Abstract
Background
Two platyhelminths of biomedical and commercial significance are Schistosoma mansoni (blood fluke) and Fasciola hepatica (liver fluke). These related trematodes are responsible for the chronic neglected tropical diseases schistosomiasis and fascioliasis, respectively. As no vaccine is currently available for anti-flukicidal immunoprophylaxis, current treatment is mediated by mono-chemical chemotherapy in the form of mass drug administration (MDA) (praziquantel for schistosomiasis) or drenching (triclabendazole for fascioliasis) programmes. This overreliance on single chemotherapeutic classes has dramatically limited the number of novel chemical entities entering anthelmintic drug discovery pipelines, raising significant concerns for the future of sustainable blood and liver fluke control.
Methodology/ Principle Findings
Here we demonstrate that 7-keto-sempervirol, a diterpenoid isolated from Lycium chinense, has dual anthelmintic activity against related S. mansoni and F. hepatica trematodes. Using a microtiter plate-based helminth fluorescent bioassay (HFB), this activity is specific (Therapeutic index = 4.2, when compared to HepG2 cell lines) and moderately potent (LD50 = 19.1 μM) against S. mansoni schistosomula cultured in vitro. This anti-schistosomula effect translates into activity against both adult male and female schistosomes cultured in vitro where 7-keto-sempervirol negatively affects motility/behaviour, surface architecture (inducing tegumental holes, tubercle swelling and spine loss/shortening), oviposition rates and egg morphology. As assessed by the HFB and microscopic phenotypic scoring matrices, 7-keto-sempervirol also effectively kills in vitro cultured F. hepatica newly excysted juveniles (NEJs, LD50 = 17.7 μM). Scanning electron microscopy (SEM) evaluation of adult F. hepatica liver flukes co-cultured in vitro with 7-keto-sempervirol additionally demonstrates phenotypic abnormalities including breaches in tegumental integrity and spine loss.
Conclusions/ Significance
7-keto-sempervirol negatively affects the viability and phenotype of two related pathogenic trematodes responsible for significant human and animal infectious diseases. This plant-derived, natural product is also active against both larval and adult developmental forms. As such, the data collectively indicate that 7-keto-sempervirol is an important starting point for anthelmintic drug development. Medicinal chemistry optimisation of more potent 7-keto-sempervirol analogues could lead to the identification of novel chemical entities useful for future combinatorial or replacement anthelmintic control.
Author Summary
Schistosomiasis and fascioliasis are caused by two related trematodes found within the phylum Platyhelminthes (flatworms), and are classified as neglected diseases of poverty due to their effects on people living in the most underprivileged areas of the world. With no vaccine currently near development, and the existing strategy for global control based on the over-reliance on single-class chemotherapies, there is an urgent requirement for the identification of next generation anthelmintics. Here we demonstrate that 7-keto-sempervirol, a natural product derived from Lycium chinense, displays dual anthelmintic activity towards both Schistosoma mansoni (causative agent of schistosomiasis) and Fasciola hepatica (causative agent of fascioliasis). Utilising objective and phenotypic matrices, we show this activity to be selective (compared to a human cell line) and moderately potent against S. mansoni and F. hepatica larvae. This anti-larval effect translates into additional activity against both S. mansoni and F. hepatica adults where 7-keto-sempervirol induces phenotypic abnormalities including tegumental damage, motility disruption and oviposition inhibition. Due to 7-keto-sempervirol’s anthelmintic activity against multiple life stages of two parasitic trematodes, we contend that this starting chemical scaffold could be used to develop more effective compounds useful in controlling important parasites of biomedical and commercial relevance.
Introduction
Schistosomiasis and fascioliasis are Neglected Tropical Diseases (NTDs) caused by related parasitic blood (Schistosoma sp. including Schistosoma mansoni) and liver (Fasciola sp including Fasciola hepatica) flukes found within the phylum Platyhelminthes. These NTDs are responsible for chronic conditions of biomedical and veterinary significance and collectively affect a considerable proportion of the world’s human and animal populations. Globally, approximately 200 million people are currently afflicted by schistosomiasis, with this chronic disease being most prominent in tropical/subtropical regions of poverty-stricken, rural areas [1]. While fascioliasis is one of the most important parasitic diseases of ruminant livestock animals, it also negatively impacts 2.4 to 17 million humans worldwide by inducing chronic liver pathologies in infected individuals [2].
Control of these two NTDs remains largely centred on the use of large-scale chemotherapy administered to infected individuals in high prevalence areas. Praziquantel (PZQ) is presently the gold standard drug of choice for schistosomiasis control due to its safety, low cost, and activity towards the adult life stage of the three major, human-infective species (S. mansoni, Schistosoma haematobium and Schistosoma japonicum) [3]. For fascioliasis chemotherapy, triclabendazole (TCBZ) remains the drug of choice and is the only available compound effective against both adult and juvenile liver fluke lifecycle stages [4]. In both cases, the over-reliance on a single drug class for maintaining the future of blood and liver fluke control has generated significant concerns that drug resistant flukes could eventually develop (Schistosoma) or increase in prevalence (Fasciola). Although potential new replacement or combinatorial anthelmintic compounds have recently been identified [5–11], concerns have been raised regarding the insufficient activity in this research area due to the perceived sustainability of current options [12,13].
Moving forward, it is becoming evident that a relatively untapped source of chemical entities for developing new anthelmintics is plants and their natural products [14–16]. Plants possess a diverse arsenal of chemical reserves that have evolved to aid in plant protection and competition, and this has been exploited for medicinal uses for centuries [17]. For example, while artemisinin (derived from Artemisia annua) and derivatives represent the current, frontline anti-malarials [18], they also display significant activity against Schistosoma and Fasciola [7,19]. Further interrogation of the chemical diversity found in plants suggests that terpenoids (terpenes) are the most abundant and numerous of the plant secondary products and they possess features, including considerable structural variation [20], that may be exploitable as next-generation anthelmintics. In clear support of this, previous studies have demonstrated that topical terpenoid application to the skin effectively prevents schistosome penetration, providing an innovative chemoprophylactic method to avert schistosomiasis [21,22]. However, it is presently unclear how terpenoids affect schistosome biology or, indeed, if they have activity against other parasitic trematodes, including F. hepatica, due to lack of detailed investigations.
In this study a diterpenoid (7-keto-sempervirol) extracted and purified from Lycium chinense (common name Wolfberry), a plant traditionally used in Asian medicine since 1000 AD [23], was thoroughly investigated for its anthelmintic properties against S. mansoni and F. hepatica larvae and adults. Here, we demonstrate that 7-keto-sempervirol selectively kills larval stages of each trematode and induces tegumental damage, as well as motility disruption, in both hermaphroditic and dioecious adults. Furthermore, 7-keto-sempervirol dramatically inhibits the developmental maturation and oviposition of phenotypically normal S. mansoni eggs. Collectively these findings suggest that diterpenoids, such as 7-keto-sempervirol, have broad activities against related trematodes and should be further investigated as starting points for combating both schistosomiasis and fascioliasis.
Methods
Ethics statement
All procedures performed on mice adhered to the United Kingdom Home Office Animals (Scientific Procedures) Act of 1986 (project license PPL 40/3700) as well as the European Union Animals Directive 2010/63/EU and were approved by Aberystwyth University’s (AU) Animal Welfare and Ethical Review Body (AWERB).
Compound storage and handling
The diterpenoid 7-keto-sempervirol was isolated from the root of the temperate plant Lycium chinense (The Organic Herb Trading Company, Milverton, UK). Two kilograms of ground material was extracted in CH2Cl2 using a Soxhlet system. Nine fractions were obtained using Biotage 75 flash chromatography (silica gel, eluted with a step gradient of increasing polarity: n-hexane—ethylacetate—methanol). 7-keto-sempervirol was isolated from these fractions via reverse-phase preparative HPLC (C18 preparative column, eluted with a gradient- water: acetonitrile: 0.1% trifluroacetic acid in acetonitrile, with UV detection). The compound structure was then determined by UV, 1H NMR, 13C NMR and LC-MS analyses and also through direct comparison with the literature. The compound auranofin (Sigma-Aldrich, UK), a thioredoxin glutathione reductase (TGR) inhibitor capable of killing schistosomes, was used at 70 μM (final concentration) [24] as a dead control for the Helminth Fluorescent Bioassay (HFB) [25]. Both compounds (auranofin and 7-keto-sempervirol) were stored at -80°C in DMSO until use.
