Abstract
Background
The prevalence of allergic diseases doubled in developed countries in the last several decades. Cyclooxygenase (COX) inhibiting drugs augmented allergic diseases in mice by increasing allergic sensitization and memory immune responses. However, whether COX inhibition can promote allergic airway diseases by inhibiting immune tolerance is not known.
Objective
To determine the role of the COX pathway and PGI2 signaling through the PGI2 receptor (IP) in aeroallergen-induced immune tolerance.
Methods
Wild type (WT) BALB/c mice and IP KO mice were aerosolized with ovalbumin (OVA) to induce immune tolerance prior to immune sensitization with an intraperitoneal injection of OVA/alum. The COX inhibitor indomethacin or vehicle was administered in drinking water to inhibit enzyme activity during the sensitization phase. Two weeks after sensitization, the mice were challenged with OVA aerosols. Mouse bronchoalveolar lavage fluid was harvested for cell counts and Th2 cytokine measurements.
Results
WT mice treated with indomethacin had greater numbers of total cells, eosinophils, and lymphocytes, and increased IL-5 and IL-13 protein expression in BAL fluid compared to vehicle-treated mice. Similarly, IP KO mice had augmented inflammation and Th2 cytokine responses compared to WT mice. In contrast, the PGI2 analog cicaprost attenuated the anti-tolerance effect of COX inhibition.
Conclusion
COX inhibition abrogated immune tolerance by suppressing PGI2 IP signaling, suggesting that PGI2 signaling promotes immune tolerance and clinical use of COX inhibiting drugs may increase the risk of developing allergic diseases.
Keywords: PGI2, allergy, immune tolerance
Introduction
Allergic disease is one of the most common causes of chronic illness and affects 40–50 million Americans. In the last several decades, the reported prevalence of allergic rhinitis, asthma, and atopic eczema increased markedly in developed countries.1, 2 Mounting evidence suggests that drugs inhibiting cyclooxygenase (COX) enzymes in the arachidonic acid metabolic pathway may be contributing to the increased allergy prevalence. Epidemiological studies reveal a correlation between frequent use of COX inhibiting medications and increased risk of developing allergic disorders and asthma.3 Recently, a positive association was reported between the intake of non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs) and current asthma in adult survivors of childhood asthma.4 In addition, there was a higher prevalence of new-onset asthma in subjects who regularly used NSAIDs other than aspirin compared to nonusers.5 In animal studies, COX inhibition with indomethacin increased allergic sensitization, allergen-specific immune memory response, and augmented allergic airway inflammation and Th2 immune responses,6–10 supporting a role for the COX pathway in the development of allergic diseases. Similarly, mice deficient in COX enzymes have increased allergic inflammation compared to wild type (WT) mice.11
People without allergic diseases have antigen-specific immune tolerance to common aeroallergens. Some studies support the possibility that regulatory T cells (Treg) suppress allergic diseases and contribute to allergen-specific immune tolerance.12, 13 Treg cells are a subtype of CD4 T cells that are critical for the maintenance of self-tolerance and tolerance to foreign antigens by inhibiting effector T cell responses.14 The establishment and maintenance of tolerance induced by repeated airway exposures to low-dose ovalbumin (OVA) were dependent on Treg cells that express both cell surface and soluble TGF-β.13 Therefore, Treg cells may be a mechanism by which immune tolerance prevents the immune system from responding to innocuous environmental antigens. While we and others have reported that COX inhibition increased allergic airway inflammatory responses,6–10 the effect of COX products and COX inhibition on allergen-induced immune tolerance in the airways is not known.
Prostaglandin I2 (PGI2) is one of the lipid products formed in the COX pathway of arachidonic metabolism. The other lipid molecules produced in the COX pathway are PGE2, PGD2, PGF2α and thromboxane A2 (TXA2). PGI2 binds to the G protein-coupled receptor IP and exerts its biological activities through autocrine and paracrine mechanisms. We have previously described the immune suppressive function of PGI2 and PGI2 analogs inhibited Th1 and Th2 effector cytokine production in vitro.15, 16 Jaffar et al. reported that endothelial cell-derived PGI2 inhibited the recruitment of Th2 cells in the lung in an adoptive T cell transfer model.17 In contrast, IP knockout (KO) mice have augmented Th2 immune responses and increased airway inflammation and hyperresponsiveness to OVA.18 IP KO mice also had increased Th1 responses in a mouse model of respiratory syncytial virus (RSV) infection.19 Therefore, PGI2 modulates immune responses and possibly immune tolerance as well.
Studies have shown that the COX pathway regulates the generation and maintenance of immune tolerance.20, 21 COX-2-products produced by mucosal dendritic cells were required for the development of functional Treg cells and the maintenance of immune tolerance induced by oral ingestion of OVA.20 Inhibition of COX-2 in dendritic cells derived from mediastinal lymph nodes caused increased Th2 differentiation and IL-4 production, which was directly related to impaired Treg cell differentiation and immune tolerance.20 Regulatory T cells suppressed anti-tumor immune activity in a COX-2 dependent manner in colorectal cancer patients,21 also suggesting a role for the COX pathway in Treg function and immune tolerance. However, COX products may also modulate immune tolerance independent of Tregs. For instance, PGE2 promoted immune tolerance in the gut that was not related to Treg number or function.22 In this study, we found that COX inhibition ablated immune tolerance by suppressing IP signaling and the effect was not associated with Treg cell numbers. This has important clinical implications in that medications that inhibit PGI2 production may adversely affect immune tolerance.
Materials And Methods
Mice
Wild type BALB/c mice were obtained from The Jackson Laboratory. IP KO mice were generated by homologous recombination in embryonic stem cells and were backcrossed to a BALB/c background for 6 generations.23 Age-matched WT BALB/c mice and IP KO mice were used at 8–12 weeks old. 5-lipoxygenase (LO) KO mice on a mixed 129-C57BL/6 background were purchased from The Jackson Laboratory and then backcrossed 10 generations to BALB/c in our lab. Animal experiments were reviewed, approved by the Institutional Animal Care and Use Committee at Vanderbilt University, and were conducted according to the guidelines for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources, National Research Council.
