Abstract
Objective
Ovarian cancer is the most lethal gynecological malignancy that affects women. Recent data suggests the disease may originate in the fallopian fimbriae; however, the anatomical origin of ovarian carcinogenesis remains unclear. This is largely driven by our lack of knowledge regarding the structure and function of normal fimbriae and the relative paucity of models that accurately recapitulate the in vivo fallopian tube. Therefore, a human three-dimensional (3D) culture system was developed to examine the role of the fallopian fimbriae in serous tumorigenesis.
Methods
Alginate matrix was utilized to support human fallopian fimbriae ex vivo. Fimbriae were cultured with factors hypothesized to contribute to carcinogenesis, namely; H2O2 (1mM) a mimetic of oxidative stress, insulin (5 µg/ml) to stimulate glycolysis, and estradiol (E2, 10nM) which peaks before ovulation. Cultures were evaluated for changes in proliferation and p53 expression, criteria utilized to identify potential precursor lesions. Further, secretory factors were assessed after treatment with E2 to identify if steroid signaling induces a pro-tumorigenic microenvironment.
Results
3D fimbriae cultures maintained normal tissue architecture up to 7 days, retaining both epithelial subtypes. Treatment of cultures with H2O2 or insulin significantly induced proliferation. However, p53 stabilization was unaffected by any particular treatment, although was induced by ex vivo culturing. Moreover, E2-alone treatment significantly induced its canonical target PR and expression of IL8, a factor linked to poor outcome.
Conclusions
3D alginate cultures of human fallopian fimbriae provide an important microphysicological model, which can be further utilized to investigate serous tumorigenesis originating from the fallopian tube.
Keywords: fallopian tube, fimbriae, microphysiological modeling
Background
High-grade serous cancer (HGSC), the most lethal histotype of ovarian cancer, has been postulated to originate from the epithelium lining the fallopian tube fimbriae [1]. Traditionally, HGSC was thought to arise from the ovarian surface epithelium (OSE). However, a clear ovarian precursor has yet to be identified, and screening of high risk patients has not improved recurrence and survival in several decades [2]. A potential precursor has recently been described in the secretory epithelium of the fallopian tube fimbriae; the ‘p53 signature’[3]. These lesions are classified by stabilized p53, a protein dysregulated in ~96% of HGSC [4]. p53 signatures are thought to transform into serous tubal intraepithelial carcinomas (STICs) [3], which have been found concomitant with HGSC tumors and often harbor identical mutations in p53, suggesting a common origin [5].
Despite a potential role in the origin of HGSC, there are few models for the investigation of the human fallopian tube epithelium (FTE) that accurately recapitulate the in vivo environment. Although cell culture is a valuable model and allows for continual passage of human FTE and for targeted genetic manipulation, traditional two-dimensional culture is unable to simulate interaction with the fallopian stroma and generally does not allow for maintenance of ciliated FTE [6–8]. Although an advanced FTE model, which retains ciliated cells has been reported, this model manipulates the architecture and eliminates the stromal cells [9]. Further, human FTE cells require artificial immortalization via SV40-T antigen [6–8], which sequesters p53 to the nucleus, functionally silencing p53 or equivalent siRNA molecules [10]. This is counter to the majority of p53 alterations seen in HGSC, where mutation allows for p53 gain-of-function rather than silencing [11]. Thus by immortalizing human FTE for in vitro research, the cells become a less accurate model of important preneoplastic changes suggested to occur in HGSC carcinogenesis. Moreover, although transgenic murine models have been developed using fallopian specific promoters driving Cre recombinase [12], the murine anatomy is different from that of the human, with continuation of the oviduct into a bursal sac and the absence of a fimbriated end. Thus, the development of a human 3D fallopian model is a critical goal for our field.
Irrespective of the site of origin, reducing ovulation through use of oral contraception is protective against ovarian cancer [13], suggesting a role for an ovulatory factor(s) in the initiation of the disease. However, the impact of ovulatory factors on FTE and how they might promote HGSC is unclear. Unopposed estrogen (E2) signaling is an aspect of ovulation linked to increased risk of ovarian cancer, as compared to combined E2 and progesterone signaling experienced during pregnancy, breastfeeding, and while taking the oral contraceptive pill [13]. Oxidative stress is also enhanced during ovulation, and is known to induce DNA damage in oviductal epithelium [14]. Yet, the impact of oxidative stress and E2 signaling on p53 stabilization and proliferation in fimbriae, hypothesized to be the earliest transformative changes in the FTE [3], is unknown.
The purpose of this study was to develop an ex vivo three-dimensional (3D) model of the human fallopian tube, which maintains tissue architecture and more accurately recapitulates the in vivo environment. This model allows for investigation of specific ovulatory components, and their effects on FTE proliferation. Insulin, a common culture supplement and a known mitogen, was utilized as a positive control to validate the system. The impact of E2 on fallopian samples was further investigated, to define the impact of ovarian hormones and how E2 might promote a transformative microenvironment. Finally, p53 stabilization, the hallmark of the purported precursor to HGSC, was evaluated after extended culture and treatment with ovulatory factors.
Materials and Methods
Tissue collection
Fallopian fimbriae were collected with consent prior to surgery at the University of Illinois at Chicago (UIC IRB #2012-0539). Patients utilized in this study were undergoing salpingectomy for a variety of gynecological purposes (outlined in Supplementary Table 1). Resulting tissues were deemed morphologically normal and considered benign as determined by gross examination by the University of Illinois at Chicago Pathology Department. A total of 12 samples from patients ranging from 28–62 years of age (average age of 43).
