insoluble fractions, and the Aβ42/Aβ40 ratio is increased (Figure 4B). Furthermore, Aβ immunostaining disclosed accelerated amyloid deposition in the cerebral cortex of Psen1L435F/+; APPMT mice compared to Psen1+/+; APPMT mice at 9 months of age (Figure 4C). Thus, although the L435F mutation abolishes the γ-secretase activity of mutant PS1 protein, heterozygosity for the mutation nevertheless enhances the Aβ42/Aβ40 ratio and promotes amyloid deposition.
Memory impairment caused by the L435F mutation
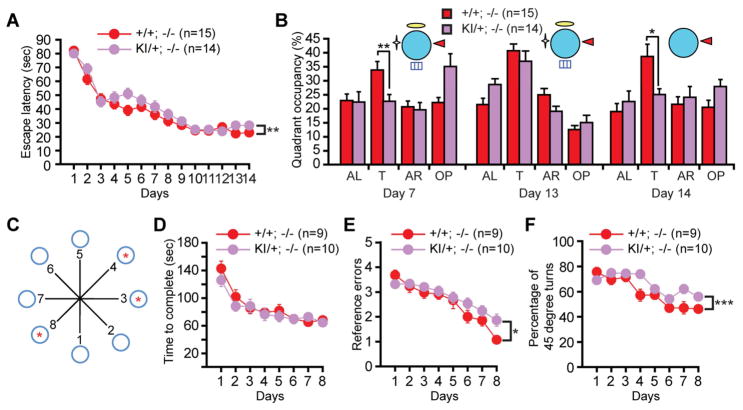
To determine the impact of the L435F mutation on hippocampal learning and memory, we assessed the performance of Psen1L435F/+; Psen2−/− (KI/+; −/−) mice and Psen1+/+; Psen2−/− (+/+; −/−) control littermates using the Morris water maze and radial arm maze tasks. Previous work has shown that PS2 expression is up-regulated in the absence of PS1 (Watanabe et al., 2014), which may compensate for behavioral and synaptic deficits caused by PS1 deficiency. We therefore examined the impact of the L435F mutation on behavioral and synaptic phenotypes in a Psen2−/− background. We first analyzed hippocampal reference memory in the hidden-platform Morris water maze task. Psen1L435F/+; Psen2−/− mice exhibited significantly higher latencies across the 14-day training period (p<0.01, Figure 5A), and displayed significantly lower target quadrant occupancy in the probe test at day 7 (p<0.01, Figure 5B), indicating impaired reference memory acquisition. Both genotypic groups showed similar target quadrant occupancies in the probe trial at day 13 (Figure 5B), suggesting that additional training overcame this reference memory deficit.
Figure 5. Memory impairment caused by the L435F mutation.
(A) Psen1L435F/+; Psen2−/− (KI/+; −/−) mice (n=14) showed significantly higher escape latencies than Psen1+/+; Psen2−/− (+/+; −/−) littermates (n=15) during the 14-day training period (F1, 27 = 8.06; p<0.01) in the Morris water maze test.
(B) Psen1L435F/+; Psen2−/− mice show significantly reduced target quadrant occupancy in the day 7 probe test, but both genotypic groups show similar target quadrant occupancy in the day 13 probe test. Psen1L435F/+; Psen2−/− mice display significantly reduced quadrant occupancy in the partial-cue probe test on day 14. T, target quadrant; AL, adjacent left quadrant; AR, adjacent right quadrant; OP, opposite quadrant.
(C) Naïve Psen1L435F/+; Psen2−/− mice (n=10) and littermate controls (n=9) were subjected to 8 days of training to locate the three baited arms (indicated by red *) following fasting in the radial arm maze test.
(D) Psen1L435F/+; Psen2−/− and control mice require similar amounts of time to complete the food search (F1, 17 = 1.18, p > 0.05) during the 8-day radial arm maze training.
(E) Psen1L435F/+; Psen2−/− mice display significantly more reference errors (F1, 17 = 4.59, p < 0.05) during the 8-day training period.
(F) Psen1L435F/+; Psen2−/− mice show significantly higher percentages of 45 degree turns (F1, 17 = 13.63, p < 0.001) during the 8-day training period. Data are represented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
See also Figure S4.
The hippocampus mediates pattern completion during memory recall, an ability to retrieve stored memory traces in response to incomplete sensory cues (Nakazawa et al., 2002). Neuropsychological analysis has shown evidence for impaired behavioral pattern completion in AD patients (Ally et al., 2013). To assess pattern completion, we removed three of the four distal spatial cues and administered another probe trial at day 14. Despite their normal quadrant occupancy under full-cue conditions on day 13, Psen1L435F/+; Psen2−/− mice displayed reduced target quadrant occupancy under partial-cue conditions (p<0.05, Figure 5B), suggesting impaired hippocampal pattern completion. Psen1L435F/+; Psen2−/− mice exhibited normal escape latency, path length and swim speed in the visible platform water maze task (Figure S4), indicating that the observed learning and memory impairments are not attributable to deficits in vision, motivation or sensorimotor abilities.
To obtain an independent assessment of hippocampal memory, we further subjected naïve Psen1L435F/+; Psen2−/− and littermate control mice to a spatial discrimination version of the radial arm maze task (Dillon et al., 2008; Rossi-Arnaud et al., 1991). Psen1L435F/+; Psen2−/− and their littermate controls were trained to locate three baited arms over 8 consecutive days (Figure 5C). Although both genotypic groups required similar amounts of time to complete the task (Figure 5D), Psen1L435F/+; Psen2−/− mice showed more reference memory errors (p<0.05, Figure 5E) and a higher proportion of 45° turns into adjacent arms (p<0.001, Figure 5F). Thus, multiple behavioral tasks revealed hippocampal spatial memory deficits caused by the L435F KI mutation.