Induction of immune tolerance and allergic inflammation
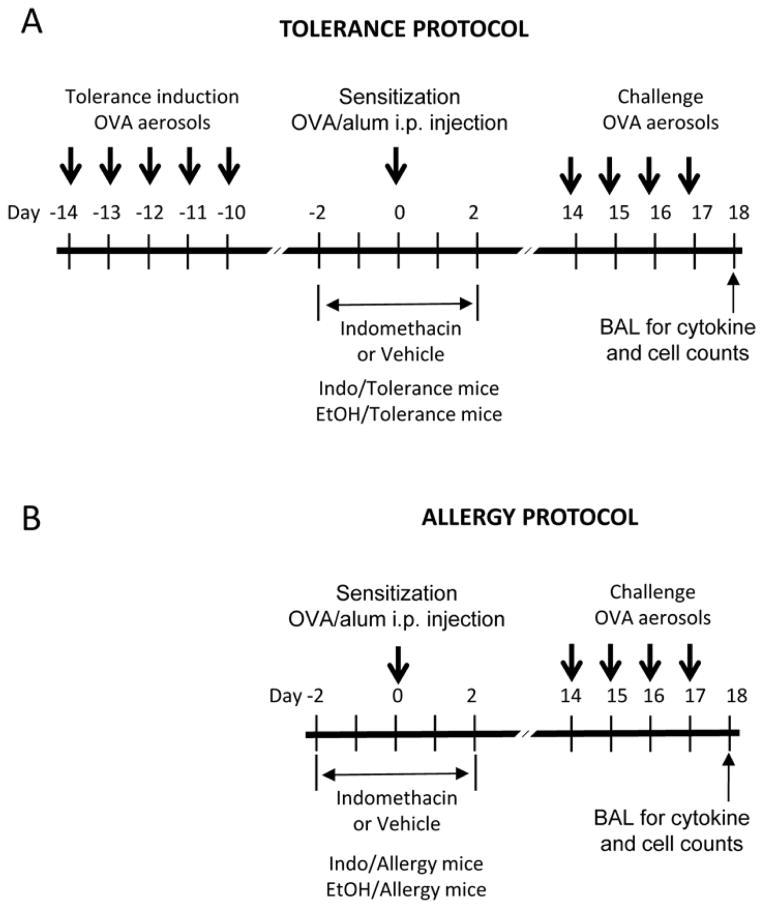
The mouse immune tolerance protocol (Figure 1) was modified from the method reported by Ostroukhova and colleagues.24 The “tolerance protocol” consisted of 3 phases: tolerance induction, OVA sensitization, and OVA challenge. For this protocol, wild type BALB/c, IP KO and 5-LO KO mice were exposed to aerosols of 1% OVA/PBS solution using an ultrasonic nebulizer for 40 min per day from day −14 to day −10 (5 days) to induce immune tolerance. At day 0, mice were OVA sensitized by an intraperitoneal (i.p.) injection of 0.1 ml OVA/alum solution (10 μg OVA formulated with 20 mg of aluminum hydroxide). Starting at day 14, the mice were challenged with an OVA aerosol for 40 min per day for 4 days. The “allergy protocol” consisted of sensitization with an intraperitoneal injection of OVA/alum at day 0 and OVA challenge with 4 days of OVA aerosols from days 14–17.
Figure 1.
Tolerance and allergy protocols.
In some experiments, WT BALB/c and 5-LO KO mice were treated with the COX inhibitor indomethacin (30 μg/ml) or vehicle (1% ethanol) in drinking water during sensitization (Figure 1) phase from day −2 to day 2. Some groups of mice were also treated with intraperitoneal injections of cicaprost (a gift from Dr. M. Huebner, Schering-Plough Corporation). The mice were injected twice daily from day −2 to day 2 with 300 ng cicaprost in 100 μl of PBS per mouse per injection.
BAL cell analysis
On day 18, bronchoalveolar lavage (BAL) fluid was collected 9 and white blood cells in the BAL were immediately counted for total number of cells. The BAL cells were also used for cytospin preparation. The cytospin slides were stained using the DiffQuik kit (American Scientific Products) for differential cell counts to enumerate macrophages, eosinophils, lymphocytes and neutrophils.
Histological analyses of Lung Sections
The mice were sacrificed on day 18 and the lung block was removed. Lung tissue was fixed in 10% formalin solution, paraffin-embedded, cut in 6 μm sections, mounted, and stained with hematoxylin and eosin for routine histology to evaluate inflammation. Slides were examined by a pathologist blinded to experimental groups to score interstitial inflammation using 0–3 scoring system: 0 - no inflammatory; 1 - a few inflammatory cells; 2 - increased accumulation of inflammatory cells; 3 - abundant accumulation of inflammatory cells.
Cytokine and IgE measurements by ELISA
The levels of IL-5 and IL-13 in BAL fluid were measured by Quantikine and Duoset ELISA kits (R&D Systems) according to the manufacturer’s instructions. Total IgE was determined by the Clonotyping ELISA kits (Southern Biotech). Ovalbumin-specific IgE in mouse sera was quantified as following. Immulon 2HB plates were coated with 2 μg ovalbumin in bicarbonate buffer (pH 9.6) per well overnight at 4 °C and blocked with 1% BSA. Serum samples were diluted in 0.1% Tween 20-PBS and incubated for 2 hours at room temperature. OVA-specific-IgE was detected by using goat-anti-mouse IgE conjugated with HRP (Southern Biotech). Plates were developed with TMB substrate solution (R&D Systems) and reactions were stopped with 1N HCl. Absorbance values at OD450 were measured. Concentrations of ovalbumin-specific IgE were determined using a standard curve made with serum from hyper-immunized mice.
Measurement of LTE4
The urine of WT BALB/c mice was collected at the last day of indomethacin treatment (Figure 1A). The amounts of LTE4 were quantitated by gas chromatography-mass spectrometry (GC-MS) as previously described.25 Creatinine in the urine was measured by a chemical assay based on Jaffe’s reaction according to the manufacturer’s instructions (Exocell Inc) for LTE4 normalization.
Flow cytometry
Mononuclear cells were isolated from mouse spleens, mediastinal lymph nodes (MLN) and lungs. The lung cells were stimulated with PMA (50 ng/ml), ionomycin (1 μg/ml) for 20 h and GolgiStop (BD Biosciences) for 6 h for IL-10 staining. The cells were stained with LIVE/DEAD® Fixable Blue Dead Cell Stain Reagent (Invitrogen) and with fluorochrome-conjugated antibodies against CD4 (RM4-5, BD Bioscience), CD25 (PC61.5, eBioscience), FoxP3 (150D/E4, eBioscience), and IL-10 (JES5-2A5) for flow cytometric analyses of Treg cells.
Statistical analysis
The p values were calculated by using unpaired Student’s t-test or one-way ANOVA with Bonferroni post hoc test. Values of p < 0.05 were considered significant.