Schistosoma mansoni schistosomula culture
S. mansoni (NRMI strain) cercariae were shed from infected Biomphalaria glabrata snails (NMRI strain) by exposure to 2 hours of light in an artificially heated room (26°C). Collected cercariae were mechanically transformed into schistosomula as previously described [26] and resuspended in culture media comprising DMEM (Dulbecco’s Modified Eagle Medium, Sigma-Aldrich, UK) lacking phenol red, but containing 4.5 g/l glucose, 2 mM L-glutamine, 200 U/ml penicillin and 200 μg/ml streptomycin (all Sigma-Aldrich, UK). Schistosomula were then transferred to a black sided, flat-bottom (optically clear) 96-well microtiter plate (Star Lab, UK) at a density of 1000 parasites per 100 μl well. The plate was then incubated at 37°C and 5% CO2 for 24 hr to allow parasite equilibration.
Adult Schistosoma mansoni culture
S. mansoni adult parasites were recovered by hepatic portal perfusion from Tuck Ordinary mice (Harlan Laboratories, UK) experimentally infected seven weeks earlier with 200 cercariae. Washed adult worms were cultured in DMEM containing phenol red, 4.5 g/l glucose, supplemented with 10% foetal calf serum, 2 mM L-glutamine, 200 U/ml penicillin, 200 μg/ml streptomycin (all Sigma-Aldrich, UK). Schistosome cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2 for 24 hr prior to further manipulations. For egg laying experiments, five adult worm pairs per ml of culture medium (48-well tissue culture plates) were cultivated as above for a total of 72 hr (in the presence/absence of 7-keto-sempervirol). Eggs were counted after 72 hr and classified as normal (oval and containing a fully-formed lateral spine) or abnormal (lacking an oval shape and fully formed lateral spine).
Fasciola hepatica newly excysted juvenile (NEJ) culture
F. hepatica metacercariae (Baldwin Aquatic, Inc., OR, USA) were transformed into newly excysted juveniles (NEJs) and equilibrated for 4 hr in Fasciola saline according to established methodologies [27]. After equilibration, NEJs were distributed into black sided, flat-bottom (optically clear) 96-well microtiter plates (Star Lab) at a density of 100 parasites per 100 μL well and cultured at 37°C in a humidified atmosphere containing 5% CO2 subject to further treatments.
Adult Fasciola hepatica culture
F. hepatica adult flukes were recovered from sheep livers (Ridgeway Research, UK), washed and cultured in media comprising DMEM (lacking phenol red) containing 4.5 g/l glucose (Sigma, UK). This was supplemented with 2.2 mM Ca(C2H3O2)2, 2.7 mM MgSO4, 61 mM glucose, 15 mM HEPES, 1 μM serotonin and 5 μg/ml gentamycin (all Sigma, UK). Adult fluke were placed in 12-well tissue culture plates, 1 parasite per well in 1 ml of culture media and cultivated for 48 hr at 37°C in a humidified atmosphere containing 5% CO2.
HepG2 cell culture and CellTitre-Glo assay
The human HepG2 cell line purchased from the European collection of cell cultures (ECACC 85011430) was grown to confluency in culture media comprising EMEM (Eagle’s Minimum Essential Medium) supplemented with 10% bovine calf serum, 2 mM L-glutamine, 1% non-essential amino acid solution (all Sigma Aldrich, UK) and 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen, UK). Cells were resuspended by trypsinisation (0.25% v/v for 5 min), rinsed and distributed into black sided, flat-bottom (optically clear) 96-well microtiter plates (StarLab, UK) at 50 μl/well (1x105 cells/ well). The plate was equilibrated in a humidified atmosphere containing 5% CO2 at 37°C for 2 hrs and then test compounds were added to relevant wells to create a total well volume of 100 μl/well. Live controls included cells incubated in the same concentration (1% v/v) of DMSO that was used in the experimental (compound treated) wells. Dead controls included cells incubated with 1% v/v Triton X-100 (Sigma-Aldrich) [28]. Tissue culture plates were returned to a humidified atmosphere containing 5% CO2 at 37°C for a further 24 hrs.
The CellTitre-Glo reagents were prepared according to the manufacturer’s instructions (Promega, UK). Tissue culture plates were equilibrated to RT for 30 min before CellTitre-Glo reagents were distributed into each plate well at 100 μl/well (total volume 200 μl/well) and mixed on an orbital shaker for 2 min. Cells were then stabilised for 10 min at RT and bubbles that may have formed were removed using a sterilised needle. After this time luminescent signal was read utilising the BMG Labtech Polarstar Omega Plate Reader and exported into Microsoft Excel for further analysis and conversion into percentage viability.
Helminth Fluorescent Bioassay (HFB)
The HFB was utilised to objectively determine the viability of schistosomula and NEJ parasites co-cultured in the presence of 7-keto-sempervirol, auranofin (70 μM) [8,29], DMSO (1% v/v) or media only. HFB methodology applied to schistosomula was performed as previously described [25] with a minor alteration. Here, as indicated in the schistosomula culture methods, parasite viability was measured in the absence of foetal calf serum (FCS). A slight modification of the HFB was also applied to NEJs and included assaying only 100 parasites/well (as opposed to 1000 schistosomula/well). A total of 100 μl of test substance (varying concentrations) was added to each well containing 100 μl of suspended parasites and cultured for 24 hr in a humidified atmosphere at 37°C before the HFB was performed.
Phenotypic measurements
The in vitro activity of 7-keto-sempervirol on S. mansoni adult worms and F. hepatica NEJs was assessed by measuring motility disturbances and morphological variations in comparison to an appropriate live control (DMSO, 1% v/v). The scoring matrix used to assess S. mansoni adult worm viability was based on the standard operating procedure for compound screening at the Special Programme for Research and Training in Tropical Diseases, World Health Organization, WHO-TDR as previously described [30]. Motility was numerically scored from 0–4 with 0 being total absence of all motility, 1 indicating absence of motility other than gut peristalsis, 2 representing minimal activity such as occasional head and tail movement, 3 demonstrating slow activity and 4 signifying normal activity. Morphological descriptors of the parasite were also recorded during phenotypic assessment and included ‘knots’ developing along the normally cylindrical body line of the adult worms and the sloughing, blebbing or tubercle swelling of the tegument. Scoring matrix assessment of the worms was performed at 24, 48 and 72 hr post treatment.
NEJ phenotyping (a supportive metric of the HFB) was performed using values derived from both movement indices and morphologic formats as previously described [31,32]. The movement score ranges from 1–5 with 1 representing good/normal movement and 5 representing a complete absence of movement. The morphologic score ranges from 1–6 with 1 representing a good/normal phenotype and 6 signifying a severely dissolved/granulated parasite. This scoring matrix assessment (values derived from summation of both movement and morphology metrics) was conducted at 24 hr post treatment.
Scanning electron, laser confocal scanning and high content imaging microscopy
Prior to scanning electron microscopy (SEM) analysis, adult schistosomes and liver flukes were first fixed in 2.5% (w/v) gluteraldehyde in phosphate buffered saline (PBS) for 24 hr at 22–24°C. After fixation, worms were washed 3 times in 1 X PBS, pH 7.5 and stored in the same buffer at 4°C until use [33]. Fixed parasite material was subsequently washed twice in double distilled water and dehydrated in ascending acetone percentages (30, 50, 70, 80, 90, 95, and 100%) for 15 minutes each as previously described [33,34]. Dehydrated worms were then critically dried (Polaron Critical Point Dryer E3000) for 1 hr in 100% acetone (critical point of CO2 is 7.38 MPa at a temperature of 31°C). Critically dried worms were mounted on aluminium stubs, sputter coated with platinum/palladium and observed under a Hitachi S-4700 field emission scanning electron microscope [34].