Synaptic dysfunction caused by the L435F mutation
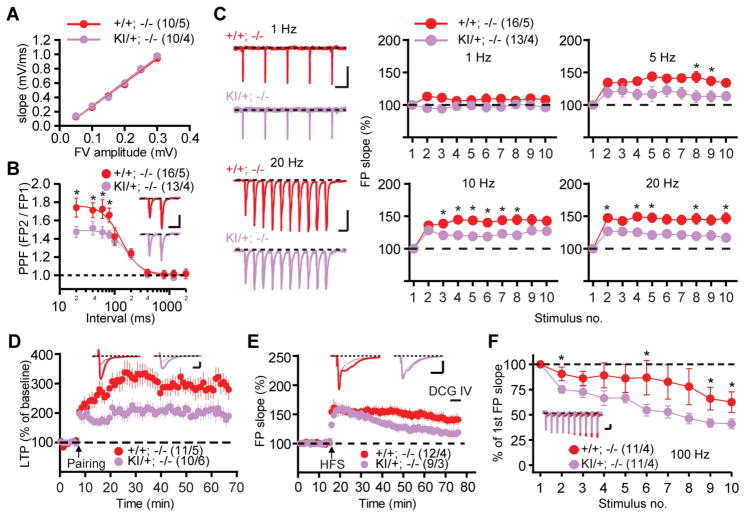
We then performed electrophysiological analysis on Psen1L435F/+; Psen2−/− mice and Psen1+/+; Psen2−/− littermate controls to determine the effect of the L435F mutation on hippocampal synaptic function and to investigate the cellular mechanism underlying the identified memory defects. Whereas basal synaptic transmission was normal in the Schaffer collateral pathway (Figure 6A), Psen1L435F/+; Psen2−/− mice displayed impaired short-term plasticity, as indicated by reduced paired-pulse facilitation (Figure 6B) and frequency facilitation (Figure 6C). As the impairment in frequency facilitation was already maximal at the 5-Hz stimulation frequency, we tested whether the observed deficits in short-term synaptic plasticity at this frequency of presynaptic activation could affect the inducibility of long-term potentiation (LTP) at the Schaeffer collateral-CA1 synapses. Using voltage-clamp recording, we found that LTP induced by pairing presynaptic stimuli with postsynaptic depolarization was significantly reduced in Psen1L435F/+; Psen2−/− mice compared to littermate controls (Figure 6D), whereas NMDAR-mediated EPSCs were unaffected (Figure S5). These impairments resemble the synaptic phenotypes previously identified in mice with conditional Psen1/2 inactivation (Saura et al., 2004; Zhang et al., 2009; Zhang et al., 2010).
Figure 6. Synaptic dysfunction caused by the L435F mutation.
(A) Normal basal synaptic transmission in Psen1L435F/+; Psen2−/− mice. The synaptic input-output relationship was obtained by plotting the fiber volley amplitude against the initial slope of the evoked fEPSP. Each point represents data averaged across all slices for a narrow bin of FV amplitude. The lines represent the best linear regression fit.
(B) Reduced paired-pulse facilitation (PPF) in Psen1L435F/+; Psen2−/− mice. The graph depicts the paired-pulse response ratio obtained at different inter-stimulus intervals (in ms). Scale bar: 1 mV, 50 ms.
(C) Synaptic facilitation elicited by stimulus trains of the indicated frequencies in Psen1L435F/+; Psen2−/− and littermate control mice. Frequency facilitation is significantly decreased in the 5–20 Hz range in Psen1L435F/+; Psen2−/− mice. Scale bar: 1 Hz: 1 mV, 500 ms; 20 Hz: 1 mV, 50 ms. FP, field potential.
(D) Pairing-induced LTP at the Schaffer collateral-CA1 synapses in slices from Psen1L435F/+; Psen2−/− mice and littermate controls. Representative traces before (thin) and after (thick) LTP induction are shown. Insets show the average of 15 EPSCs recorded before and 45–50 min after the induction. Scale bar: 20 ms, 50 pA.
(E) LTP induced at the recurrent commissural/associational (C/A)-CA3 synapses in slices from Psen1L435F/+; Psen2−/− mice and littermate controls. LTP was induced with 3 trains of high frequency stimulation (HFS). Representative traces before (thin) and after (thick) LTP induction are shown. Superimposed traces are averages of four consecutive responses 1 min before and 50 min after HFS. The lack of response to DCG IV, which selectively inhibits mossy fiber LTP, confirms that the responses are obtained from (C/A)-CA3 synapses. Scale bar: 10 ms, 0.5 mV.
(F) Increased synaptic depression at (C/A)-CA3 synapses during LTP-inducing stimulation in Psen1L435F/+; Psen2−/− mice. Insets show representative fEPSP traces obtained during single stimulation train. Scale bar, 10 ms, 2 mV.
The values in parentheses indicate the number of neurons or slices (left) and the number of mice (right) used in each experiment. Data are represented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
See also Figure S5.
We further assessed synaptic plasticity at commissural/associational (C/A)-CA3 synapses, as pattern completion is thought to be mediated by the recurrent collateral connectivity of CA3 neurons (Nakazawa et al., 2002). We found that LTP was impaired at (C/A)-CA3 synapses in Psen1L435F/+; Psen2−/− mice (Figure 6E). Moreover, short-term depression during the initial phase of the LTP-inducing stimulus train was increased at (C/A)-CA3 synapses of Psen1L435F/+; Psen2−/− mice (Figure 6F). These data indicate that the L435F mutation impairs short-term and long-term synaptic plasticity at hippocampal CA1 and CA3 synapses.