Results
COX inhibition abrogated the immune tolerance induced by repeated airway OVA exposure in WT mice
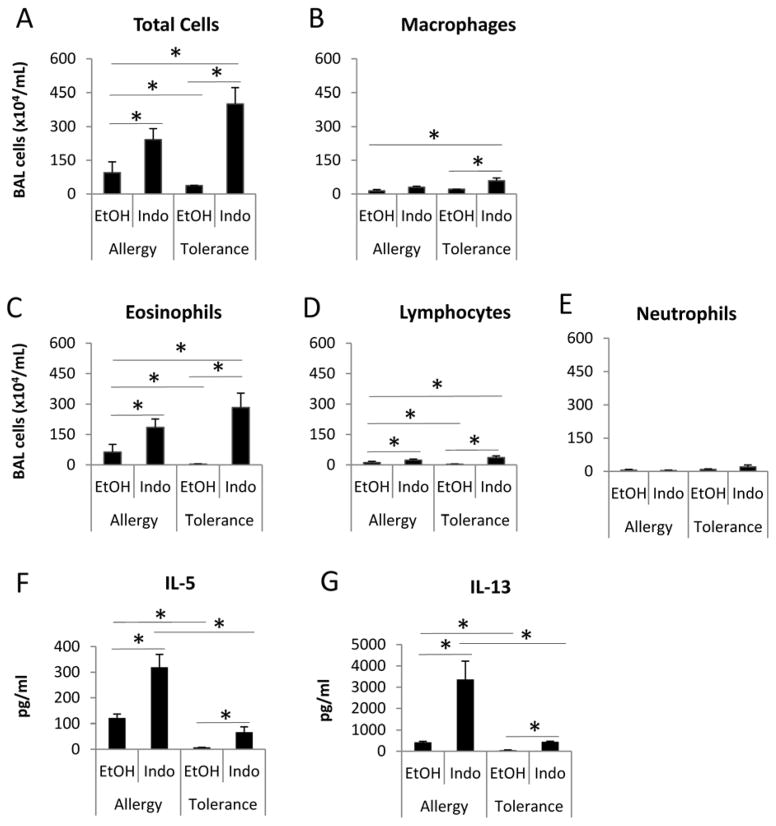
WT BALB/c mice underwent the tolerance and allergy protocols and were treated with either the COX inhibitor indomethacin (Figure 1A, Indo/Tolerance mice, Figure 1B, Indo/Allergy mice) or vehicle solution (ethanol, EtOH) (Figure 1A, EtOH/Tolerance mice; Figure 1B, EtOH/Allergy mice) during sensitization from day −2 to day 2 to determine the effect of COX inhibition on immune tolerance and allergic inflammation. We found that mice in EtOH/Tolerance group had significant decreases in the numbers of total cells, eosinophils and lymphocytes in BAL compared to EtOH/Allergy mice (Figure 2A, 2C & 2D), indicating that the initial OVA aerosols induced immune tolerance, which suppressed inflammation in the lung in OVA sensitized and challenged mice. Indo/Tolerance mice had significantly greater numbers of total cells, macrophages, eosinophils and lymphocytes (Figure 2A~ 2D), relative to the EtOH/Tolerance group, indicating that COX inhibition with indomethacin abrogated immune tolerance and led to allergic inflammation in the lung after sensitization and challenge. Mice in the Indo/Tolerance group also had significant increases in the numbers of total cells, macrophages, eosinophils and lymphocytes than EtOH/Allergy mice (Figure 2A~2D), indicating that COX inhibition not only abrogated immune tolerance, but further augmented allergic inflammation in the lung to a level comparable to that in Indo/Allergy mice. Indo/Allergy mice had significantly greater numbers of total cells, eosinophils and lymphocytes than EtOH/Allergy mice (Figure 2A, 2C & 2D), indicating a stimulatory effect of COX inhibition on allergic inflammation, consistent with our published findings.10
Figure 2.
COX inhibition abrogated OVA-induced immune tolerance in BALB/c mice. BAL fluid was harvested at day 18 and assessed for cell counts and cytokines. * p<0.05, n=4–5 mice per group. Data are representative of 3 experiments.
We also found that mice in Indo/Tolerance group had significant increases in IL-5 and IL-13 protein expression in BAL compared to mice in EtOH/Tolerance group in which IL-5 and IL-13 protein expression was undetectable (Figure 2F & 2G). The mice in Indo/Tolerance group had comparable levels of IL-5 and IL-13 in the BAL to the mice in EtOH/Allergy group (Figure 2F & 2G), indicating that COX inhibition with indomethacin abrogated immune tolerance and caused Th2 immune responses after OVA sensitization and challenge. However, the levels of IL-5 and IL-13 in the BAL of Indo/Tolerance mice were significantly lower than Indo/Allergy mice (Figure 2F & 2G), suggesting that although the inflammatory cell numbers in BAL were comparable between Indo/Tolerance and EtOH/Allergy groups, COX inhibition did not reverse the Th2 cytokine production in the tolerance mice to the levels of indomethacin-treated allergy mice (Indo/Tolerance mice vs. Indo/Allergy mice). Consistent with our previously published findings, mice in Indo/Allergy group had significantly increased levels of IL-5 and IL-13 than that in EtOH/Allergy group, indicating a stimulatory effect of COX inhibition on Th2 responses.9, 10, 26 Taken together, our results suggest that COX inhibition during sensitization phase abrogated immune tolerance as measured by airway inflammatory cell numbers and composition and Th2 cytokines.
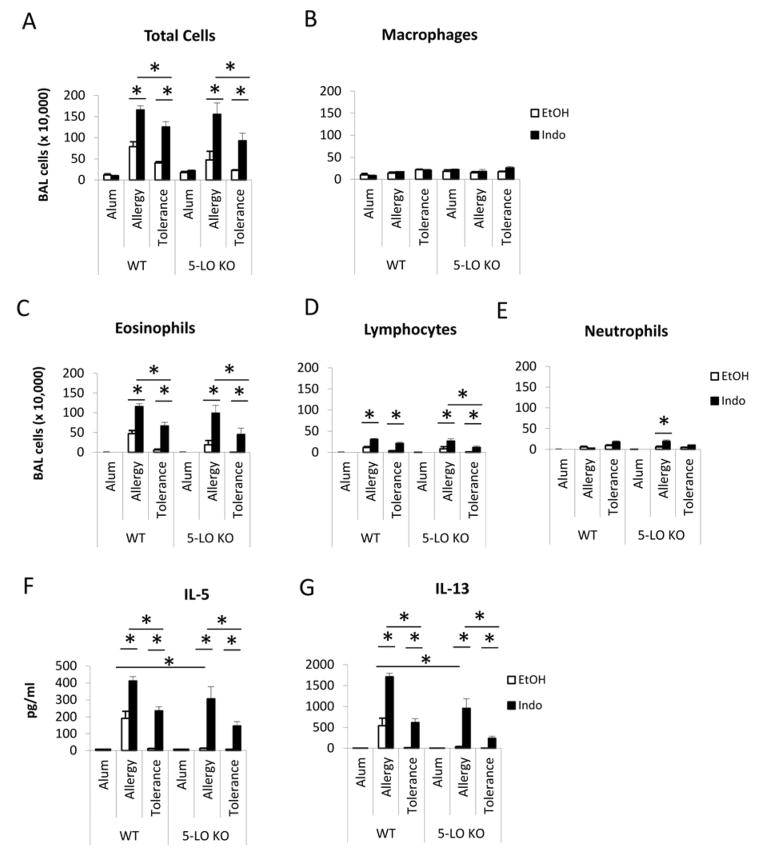
To determine whether cysteinyl leukotrienes, a 5-lipooxygenase (5-LO) product of arachidonic acid metabolism, is elevated and contribute to increased allergic inflammation under COX inhibition condition, WT mice were subjected to the tolerance induction phase and OVA/alum sensitization phase, and treated with either EtOH or indomethacin (Figure 1A). Mouse urine was collected at the last day of indomethacin treatment for LTE4 measurement. The LTE4 levels were not significantly different between WT/EtOH and WT/Indo mice (131±34 pg/mg creatinine vs. 114±31 pg/mg creatinine, p=0.74, n=6–8 mice). To further investigate the role of 5-LO pathway in indomethacin-abrogated immune tolerance, we used 5-LO KO mice in the immune tolerance protocol and treated the mice with EtOH or indomethacin during the sensitization phase. Compared to 5-LO KO EtOH/Tolerance mice, 5-LO KO Indo/Tolerance mice had significantly increased numbers of total cells, eosinophils, and lymphocytes (Figure E1A–E1D), and increased IL-5 and IL-13 production in BAL after OVA challenge (Figure E1F & E1G). This data support that COX inhibition abrogated immune tolerance in a mechanism that is independent of 5-LO expression and cysteinyl leukotriene generation. 5-LO KO EtOH/Allergy mice had lower levels of IL-5 and IL-13 in BAL (Figure E1F & E1G) compared with WT EtOH/Allergy mice, indicating that lipid products derived in the 5-LO pathway contributed to Th2 responses in the allergic airway inflammation. Indomethacin treatment increased the numbers of total, eosinophils and lymphocytes, and augmented IL-5 and IL-13 responses in BAL, consistent with our previous findings.