For laser scanning confocal microscopy (LSCM), adult schistosomes were first fixed in a solution containing 2% (v/v) acetic acid, 25% (v/v) formalin, 48% (v/v) ethanol and 25% (v/v) H2O at room temperature for 24 hr. After fixation, worms were stained with Langeron’s Carmine as previously described [35]. Worms were then mounted on glass microscope slides in DPX (distyrene, plasticiser, xylene) as previously described [36] and observed using a Leica TCS SP5II laser scanning confocal microscope, equipped with a 40X oil immersion objective and a 488 nm Argon laser and a 561nm DPSS laser.
S. mansoni eggs laid by adult females after 72 hr in vitro cultures (+/- 7-keto-sempervirol) were fixed in a 10% formaldehyde solution to prepare the biological tissue for microscopy. Fixed eggs were then phenotypically assessed by an ImageXpress micro XL High Content Imager (Molecular Devices, UK). Fluorescent images were obtained using a FITC filter (40x magnification). Schistosomula subjected to the HFB were visualised on the ImageXpress micro XL High Content Imager using FITC (to identify fluorescein diacetate positive parasites) and TRITC (to identify propidium iodide positive parasites) filters (10X magnification).
Statistical analysis
A One Way ANOVA was utilised to identify any significant differences between more than three treatment groups followed by post hoc testing with the Tukey’s test to identify significantly different means (means that do not share a letter are significantly different). Student’s t-test was utilised to determine significant differences between two treatment groups.
Results
Anti-schistosomal 7-keto-sempervirol properties
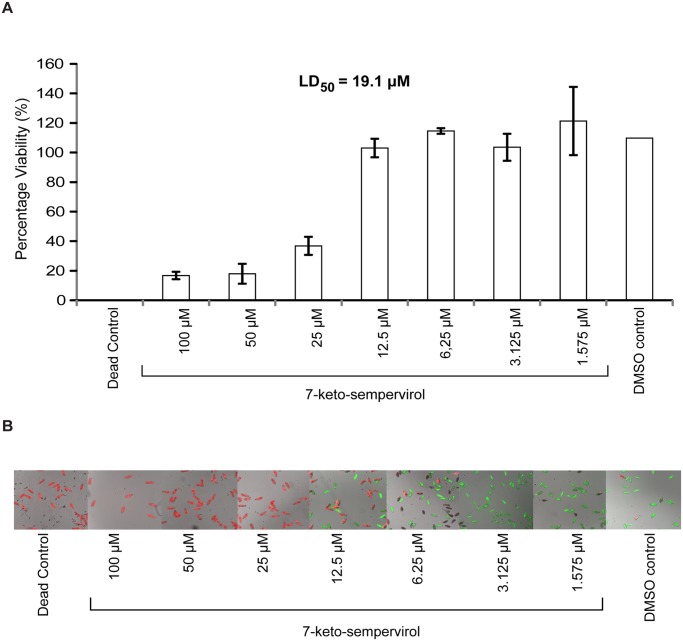
Due to the previously described action of select terpenes on schistosome viability [21,22] and definitive host penetration [37], we became interested in assessing the potential anthelmintic activity of 7-keto-sempervirol, a diterpenoid purified from Lycium chinense (Fig. 1). Employing the HFB [25], a titration series of 7-keto-sempervirol (100 μM–1.575 μM) was first used to assess the ability of this diterpenoid to affect schistosomula viability during in vitro co-culture (Fig. 2). At high 7-keto-sempervirol concentrations (100–25 μM), almost all of the schistosomula were killed or severely affected as indicated by low percent viability values (Fig. 2A) and fluorescent microscopic images of individual parasites (PI positive parasites > FDA positive parasites) (Fig. 2B). Lower 7-keto-sempervirol concentrations (between 6.25 μM–1.575 μM) had little effect on schistosomula viability and phenotype when compared to the DMSO control parasites. Based on these titration experiments, an LD50 of 19.1 μM was derived for 7-keto-sempervirol on the schistosomula lifecycle stage. A parallel set of titration experiments was additionally performed with the human HepG2 cell line (S1 Fig.) and demonstrated a 7-keto-sempervirol derived LD50 of 80 μM. 7-keto-sempervirol, therefore, demonstrates a therapeutic index of 4.2 towards the intra-mammalian schistosomula lifecycle stage.
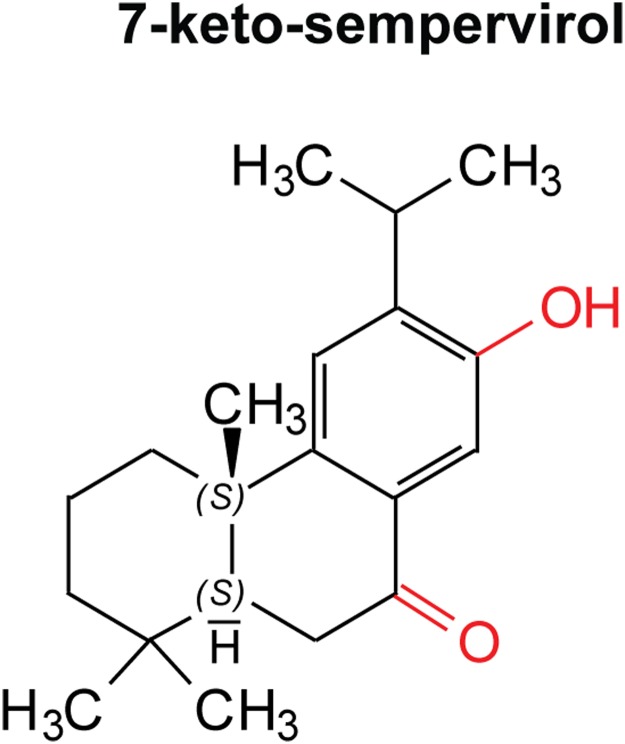
Fig 1. 7-keto-sempervirol is a diterpenoid phenol derived from Lycium chinense.
This compound possesses a diterpenoid (C20) (4 isoprene units) scaffold consisting of a single phenyl group, one hydroxyl group (red) and one carbonyl group (red). In light of Lipinski’s rule of 5 (RO5) [57], this compound only possesses one violation to the rule (log p > 5), however, three out of the four core rules are satisfied.
Fig 2. The diterpenoid 7-keto-sempervirol displays lethal activity against Schistosoma mansoni schistosomula.
Schistosomula (96 well plate format; 1000 parasites/well; triplicate wells for each concentration assessed) were co-cultivated with a 50% dilution series of 7-keto-sempervirol (100, 50, 25, 12.5, 6.25, 3.125, 1.563 μM) at 37°C and 5% CO2 for 24 hr and subjected to the helminth fluorescent bioassay (HFB). A) Fluorescent data derived from wells were converted into corresponding Log10 values and the mean percentage viability was transformed into probit values to create a dose-response curve as previously described [25]. An LD50 of 19.1 μM was calculated from this dose-response curve. Error bars represent the standard deviation of the mean (SD). B) High content imaging of fluorescently labelled parasites (fluorescein diacetate, green and propidium iodide, red) co-cultured with titrated 7-keto-sempervirol supports the HFB measurements. The concentration of fluorophores employed for high content imaging were the same as previously described for the HFB [25]. Dead control = parasites co-cultured with 70 μM auranofin. DMSO control = parasites co-cultured with 1% (v/v) DMSO. Parasites were imaged at 10 x magnification on a high content imaging system processed by the MetaXpress version 5.10.46 software utilising both FITC and TRITC filters.