The L435F mutation causes age-dependent neurodegeneration
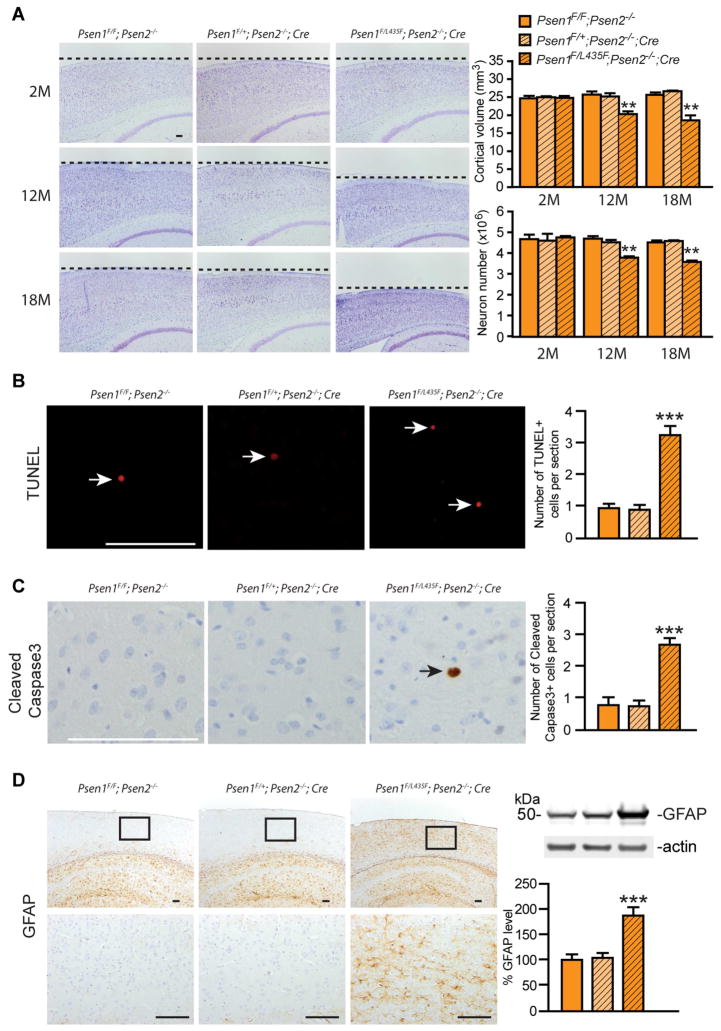
Conditional inactivation of PS in the adult cerebral cortex using the Camk2a-Cre transgene causes severe, age-dependent neurodegeneration (Saura et al., 2004; Wines-Samuelson et al., 2010). Further analysis of Psen conditional knockout mice with varying Psen2 gene dosage showed that partial inactivation of PS also results in age-dependent neurodegeneration, though with lesser severity and a later age of onset (Watanabe et al., 2014). To examine the impact of the L435F mutation on neuronal survival, relative to the Psen1 wild-type allele, we generated Psen1F/L435F; Psen2−/−; Camk2a-Cre mice and littermate Psen1F/+; Psen2−/−; Camk2a-Cre and Psen1F/F; Psen2−/− mice. Remarkably, Psen1F/L435F; Psen2−/−; Camk2a-Cre mice display age-dependent neurodegeneration throughout the cerebral cortex, with marked reductions in cortical volume (31.5±5.2%) and neuron number (22.1±1.4%) at 18 months of age (Figure 7A), whereas Psen1F/+; Psen2−/−; Camk2a-Cre mice, which has one wild-type Psen1 allele, show no neurodegeneration (Figure 7A). Moreover, increased apoptosis was identified by TUNEL staining (Figure 7B) and cleaved Caspase3 immunostaining (Figure 7C) in the neocortex of Psen1F/L435F; Psen2−/−; Camk2a-Cre mice.
Figure 7. The L435F mutation causes age-dependent neurodegeneration.
(A) Comparison of brain morphology between Psen1F/L435F; Psen2−/−; Cre mice and littermate controls at 2, 12 and 18 months of age. Left: Images of Nissl stained comparable sagittal brain sections. Right: Cortical volume and neuron number obtained by stereological quantification following Nissl staining and NeuN immnostaining, respectively. Cortical volume and neuron number are normal at 2 months of age but significantly reduced at 12 (~22%) and 18 (~32%) months of age in Psen1F/L435F; Psen2−/−; Cre mice relative to controls (n≥3 for each genotype at each age). Scale bar: 0.1 mm.
(B) More TUNEL-positive cells are observed in the cerebral cortex of Psen1F/L435F; Psen2−/−; Cre mice (n=3), compared to control mice (n=4) at 18 months of age. Scale bar: 0.1 mm.
(C) More active caspase3-positive cells are detected in the cerebral cortex of Psen1F/L435F; Psen2−/−; Cre mice (n=3), relative to control mice (n=4) at 18 months of age. Scale bar: 0.1 mm.
(D) Increased GFAP immunoreactivity in the cortex of Psen1F/L435F; Psen2−/−; Cre mice at 12 months of age indicates astrogliosis. Bottom images show higher magnification views of the boxed area in the cortex. Western analysis also shows increased levels of GFAP in the cortex of Psen1F/L435F; Psen2−/−; Cre mice (KI) compared to littermate control Psen1F/F; Psen2−/− and Psen1F/+; Psen2−/−; Cre mice (n=8 for each genotype). Data are represented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001. Scale bar: 0.1mm.
See also Figure S6.
We further evaluated astrogliosis and microgliosis, as inflammatory responses often accompany neurodegeneration. Immunohistochemical and western analyses reveal enhanced GFAP levels in the cortex of Psen1F/L435F; Psen2−/−; Camk2a-Cre mice (Figure 7D), indicating the occurrence of astrogliosis in these mice. We also found that immunoreactivity for the microglial marker Iba1 is elevated in the neocortex and hippocampus of Psen1F/L435F; Psen2−/−; Camk2a-Cre mice (Figure S6), indicating that neurodegeneration is also associated with microgliosis. These data demonstrate that the L435F mutation abolishes the ability of PS1 to support neuronal survival during aging.