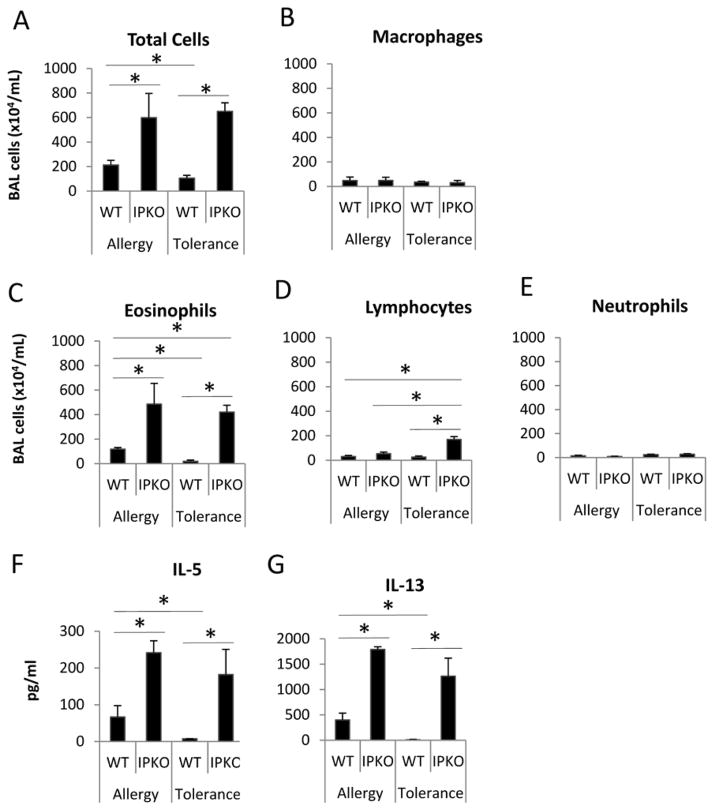
IP deficiency abrogated immune tolerance induced by repeated airway OVA exposures
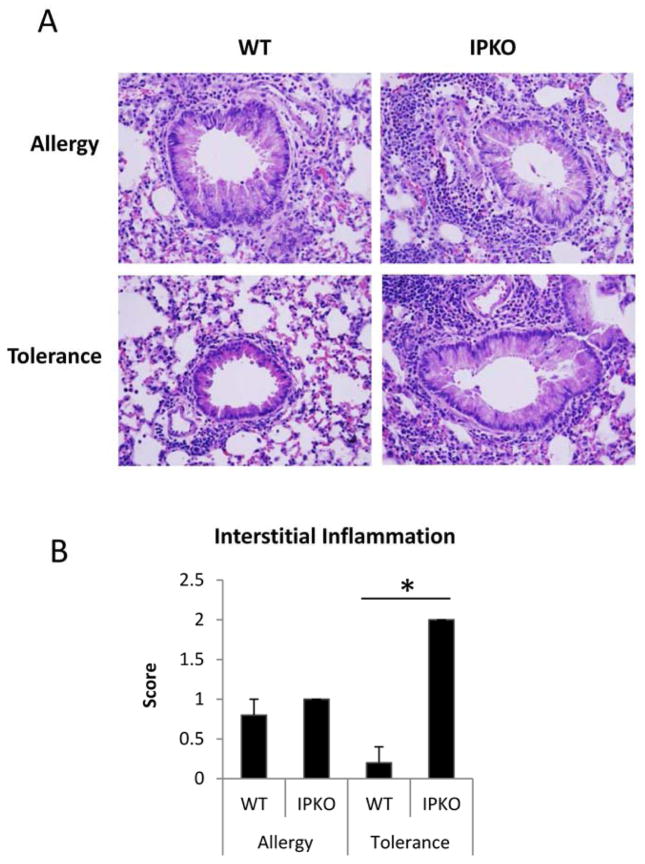
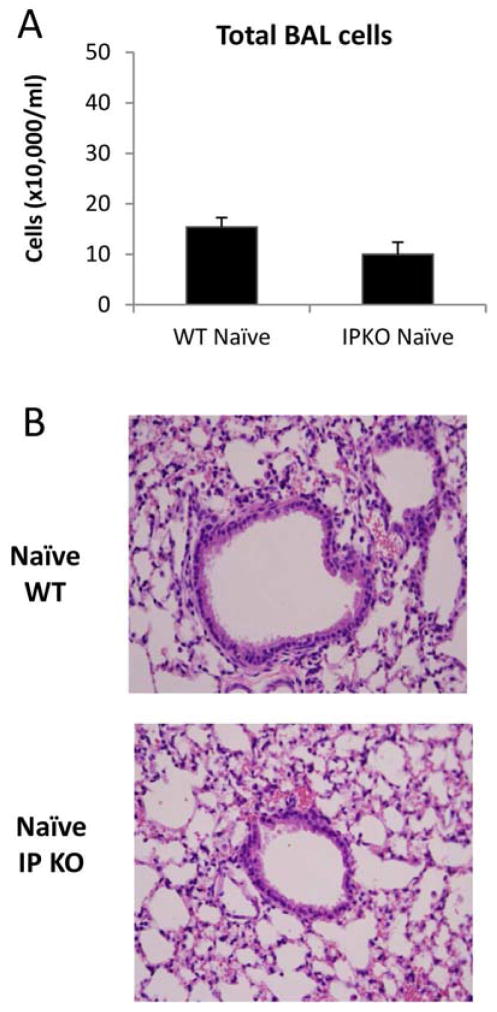
As shown in Figure 3, WT/Tolerance mice had no eosinophilia in BAL (Figure 3C), minimum levels of lung inflammation (Figure E2) and undetectable levels of IL-5 and IL-13 (Figure 3F & 3G), indicating that immune tolerance was induced and maintained. In contrast, IP KO/Tolerance mice had significantly greater numbers of total cells, eosinophils, and lymphocytes (Figure 3A, 3C & 3D), greater levels of lung inflammation (Figure E2), and significantly increased IL-5 and IL-13 protein expression (Figure 3F & 3G) in BAL compared to WT/Tolerance mice. In addition, IP KO/Tolerance mice had comparable numbers of total cells and eosinophils (Figure 3A & 3C), greater numbers of lymphocytes (Figure 3D), more lung inflammation (Figure E2), and similar levels of BAL IL-5 and IL-13 protein expression (Figure 3F &3G) compared to IP KO/Allergy mice. These data indicate that IP deficiency abrogated immune tolerance. IP KO/Allergy mice had greater numbers of total cells and eosinophils (Figure 3A & 3C) and increased IL-5 and IL-13 production (Figure 3F &3G) in BAL compared to WT/Allergy mice, consistent with increased Th2 cytokine responses and allergic airway inflammation in the absence of the IP.27 To exclude the possibility that IP KO mice had greater baseline inflammation than WT mice, we harvested blood, BAL and lungs of naïve WT and IP KO mice and determined IL-5 and IL-13 levels in BAL fluid, the levels of total serum IgE and OVA-specific IgE, and lung histology. We found that IL-5, IL-13, total IgE and OVA-specific IgE were undetectable (data not shown), and there was no active inflammation in the lungs (Figure E3) for both WT and IP KO mice.