3D culture optimization and treatment
Tissues were micro-dissected in alpha-MEM (Gibco, Carlsbad, CA) with 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA). Individual fimbriae were separated into ~50mm3 pieces. A portion of the tissue was fixed in 2% paraformaldehyde for use as an uncultured control. For optimization studies, fimbriae were cultured without matrix, encapsulated in 0.5% alginate, or encapsulated in 0.5% alginate with 1 mg/ml collagen and 0.1% fibronectin, as previously described for murine cultures [15]. For subsequent treatments, 0.5% alginate encapsulated fimbriae were randomly assigned to treatment groups, with at least five fimbriae per condition, per patient, in a 24-well plate containing alpha-MEM and 1% penicillin-streptomycin. Tissues were treated with 1 µl/ml ethanol (vehicle), 10 nM E2 (Sigma-Aldrich, St. Louis, USA), 1 mM H2O2 (Fisher Scientific, Pittsburgh, PA), or 5 µg/ml insulin (via ITS (insulin; transferrin 5 µg/ml; selenite 5 ng/ml) Roche, Indianapolis, IN), and cultured for 2 or 7 days. Prior to fixation, fimbriae were labeled with 10 µM bromodeoxyurine (BrdU, Sigma-Aldrich) for 24 hours to denote proliferating cells. Fimbriae cultures were fixed (2% paraformaldehyde) followed by dehydration in ethanol and xylene, and embedment in paraffin.
Tissue preparation and immunohistochemistry
Sections (5 µm) were cut and stained via hematoxylin and eosin for morphological analysis or immunohistochemistry was preformed to localize proteins of interest as previously described [14]. Briefly, slides were rehydrated through an ethanol gradient, prior to 0.1M sodium citrate retrieval and peroxidase block. Tissues probed for BrdU were exposed to 4M HCl and 0.1M NaB4O7 (Fisher Scientific) to denature DNA. All immunohistochemical reagents were obtained from Vector Laboratories, Inc (Burlingame, CA) unless otherwise stated. Tissues were blocked in 3% bovine serum albumin (Gemini, West Sacramento, CA)-TBS / 10% serum and incubated with a primary antibody 1:50 acetylated tubulin (Cell Signaling, Cambridge, MA); 1:200 BrdU (AbCam, Cambridge, MA); 1:100 cytokeratin 8 (CK8, Developmental Studies Hybridoma Bank, Iowa City, IA); 1:50 p53 (Santa Cruz, Santa Cruz, CA); 1:100 PAX8 (Proteintech, Chicago, IL); 1:100 pH2AX (Cell Signaling); or 1:75 PR (Santa Cruz)) overnight at 4°. Tissues were washed in TBS-0.1% Tween and incubated with a secondary antibody (1:200), before being probed with ABC peroxidase standard, followed by detection with 3,3’-diaminobenzidine (DAB) and counterstained with hematoxylin.
Image capture and analysis
Immunohistochemistry images were taken via a Nikon E600 microscope, DXM1200 digital camera and NIS Elements software (Nikon Instruments, Melville, NY). For proliferation analysis, concurrent sections were stained for CK8 and BrdU. BrdU sections were imaged and epithelial cells (CK8 positive) were quantified for proliferation via ImageJ software (NIH, Bethesda, MD). At least three fimbriae with 200 or more FTE were quantified for each treatment. Analysis of p53 staining was similar, with at least three fimbriae per treatment, per patient analyzed. Samples with p53 expression were quantified utilizing adjacent sections stained for the secretory cell marker PAX8 in a qualitative manner as described.
ELISA
IL8, VEGF-A, and FGF2 were detected in fallopian culture medium by enzyme-linked immunosorbent assay for human IL8 (EMD Millipore, Billerica, MA, USA), VEGF-A (RayBiotech, GA,USA), or FGF2 (Abcam) respectively using the manufacturers’ protocols. The sensitivity for IL8, VEGF-A, and FGF2 are 4.4pg/ml, 10pg/ml, and 2pg/ml, respectively. Results were normalized to total protein content as determined by western blotting and Ponceau staining to account for difference in tissue size between treatment groups. Briefly, conditioned medium (20µl) was run on a 10% SDS-PAGE gel and transferred to nitrocellulose membrane (Fisher Scientific). Ponceau (Sigma-Aldrich) staining and subsequent densitometry via ImageJ software was performed.
Statistical Analysis
Proliferation and ELISA data are displayed as mean ± standard error, with significance determined via paired t-test utilizing Prism software (GraphPad, La Jolla, CA). All data sets were analyzed for significant outliers by Grubbs’ test of deviation.
Results
3D fimbriae cultures retain tissue architecture and epithelial subtypes
Optimization was performed to identify culture conditions that best supported fallopian architecture ex vivo. Previous studies identified alginate as an ideal matrix for the maintenance of baboon and murine ovaries and oviducts [15, 16], therefore 0.5% alginate was utilized to encapsulate human fallopian fimbriae alongside fimbriae with no culture matrix, and 0.5% alginate supplemented with extracellular matrix (1 mg/ml collagen and 0.1% fibronectin) (Supp. Fig. 1). Culture of human fimbriae revealed the alginate matrix maintained tissue architecture and cell morphology up to 7 days. Samples without matrix were also intact after 7 days, but had flattening of the FTE in some areas. No additional benefit was seen with supplementary ECM. Therefore, alginate hydrogel (0.5%) was utilized for subsequent experiments.