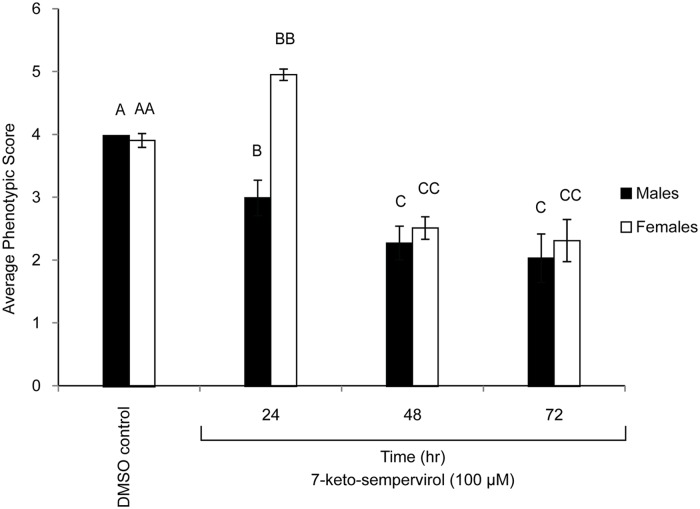
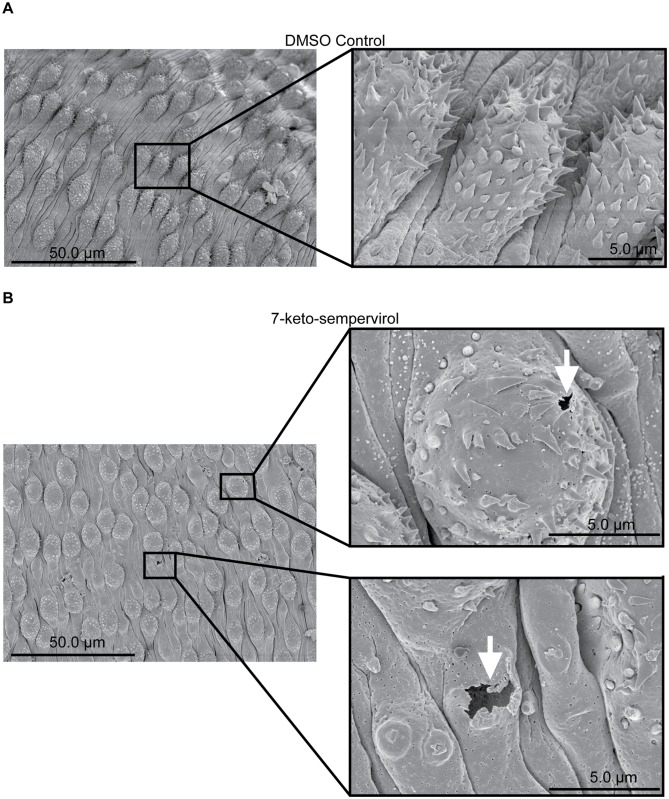
Based on its anti-schistosomula activity, 7-keto-sempervirol’s ability to affect adult schistosome motility, surface-tegument morphology and egg development was subsequently measured using both WHO-adopted indices and microscopic measures. Here, 7-keto-semperivol displayed a significant effect on both male and female worm motility at the highest concentration used in this study (100 μM) at 24 hr and 48 hr post treatment compared to the DMSO (24 hr) control group (Fig. 3). For both genders, there was no significant difference between 48 hr and 72 hr treatments at this concentration (Fig. 3) nor in DMSO treated worms cultivated for 48 hr or 72 hr (S2 Fig.). Interestingly, female worms (unlike males) displayed a 7-keto-semperivol-induced hyperactivity at 24 hr post-treatment (Fig. 3). There were motility and phenotypic discrepancies observed for some individuals cultured in the presence of 10 μM 7-keto-sempervirol, but these differences were not significantly different compared to the DMSO control group (S3 Fig.). Scanning electron microscopy (SEM) of adult male worms co-cultured in the presence of 7-keto-sempervirol (100 μM for 72 hr) further revealed tubercle swelling, spine loss/shortening and surface holes across the tegument (Fig. 4).
Fig 3. Adult Schistosoma mansoni motility is reduced in the presence of 7-keto-sempervirol.
World Health Organisation motility metrics were used for scoring adult S. mansoni male and female worm phenotypes (5 worm pairs/ml) co-cultured (37°C and 5% CO2) with 7-keto-sempervirol (100 μM final concentration) for 24 hr, 48 hr and 72 hr. Phenotypes derived from a control group (media containing 1% v/v DMSO) of male and female worms cultivated for 24 hr (worm pairs cultured for 48 hr or 72 hr were identical to the 24 hr worms, S2 Fig.) is also illustrated. Mean motility values are indicated as histograms (n = 5 replicates/time-point) and the error bars represent the standard deviation of the mean (SD). Different letters indicate a significant difference between the mean phenotypic scores calculated by ANOVA and Tukey’s post-hoc tests.
Fig 4. The diterpenoid 7-keto-sempervirol induces surface tegumental damage in adult Schistosoma mansoni males.
Scanning Electron Microscopic (SEM) images of adult S. mansoni male worms cultured for 72 hr at 37°C and 5% CO2 in the presence (100 μM) or absence (1% v/v DMSO) of 7-keto-sempervirol. A) (left) 1.00K x magnification of a control (1% v/v DMSO) adult male surface (anterior end) and corresponding (right) 6.00K x magnification of normal tubercles. B) (left) 1.00K x magnification of a 7-keto-sempervirol treated (100 μM), adult male surface (anterior end) and enlarged (right) 9.00K x magnification of two indicated (black boxes) areas of tegumental damage. Holes in the surface tegument/tubercles are indicated by white arrows. These images are representative of 5 adult worms/condition.
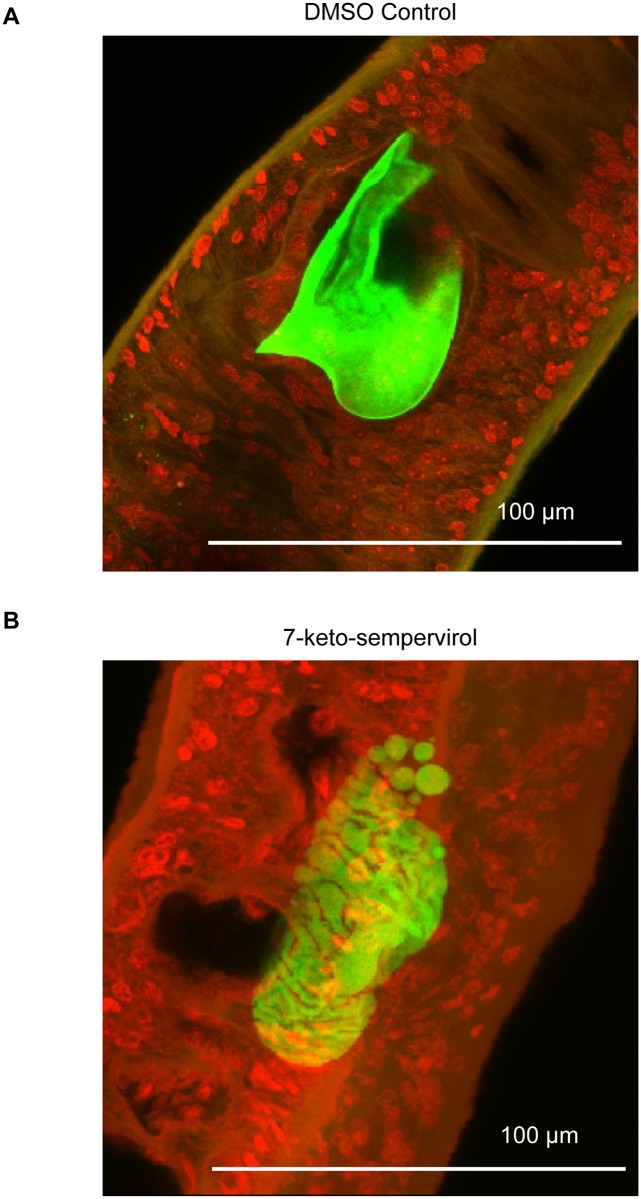
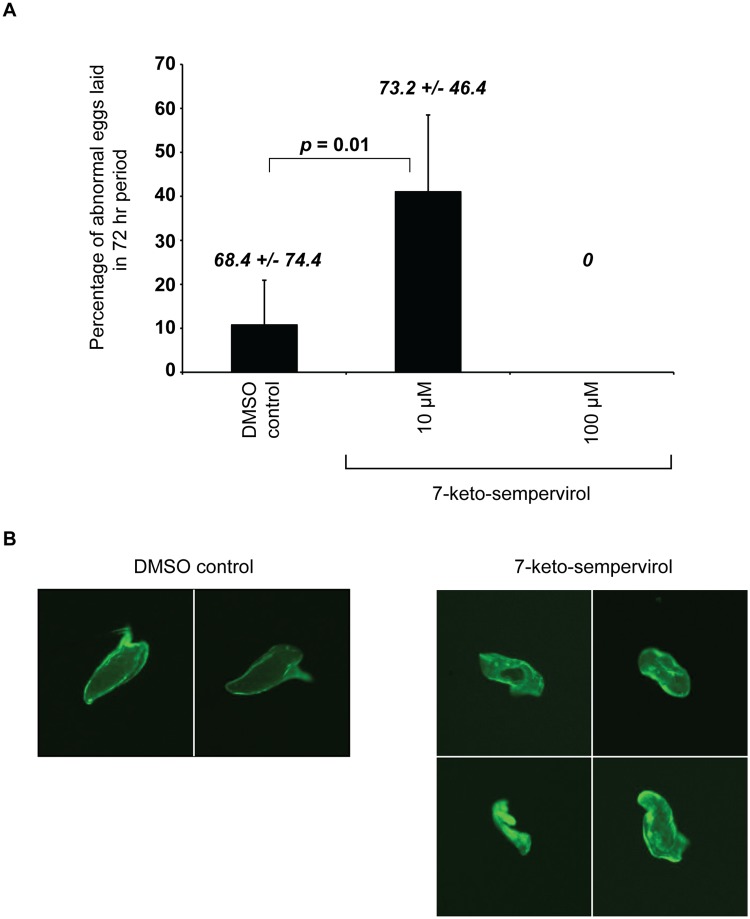
Laser scanning confocal microscopy (LSCM) of adult females cultured in the presence of 7-keto-sempervirol (100 μM for 24 hr) indicated the presence of irregularly shaped in utero eggs (Fig. 5). When compared to control eggs (parasites treated with 1% v/v DMSO, Fig. 5A), these abnormal eggs lacked regular autofluorescence as well as fully formed eggshells and were missing the characteristic lateral spines indicative of the species (Fig. 5B). Due to these phenotypic deficiencies in egg development, the effect that 7-keto-sempervirol had on in vitro schistosome oviposition was also assessed (Fig. 6). Here, 7-keto-sempervirol induced a concentration-dependent (100 μM > 10 μM) ability to inhibit the deposition of phenotypically normal schistosome eggs with a complete lack of oviposition observed in wells containing the highest amount of compound (100 μM) (Fig. 6A). When compared to control wells (schistosomes co-cultured with 1% v/v DMSO), eggs deposited in wells containing 7-keto-sempervirol (10 μM) displayed a range of abnormal phenotypes (Fig. 6B) similar to those observed in utero (Fig. 5B). These phenotypes included non-oval shapes, lack of lateral spines and irregular autofluorescence.