Figure 3.
IP deficiency abrogated OVA-induced immune tolerance. BAL fluid was harvested at day 18 for cell counts and cytokine measurements. * p<0.05, n=5 mice per group. Data are representative of 3 experiments.
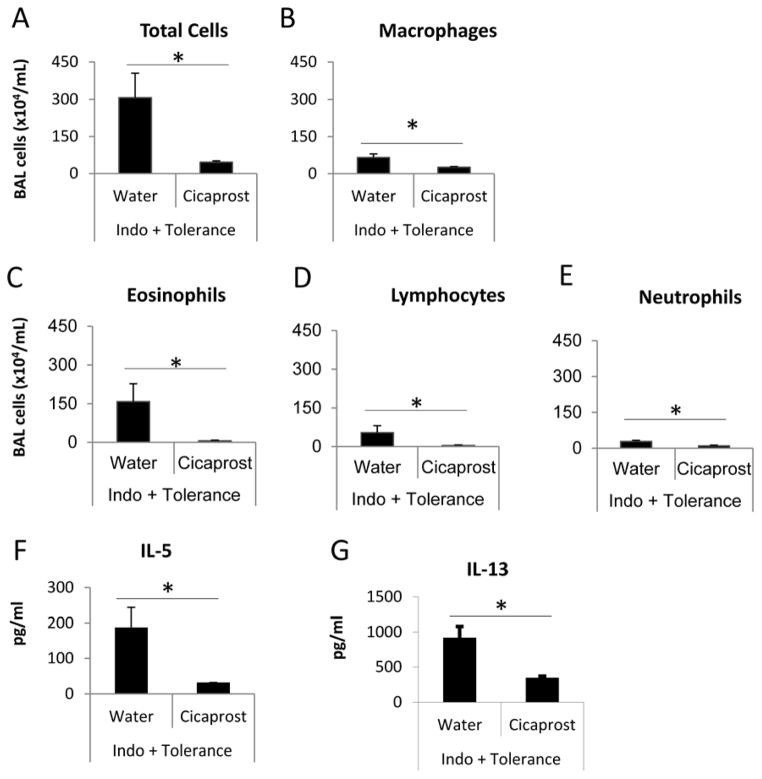
Cicaprost restored immune tolerance in indomethacin-treated mice
The PGI2 analog cicaprost or water (vehicle) was administered by intraperitoneal injections to WT BALB/c mice that were undergoing the tolerance protocol during sensitization phase. In the same time period, the mice were also treated with indomethacin or EtOH in drinking water (Figure 1A). Cicaprost significantly decreased the numbers of total cells, macrophages, eosinophils, lymphocytes and neutrophils (Figure 4A–4E) and inhibited IL-5 and IL-13 protein expression (Figure 4F & 4G) in BAL, compared to water treatment.
Figure 4.
PGI2 analog cicaprost restored the immune tolerance in the mice treated with COX inhibitors. BALB/c mice underwent the tolerance protocol and treated with EtOH or indomethacin in drinking water during sensitization phase from day −2 to day 2. The mice received cicaprost or water (vehicle) i.p. injections twice daily from day −2 to day 2. BAL fluid was harvested at day 18 for cell counts and cytokine measurements. * p<0.05, n=4–5 mice per group. Data are representative of 2 experiments.
COX inhibition and IP deficiency increased levels of serum IgE
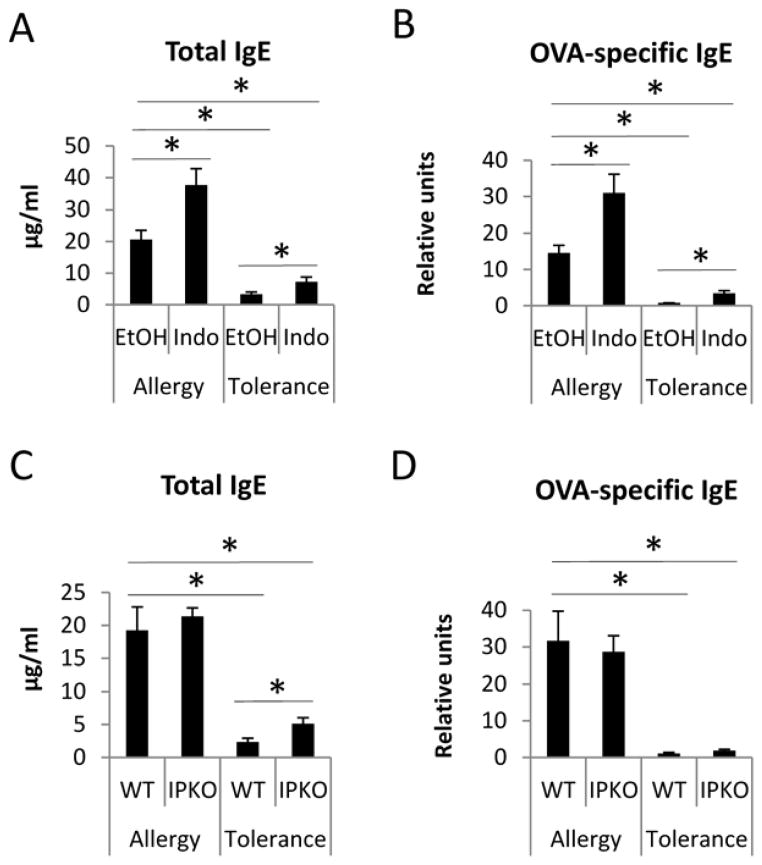
WT mice treated with indomethacin during the tolerance protocol had increased levels of total IgE and OVA-specific IgE compared to EtOH-treated mice subjected to the tolerance protocol (Figure 5A and 5B), indicating COX inhibition modulated humoral immune tolerance. Indomethacin also increased total and OVA-specific IgE compared to EtOH-treated mice that underwent the allergy protocol (Figure 5A and 5B), which is consistent with our previous findings.9 Similarly, IP KO mice that underwent the tolerance protocol had greater levels of total IgE than WT mice, suggesting a negative regulation of IP signaling on allergic inflammation and/or immune tolerance. Indo/Tolerance mice had significantly lower levels of total and OVA-specific IgE than EtOH/Allergy mice (Figure 5A & 5B). IP KO/Tolerance mice had significantly lower levels of total and OVA-specific IgE than WT/Allergy mice (Figure 5C&5D). These results indicate that tolerance developed in WT Indo/Tolerance and IP KO/Tolerance mice limited the magnitude of B cell responses.