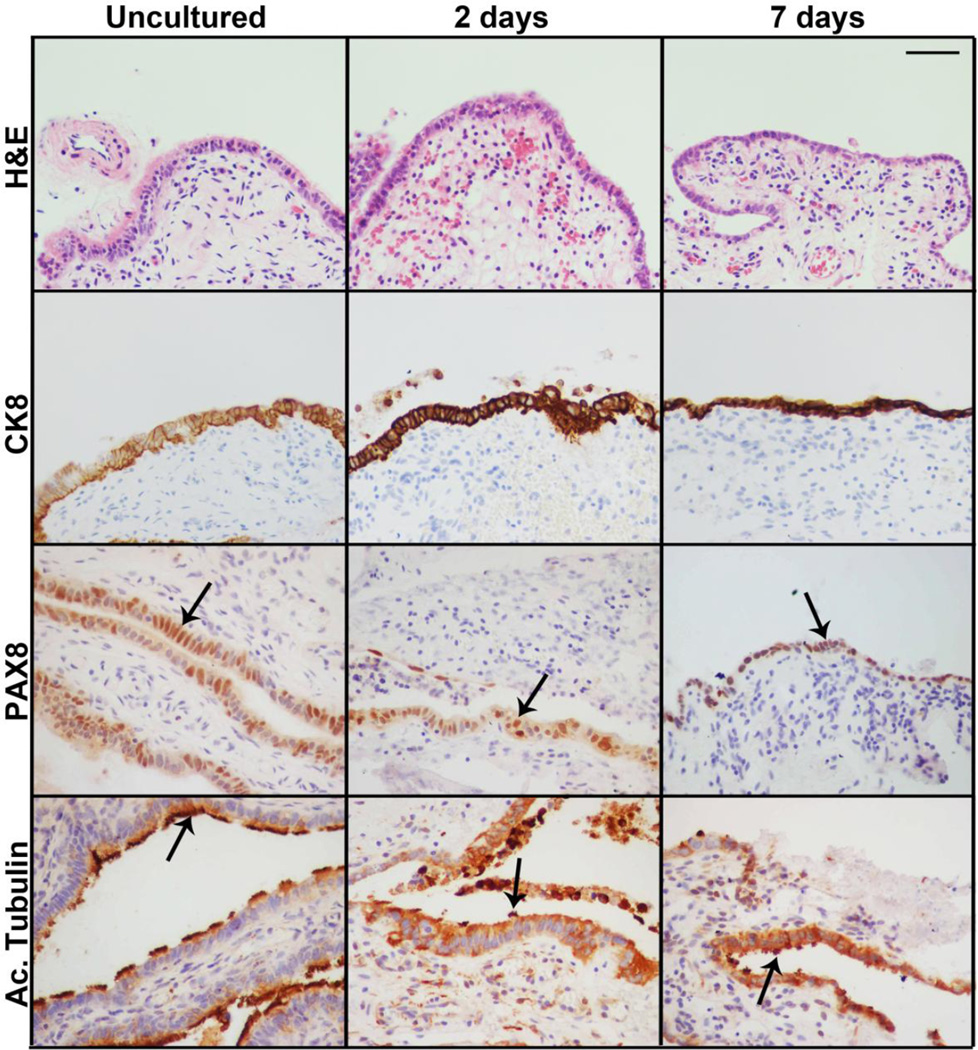
Characterization after 2 and 7 days in culture indicated that alginate maintained FTE viability and contact with the fallopian stroma (Figure 1). Both epithelial and stromal compartments appeared morphologically similar to uncultured tissues, as indicated by H&E staining and the epithelial marker CK8. Further, unlike extended culture of fallopian epithelium in 2D where ciliated epithelia are lost, 3D fimbriae cultures retain both FTE subtypes; ciliated (acetylated tubulin) and secretory (PAX8), allowing for investigation of the role of both epithelial subtypes in fallopian function and pathophysiology.
Figure 1. 3D human fimbriae culture system supports secretory and ciliated epithelium.
Human fimbriae cultures encapsulated in alginate hydrogels retained normal tissue architecture for up to 7 days in culture, as demonstrated by comparative H&E staining. FTE was maintained as identified by cytokeratin 8 (CK8) staining. Further, both epithelial subtypes; secretory (PAX8) and ciliated (Ac. Tubulin). Scalebar equals 50 µm.