Fig 5. Schistosoma mansoni egg-shell formation is inhibited by 7-keto-sempervirol.
Adult S. mansoni female worms were cultured (+/- 100 μM 7-keto-sempervirol) for 24 hr at 37°C and 5% CO2. A) 40x laser scanning confocal microscopic image of an egg derived from a control female (1% v/v DMSO) demonstrating regular auto-fluorescence and a well-formed lateral spine (normal architecture of a S. mansoni egg). B) 40x laser scanning confocal microscopic image of an egg derived from one adult female worm treated with 100 μM 7-keto-sempervirol. Images are representative of typical in utero egg phenotypes obtained from 5 worms/condition.
Fig 6. Schistosoma mansoni oviposition is inhibited by 7-keto-sempervirol.
Adult worm pairs (5 pairs/ml) were co-cultured (37 oC and 5% CO2) with 1% v/v DMSO (DMSO control), 10 μM 7-keto-sempervirol or 100 μM 7-keto-sempervirol for 72 hr. A) Bar charts represent the mean percentage of abnormal eggs (lacking a fully formed lateral spine) produced after 72 hr (n = 5 replicates/treatment). Error bars represent the standard deviation of the mean (SD). After 72 hr, a total of 68.4 +/- 74.4 eggs (normal and abnormal) were enumerated in the worm cultures containing 1% DMSO, 73.2 +/- 46.4 eggs (normal and abnormal) were observed in the cultures containing 10 μM 7-keto-sempervirol and zero eggs were counted in the wells containing 100 μM 7-keto-sempervirol. Student’s t-test indicates that a significant difference exists between the percentage of abnormal eggs laid between the 1% v/v DMSO and 10 μM 7-keto-sempervirol treatments (p = 0.01). B) Fluorescent images of representative eggs deposited in vitro demonstrate normal egg architecture in the DMSO control wells (DMSO control) and abnormal egg architecture in the 10 μM 7-keto-sempervirol wells (7-keto-sempervirol).
Anti-Fasciola 7-keto-sempervirol activities
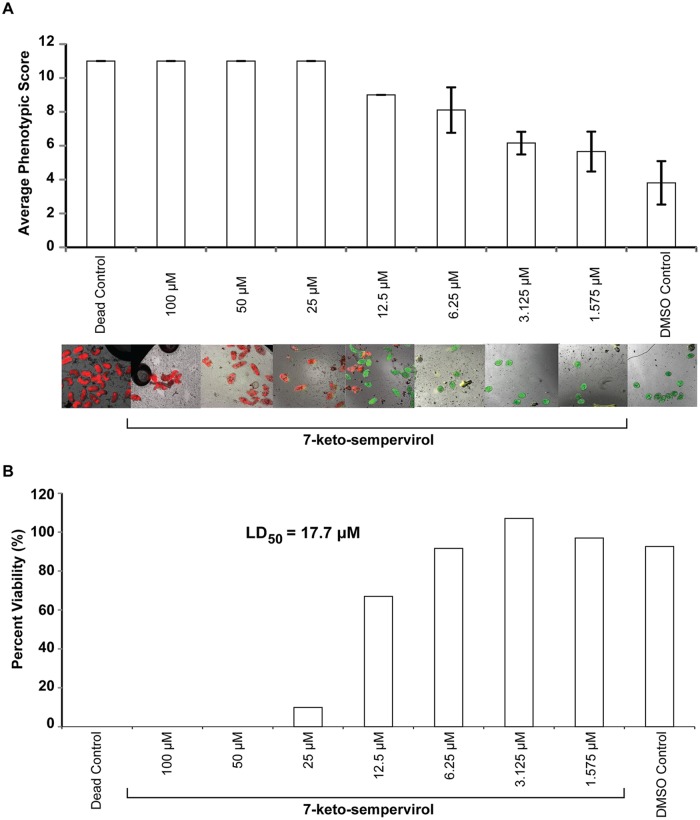
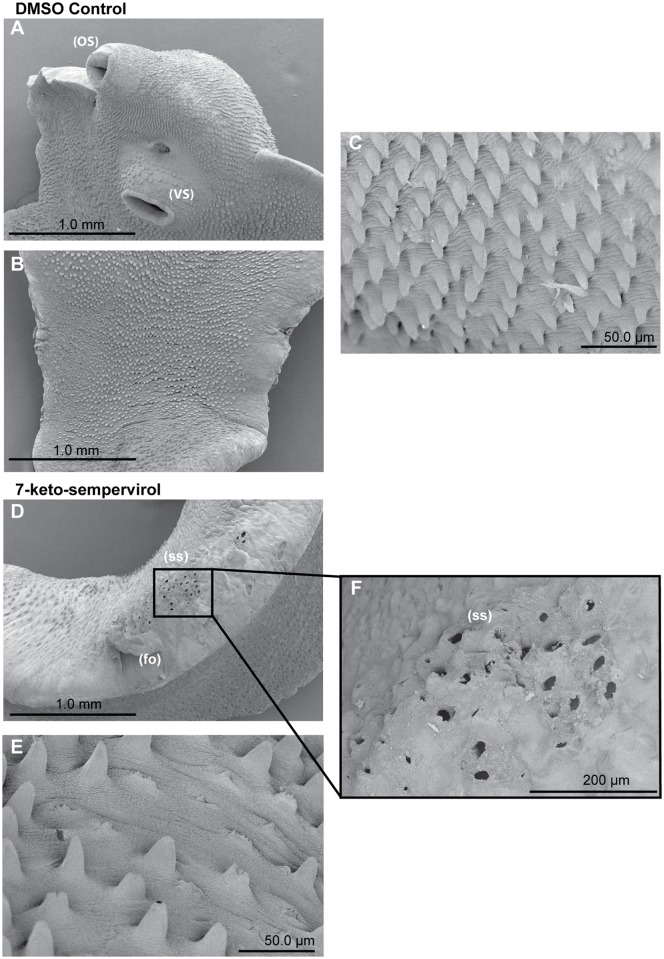
To assess 7-keto-sempervirol’s activity on F. hepatica NEJs, two complementary methodologies were employed (Fig. 7). The first methodology, using well-established motility and phenotypic metrics [31,32], indicated that 7-keto-sempervirol induced a negative concentration-dependent effect on NEJ movement and viability (Fig. 7A). This finding was supported by fluorescent microscopy of NEJs co-stained with the discriminatory viability dyes FDA and PI (Fig. 7A). The second methodology, using the HFB as a more objective method for determining NEJ viability [25], confirmed this concentration-dependent effect and established an LD50 of 17.7 μM for 7-keto-sempervirol against F. hepatica NEJs (Fig. 7B). When compared to the mammalian HepG2 cell line (S1 Fig.), 7-keto-sempervirol displayed an anti-NEJ therapeutic index of 4.5. To identify whether 7-keto-sempervirol also affected the surface integrity of F. hepatica adults, similar to S. mansoni (Fig. 4), SEM analyses were performed on adult liver flukes co-cultured with this diterpenoid (Fig. 8). Here, in comparison to control flukes incubated with 1% (v/v) DMSO (Fig. 8A-C), prolonged (48 hr) exposure to 7-keto-sempervirol (50 μM) induced substantial spine shortening and spine loss that was apparent on both dorsal and ventral sides of the organism (Fig. 8D-F).