Figure 5.
COX inhibition and IP deficiency increased levels of total serum IgE. IgE and OVA-specific IgE of mice harvested at day 18 were determined by ELISA. * p<0.05, n=4–5 mice per group. Data are representative of 3 experiments.
The abrogation of immune tolerance in IP KO mice was not associated with decreased number of Treg cells
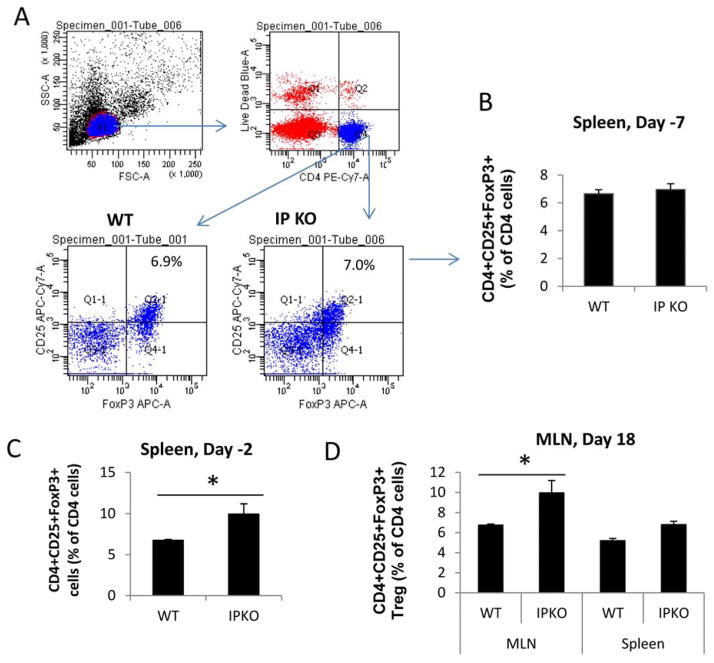
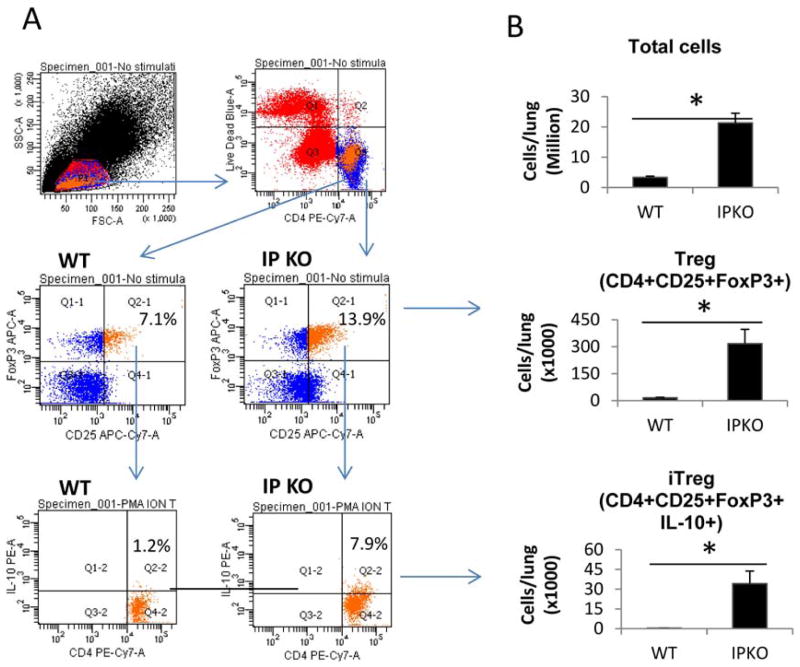
To test the hypothesis that IP deficiency abrogates immune tolerance by increasing Treg cell numbers, we analyzed Treg cells in the spleen of WT/Tolerance and IP KO/Tolerance mice at different time points during the tolerance protocol. We found similar percentages of Treg cells as defined as CD4+CD25+FoxP3+ cells in the spleens of WT/Tolerance and IP KO/Tolerance mice at 3 days after the tolerance induction phase (day −7 in the tolerance protocol) (Figure E4A and E4B). By contrast, there were greater percentages of Treg cells in the spleen of IP KO/Tolerance mice than in WT/Tolerance mice at 8 days after tolerance induction (day −2) (Figure E4C). The percentage of Treg cells in MLNs of IP KO/Tolerance mice at day 18 was also greater than in WT/Tolerance mice (Figure E4D). We also analyzed Treg cells and IL-10-producing Treg cells (inducible Treg, iTreg) in WT and IP KO mouse lungs at day 18. We found that the percentages and the numbers of Treg and iTreg cells in IP KO lungs were greater than in WT lungs (Figure E5A and E5B). Therefore, the failure to induce tolerance in IP KO mice was not associated with a decrease in Treg number.
Discussion
In this study, we have shown that the COX pathway has an important regulatory function in the maintenance of aeroallergen-induced immune tolerance. In mice in which antigen-specific tolerance was induced by repeated airway OVA exposures, COX inhibition during the sensitization phase abrogated immune tolerance. Given our previous studies implicating the IP in regulation of the immune response, we focused on elucidating the role of PGI2 signaling in this tolerance model. In IP KO mice, immune tolerance was not induced by the tolerance protocol, consistent with an important role for PGI2/IP signaling in the development and/or maintenance of immune tolerance. We also found that the PGI2 analog, cicaprost, markedly attenuated the suppressive effect of pharmacological inhibition of prostaglandin formation on tolerance and reduced the inflammatory and Th2 responses in Indo/Tolerance mice. These findings raise the possibility that non-steroidal anti-inflammatory drugs, such as indomethacin, may have detrimental effect on immune tolerance to aeroallergens.