Insulin and H2O2 induce epithelial proliferation in fimbriae cultures
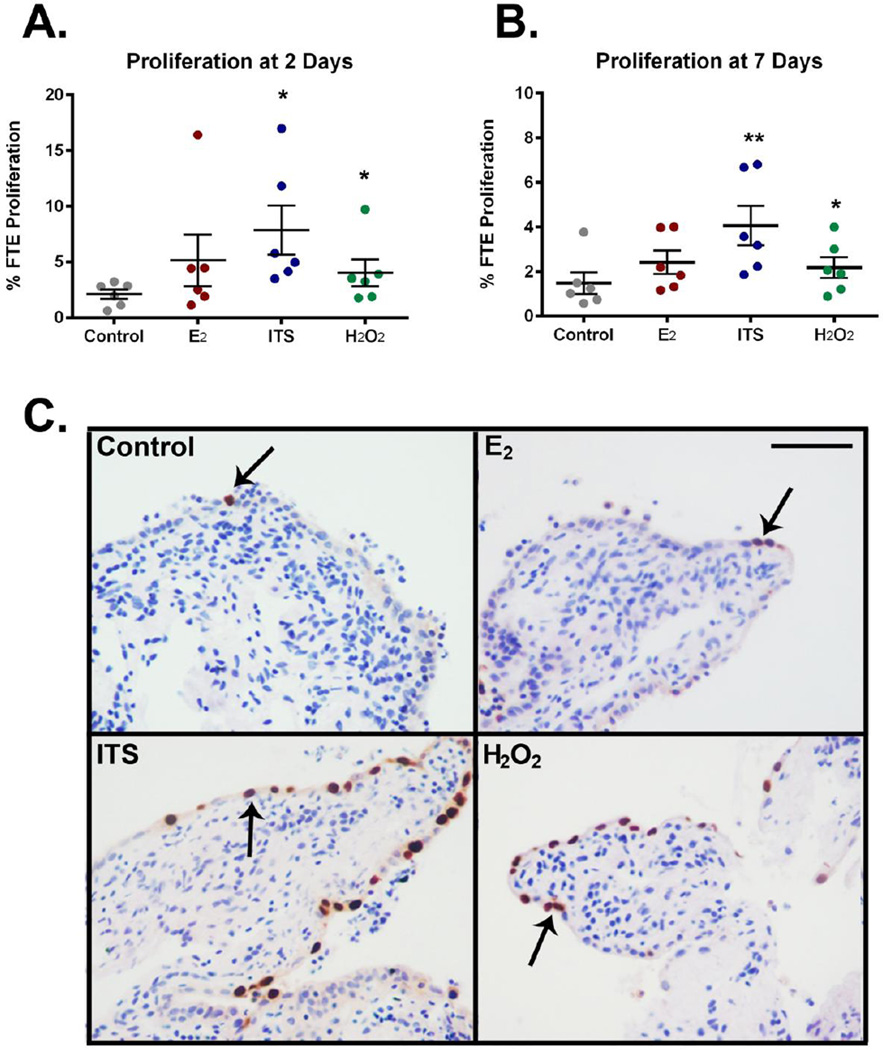
Proliferation is an important aspect of normal physiology and uncontrolled proliferation is a hallmark of tumorigenesis. To clarify the regulation of human FTE proliferation, ovulatory factors hypothesized to be involved in serous carcinogenesis were investigated (n=6). Insulin treatment, contained within the common culture supplement ITS, induced FTE proliferation after both 2 and 7 days in culture (7.8±2.2% and 4.1±0.9% respectively) compared to vehicle control treated 3D cultures (2.1±0.4% and 1.5±0.5%, Figure 2A and 2B). Proliferation in control cultures was similar to basal proliferation levels in normal in vivo fimbriae (~1–3%) [17]. The oxidative stress mimetic, H2O2, induced proliferation at 2 and 7 days (4.0±1.1% and 2.2±0.5%, respectively). However, treatment with the steroid hormone E2 did not significantly affect epithelial proliferation (5.2±2.3% and 2.4±0.5%, day 2 and day 7 respectively). FTE proliferation was determined as a percentage of total FTE cells 24 hrs post-BrdU labeling (Figure 2C). BrdU labeling results were supported by Ki67 immunostaining (Supplemental Figure 2).
Figure 2. 3D Fimbriae proliferation in response to estrogen, insulin, and hydrogen peroxide.
Proliferation was quantified in the FTE of human fimbriae treated with 10nM estradiol (E2), 1× ITS, 1mM H2O2 or vehicle (Control) for A) 2 and B) 7 days. ITS and H2O2 demonstrated enhanced proliferation after 2 and 7 days in culture. E2 did not alter FTE growth. C) Proliferation was determined by 24 hour BrdU labeling (arrows) n=6. Error bars equal mean ± SEM. Scalebar equals 50 µm. * p≤0.05 **p≤0.01
E2 regulates fallopian tissue and induces secretion of the pro-tumorigenic factor IL8
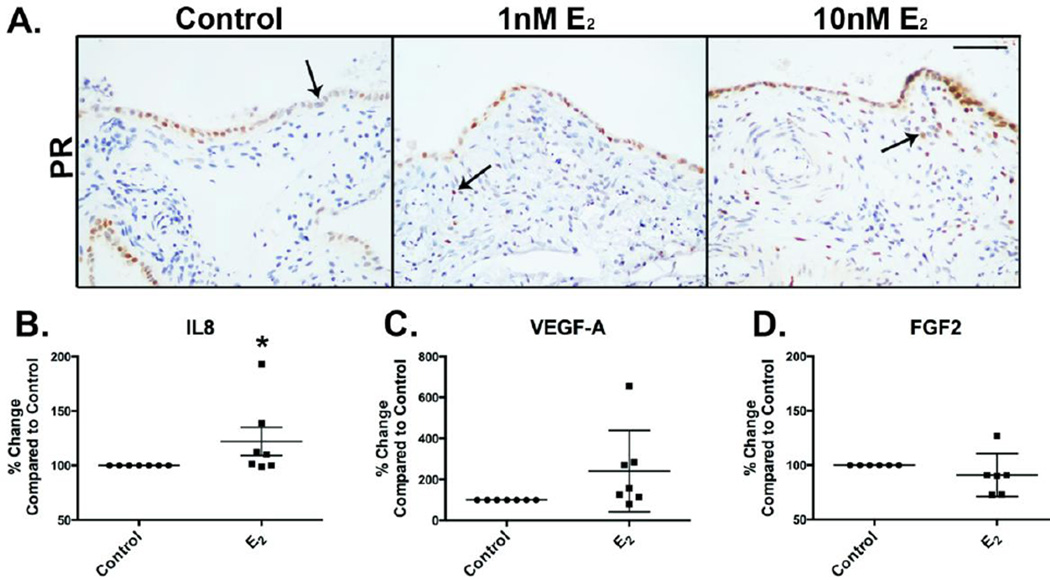
Although E2 treatment did not induce proliferation, it was functional in fimbriae samples, with the induction of its conical target, progesterone receptor (PR) at both 1nM and 10nM concentrations compared to vehicle control treated tissues (Figure 3A). PR expression was weak and limited to epithelial cells in control tissues and heightened in both the FTE and underlying fallopian stroma post-E2 treatment, further demonstrating the necessity of studying these cell types in association, as steroidogenic responses are induced in both tissue compartments. To clarify the link between E2 signaling and ovarian cancer risk, fallopian tissues were treated with 10nM E2 for 7 days and the conditioned medium compared to culture medium from samples treated with vehicle. These experiments identified a subtle, yet significant increase in the pro-inflammatory, angiogenic cytokine interleukin 8 (IL8, Figure 3B), in E2 treated samples (22% ± 13% increase) compared to vehicle control (n=7). The modest increase in IL8 induced by E2 was specific, as other pro-tumorigenic factors, including vascular endothelial growth factor (VEGF-A, 140% ± 75% increase compared to vehicle control, n=7, Figure 3C), or fibroblast growth factor 2 (FGF2, 9% ± 8% decrease compared to vehicle control, n=6, Figure 3D), which were not significantly altered post-treatment. Immunostaining was performed to determine localization of IL8 in tissues. This demonstrated a weak diffuse staining pattern in 3D cultured FTE samples that was primarily localized to the stromal compartment of the cultures (Supplemental Figure 3). Although modest, these data confirm human fimbriae remain metabolically active ex vivo and suggest a potential mechanism whereby E2 might contributes to a pro-tumorigenic microenvironment.
Figure 3. E2 induces PR expression and IL8 secretion from 3D cultured human fimbriae.
A) Progesterone receptor (PR) expression is limited to a portion of the FTE in control (vehicle treated) samples. Epithelial and stromal expression is induced by treatment with 1 or 10 nM E2 for 7 days (arrows). B) Treatment of fallopian cultures with 10nM E2 demonstrated induction of the pro-tumorigenic cytokine IL8 compared to control (vehicle treated). No change was seen in other pro-inflammatory cytokines; C) VEGF-A and D) FGF2. Scalebar equals 50 µm.
p53 stabilization is not enhanced by ovulatory factors ex vivo
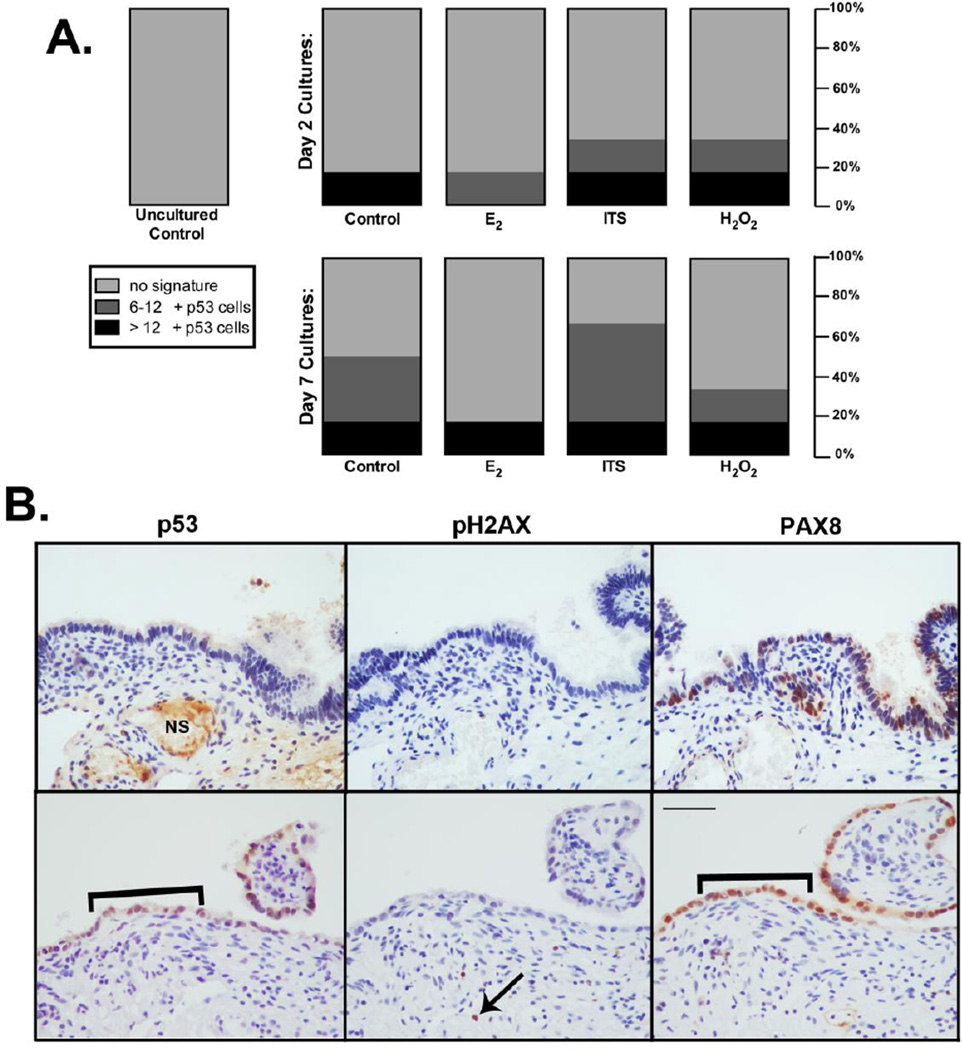
p53 induction in 3D cultures was evaluated, as its stabilization in secretory FTE is hypothesized to be a potential precursor to HGSC. As with the proliferation studies, 3D fimbriae samples were treated for 2 and 7 days with insulin, H2O2, and E2 and compared with uncultured and vehicle control treated tissues (n=8, Figure 4A). Although no factor notably induced p53 stabilization at either time point, the ex vivo culture process alone appeared to induce p53 expression. Similar to in vivo ‘p53 signatures’, p53 stabilization was often limited to the secretory FTE, as noted by PAX8 expression (Figure 4B). p53 expression was quantified by the number of consecutive positive cells (as with the SEE-FIM protocol), with 12 cells denoting a full p53 signature. These data demonstrate an ex vivo ‘forgery’ of the p53 signature can be produced that is similar to in vivo signatures originally defined in high-risk patients. Moreover, it was identified that these areas of p53 stabilization were not always concomitant with DNA damage (pH2AX staining), indicating p53 expression in the fallopian tube was not always in response to damage repair. p53 and pH2AX staining were not apparent in uncultured normal fallopian tissues, as has previously been demonstrated [9].