Fig 7. The diterpenoid 7-keto-sempervirol displays lethal activity against Fasciola hepatica newly excysted juveniles (NEJs).
Complementary phenotypic-based [31,32] and fluorescent-mediated tests [25] were used to assess NEJ viability. A) The phenotypic assay involved co-culturing 20 NEJs (96-well plate format) with a 50% dilution series of 7-keto-sempervirol (100, 50, 25, 12.5, 6.25, 3.125, 1.563 μM) at 37°C and 5% CO2 for 24 hr. Mean phenotypic score values (11 = severely affected, 0 = not affected) are indicated by bar charts (n = 20 individuals scored/experimental treatment) with error bars representing the standard deviation of the mean (SD). A collection of fluorescent high content images (10 x) was obtained similar to those collected for schistosomula (Fig. 2). B) The fluorescent assay utilised the HFB [25] and co-cultured 100 NEJs/experimental treatment (96-well plate format) with the same dilution series of 7-keto-sempervirol used in the phenotypic-based test. Each concentration was converted into corresponding Log10 values and the percentage viability was transformed into probit values to create a dose-response curve. An LD50 of 17.7 μM was calculated from this dose-response curve. One representative HFB is illustrated here. Dead control = NEJs co-cultured with 70 μM auranofin. DMSO control = NEJs co-cultured with 1% v/v DMSO.
Fig 8. The diterpenoid 7-keto-sempervirol induces damage, shortening and loss of adult Fasciola hepatica tegumental spines.
SEM images of adult F. hepatica 48 hr post-treatment with culture media containing DMSO (1% v/v) or 100 μM 7-keto-sempervirol. A) Normal fluke ventral surface architecture including oral sucker (OS) and ventral sucker (VS) is unaffected after cultivation with culture media containing 1% (v/v) DMSO. B) Fluke dorsal surface architecture is also unaffected after cultivation with culture media containing 1% (v/v) DMSO. C) Fluke ventral spine phenotype after cultivation with culture media containing 1% (v/v) DMSO. D) Dorsal spine sockets (ss) and folding (fo) after cultivation with 100 μM 7-keto-sempervirol. E) Ventral spines after cultivation with 100 μM 7-keto-sempervirol. F) Higher magnification of (ss) region outlined by black box in (D).
Discussion
Current control efforts aimed at reducing disease prevalence of schistosomiasis and fascioliasis are predominantly based on mono-chemotherapy administration of praziquantel (PZQ) and triclabendazole (TCBZ), respectively. Nevertheless, worries about the development of PZQ resistant blood flukes and the spread of TCBZ resistant liver flukes coupled to the slow progression of immunoprophylactic vaccines, have notably increased the number of new anthelmintic drug discovery projects initiated throughout the last decade. While our efforts in this area have successfully leveraged interdisciplinary techniques to identify drug targets [38–42] and to develop high-throughput drug screening methodologies [25], we have only recently begun characterising the detailed anthelmintic activities of defined chemical entities. Here, we report the findings from one such investigation and demonstrate that a diterpenoid (7-keto-sempervirol) derived from Lycium chinense has key properties useful in the development of a broad-spectrum agent active against both blood and liver flukes.
First amongst the examined properties was the ability of 7-keto-sempervirol to kill, in a concentration dependent manner, both S. mansoni schistosomula (Fig. 2) and F. hepatica newly excysted juveniles (NEJs) (Fig. 7) during in vitro culture. While this anti-larval effect was not overly potent (LD50 = 17.7 μM for schistosomula and LD50 = 19.1 μM for NEJs), it was selective (therapeutic index = 4.2–4.5) and reproducible (e.g. two independent measurements of NEJ viability provided confirmatory results, Fig. 7). Importantly, this moderate anti-larval effect translated into pronounced phenotypic abnormalities associated with the tegumental surface of both fluke species (Figs. 4 and 8). Here, and similar to artemisinin’s effect on S. mansoni [7] and artemether/artesunate’s effect on F. hepatica [43], 7-keto-sempervirol induced tubercle swelling, tegumental breaches and spine loss in both flukes. This degree of surface damage (also seen in [9,33,44]), if replicated in vivo, would severely compromise the barrier function of this protective layer [45] and negatively affect the ability of both flukes to remain in hostile definitive host environments (blood and bile). Whether 7-keto-sempervirol’s ability to disrupt fluke surface architecture is similar in action to the protonophoric properties of membrane-disruptive, plant-derived quinones (anti-tumour derived diterpenoids) [46] is currently unknown, but is consistent with our observations. Further work is required to develop this line of investigation.
Another interesting property of 7-keto-sempervirol is its ability to progressively paralyse adult schistosomes during in vitro culture (Fig. 3). This feature, in addition to tegumental alterations, is similar to the effects induced by praziquantel [47] treatment of adult schistosomes and suggests that this diterpenoid may also perturb calcium homeostasis [3]. Interestingly, the antifungal activities of two related mono-terpenoid phenols (carvacrol and thymol) also involve dis-regulation of intracellular calcium balances and suggest a common mechanism of action amongst terpenes [48] and praziquantel. In support of this, treatment of Saccharomyces cerevisiae with either carvacrol or thymol leads to the differential expression of similar gene categories as those found in praziquantel treated schistosomes [47]. These include genes associated with drug transportation across membranes, ribosomal biogenesis, autophagy, heat shock responses, rRNA processing, tRNA processing and pyrimidine metabolism [48]. Whether 7-keto-sempervirol is capable of differentially regulating a similar repertoire of schistosome gene products is currently unknown, but the time-dependent paralysis of adults co-cultured with this diterpenoid in vitro would suggest that this hypothesis is likely. Gender specific transcriptomic responses to 7-keto-sempervirol may also exist, similar to those induced by praziquantel [47], and could explain the differential sensitivity and hyperactivity observed in females (but not males) at 24 hr post treatment (Fig. 3). While 7-keto-sempervirol/adult F. hepatica co-cultures were not kinetically studied in this investigation, it would be useful to ascertain in future experiments if the diterpenoid-induced, time-dependent paralysis of dioecious blood flukes translates to hermaphroditic liver flukes.