The anti-tolerance effect of COX inhibition was reversed by cicaprost injections during the sensitization phase, revealing that IP signaling was a major component in COX pathway important for the maintenance of OVA-specific tolerance and suppression of allergic airway inflammation in this mouse tolerance model. This is consistent with the immune suppressive functions of PGI2 on Th2 immunity and allergic inflammation reported by other and our research groups.15, 27 Since cicaprost was administered only during the sensitization (OVA/alum i.p. injection) phase, cicaprost in this tolerance model seems to affect immune tolerance, immune sensitization and immune response systemically, while it may also act locally in the lung during OVA challenge phase as reported by Idzko et al.28
COX inhibition with indomethacin significantly increased airway inflammation and eosinophilia in the airway and the levels of IL-5 and IL-13 cytokines in BAL and the amounts of total IgE and OVA-specific IgE in the serum compared to the vehicle control in the mice that underwent the tolerance protocol. Therefore, COX inhibition effectively abrogated the immune tolerance. However, the increased airway eosinophilia in WT Indo/Tolerance group compared with WT EtOH/Tolerance group (Figure 1C) was not proportionally correlated with Th2 cytokine levels (Figure 1F and 1G). The airway cellular data (Figure 2A~2D) conclusively reveal that COX inhibition abrogated tolerance, and the significant increase in IL-5 and IL-13 in the Indo/Tolerance group compared to the EtOH/Tolerance group (Figure 2F & 2G) (even though it is substantially less than that of the Indo/Allergy group) may have been sufficient to induce the airway eosinophilia seen. In contrast to WT mice, we found that the augmentation of inflammatory cell infiltration and the increases of IL-5 and IL-13 production in BAL of IP KO mice were correlated better (Figure 3). To explain the difference of inflammation-cytokine patterns between WT and IP KO mice, we speculate that indomethacin administered during the sensitization phase did not have an effect on PGI2 formation during the OVA challenge phase in the lung and PGI2 formed in the lung during the OVA challenge phase might have suppressive function on Th2 cytokine responses, leading to lower IL-5 and IL-13 levels in WT mice. Such PGI2-mediated suppression was absent in IP KO mice because of IP-deficiency.
COX-2 inhibition with NS-398 has been previously shown to suppress immune tolerance induced by oral ingestion of OVA.20 In that study, COX expression by DCs in mesenteric lymph nodes was associated with intestinal immune tolerance, induction of Treg cells and decreased production of IL-4 and Gata3 expression by T cells.20 During COX-2 inhibition, IL-4 was upregulated and responsible for the inhibition of oral tolerance and impeded the conversion of naïve T cells into mucosal Treg cells.20 In our study, we did not find detectable levels of IL-4 in BAL and in the supernatant of the lung homogenate in either WT or IP KO mice after the mice underwent the tolerance protocol (data not shown), suggesting that IL-4 is not responsible for the diminished immune tolerance.
In this study, we noticed that the inflammatory cell infiltration in the airway did not always correlate with the level of serum IgE. WT Indo/Tolerance mice had greater numbers of total and eosinophils in BAL (Figure 2A and 2C) and higher levels of serum IgE and OVA-specific IgE (Figure 5A and 5B), compared to WT EtOH/Tolerance mice, indicating a break of immune tolerance. However, the levels of IgE and OVA-specific IgE in Indo/Tolerance mice were lower than that of EtOH/Allergy and Indo/Allergy mice. Indomethacin treatment of the tolerance mice (Indo/Tolerance) did not reverse the IgE response to the levels of EtOH/Allergy or Indo/Allergy mice, but induced greater numbers of BAL inflammatory cells than that in EtOH/Allergy mice, and comparable numbers of cells to that of Indo/Allergy mice (Figure 2A–D). Similarly, the serum IgE levels of IP KO/Tolerance mice were much lower than WT/Allergy mice (Figure 3 & 5), although IP KO/Tolerance mice had comparable numbers of inflammatory cells and Th2 cytokine responses in BAL as WT/Allergy mice. These results suggest an IgE-independent mechanism for inflammatory infiltration to the lung. Such mechanism is supported by our published findings showing the development of allergic airway inflammation in the absence of IgE response in indomethacin-treated IL-4KO, IL-4R KO and STAT6 KO mice.9
Airway exposure of aeroallergens resulted in the generation of Treg cells and antigen-specific immune tolerance.29 Although Treg cells are an important component of immune tolerance, we found that IP KO mice had the same or greater percentages of Treg cells in the spleen, MLNs and lung compared to WT mice. Therefore, the anti-tolerance effect of IP deficiency appears not to be mediated by down-regulation of Treg cells. Possibly the increased number of Treg cells in IP/Tolerance mice is an effort to suppress the increased inflammation in these mice compared to WT/Tolerance mice. Chinen and colleagues reported an essential role of PGE2 in maintenance of immune tolerance to commensal microorganisms in the gut22. The PGE2-mediated immune tolerance requires the cytokine signaling of SOCS1 and was independent of Treg cells.22 These results are consistent with ours and it is possible that, similar to PGE2, PGI2 signaling may promote immune tolerance through Treg-independent mechanisms.
In conclusion, COX inhibition abrogated immune tolerance by suppressing COX-dependent IP signaling, suggesting that the frequent use of COX inhibiting drugs may attenuate aeroallergen-specific immune tolerance. Our results also suggest a possible role for PGI2 analogs in allergen immunotherapy and tolerance induction.
Extended Data
Figure E1.
WT and 5-LO KO mice were subjected to allergy and immune tolerance protocols and treated with EtOH and indomethacin. BAL cell counts (A–E), and IL-5 and IL-13 cytokine levels (F–G) in BAL at day 18 were shown. * p<0.05, n=4–5 mice per group. Data are representative of 3 experiments.
Figure E2.
IP KO mice had increased lung inflammation in the tolerance protocol compared to WT mice. Lung sections of mice harvested at day 18 were H&E stained (A) and scored for Inflammation (B). * p<0.05, n=5 mice per group.
Figure E3.
BAL cell counts and H&E stained lung sections of naïve WT and IP KO mice.
Figure E4.
IP deficiency increased the percentage of Treg cells in the spleen and MLN compared to WT mice subjected to the tolerance protocol. Mouse spleens were harvested at (A) day −7 (3 days after tolerance induction phase), (B) day −2 (8 days after tolerance induction phase), or day 18 (1 day after OVA challenge phase). MLNs were also harvested on day 18. Treg cells (CD4+CD25+FoxP3+ cells) were shown as percentage of gated live CD4 T cells. * p<0.05, n=5 mice per group.
Figure E5.
IP deficiency increased the number of Treg and inducible Treg (iTreg) cells in the lung compared to WT mice subjected to the tolerance protocol. Mouse lungs were harvested 1 day after OVA challenge (day 18). Treg cells (CD4+CD25+FoxP3+) and iTreg (IL-10+CD4+CD25+FoxP3+) cells were determined by flow cytometry. * p<0.05, n=5 mice per group.
Clinical Implications.
Frequent use of COX inhibiting drugs may attenuate aeroallergen-specific immune tolerance. PGI2 and its analogs that are clinically used for treating pulmonary hypertension may have a therapeutic potential for allergic diseases.
Acknowledgments
This study was supported by National Institute of Health grants R01 AI 111820, 2I01BX000624, U19 AI 095227-02, R01 HL 090664-04 and R56 AI076411 and Veteran Affairs grant 2I01BX000624.
Abbreviations used
- 5-LO
5-lipoxygenase
- COX
Cyclooxygenase
- KO
Knockout
- IP
PGI2 receptor
- OVA
Ovalbumin
- PGI2
Prostaglandin I2
- Treg
Regulatory T cells
- WT
Wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jarvis D, Burney P. ABC of allergies. The epidemiology of allergic disease. BMJ. 1998;316:607–10. doi: 10.1136/bmj.316.7131.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eder W, Ege MJ, von ME. The asthma epidemic. N Engl J Med. 2006;355:2226–35. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 3.Shaheen SO, Newson RB, Henderson AJ, Headley JE, Stratton FD, Jones RW, et al. Prenatal paracetamol exposure and risk of asthma and elevated immunoglobulin E in childhood. Clin Exp Allergy. 2005;35:18–25. doi: 10.1111/j.1365-2222.2005.02151.x. [DOI] [PubMed] [Google Scholar]
- 4.Marquis A, Strippoli MP, Spycher BD, Rebholz CE, von der Weid NX, Kuehni CE. Paracetamol, nonsteroidal anti-inflammatory drugs, and risk of asthma in adult survivors of childhood cancer. J Allergy Clin Immunol. 2011;127:270–2. doi: 10.1016/j.jaci.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Thomsen SF, Kyvik KO, Skadhauge LR, Steffensen I, Backer V. Regular use of non-steroidal anti-inflammatory drugs increases the risk of adult-onset asthma: a population-based follow-up study. Clin Respir J. 2009;3:82–4. doi: 10.1111/j.1752-699X.2008.00113.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhou W, Newcomb DC, Moore ML, Goleniewska K, O’Neal JF, Peebles RS., Jr Cyclooxygenase inhibition during allergic sensitization increases STAT6-independent primary and memory Th2 responses. J Immunol. 2008;181:5360–7. doi: 10.4049/jimmunol.181.8.5360. [DOI] [PubMed] [Google Scholar]
- 7.Carey MA, Germolec DR, Bradbury JA, Gooch RA, Moorman MP, Flake GP, et al. Accentuated T helper type 2 airway response after allergen challenge in cyclooxygenase-1−/− but not cyclooxygenase-2−/− mice. Am J Respir Crit Care Med. 2003;167:1509–15. doi: 10.1164/rccm.200211-1383OC. [DOI] [PubMed] [Google Scholar]
- 8.Laouini D, Elkhal A, Yalcindag A, Kawamoto S, Oettgen H, Geha RS. COX-2 inhibition enhances the TH2 immune response to epicutaneous sensitization. J Allergy Clin Immunol. 2005;116:390–6. doi: 10.1016/j.jaci.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto K, Sheller JR, Morrow JD, Collins RD, Goleniewska K, O’Neal J, et al. Cyclooxygenase inhibition augments allergic inflammation through CD4-dependent, STAT6-independent mechanisms. J Immunol. 2005;174:525–32. doi: 10.4049/jimmunol.174.1.525. [DOI] [PubMed] [Google Scholar]
- 10.Peebles RS, Jr, Hashimoto K, Morrow JD, Dworski R, Collins RD, Hashimoto Y, et al. Selective cyclooxygenase-1 and -2 inhibitors each increase allergic inflammation and airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 2002;165:1154–60. doi: 10.1164/ajrccm.165.8.2106025. [DOI] [PubMed] [Google Scholar]
- 11.Gavett SH, Madison SL, Chulada PC, Scarborough PE, Qu W, Boyle JE, et al. Allergic lung responses are increased in prostaglandin H synthase-deficient mice. J Clin Invest. 1999;104:721–32. doi: 10.1172/JCI6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gereke M, Jung S, Buer J, Bruder D. Alveolar type II epithelial cells present antigen to CD4(+) T cells and induce Foxp3(+) regulatory T cells. Am J Respir Crit Care Med. 2009;179:344–55. doi: 10.1164/rccm.200804-592OC. [DOI] [PubMed] [Google Scholar]
- 13.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, et al. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W, Blackwell TS, Goleniewska K, O’Neal JF, FitzGerald GA, Lucitt M, et al. Prostaglandin I2 analogs inhibit Th1 and Th2 effector cytokine production by CD4 T cells. J Leukoc Biol. 2007;81:809–17. doi: 10.1189/jlb.0606375. [DOI] [PubMed] [Google Scholar]
- 16.Zhou W, Hashimoto K, Goleniewska K, O’Neal JF, Ji S, Blackwell TS, et al. Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. J Immunol. 2007;178:702–10. doi: 10.4049/jimmunol.178.2.702. [DOI] [PubMed] [Google Scholar]
- 17.Jaffar Z, Ferrini ME, Buford MC, FitzGerald GA, Roberts K. Prostaglandin I2-IP signaling blocks allergic pulmonary inflammation by preventing recruitment of CD4+ Th2 cells into the airways in a mouse model of asthma. J Immunol. 2007;179:6193–203. doi: 10.4049/jimmunol.179.9.6193. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi Y, Tokuoka S, Masuda T, Hirano Y, Nagao M, Tanaka H, et al. Augmentation of allergic inflammation in prostanoid IP receptor deficient mice. Br J Pharmacol. 2002;137:315–22. doi: 10.1038/sj.bjp.0704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto K, Graham BS, Geraci MW, FitzGerald GA, Egan K, Zhou W, et al. Signaling through the prostaglandin I2 receptor IP protects against respiratory syncytial virus-induced illness. J Virol. 2004;78:10303–9. doi: 10.1128/JVI.78.19.10303-10309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broere F, du Pre MF, van Berkel LA, Garssen J, Schmidt-Weber CB, Lambrecht BN, et al. Cyclooxygenase-2 in mucosal DC mediates induction of regulatory T cells in the intestine through suppression of IL-4. Mucosal Immunol. 2009;2:254–64. doi: 10.1038/mi.2009.2. [DOI] [PubMed] [Google Scholar]
- 21.Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjornbeth BA, et al. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008;57:813–21. doi: 10.1007/s00262-007-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chinen T, Komai K, Muto G, Morita R, Inoue N, Yoshida H, et al. Prostaglandin E2 and SOCS1 have a role in intestinal immune tolerance. Nat Commun. 2011;2:190. doi: 10.1038/ncomms1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296:539–41. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 24.Ostroukhova M, Qi Z, Oriss T, Dixon-McCarthy B, Ray P, Ray A. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J Clin Invest. 2006;116:996–1004. doi: 10.1172/JCI26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrow J, Prakash C, Awad J, Duckworth T, Zackert W, Blair I, et al. Quantification of the major urinary metabolite of prostaglandin D2 by a stable isotope dilution mass spectrometric assay. Analytical biochemistry. 1991;193:142–8. doi: 10.1016/0003-2697(91)90054-w. [DOI] [PubMed] [Google Scholar]
- 26.Peebles RS, Jr, Dworski R, Collins RD, Jarzecka K, Mitchell DB, Graham BS, et al. Cyclooxygenase inhibition increases interleukin 5 and interleukin 13 production and airway hyperresponsiveness in allergic mice. Am J Respir Crit Care Med. 2000;162:676–81. doi: 10.1164/ajrccm.162.2.9911063. [DOI] [PubMed] [Google Scholar]
- 27.Nagao K, Tanaka H, Komai M, Masuda T, Narumiya S, Nagai H. Role of prostaglandin I2 in airway remodeling induced by repeated allergen challenge in mice. Am J Respir Cell Mol Biol. 2003;29:314–20. doi: 10.1165/rcmb.2003-0035OC. [DOI] [PubMed] [Google Scholar]
- 28.Idzko M, Hammad H, van Nimwegen M, Kool M, Vos N, Hoogsteden HC, et al. Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. J Clin Invest. 2007;117:464–72. doi: 10.1172/JCI28949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, et al. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]