Figure 4. p53 expression is induced in 3D human fimbriae cultures.
A) p53 expression was evaluated in FTE of 3D human fimbriae samples cultured for 2 and 7 days with vehicle (control), 10 nM E2, 1× ITS, or 1mM H2O2. p53 expression was not induced by an specific treatment. However, p53 expression did appear to be induced by culturing alone. B) p53 signatures were not identified in uncultured samples, but were noted in ex vivo cultured fimbriae (brackets) primarily in secretory epithelium (PAX8), but not always coincidental with DNA damage (pH2AX) although damage was seen in stromal cells (arrow). Scalebar equals 50 µm. NS= non-specific staining.
Discussion
Recently, evidence has been compiled which supports the fallopian tube, in particular the FTE, as an origin for HGSC. However, this data has been extrapolated from immunohistochemical analysis of patient tissue [18], animal models [12], and human FTE artificially immortalized in vitro [6, 19], models which do not fully address how HGSC might initiate from the FTE. For this reason, a 3D culture system was developed from primary human fimbriae that utilizes alginate to support tissue architecture and maintain epithelial cell types for ex vivo examination. Although, 3D FTE modeling has been previously described [20], this system was generated from secretory FTE alone, aggregated, in the absence of stroma and ciliated FTE, thus disrupting normal tissue composition and crosstalk, which are likely involved in early tumorigenesis. The development of an ex vivo system which closely mimics the in vivo microenvironment has allowed for examination of normal fallopian function and the role of the FTE in transformation leading to HGSC formation.
Our 3D fimbriae model was utilized to investigate ovulatory factors identified as regulators of normal fallopian physiology, which are also linked to heightened risk of ovarian cancer development: E2 and the oxidative stress mimetic, H2O2. Insulin, in the form of the common media supplement ITS, was also utilized as it is a known mitogen and served as a positive control demonstrating that 3D cultures were metabolically active. Although induction of FTE proliferation was expected after insulin treatment, the role of insulin in HGSC development is also intriguing. Long term use of insulin carries increased risk of ovarian cancer development [21]. Further, polycystic ovarian syndrome, which presents with hyperinsulinemia, also increases risk [22]. Insulin in the form of ITS, does regulate fallopian proliferation, therefore the role of glycolysis in early carcinogenesis may warrant further investigation. Moreover, the impact of insulin as an in vitro culture supplement should be considered, as it is currently utilized in some models [20] and may distort proliferation rates, which are low (~1–3%) in normal FTE [17].
E2 only hormone replacement therapy carries heightened risk of HGSC development, which is enhanced with duration of treatment [23]. However, it remains unclear how E2 might promote tumorigenesis originating from the fallopian tube. 3D cultures indicated that E2 is not mitogenic in human FTE, which is consistent with our previous findings in baboon oviductal cells [14], although variability between samples was noted. Variability was not linked to age, menstrual status, or expression of estrogen receptors. However, E2 did produce functional changes in fallopian fimbriae, namely the induction of PR and a subtle increase in secretion of the pro-tumorigenic chemokine IL8. PR expression in normal fimbriae has previously been demonstrated in response to enhanced E2 during follicular phase [24] and demonstrates that 3D cultures remain metabolically active. Increased IL8 expression is associated with poor prognosis in ovarian cancer patients [25, 26] and is thought to play a role in angiogenesis, adhesion, and migration of ovarian cancer cells [27]. Further, enhanced IL8 expression is also predictive of poor response to several cancer therapeutics [28]. Induction of IL8 in 3D fimbriae cultures demonstrates that although E2 may not induce proliferation, it may alter the fallopian microenvironment. Previous publications reported upregulation of IL8 in fallopian cells treated with follicular fluid collected from hyperstimulated patients [29]. Although E2 modestly induced IL8 secretion in our culture system, it did not stabilize p53, which was enhanced in FTE treated with follicular fluid [29] suggesting a different ovulatory factor may be involved in p53 accumulation, or alternatively that hormone concentrations in hyperstimulated follicular fluid are enhanced beyond the levels utilized in this study. Interestingly, IL8 is implicated in fallopian ectopic pregnancy [30], suggesting this 3D microphyisiological model may be ideal for examining both reproductive and tumorigenic physiology.
As with E2 treatment, neither oxidative stress nor insulin was able to significantly promote p53 expression, despite inducing FTE proliferation. pH2AX and p53 expression were not evident in sections of uncultured control tissue, as has previously been demonstrated in studies examining normal fimbriae tissues [9], although p53 signatures have been demonstrated in ~50% of fimbriae if extensively examined via SEE-FIM [31]. Ex vivo ‘p53 signatures’ were identified post-culture in several 3D fimbriae tissues, as identified by heightened p53 expression in 12 or more secretory FTE cells, but these signatures were not always coincident with DNA damage (although pH2AX staining was evident in other cells). Studies utilizing follicular fluid on FTE cells did induce pH2AX and p53 stabilization [29]. However, data from our lab have demonstrated 1mM H2O2 is sufficient to induce pH2AX expression and increase p53 mRNA expression in 2D OSE models but not in 3D ovarian cultures [32]. These conflicting results demonstrate the differences between 2D and 3D models, suggesting normal reproductive epithelial contact with stroma may be protective. In support of this theory, treatment of 2D oviductal cells with 1mM H2O2 (the concentration utilized in this study) does induce damage [32]. These data further highlight the necessity of stromal-epithelial interaction for normal tissue response, and suggests a role for fallopian stroma in response to ovulatory stressors. Further, stabilization of p53 in the absence of cellular damage was noted in this study. Although, data from high-risk patients, continues to support a possible role for ‘p53 signatures’ in the pathogenesis of HGSC [1, 33], these potential precursors can also be found in ~50% of normal women [31]. Additionally, a portion of established fallopian precursor malignancies, STICs, do not have stabilized p53 expression [34]. These data and our culture system suggest p53 stabilization alone in the absence of damage may be stochastic and part of normal fallopian physiology. Additional changes to fallopian tube cells beyond mutation and stabilization in p53 may be required to fully define pre-neoplastic lesions that will progress to STICs and possibly HGSC.
This study has developed a novel 3D fimbriae model for the study of the fallopian tube as an origin for HGSC formation, in which an ex vivo ‘p53 signature’ can be forged. Initial cultures were only examined for 7 days, thus limiting their ability to determine long term changes to the microenvironment. Despite these limitations, E2 was able to promote IL8 secretion from human fimbriae, while H2O2 and insulin induce proliferation of the FTE. This 3D model can be further utilized to decipher the importance of p53 expression in the fallopian tube, and what factor/s are necessary for consistent p53 stabilization and DNA damage in the FTE, further clarifying how these changes might lead to HGSC formation. Defining the origin of HGSC is critical for expediting ovarian cancer research, as cancerous changes might be hidden or misinterpreted if compared to the wrong normal tissue.
Supplementary Material
Highlights.
3D fimbriae cultures retain tissue architecture and remain metabolically active
H2O2 and insulin promote epithelial proliferation and E2 induces IL8 secretion
p53 expression in secretory cells can be induced artificially in culture
Acknowledgements
We would like to acknowledge Rosemarie Tagare and Brian Talon for their assistance with immunohistochemical staining and imaging.
Funding
Generous funding for this work was provided by the American Cancer Society Illinois Division RSG-12-230-01-TBG and the NIH (UH2TR000498-01 and UH3TR001207).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Author Contribution
SLE drafted the manuscript. SLE, JZ, and SMQ performed experiments. JAS and RMK consented and collected human fallopian tissues. JJK, TKW, and JEB aided in study concept, experimental design and manuscript preparation.
References
- 1.Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, Gille JJ, Jongsma AP, Pals G, Kenemans P, Verheijen RH. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Facts and Figures. American Cancer Society. 2013 [Google Scholar]
- 3.Mehra K, Mehrad M, Ning G, Drapkin R, McKeon FD, Xian W, Crum CP. STICS, SCOUTs and p53 signatures; a new language for pelvic serous carcinogenesis. Front Biosci (Elite Ed) 2011;3:625–634. doi: 10.2741/e275. [DOI] [PubMed] [Google Scholar]
- 4.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhn E, Kurman RJ, Vang R, Sehdev AS, Han G, Soslow R, Wang TL, Shih Ie M. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma--evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226:421–426. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci U S A. 2011;108:7547–7552. doi: 10.1073/pnas.1017300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jazaeri AA, Bryant JL, Park H, Li H, Dahiya N, Stoler MH, Ferriss JS, Dutta A. Molecular requirements for transformation of fallopian tube epithelial cells into serous carcinoma. Neoplasia. 2011;13:899–911. doi: 10.1593/neo.11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shan W, Mercado-Uribe I, Zhang J, Rosen D, Zhang S, Wei J, Liu J. Mucinous adenocarcinoma developed from human fallopian tube epithelial cells through defined genetic modifications. Cell Cycle. 2012;11:2107–2113. doi: 10.4161/cc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levanon K, Ng V, Piao HY, Zhang Y, Chang MC, Roh MH, Kindelberger DW, Hirsch MS, Crum CP, Marto JA, Drapkin R. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–1113. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mietz JA, Unger T, Huibregtse JM, Howley PM. The transcriptional transactivation function of wild-type p53 is inhibited by SV40 large T-antigen and by HPV-16 E6 oncoprotein. EMBO J. 1992;11:5013–5020. doi: 10.1002/j.1460-2075.1992.tb05608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 12.Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, Chen JY, Ohman AW, Stepule CD, Kwak S, Karst AM, Hirsch MS, Setlur SR, Crum CP, Dinulescu DM, Drapkin R. Transformation of the fallopian tube secretory epithelium leads to highgrade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24:751–765. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sueblinvong T, Carney ME. Current understanding of risk factors for ovarian cancer. Curr Treat Options Oncol. 2009;10:67–81. doi: 10.1007/s11864-009-0108-2. [DOI] [PubMed] [Google Scholar]
- 14.King SM, Hilliard TS, Wu LY, Jaffe RC, Fazleabas AT, Burdette JE. The impact of ovulation on fallopian tube epithelial cells: evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr Relat Cancer. 2011;18:627–642. doi: 10.1530/ERC-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King SM, Quartuccio S, Hilliard TS, Inoue K, Burdette JE. Alginate hydrogels for three-dimensional organ culture of ovaries and oviducts. J Vis Exp. 2011 doi: 10.3791/2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, Kiesewetter SE, Shea LD, Woodruff TK. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011;84:689–697. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George SH, Milea A, Shaw PA. Proliferation in the normal FTE is a hallmark of the follicular phase, not BRCA mutation status. Clin Cancer Res. 2012;18:6199–6207. doi: 10.1158/1078-0432.CCR-12-2155. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. The Journal of Pathology. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 19.Fotheringham S, Levanon K, Drapkin R. Ex vivo culture of primary human fallopian tube epithelial cells. J Vis Exp. 2011 doi: 10.3791/2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrenson K, Notaridou M, Lee N, Benjamin E, Jacobs IJ, Jones C, Gayther SA. In vitro three-dimensional modeling of fallopian tube secretory epithelial cells. BMC Cell Biol. 2013;14:43. doi: 10.1186/1471-2121-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case-control analysis. Gynecol Oncol. 2011;123:200–204. doi: 10.1016/j.ygyno.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol. 1996;88:554–559. doi: 10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]
- 23.Lacey JV, Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, Schatzkin A, Schairer C. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–341. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 24.Amso NN, Crow J, Shaw RW. Comparative immunohistochemical study of oestrogen and progesterone receptors in the fallopian tube and uterus at different stages of the menstrual cycle and the menopause. Hum Reprod. 1994;9:1027–1037. doi: 10.1093/oxfordjournals.humrep.a138628. [DOI] [PubMed] [Google Scholar]
- 25.Ivarsson K, Runesson E, Sundfeldt K, Haeger M, Hedin L, Janson PO, Brannstrom M. The chemotactic cytokine interleukin-8--a cyst fluid marker for malignant epithelial ovarian cancer? Gynecol Oncol. 1998;71:420–423. doi: 10.1006/gyno.1998.5198. [DOI] [PubMed] [Google Scholar]
- 26.Penson RT, Kronish K, Duan Z, Feller AJ, Stark P, Cook SE, Duska LR, Fuller AF, Goodman AK, Nikrui N, MacNeill KM, Matulonis UA, Preffer FI, Seiden MV. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer. 2000;10:33–41. doi: 10.1046/j.1525-1438.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Xu RC, Zhang XL, Niu XL, Qu Y, Li LZ, Meng XY. Interleukin-8 secretion by ovarian cancer cells increases anchorage-independent growth, proliferation, angiogenic potential, adhesion and invasion. Cytokine. 2012;59:145–155. doi: 10.1016/j.cyto.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Gales D, Clark C, Manne U, Samuel T. The Chemokine CXCL8 in Carcinogenesis and Drug Response. ISRN Oncol. 2013;2013:859154. doi: 10.1155/2013/859154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bahar-Shany K, Brand H, Sapoznik S, Jacob-Hirsch J, Yung Y, Korach J, Perri T, Cohen Y, Hourvitz A, Levanon K. Exposure of fallopian tube epithelium to follicular fluid mimics carcinogenic changes in precursor lesions of serous papillary carcinoma. Gynecologic oncology. 2014;132:322–327. doi: 10.1016/j.ygyno.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Balasubramaniam ES, Van Noorden S, El-Bahrawy M. The expression of interleukin (IL)-6, IL-8, and their receptors in fallopian tubes with ectopic tubal gestation. Fertil Steril. 2012;98:898–904. doi: 10.1016/j.fertnstert.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Mehra KK, Chang MC, Folkins AK, Raho CJ, Lima JF, Yuan L, Mehrad M, Tworoger SS, Crum CP, Saleemuddin A. The impact of tissue block sampling on the detection of p53 signatures in fallopian tubes from women with BRCA 1 or 2 mutations (BRCA+) and controls. Mod Pathol. 2011;24:152–156. doi: 10.1038/modpathol.2010.171. [DOI] [PubMed] [Google Scholar]
- 32.King SM, Quartuccio SM, Vanderhyden BC, Burdette JE. Early transformative changes in normal ovarian surface epithelium induced by oxidative stress require Akt upregulation, DNA damage and epithelial-stromal interaction. Carcinogenesis. 2013;34:1125–1133. doi: 10.1093/carcin/bgt003. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 34.Cass I, Walts AE, Barbuto D, Lester J, Karlan B. A cautious view of putative precursors of serous carcinomas in the fallopian tubes of BRCA mutation carriers. Gynecol Oncol. 2014 doi: 10.1016/j.ygyno.2014.07.084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.