A final anthelmintic property examined for 7-keto-sempervirol, based on its established capacity to damage surface membranes (Figs. 4 & 8) and impede schistosome motility (Fig. 3), was its potential to affect egg production during in vitro cultures (Figs. 5 & 6). As oviposition is required for schistosome lifecycle transmission and is responsible for chronic definitive host immunopathology, identification and progression of compounds that inhibit this process (even if they do not kill the parasite) are likely to benefit schistosomiasis control. Interestingly, the concentration-dependent (100 μM > 10 μM), 7-keto-sempervirol mediated, inhibition of phenotypically-normal egg maturation (Fig. 5 & 6B) and egg production (Fig. 6A) observed here are strikingly similar to those findings reported for schistosome pairs co-cultured with kojic acid [41]. Kojic acid exerts its effects on eggshell sclerotisation (hardening or tanning) and schistosome oviposition by inhibiting the phenol oxidase activities of S. mansoni tyrosinases 1 and 2 (SmTYR1/SmTYR2). Whether 7-keto-sempervirol also targets the phenol oxidase activity of SmTYR1/SmTYR2 is currently unknown. However, as egg maturation, sclerotisation and oviposition are complex processes involving cytosine methylation [40], tyrosine kinase-mediated phosphorylation [49] serine/threonine kinase-mediated phosphorylation [50], fatty acid oxidation [51] and TGF-beta signalling [52], 7-keto-sempervirol could theoretically act upon any component of these (or other) diverse biological processes. Limitations in obtaining sufficient quantities of adult F. hepatica prohibited the assessment of 7-keto-sempervirol’s effect on liver fluke egg production. Nevertheless, as the process of eggshell formation is thought to occur via similar mechanisms in both schistosomes and liver flukes [53], it is likely that 7-keto-sempervirol will also inhibit F. hepatica oviposition.
Here, we provide complementary evidence that supports the further progression of 7-keto-sempervirol as a dual anthelmintic against S. mansoni and F. hepatica parasites. Although these findings add value to the growing medicinal properties described for diterpenoids [54–56] and expand upon their anti-schistosomal chemoprophylactic characteristics [21,22], improving their selective potency is a necessary next step in their development as wide-acting anthelmintics. Whilst defining a specific mechanism of 7-keto-sempervirol action and experimental animal model verification was beyond the scope of this investigation, our results suggest that membrane biogenesis/maintenance and calcium homeostasis/stress are likely contributing to the diverse in vitro phenotypes observed in both fluke species. Activity against both larvae and adult fluke stages broaden the window of therapeutic opportunity and, if replicable in vivo, would provide a useful alternative to currently used anthelmintics within the biomedical and animal health landscapes.
Supporting Information
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Ms. Julie Hurst, Ms. Kezia Whatley, Ms. Fanny Nowacki and Drs. Kathrin Geyer, Iain Chalmers and Sabrina Munshi for help in maintaining the S. mansoni life cycle. We also acknowledge Drs. Stephen Wade, Dylan Phillips and Kathrin Geyer for assisting with the microscopy and Dr. Russell Morphew and Ms. Rebekah Stuart for guiding the F. hepatica cultivation.
Data Availability
All relevant data are within the paper.
Funding Statement
We thank the Welsh Government Knowledge Economy Skills Scholarship (KESS) and Academia for Business (A4B) schemes as well as the BBSRC Follow On Fund (BB/I532937/1) for financially supporting this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, et al. (2012) A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Negl Trop Dis 6: e1582 10.1371/journal.pntd.0001582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mas-Coma S, Valero MA, Bargues MD (2014) Fascioliasis. Adv Ecp Med Bio 766: 77–114. [DOI] [PubMed] [Google Scholar]
- 3. Cioli D, Pica-Mattoccia L, Basso A, Guidi A (2014) Schistosomiasis control: praziquantel forever? Mol Biochem Parasitol 195: 23–29. 10.1016/j.molbiopara.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 4. Fairweather I (2005) Triclabendazole: new skills to unravel an old(ish) enigma. J Helminthol 79: 227–234. [DOI] [PubMed] [Google Scholar]
- 5. Rojo-Arreola L, Long T, Asarnow D, Suzuki BM, Singh R, et al. (2014) Chemical and genetic validation of the statin drug target to treat the helminth disease, schistosomiasis. PloS One 9: e87594 10.1371/journal.pone.0087594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang HH, Rigouin C, Williams DL (2012) The redox biology of schistosome parasites and applications for drug development. Curr Pharm Design 18: 3595–3611. [PMC free article] [PubMed] [Google Scholar]
- 7. Keiser J, Utzinger J (2007) Artemisinins and synthetic trioxolanes in the treatment of helminth infections. Curr Opin Infect Dis 20: 605–612. [DOI] [PubMed] [Google Scholar]
- 8. Sayed AA, Simeonov A, Thomas CJ, Inglese J, Austin CP, et al. (2008) Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat Med 14: 407–412. 10.1038/nm1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKinstry B, Fairweather I, Brennan GP, Forbes AB (2003) Fasciola hepatica: tegumental surface alterations following treatment in vivo and in vitro with nitroxynil (Trodax). Parasitol Res 91: 251–263. [DOI] [PubMed] [Google Scholar]
- 10. Kirchhofer C, Vargas M, Braissant O, Dong Y, Wang X, et al. (2011) Activity of OZ78 analogues against Fasciola hepatica and Echinostoma caproni . Acta Trop 118: 56–62. 10.1016/j.actatropica.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pierce RJ, Dubois-Abdesselem F, Lancelot J, Andrade L, Oliveira G (2012) Targeting schistosome histone modifying enzymes for drug development. Curr Pharm Design 18: 3567–3578. [PubMed] [Google Scholar]
- 12. Geary TG (2012) Are new anthelmintics needed to eliminate human helminthiases? Curr Opin Infect Dis 25: 709–717. 10.1097/QCO.0b013e328359f04a [DOI] [PubMed] [Google Scholar]
- 13. Cabada MM, White AC Jr. (2012) New developments in epidemiology, diagnosis, and treatment of fascioliasis. Curr Opin Infect Dis 25: 518–522. 10.1097/QCO.0b013e3283567b7e [DOI] [PubMed] [Google Scholar]
- 14. Kayser O, Kiderlen AF, Croft SL (2003) Natural products as antiparasitic drugs. Parasitol Res 90 Suppl 2: S55–62. [DOI] [PubMed] [Google Scholar]
- 15. Li JW, Vederas JC (2009) Drug discovery and natural products: end of an era or an endless frontier? Science 325: 161–165. 10.1126/science.1168243 [DOI] [PubMed] [Google Scholar]
- 16. Murthy PK, Joseph SK, Murthy PS (2011) Plant products in the treatment and control of filariasis and other helminth infections and assay systems for antifilarial/anthelmintic activity. Planta Med 77: 647–661. 10.1055/s-0030-1250452 [DOI] [PubMed] [Google Scholar]
- 17. da Rocha AB, Lopes RM, Schwartsmann G (2001) Natural products in anticancer therapy. Curr Opin Pharm 1: 364–369. [DOI] [PubMed] [Google Scholar]
- 18. van Vugt M, van Beest A, Sicuri E, van Tulder M, Grobusch MP (2011) Malaria treatment and prophylaxis in endemic and nonendemic countries: evidence on strategies and their cost-effectiveness. Fut Microbiol 6: 1485–1500. [DOI] [PubMed] [Google Scholar]
- 19. Liu R, Dong HF, Guo Y, Zhao QP, Jiang MS (2011) Efficacy of praziquantel and artemisinin derivatives for the treatment and prevention of human schistosomiasis: a systematic review and meta-analysis. Parasit Vectors 4: 201 10.1186/1756-3305-4-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Radulovic NS, Blagojevic PD, Stojanovic-Radic ZZ, Stojanovic NM (2013) Antimicrobial plant metabolites: structural diversity and mechanism of action. Curr Med Chem 20: 932–952. [PubMed] [Google Scholar]
- 21. Gilbert B, de Souza JP, Fortes CC, Santos D, Seabra A, et al. (1970) Chemoprophylactic agents in schistosomiasis: active and inactive terpenes. J Parasitol 56: 397–398. [PubMed] [Google Scholar]
- 22. Mors WB, dos Santos Filho MF, Monteiro HJ, Gilbert B, Pellegrino J (1967) Chemoprophylactic agent in schistosomiasis: 14,15-epoxygeranylgeraniol. Science 157: 950–951. [DOI] [PubMed] [Google Scholar]