DISCUSSION
Large numbers of PSEN mutations are linked to FAD, but how these mutations cause the disease remains unresolved. Earlier studies in patient plasma, transgenic mice and cell lines found that PSEN mutations increased Aβ42 levels and/or the Aβ42/Aβ40 ratio (Borchelt et al., 1996; Duff et al., 1996; Hardy and Selkoe, 2002; Scheuner et al., 1996), leading to the hypothesis that PSEN mutations cause FAD via a gain-of-function mechanism mediated by selective overproduction of Aβ42 (Hardy and Selkoe, 2002). Interestingly, genetic rescue studies in C. elegans showed that PSEN mutations resulted in partial loss of PS activity (Levitan et al., 1996). However, overexpression of the Psen1 A246E transgene rescued the phenotypes of Psen1−/− mice (Davis et al., 1998; Qian et al., 1998), and KI mice carrying the Psen1 M146V mutation did not exhibit phenotypes of Psen1−/− mice (Guo et al., 1999), though they exhibited hippocampal memory deficits (Sun et al., 2005). These findings suggested that PSEN mutations might not impair PS function in the mammalian brain.
Subsequent genetic studies yielded the surprising discovery that loss of PS function in the adult cerebral cortex recapitulates key features of AD, including progressive memory deficits and age-dependent, widespread neurodegeneration (Beglopoulos et al., 2004; Saura et al., 2004; Wines-Samuelson et al., 2010). Furthermore, similar genetic analysis of another γ-secretase component, Nicastrin, confirmed the importance of γ-secretase in memory, synaptic function and neuronal survival (Lee et al., 2014; Tabuchi et al., 2009). In principle, there are two potential explanations for these unexpected in vivo findings: the essential roles played by PS in the maintenance of memory and synaptic function and protection of cortical neurons from neurodegeneration may represent mere coincidence with no relevance to FAD; or alternatively, pathogenic PSEN mutations compromise the essential physiological roles of PS in memory, synaptic function and neuronal survival, leading over time to neurodegeneration and dementia in FAD.
The current study was designed to address this important unanswered question as to whether PSEN mutations indeed compromise the essential functions of PS in the brain, particularly with respect to adult brain phenotypes relevant to FAD. We chose two independent mutations PSEN1 L435F and C410Y, both of which were clinically and neuropathologically confirmed in multiple early-onset FAD families (Campion et al., 1999; Campion et al., 1995; Goudsmit et al., 1981; Heilig et al., 2010; Poorkaj et al., 1998; Rogaeva et al., 2001; Sherrington et al., 1995). Each mutation was introduced into the genomic locus of the Psen1 gene, so that the FAD mutations are expressed under the control of the endogenous regulatory elements as in human patients. Thus, these KI mice are genetically equivalent to human FAD patients carrying the same PSEN1 mutation.
To our great surprise, both homozygous L435F and C410Y KI/KI mice are phenotypically indistinguishable from Psen1−/− mice (Shen et al., 1997), and exhibit perinatal lethality with complete penetrance (Figure 1, Table S1). However, in contrast to the absence of Psen1 mRNA and protein in Psen1−/− mice, levels of Psen1 mRNA are normal in the brain of L435F and C410Y KI/KI mice (Figure 1). Levels of PS1 NTFs and CTFs are drastically reduced in the brain of L435F and C410Y KI/KI mice with a greater reduction by the L435F mutation (Figure 1). This minor difference between the two mutations correlates with the severity of the phenotypes in L435F and C410Y KI/KI newborn pups (Figure 1), and is consistent with earlier reports regarding the functional impairment of the presenilinase and γ-secretase activity caused by these two mutations (Heilig et al., 2013; Heilig et al., 2010). Corresponding to these changes, PS1 holoprotein accumulates in the L435F and C410Y KI/+ and KI/KI brains in a KI allele dosage-dependent manner (Figure 1). Importantly, PS1 holoprotein appears to participate in the stable γ-secretase complex, as levels of other γ-secretase components are unaffected in KI/KI mice but are reduced in Psen1−/− mice (Figure S1). Thus, the L435F and C410Y mutations do not affect Psen1 mRNA expression but drastically reduce presenilinase activity and the production of the functional PS1 NTFs and CTFs.
Further analysis revealed impaired neurogenesis and reduced neural progenitor population in L435F and C410Y KI/KI brains (Figure 2), similar to Psen1-null embryos (Handler et al., 2000; Shen et al., 1997). Notch signaling evaluated by the expression of the Notch target Hes5 is reduced in L435F and C410Y KI/KI embryonic brains (Figure 2). NICD production, measured by an in vitro γ-secretase assay, is reduced in L435F and C410Y KI/+ brains, and is abolished in L435F and C410Y KI/KI brains (Figure 2), indicating that both L435F and C410Y mutations eliminate γ-secretase activity. This interpretation is further supported by the findings that the CTFs of APP and N-cadherin, both of which are γ-secretase substrates, accumulate dramatically in L435F and C410Y KI/KI brains to a level comparable to Psen1−/− brains (Figure 3, Figure S2), and that γ-secretase-mediated production of Aβ40 and Aβ42 is abolished in KI/KI brains (Figure 3). These data demonstrate that the L435F and C410Y mutations abolish γ-secretase activity, leading to accumulation of γ-secretase substrates and impairment of Notch signaling and neurogenesis. This study demonstrates that FAD mutations in the PSEN genes can cause complete loss of its function and γ-secretase activity in vivo, and can recapitulate Psen1-null phenotypes in homozygosity.
The loss of PS1 and γ-secretase function brought about in vivo by the L435F and C410Y mutations is remarkably consistent with their previously reported effects in a sensitive cell-based system (Heilig et al., 2013; Heilig et al., 2010). These mutations are likely particularly deleterious to γ-secretase activity due to their predicted proximity to the enzyme active site (Sato et al., 2008) and the introduction of bulky aromatic side chains, which likely disrupts the structure of γ-secretase. Analysis of a panel of FAD mutations in the same cell-based system demonstrated uniform impairment of γ-secretase activity, with the severity varying among mutations (Heilig et al., 2013; Heilig et al., 2010). Thus, we suggest that the loss of in vivo function conferred by the L435F and C410Y mutations analyzed here represents a general property of FAD mutations. Our findings may differ from previously reported PSEN1 KI (e.g. M146V) and transgenic mice due to the varying severity of different PSEN1 mutations on impairment of γ-secretase activity and to overexpression of mutant PS1 transgene that could compensate for the partial loss of PS1 function.
In contrast to abolished production of Aβ40 and Aβ42 in KI/KI embryonic brains, their production is reduced in KI/+ embryonic and adult brains measured by in vitro γ-secretase assay (Figure 3). Steady-state levels of Aβ40 and Aβ42 measured by ELISA are also reduced in the KI/+ adult brain, and the reduction of Aβ40 is slightly greater than that of Aβ42, leading to a modest but significant increase (~15%) of the Aβ42/Aβ40 ratio (Figure 3). Similar results (Figure 4, Table S2) were obtained when L435F KI/+ mice were crossed to APP transgenic mice overexpressing (~2-fold) either human wild-type (I5 line) or mutant (J20 line) APP containing both the Swedish and the Indiana mutations (Mucke et al., 2000). Strikingly, heterozygosity for the L435F KI mutation markedly accelerates amyloid deposition in mutant APP transgenic mice (Figure 4). This finding demonstrates that PSEN mutations can act through a loss-of-function mechanism to elevate the Aβ42/Aβ40 ratio and thereby promote amyloid deposition. Since production of Aβ40 and Aβ42 measured by in vitro γ-secretase assay is reduced to comparable extents by the heterozygous L435F KI mutation (Figure 3), the greater reduction in steady-state accumulation of Aβ40 relative to Aβ42 may reflect their differential clearance.
Synaptic dysfunction is thought to be a pathogenic precursor of frank neurodegeneration, and the hippocampus is particularly vulnerable in AD. Relative to wild-type PS1, the L435F mutation leads to impaired short-term synaptic plasticity and LTP in the hippocampal Schaeffer collateral pathway (Figure 6, Figure S5). These synaptic deficits are compatible with the spatial memory deficits observed in the hidden platform version of the water maze, the radial arm maze and the pattern completion tasks (Figure 5, Figure S4). Similar synaptic and memory deficits were also observed in Psen conditional knockout mice (Saura et al., 2004; Zhang et al., 2009; Zhang et al., 2010). Consistent with the pattern completion deficits observed in the KI mice (Figure 5), LTP is also impaired in the commissural/associational pathway in hippocampal area CA3, which is thought to play a crucial role in this process (Figure 6, Figure S5). The pattern completion deficit exhibited by L435F KI mice parallels the impaired behavioral pattern completion reported in AD patients (Ally et al., 2013).
Importantly, compared to the wild-type Psen1 allele, the L435F mutant allele is incapable of supporting normal neuronal survival and preventing neurodegeneration in the aging brain, and causes age-dependent neurodegeneration, as shown by progressive loss of cortical neurons and increases of apoptosis as well as astrogliosis and microgliosis (Figure 7, Figure S6). Thus, this study provides an animal model in which a pathogenic FAD mutation causes progressive and widespread neurodegeneration. Our findings are consistent with prior reports indicating that PSEN mutations could enhance apoptosis in cultured cells (Vito et al., 1996; Wolozin et al., 1996). Interestingly, the extent of neurodegeneration associated with the Psen1 L435F mutation is less severe compared to that in Psen conditional knockout mice, raising the possibility that PS1 holoprotein may partially support neuronal survival in the aging brain despite its essentially complete loss of γ-secretase activity. Further studies will be needed to elucidate the underlying mechanism.
More than 80% of identified FAD mutations reside within the PSEN genes, but the pathogenic mechanism remains unresolved. While earlier studies supported a toxic gain-of-function pathogenic mechanism based on excessive production of Aβ42 (Borchelt et al., 1996; Duff et al., 1996; Scheuner et al., 1996), this hypothesis is at odds with several lines of experimental data. For example, overproduction of Aβ42, even at massive levels (e.g. J20 mice overproduce Aβ42 >40-fold at 2 months and >6400-fold at 17 months), did not produce significant neurodegeneration in mouse models (Irizarry et al., 1997; Saura et al., 2005). Human FAD patients carrying PSEN1 mutations develop the disease at earlier ages than APP mutation carriers (Ryman et al., 2014), even though PSEN1 mutations have modest effects on the Aβ42/Aβ40 ratio in mice (e.g. ~15% increase for the L435F mutation while overall production of Aβ42 and Aβ40 is reduced). Furthermore, FAD PSEN mutations cause loss of PS activity in C. elegans (Levitan et al., 1996) and in cultured cells (Heilig et al., 2013; Heilig et al., 2010; Song et al., 1999), and loss of PS in the adult mouse brain recapitulates widespread age-dependent neurodegeneration, gliosis, memory loss and synaptic dysfunction (Beglopoulos et al., 2004; Saura et al., 2004; Wines-Samuelson et al., 2010). These observations suggest that the pathogenic mechanism underlying PSEN mutations is more complex than initially anticipated. Our in vivo analysis of two independent clinical PSEN1 mutations provides important experimental support for the Presenilin hypothesis, which posits that loss of PS function plays an important role in the pathogenesis of FAD (Kelleher and Shen, 2010; Shen and Kelleher, 2007).
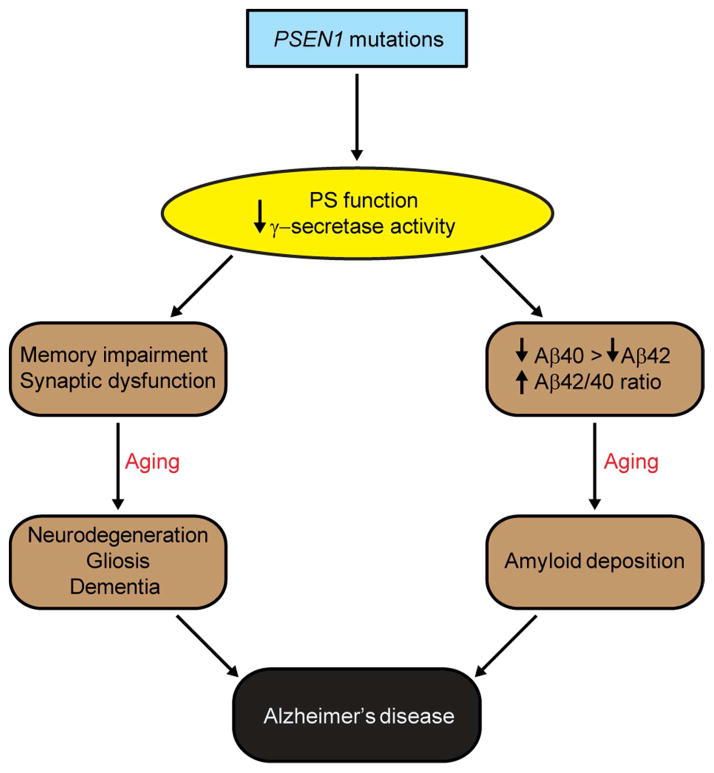
Through the development and multidisciplinary analysis of KI mice expressing PSEN1 mutations, the current study reveals two parallel pathways affected by PSEN mutations that are relevant to FAD pathogenesis (Figure 8). Pathogenic PSEN mutations cause synaptic dysfunction, memory impairment, and age-dependent neurodegeneration via a loss of essential PS functions. In addition, loss of PS function conferred by PSEN mutations promotes amyloid pathology by inhibiting γ-secretase activity, decreasing Aβ production, and increasing the Aβ42/40 ratio due to a greater relative reduction in Aβ40 accumulation (Figure 8). An intriguing implication of our findings is that therapeutic strategies aimed at restoring, rather than inhibiting, normal PS function in the brain may hold promise for the effective treatment of AD.
Figure 8. A schematic model for FAD.
Pathogenic PSEN1 mutations cause loss of PS1 function and γ-secretase activity, leading to AD-relevant functional and neuropathological changes, including memory impairment, synaptic dysfunction, age-dependent neurodegeneration, gliosis and dementia. In parallel, the loss of PS function produced by pathogenic PSEN1 mutations also reduces Aβ production and increases the Aβ42/Aβ40 ratio due to the greater reduction of Aβ40 production than Aβ42, thereby promoting amyloid plaque deposition. Thus, PSEN mutations produce the full spectrum of AD phenotypes through a loss-of-function mechanism.
EXPERIMENTAL PROCEDURES
Brief description of the experimental procedures is included below, and full experimental details can be found in Supplemental Information.
Generation of Psen1 L435F and C410Y KI Mice
Targeting vectors for the generation of the L435F and C410Y KI alleles were constructed by PCR amplification of the 5′ and 3′ homologous sequences from genomic DNA followed by site-directed mutagenesis to introduce the mutation (1303C->T in exon 12 for L435F, 1229G->A in exon 11 for C410Y). The targeting vector was transfected into ES cells, and the ES cells carrying the correct homologous recombination events were screened and confirmed by Southern analysis. The correctly targeted ES cells were injected into blastocysts to generate chimeric mice. Mice carrying the targeted allele were crossed with Camk2a-Cre transgenic mice (Saura et al., 2004) to remove the floxed neomycin cassette and produce heterozygous KI mice. L435F KI/+ mice were crossed with Psen2−/− (Steiner et al., 1999) to generate Psen1L435F/+; Psen2−/− mice, which were further crossed with Psen1F/F; Psen2−/−; Camk2a-Cre mice to generate Psen1F/L435F; Psen2−/−; Camk2a-Cre mice. Mice were maintained on the C57BL6/J-129 hybrid genetic background and littermate controls were used for all analysis. Electrophysiological, behavioral, histological and biochemical analyses were performed in a genotype-blind manner. All procedures relating to animal care and treatment conformed to institutional and NIH guidelines.
In vitro γ-Secretase Assay
γ-Secretase activities were measured by cell-free in vitro assay using CHAPSO-solubilized brain fractions and bacterially expressed recombinant proteins as substrates (Takahashi et al., 2003). See Supplemental Information for full experimental details.
ELISA
Aβ40 and Aβ42 generated in in vitro assays were measured using the 11A50-B10/4G8 and 12F4/4G8 sandwich ELISA, respectively (Watanabe et al., 2012), and the Aβ antibodies were purchased from Covance. Endogenous mouse and human Aβ40 and Aβ42 were measured by MSD 96-well MULTI-SPOT Human/Rodent (4G8) Abeta Triplex Ultra-sensitive Assay (MesoScale Discovery). See Supplemental Information for full experimental details.
Behavioral Analysis
Littermate male mice were used in the behavioral tests. For the Morris water maze tasks, mice were trained for 14 days with four trials daily, and the full-cue probe tests were administered at days 7 and 13 and the partial-cue probe test was administered at day 14. For the radial arm maze, all mice were single housed and fed restrictively to reduce and maintain their body weight by 20% before and during the 8-day training session. See Supplemental Information for full experimental details.
Electrophysiological Analysis
Acute hippocampal slices (400μm) were prepared and recorded as described previously (Zhang et al., 2009). See Supplemental Information for full experimental details of field and whole-cell recording at hippocampal Schaffer collateral-CA1 synapses and recurrent commissural/associational-CA3 synapses.
Histological analysis
Embryonic brains were dissected at E16.5 and processed for serial paraffin sections (10μm). Every fifth section was stained with hematoxylin and eosin, and comparable sections from both genotypic groups were compared. The number of Ki67-immunoreactive cells was quantified using 4 comparable sections per brain and 4 brains per genotype. Adult brains were perfused with PBS, fixed in 4% PFA, processed for paraffin embedding, and serially sectioned (10μm). Adult brain sections were stained with 0.5 % cresyl violet (Nissl) or immunostained with antibodies against NeuN (1:300; Millipore), cleaved Caspase-3 (1:100, Cell Signaling Technology), GFAP (1:500; Sigma), Iba1 (1:250; Wako), Aβ (R1282; 1:1000).
Statistical Analysis
Statistical analysis was performed using one-way ANOVA or two-tailed unpaired Student’s t-test for all comparisons of the biochemical, behavioral and electrophysiological results, except that Two-way ANOVA was used to determine the genotypic effects on latencies in the water maze, reference errors and percentages of 45 degree turns in the radial arm maze, and EPSCs of NMDAR. A value of p < 0.05 was considered significant. All data are represented as mean ± SEM.
Supplementary Material
Highlights.
FAD-linked Presenilin-1 mutations cause complete loss of PS1 function in vivo
FAD-linked Presenilin-1 mutations abolish γ-secretase activity
FAD-linked Presenilin-1 mutations impair hippocampal memory and synaptic function
FAD-linked Presenilin-1 mutations cause age-dependent neurodegeneration
Acknowledgments
We would like to thank T. Südhof for critical reading of the manuscript, L. Mucke for APP transgenic mice, T. Iwatsubo and T. Tomita for the pTrcHis2A-C100-FmH and pTrcHis2A-N102-FmH plasmids, E. Heilig, T. Ding, H. Zhao for assistance, and other lab members for helpful discussions. This work was supported by grants from the National Institutes of Health (R01NS041783 and R01NS042818 to JS, and R01NS075346 to RJK).
Footnotes
AUTHOR CONTRIBUTIONS
J.S. and R.J.K. conceived and directed the project, D.X., J.S., and R.J.K. designed experiments, D.X. performed most experiments and generated all figures; H.W., B.W., S.H.L., Y.L., E.T., and V.Y.B. performed experiments and contributed to figures; D.X., J.S., and R.J.K wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ally BA, Hussey EP, Ko PC, Molitor RJ. Pattern separation and pattern completion in Alzheimer’s disease: Evidence of rapid forgetting in amnestic mild cognitive impairment. Hippocampus. 2013 doi: 10.1002/hipo.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beglopoulos V, Sun X, Saura CA, Lemere CA, Kim RD, Shen J. Reduced beta-amyloid production and increased inflammatory responses in presenilin conditional knock-out mice. J Biol Chem. 2004;279:46907–46914. doi: 10.1074/jbc.M409544200. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, et al. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Campion D, Dumanchin C, Hannequin D, Dubois B, Belliard S, Puel M, Thomas-Anterion C, Michon A, Martin C, Charbonnier F, et al. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet. 1999;65:664–670. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion D, Flaman JM, Brice A, Hannequin D, Dubois B, Martin C, Moreau V, Charbonnier F, Didierjean O, Tardieu S, et al. Mutations of the presenilin I gene in families with early-onset Alzheimer’s disease. Hum Mol Genet. 1995;4:2373–2377. doi: 10.1093/hmg/4.12.2373. [DOI] [PubMed] [Google Scholar]
- Davis JA, Naruse S, Chen H, Eckman C, Younkin S, Price DL, Borchelt DR, Sisodia SS, Wong PC. An Alzheimer’s disease-linked PS1 variant rescues the developmental abnormalities of PS1-deficient embryos. Neuron. 1998;20:603–609. doi: 10.1016/s0896-6273(00)80998-8. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, et al. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Goudsmit J, White BJ, Weitkamp LR, Keats BJ, Morrow CH, Gajdusek DC. Familial Alzheimer’s disease in two kindreds of the same geographic and ethnic origin. A clinical and genetic study. J Neurol Sci. 1981;49:79–89. doi: 10.1016/0022-510x(81)90190-8. [DOI] [PubMed] [Google Scholar]
- Guo Q, Fu W, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- Haleem K, Lippa CF, Smith TW, Kowa H, Wu J, Iwatsubo T. Presenilin-1 C410Y Alzheimer disease plaques contain synaptic proteins. Am J Alzheimers Dis Other Demen. 2007;22:137–144. doi: 10.1177/1533317506298051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler M, Yang X, Shen J. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development. 2000;127:2593–2606. doi: 10.1242/dev.127.12.2593. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Heilig EA, Gutti U, Tai T, Shen J, Kelleher RJ., 3rd Trans-dominant negative effects of pathogenic PSEN1 mutations on gamma-secretase activity and Abeta production. J Neurosci. 2013;33:11606–11617. doi: 10.1523/JNEUROSCI.0954-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig EA, Xia W, Shen J, Kelleher RJ., 3rd A presenilin-1 mutation identified in familial Alzheimer disease with cotton wool plaques causes a nearly complete loss of gamma-secretase activity. J Biol Chem. 2010;285:22350–22359. doi: 10.1074/jbc.M110.116962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Shen J. Genetics. Gamma-secretase and human disease. Science. 2010;330:1055–1056. doi: 10.1126/science.1198668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Shen J. Presenilins are required for maintenance of neural stem cells in the developing brain. Mol Neurodegener. 2008;3:2. doi: 10.1186/1750-1326-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Price JC, Mathis CA, Tsopelas ND, Lopresti BJ, Ziolko SK, Bi W, Hoge JA, Cohen AD, Ikonomovic MD, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Sharma M, Sudhof TC, Shen J. Synaptic function of nicastrin in hippocampal neurons. Proc Natl Acad Sci U S A. 2014;111:8973–8978. doi: 10.1073/pnas.1408554111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan D, Doyle TG, Brousseau D, Lee MK, Thinakaran G, Slunt HH, Sisodia SS, Greenwald I. Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1996;93:14940–14944. doi: 10.1073/pnas.93.25.14940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, et al. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Moonis M, Swearer JM, Dayaw MP, St George-Hyslop P, Rogaeva E, Kawarai T, Pollen DA. Familial Alzheimer disease: decreases in CSF Abeta42 levels precede cognitive decline. Neurology. 2005;65:323–325. doi: 10.1212/01.wnl.0000171397.32851.bc. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorkaj P, Sharma V, Anderson L, Nemens E, Alonso ME, Orr H, White J, Heston L, Bird TD, Schellenberg GD. Missense mutations in the chromosome 14 familial Alzheimer’s disease presenilin 1 gene. Hum Mutat. 1998;11:216–221. doi: 10.1002/(SICI)1098-1004(1998)11:3<216::AID-HUMU6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Qian S, Jiang P, Guan XM, Singh G, Trumbauer ME, Yu H, Chen HY, Van de Ploeg LH, Zheng H. Mutant human presenilin 1 protects presenilin 1 null mouse against embryonic lethality and elevates Abeta1-42/43 expression. Neuron. 1998;20:611–617. doi: 10.1016/s0896-6273(00)80999-x. [DOI] [PubMed] [Google Scholar]
- Rogaeva EA, Fafel KC, Song YQ, Medeiros H, Sato C, Liang Y, Richard E, Rogaev EI, Frommelt P, Sadovnick AD, et al. Screening for PS1 mutations in a referral-based series of AD cases: 21 novel mutations. Neurology. 2001;57:621–625. doi: 10.1212/wnl.57.4.621. [DOI] [PubMed] [Google Scholar]
- Rossi-Arnaud C, Fagioli S, Ammassari-Teule M. Spatial learning in two inbred strains of mice: genotype-dependent effect of amygdaloid and hippocampal lesions. Behav Brain Res. 1991;45:9–16. doi: 10.1016/s0166-4328(05)80175-5. [DOI] [PubMed] [Google Scholar]
- Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, Goate A, Frommelt P, Ghetti B, Langbaum JB, et al. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology. 2014;83:253–260. doi: 10.1212/WNL.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C, Takagi S, Tomita T, Iwatsubo T. The C-terminal PAL motif and transmembrane domain 9 of presenilin 1 are involved in the formation of the catalytic pore of the gamma-secretase. J Neurosci. 2008;28:6264–6271. doi: 10.1523/JNEUROSCI.1163-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura CA, Chen G, Malkani S, Choi SY, Takahashi RH, Zhang D, Gouras GK, Kirkwood A, Morris RG, Shen J. Conditional inactivation of presenilin 1 prevents amyloid accumulation and temporarily rescues contextual and spatial working memory impairments in amyloid precursor protein transgenic mice. J Neurosci. 2005;25:6755–6764. doi: 10.1523/JNEUROSCI.1247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ, 3rd, Kandel ER, Duff K, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Song W, Nadeau P, Yuan M, Yang X, Shen J, Yankner BA. Proteolytic release and nuclear translocation of Notch-1 are induced by presenilin-1 and impaired by pathogenic presenilin-1 mutations. Proc Natl Acad Sci U S A. 1999;96:6959–6963. doi: 10.1073/pnas.96.12.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Duff K, Capell A, Romig H, Grim MG, Lincoln S, Hardy J, Yu X, Picciano M, Fechteler K, et al. A loss of function mutation of presenilin-2 interferes with amyloid beta-peptide production and notch signaling. J Biol Chem. 1999;274:28669–28673. doi: 10.1074/jbc.274.40.28669. [DOI] [PubMed] [Google Scholar]
- Struhl G, Greenwald I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999;398:522–525. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- Sun X, Beglopoulos V, Mattson MP, Shen J. Hippocampal spatial memory impairments caused by the familial Alzheimer’s disease-linked presenilin 1 M146V mutation. Neurodegener Dis. 2005;2:6–15. doi: 10.1159/000086426. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Chen G, Sudhof TC, Shen J. Conditional forebrain inactivation of nicastrin causes progressive memory impairment and age-related neurodegeneration. J Neurosci. 2009;29:7290–7301. doi: 10.1523/JNEUROSCI.1320-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Hayashi I, Tominari Y, Rikimaru K, Morohashi Y, Kan T, Natsugari H, Fukuyama T, Tomita T, Iwatsubo T. Sulindac sulfide is a noncompetitive gamma-secretase inhibitor that preferentially reduces Abeta 42 generation. J Biol Chem. 2003;278:18664–18670. doi: 10.1074/jbc.M301619200. [DOI] [PubMed] [Google Scholar]
- Vito P, Lacana E, D’Adamio L. Interfering with apoptosis: Ca(2+)-binding protein ALG-2 and Alzheimer’s disease gene ALG-3. Science. 1996;271:521–525. doi: 10.1126/science.271.5248.521. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Iqbal M, Zheng J, Wines-Samuelson M, Shen J. Partial loss of presenilin impairs age-dependent neuronal survival in the cerebral cortex. J Neurosci. 2014;34:15912–15922. doi: 10.1523/JNEUROSCI.3261-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Xia D, Kanekiyo T, Kelleher RJ, 3rd, Shen J. Familial frontotemporal dementia-associated presenilin-1 c.548G>T mutation causes decreased mRNA expression and reduced presenilin function in knock-in mice. J Neurosci. 2012;32:5085–5096. doi: 10.1523/JNEUROSCI.0317-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wines-Samuelson M, Schulte EC, Smith MJ, Aoki C, Liu X, Kelleher RJ, 3rd, Shen J. Characterization of age-dependent and progressive cortical neuronal degeneration in presenilin conditional mutant mice. PLoS One. 2010;5:e10195. doi: 10.1371/journal.pone.0010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B, Iwasaki K, Vito P, Ganjei JK, Lacana E, Sunderland T, Zhao B, Kusiak JW, Wasco W, D’Adamio L. Participation of presenilin 2 in apoptosis: enhanced basal activity conferred by an Alzheimer mutation. Science. 1996;274:1710–1713. doi: 10.1126/science.274.5293.1710. [DOI] [PubMed] [Google Scholar]
- Yu H, Saura CA, Choi SY, Sun LD, Yang X, Handler M, Kawarabayashi T, Younkin L, Fedeles B, Wilson MA, et al. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001;31:713–726. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, Sudhof TC, Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang C, Ho A, Kirkwood A, Sudhof TC, Shen J. Inactivation of presenilins causes pre-synaptic impairment prior to post-synaptic dysfunction. J Neurochem. 2010;115:1215–1221. doi: 10.1111/j.1471-4159.2010.07011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.