- 23. Potterat O (2010) Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med 76: 7–19. 10.1055/s-0029-1186218 [DOI] [PubMed] [Google Scholar]
- 24. Kuntz AN, Davioud-Charvet E, Sayed AA, Califf LL, Dessolin J, et al. (2007) Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target. PLoS Med 4: e206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peak E, Chalmers IW, Hoffmann KF (2010) Development and validation of a quantitative, high-throughput, fluorescent-based bioassay to detect Schistosoma viability. PLoS Negl Trop Dis 4: e759 10.1371/journal.pntd.0000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Colley DG, Wikel SK (1974) Schistosoma mansoni: simplified method for the production of schistosomules. Exp Parasitol 35: 44–51. [DOI] [PubMed] [Google Scholar]
- 27. McGonigle L, Mousley A, Marks NJ, Brennan GP, Dalton JP, et al. (2008) The silencing of cysteine proteases in Fasciola hepatica newly excysted juveniles using RNA interference reduces gut penetration. Int J Parasitol 38: 149–155. [DOI] [PubMed] [Google Scholar]
- 28. Dayeh VR, Chow SL, Schirmer K, Lynn DH, Bols NC (2004) Evaluating the toxicity of Triton X-100 to protozoan, fish, and mammalian cells using fluorescent dyes as indicators of cell viability. Ecotox Environ Safe 57: 375–382. [DOI] [PubMed] [Google Scholar]
- 29. Ross F, Hernandez P, Porcal W, Lopez GV, Cerecetto H, et al. (2012) Identification of thioredoxin glutathione reductase inhibitors that kill cestode and trematode parasites. PloS One 7: e35033 10.1371/journal.pone.0035033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramirez B, Bickle Q, Yousif F, Fakorede F, Mouries MA, et al. (2007) Schistosomes: challenges in compound screening. Expert Opin Drug Discov 2: S53–61. 10.1517/17460441.2.S1.S53 [DOI] [PubMed] [Google Scholar]
- 31. Duthaler U, Smith TA, Keiser J (2010) In vivo and in vitro sensitivity of Fasciola hepatica to triclabendazole combined with artesunate, artemether, or OZ78. Antimicrob Agents Chemother 54: 4596–4604. 10.1128/AAC.00828-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tansatit T, Sahaphong S, Riengrojpitak S, Viyanant V, Sobhon P (2012) Fasciola gigantica: the in vitro effects of artesunate as compared to triclabendazole on the 3-weeks-old juvenile. Exp Parasitol 131: 8–19. 10.1016/j.exppara.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 33. Manneck T, Haggenmuller Y, Keiser J (2010) Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni . Parasitol 137: 85–98. [DOI] [PubMed] [Google Scholar]
- 34. Lorsuwannarat N, Saowakon N, Ramasoota P, Wanichanon C, Sobhon P (2013) The anthelmintic effect of plumbagin on Schistosoma mansoni . Exp Parasitol 133: 18–27. 10.1016/j.exppara.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 35. Machado-Silva JR, Pelajo-Machado M, Lenzi HL, Gomes DC (1998) Morphological study of adult male worms of Schistosoma mansoni Sambon, 1907 by confocal laser scanning microscopy. Mem Inst Oswaldo Cruz 93 Suppl 1: 303–307. [DOI] [PubMed] [Google Scholar]
- 36. Franklin AL, Filion WG (1985) A new technique for retarding fading of fluorescence: DPX-BME. Stain Technol 60: 125–135. [DOI] [PubMed] [Google Scholar]
- 37. Steck EA (1981) Topical prophylaxis against schistosomiasis. USA: The United States of America as represented by the Secretary of the Army. [Google Scholar]
- 38. Fitzpatrick JM, Peak E, Perally S, Chalmers IW, Barrett J, et al. (2009) Anti-schistosomal intervention targets identified by lifecycle transcriptomic analyses. PLoS Negl Trop Dis 3: e543 10.1371/journal.pntd.0000543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swain MT, Larkin DM, Caffrey CR, Davies SJ, Loukas A, et al. (2011) Schistosoma comparative genomics: integrating genome structure, parasite biology and anthelmintic discovery. Trends Parasitol 27: 555–564. 10.1016/j.pt.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Geyer KK, Rodriguez Lopez CM, Chalmers IW, Munshi SE, Truscott M, et al. (2011) Cytosine methylation regulates oviposition in the pathogenic blood fluke Schistosoma mansoni . Nat Commun 2: 424 10.1038/ncomms1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fitzpatrick JM, Hirai Y, Hirai H, Hoffmann KF (2007) Schistosome egg production is dependent upon the activities of two developmentally regulated tyrosinases. Faseb J 21: 823–835. [DOI] [PubMed] [Google Scholar]
- 42. Hai Y, Edwards JE, Van Zandt MC, Hoffmann KF, Christianson DW (2014) Crystal structure of Schistosoma mansoni arginase, a potential drug target for the treatment of schistosomiasis. Biochem 53: 4671–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keiser J, Morson G (2008) Fasciola hepatica: tegumental alterations in adult flukes following in vitro and in vivo administration of artesunate and artemether. Exp Parasitol 118: 228–237. [DOI] [PubMed] [Google Scholar]
- 44. Stitt AW, Fairweather I (1994) The effect of the sulphoxide metabolite of triclabendazole (‘Fasinex’) on the tegument of mature and immature stages of the liver fluke, Fasciola hepatica . Parasitol 108 (Pt 5): 555–567. [DOI] [PubMed] [Google Scholar]
- 45. Skelly PJ, Alan Wilson R (2006) Making sense of the schistosome surface. Adv Parasitol 63: 185–284. [DOI] [PubMed] [Google Scholar]
- 46. Slamenova D, Masterova I, Labaj J, Horvathova E, Kubala P, et al. (2004) Cytotoxic and DNA-damaging effects of diterpenoid quinones from the roots of Salvia officinalis L. on colonic and hepatic human cells cultured in vitro . Basic Clin Pharmacol Toxicol 94: 282–290. [DOI] [PubMed] [Google Scholar]
- 47. You H, McManus DP, Hu W, Smout MJ, Brindley PJ, et al. (2013) Transcriptional responses of in vivo praziquantel exposure in schistosomes identifies a functional role for calcium signalling pathway member CamKII. PLoS Pathog 9: e1003254 10.1371/journal.ppat.1003254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rao A, Zhang Y, Muend S, Rao R (2010) Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob Agents Chemother 54: 5062–5069. 10.1128/AAC.01050-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morel M, Vanderstraete M, Hahnel S, Grevelding CG, Dissous C (2014) Receptor tyrosine kinases and schistosome reproduction: new targets for chemotherapy. Front Gen 5: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andrade LF, Mourao Mde M, Geraldo JA, Coelho FS, Silva LL, et al. (2014) Regulation of Schistosoma mansoni development and reproduction by the mitogen-activated protein kinase signaling pathway. PLoS Negl Trop Dis 8: e2949 10.1371/journal.pntd.0002949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang SC, Freitas TC, Amiel E, Everts B, Pearce EL, et al. (2012) Fatty acid oxidation is essential for egg production by the parasitic flatworm Schistosoma mansoni . PLoS Pathog 8: e1002996 10.1371/journal.ppat.1002996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Freitas TC, Jung E, Pearce EJ (2007) TGF-beta signaling controls embryo development in the parasitic flatworm Schistosoma mansoni . PLoS Pathog 3: e52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Colhoun LM, Fairweather I, Brennan GP (1998) Observations on the mechanism of eggshell formation in the liver fluke, Fasciola hepatica . Parasitol 116 (Pt 6): 555–567. [DOI] [PubMed] [Google Scholar]
- 54. Ojo-Amaize EA, Nchekwube EJ, Cottam HB, Bai R, Verdier-Pinard P, et al. (2002) Hypoestoxide, a natural nonmutagenic diterpenoid with antiangiogenic and antitumor activity: possible mechanisms of action. Cancer Res 62: 4007–4014. [PubMed] [Google Scholar]
- 55. Urzua A, Rezende MC, Mascayano C, Vasquez L (2008) A structure-activity study of antibacterial diterpenoids. Molecules 13: 882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guerrero MF, Puebla P, Carron R, Martin ML, San Roman L (2004) Vasorelaxant effect of new neo-clerodane diterpenoids isolated from Croton schiedeanus . J Ethnopharmacol 94: 185–189. [DOI] [PubMed] [Google Scholar]
- 57. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliver Rev 23: 3–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper.