Abstract
Objective
Vitamin D deficiency is associated with increased cardiovascular (CV) disease risk in the general population. We examined the association between vitamin D deficiency and CV risk in rheumatoid arthritis (RA).
Methods
We measured large artery compliance by pulse wave velocity and microvascular function by the reactive hyperemia index in patients with stable RA (n = 87). We quantified CV risk factors, serum 25-hydroxyvitamin D [25(OH)D], and interleukin 17 (IL-17), and RA disease activity by Disease Activity Score of 28 joints. We used linear regression to test associations between serum 25(OH)D and CV risk factors.
Results
The mean serum 25(OH)D level in the cohort was 27.1 ± SD 13.6 ng/ml. Fifty-nine patients (68%) were vitamin D-insufficient (25(OH)D < 30 ng/ml; mean 20.2 ± 5.9 ng/ml) and of these, 25 (29%) were vitamin D-deficient (25(OH)D < 20 ng/ml; mean 14.4 ± 3.4 ng/ml). In the whole cohort and the vitamin D-insufficient group, serum 25(OH)D was inversely associated with IL-17 (log IL-17; β = −0.83, p = 0.04; β = −0.63, p = 0.004, respectively) by univariate analysis, which persisted after adjustment for season, and in multivariate analysis after adjustment for confounders (log IL-17; β = −0.74, p = 0.04; β = −0.53, p = 0.02). In vitamin D-deficient patients, serum 25(OH)D was positively associated with microvascular function by univariate and multivariate analysis after adjustment for confounders (β = 2.1, p = 0.04; β = 2.7, p = 0.04).
Conclusion
Vitamin D deficiency in RA may affect Th17 responses and microvascular function. Maintaining normal serum vitamin D levels may protect against IL-17-mediated inflammation and vascular dysfunction in RA.
Key Indexing Terms: VITAMIN D, INTERLEUKIN 17, MICROVASCULAR FUNCTION, RHEUMATOID ARTHRITIS
Understanding of the role of vitamin D in health and disease has progressed significantly in recent years. Vitamin D is a steroid hormone with wide-ranging effects; in addition to its well-known effects on bone metabolism, vitamin D is crucial in maintaining the health and integrity of several organ systems including the immune and cardiovascular (CV) systems. Vitamin D deficiency is highly prevalent and can affect up to 50% of the population during winter months in the Northern Hemisphere.
In the general population, individuals with vitamin D [25-hydroxyvitamin D (25(OH)D)] deficiency (serum 25(OH)D < 20 ng/ml) may be at higher risk for CV disease (CVD), including coronary and peripheral artery disease, heart failure, and stroke, compared to those who are vitamin D-sufficient (25(OH)D ≥ 30 ng/ml)1,2. In addition, vitamin D may have immune modulatory effects in several chronic inflammatory diseases, including rheumatoid arthritis (RA)3. CVD is an important cause of morbidity and mortality in RA and identification and management of traditional and nontraditional CV risk factors has become a priority in this disease4,5.
We identified interleukin 17 (IL-17), an important proinflammatory cytokine in RA pathogenesis, as a novel determinant of microvascular endothelial function and arterial compliance in patients with RA6. While vitamin D influences Th17 cell development and polarization, no studies to date have examined the effects of vitamin D deficiency on IL-17 and CV risk in RA. One of the earliest manifestations of CVD in RA is endothelial dysfunction, which precedes overt atherosclerosis, and can be used as an early, surrogate marker of CV risk7,8.
The vascular endothelium plays a critical role in regulating CV homeostasis. Nitric oxide (NO) released by endothelial cells is a vasodilator and, in addition to controlling vascular tone, has inhibitory effects on tumor necrosis factor-α (TNF-α) and the expression of proinflammatory adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and chemokine monocyte chemoattractant protein-1 (MCP-1). Endothelial dysfunction occurs when there is impairment of endothelium-dependent vasodilatation due to decreased NO bioavailability in the vessel wall. Poor NO bioavailability occurs as a result of increased oxidative stress with excess production of superoxide anions and subsequent degradation of NO before it reaches target tissues. This in turn leads to vasoconstriction, increased platelet aggregation, and expression of ICAM-1, VCAM-1, and MCP-1 with subsequent leukocyte activation and recruitment9.
We hypothesized that vitamin D deficiency may increase CV risk in RA by influencing IL-17 and vascular function. We determined the association between serum 25(OH)D levels and traditional and nontraditional CV risk factors in a cohort of RA patients with low Framingham scores. We assessed microvascular endothelial function and general vascular (macrovascular) function and subclinical atherosclerotic burden using 2 measures. We used finger pulse amplitude tonometry measured before and after reactive hyperemia to measure microvascular endothelial function in the peripheral circulation, and applanation tonometry to calculate pulse wave velocity, a direct measure of arterial stiffness, to assess macrovascular function and subclinical atherosclerotic burden10.
MATERIALS AND METHODS
Patients with RA who fulfilled the 1987 American College of Rheumatology diagnostic criteria11 were recruited. For inclusion, patients had to be receiving stable doses of disease-modifying antirheumatic drugs (DMARD) and/or biologic agents for at least 3 months before enrollment; if on lipid-lowering drugs, they were required to be on stable doses of these drugs for at least 6 months. Patients were excluded if they were pregnant or breast-feeding, were smokers, or had diabetes mellitus, congestive heart failure (New York Heart Association class III or IV), acute infection or liver disease with serum hepatic transaminase levels > 3 times upper limit of normal.
The Washington University School of Medicine and University of Michigan institutional review boards approved this study, which complied with the Declaration of Helsinki.
Vascular function measurements
Procedures were performed at the University of Michigan research vascular laboratory in a temperature-controlled room, after patients fasted and withheld vasoactive drugs for at least 12 h. Resting blood pressure was measured, and body mass index (BMI) and Framingham composite score were calculated for each participant. All vascular assessments were performed by a single observer.
Arterial pulse wave velocity (PWV)
After subjects rested supine for 10 min, applanation tonometry was measured at the right carotid and femoral arteries for 10 s each, following operational guidelines of the Sphygmocor device (Atcor). Three-lead echocardiographic recordings were obtained simultaneously with the tonometry to calculate aortic PWV (a direct measure of arterial stiffness).
Microvascular endothelial function
Microvascular endothelium-dependent vasodilatation was quantified in the dominant arm with the EndoPat-2000 device (Itamar). Ten minutes after PWV determination, patients were connected to the EndoPat-2000 device finger probes. This device uses finger pulse amplitude tonometry (PAT), measured before (baseline) and after reactive hyperemia (RH: termed RH-PAT) induced by upper arm 5-min cuff occlusion. Basal resting finger PAT was recorded by the probes on 1 finger from each hand. A dominant upper arm blood pressure (BP) cuff was inflated to 50 mm Hg above systolic BP to occlude blood flow. Upon rapid cuff deflation, RH was created and RH-PAT recorded in the ipsilateral dominant hand finger for 5 min after cuff release. The device computer compared 120 s of baseline mean PAT to the RH-PAT, defined as the mean PAT from 60 to 120 s post-cuff release on the same dominant arm. These readings were standardized to contralateral hand PAT during the same time periods, providing the reactive hyperemia index (RHI; coefficient of variation = 0.3).
Assessment of insulin resistance
The Homeostatic Model Assessment (HOMA1-IR) was used to assess insulin resistance using the formula: HOMA1-IR = [fasting plasma insulin (μU/ml) × fasting plasma glucose (mg/dl)]/40512.
Measurement of serum and plasma markers of vascular damage
Commercial ELISA kits were used to quantify serum levels of ICAM-1, VCAM-1 (R&D Systems), and MCP-1 (BD-Bioscience).
Determination of serum IL-17
High-binding enzyme immunoassay/radioimmunoassay 96-well plates were coated with anti-human IL-17 diluted with carbonate buffer, sealed, incubated at 4°C overnight, washed, and blocked at room temperature for 2 h. Standards were prepared from recombinant human IL-17 (Ebioscience). Serum samples and standards were added to wells, incubated 2 h at room temperature or overnight at 4°C, and followed by washes, addition of biotinylated anti-IL17 (Ebioscience) and streptavidin horseradish peroxidase (Biolegend), and incubation for 2 h. TMB reagent (BD Biosciences) was added to each well. Samples were placed in the dark until color change, followed by addition of stop solution. Absorbance was read on a microplate reader at 450 nm wavelength. Three samples > 18.0 ng/ml were excluded from analysis.
Determination of serum 25(OH)D
Serum 25(OH)D levels were quantified by enzyme immunoassay (Immunodiagnostic Systems Ltd.); the intraassay coefficient of variation was 5.6% and interassay coefficient of variation was 6.4%. Serum samples were measured in duplicate and the average value was reported. Vitamin D deficiency was defined as serum 25(OH)D levels < 20 ng/ml and insufficiency as levels < 30 ng/ml13.
Other measurements
Lipid profile, high sensitivity C-reactive protein (hsCRP), insulin, and fasting glucose were measured in the Michigan Diabetes Research and Training Center. Rheumatoid factor (RF), anticyclic citrullinated peptide antibodies, and erythrocyte sedimentation rate (ESR) were measured at the University of Michigan Central Laboratories using standardized techniques. Framingham scores were calculated using an online calculator and expressed in percentages (US National Heart, Lung, and Blood Institute, Bethesda, MD; Website: http://hp2010.nhlbihin.net/atpiii/calculator.asp).
Statistical analysis
Serum 25(OH)D levels were analyzed as dependent variables. Continuous variables were presented using means ± SD, and categorical variables using counts and percentages. Univariate linear regression analysis was performed to evaluate the association between serum 25(OH)D levels and various CV measures. Log-transformation was performed for highly skewed variables such as ESR and IL-17. A stepwise forward regression model was then fit to further examine each predictor’s association with serum 25(OH)D in the presence of other factors. Variables with probability values < 0.15 in univariate analysis were included in multivariate analysis. The above analyses were repeated for the vitamin D-insufficient and deficient groups. A value of p < 0.05 was considered significant. All procedures were done using SAS 9.2 (SAS Institute Inc.).
To adjust for season, the lower ultraviolet light season (winter) was defined as having a vitamin D level drawn between October and March and the higher ultraviolet season (summer) between April and September in the Northern Hemisphere14.
RESULTS
Eighty-seven patients with RA enrolled in the study. The mean serum 25(OH)D level for the cohort was 27.1 ng/ml (SD 13.6). Fifty-nine patients (68%) were vitamin D-insufficient (25(OH)D < 30 ng/ml; mean 20.2 ± 5.9 ng/ml), of whom 25 (29%) were vitamin D-deficient (25(OH)D < 20 ng/ml; mean 14.4 ± 3.4 ng/ml). Their demographic and clinical features are summarized in Table 1. The cohort was predominantly female (80.5%) and white (87.4%) with smaller numbers of African Americans (9.2%), Asians (2.3%), and North American Indians (1.2%). The mean age and disease duration of the cohort were 55.2 (SD 12.1) and 13.9 (SD 11.3) years, respectively. It was found that 97.7% of the cohort tested positive for RF and over half (57%) the patients tested positive for cyclic citrullinated peptide (CCP) antibodies. The mean Disease Activity Score of 28 joints was 3.0 (SD 1.5) and ESR was 15.3 mm (SD 14.6), both measures consistent with low RA disease activity.
Table 1.
Demographic and clinical features of study participants.
| Characteristic | 25(OH)D < 30 ng/ml, n = 59 | 25(OH)D < 20 ng/ml, n = 25 (of n = 59) | All Subjects, n = 87 |
|---|---|---|---|
| Sex, n (% female) | 48 (81.4) | 23 (92) | 70 (80.5) |
| Race, n (%) | |||
| White | 50 (84.8) | 18 (72.0) | 76 (87.4) |
| African American | 6 (10.2) | 5 (20) | 8 (9.2) |
| Asian | 2 (3.4) | 1 (4.0) | 2 (2.3) |
| American Indian/Alaska Native | 1 (1.6) | 1 (4.0) | 1 (1.2) |
| Ethnicity, n (% Hispanic) | 4 (6.8) | 1 (4.0) | 6 (6.9) |
| RF-positive, n (%) | 57 (98.3) | 25 (100) | 84 (97.7) |
| Anti-CCP–positive, n (%) | 34 (58.6) | 15 (60) | 49 (57.0) |
| Age, mean yrs (SD) | 52.9 (11.6) | 49.0 (11.2) | 55.2 (12.1) |
| Anti-CCP, mean U/ml (SD) | 49.4 (56.3) | 48.3 (58.8) | 53.4 (63.7) |
| RF, mean IU/ml (SD) | 81.1 (123.1) | 83.3 (119.3) | 138.2 (333.4) |
| DAS28, mean (SD) | 2.9 (1.5) | 3.2 (1.6) | 3.0 (1.5) |
| Years since RA diagnosis, mean (SD) | 12.3 (10.1) | 11.9 (9.6) | 13.9 (11.3) |
| ESR, mean mm/h (SD) | 13.4 (12.2) | 14.3 (9.2) | 15.3 (14.6) |
CCP: cyclic citrullinated peptide; RF: rheumatoid factor; ESR: erythrocyte sedimentation rate; DAS28: Disease Activity Score 28 joints; anti-CCP: anticyclic citrullinated peptide antibodies; 25(OH)D: 25-hydroxyvitamin D; RA: rheumatoid arthritis.
Fifty-eight percent of the patients were receiving biologics, 69% traditional DMARD, and 41% were taking glucocorticoids. Details of CV risk factors including blood pressure, BMI, Framingham composite scores, vascular function, insulin resistance, markers of vascular damage, serum IL-17, lipid profile, hsCRP, fasting plasma insulin, and glucose are shown in Table 2.
Table 2.
Cardiovascular risk factors and medications. Data are mean (SD) except as indicated.
| 25(OH)D < 30 ng/ml, n = 59 | 25(OH)D < 20 ng/ml, n = 25 (of n = 59) | All Subjects, n = 87 | |
|---|---|---|---|
| SBP, mm Hg | 136.4 (17.7) | 135.2 (15.4) | 134.9 (17.9) |
| DBP, mm Hg | 81.1 (9.7) | 80.2 (11.5) | 80.9 (10.0) |
| BMI | 31.4 (7.5) | 34.2 (8.9) | 29.3 (7.2) |
| hsCRP, mg/dl | 5.1 (6.4) | 5.7 (7.4) | 6.0 (12.8) |
| Framingham composite score (%) | 2.7 (3.7) | 1.3 (1.7) | 3.6 (5.1) |
| MCP-1, pg/ml | 486.7 (202.4) | 511.5 (206.3) | 498.3 (214.0) |
| VCAM-1, ng/ml | 556.3 (212.5) | 559.4 (266.6) | 592.2 (271.9) |
| ICAM-1, ng/ml | 186.1 (69.0) | 192.4 (69.6) | 189.1 (69.4) |
| IL-17, ng/ml | 2.0 (4.3) | 2.6 (4.3) | 2.5 (5.5) |
| Total cholesterol, mg/dl | 188.5 (36.9) | 186.1 (34.2) | 185.8 (37.9) |
| Triglycerides, mg/dl | 115.2 (63.2) | 107.5 (55.6) | 109.4 (62.2) |
| HDL, mg/dl | 62.3 (17.6) | 60.3 (17.4) | 62.6 (17.3) |
| LDL, mg/dl | 112.0 (31.4) | 114.7 (28.4) | 109.4 (31.9) |
| Fasting serum insulin μU/ml | 23.6 (14.9) | 24.5 (14.1) | 20.8 (13.4) |
| Fasting glucose (mg/dl) | 96.3 (12.6) | 95.8 (12.9) | 95.3 (11.8) |
| HOMA1-IR, mg/dl × μU/ml | 5.8 (4.4) | 5.9 (3.7) | 5.1 (3.9) |
| PWV, cm-sec-1 | 8.8 (2.5) | 8.9 (2.7) | 9.3 (2.9) |
| RHI (%) | 2.1 (0.6) | 2.0 (0.6) | 2.1 (0.6) |
| Medications, n (%) | |||
| Biologics | 32 (54.2) | 14 (56.0) | 50 (57.5) |
| DMARD | 48 (81.4) | 18 (72.0) | 60 (69.0) |
| Steroids | 26 (44.1) | 13 (52.0) | 36 (41.4) |
| Vasoactive drugs | 18 (30.5) | 8 (32.0) | 27 (31.0) |
| Lipid-lowering drugs | 4 (55) | 1 (4.0) | 8 (9.2) |
SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; hsCRP: high-sensitivity C-reactive protein; MCP-1: monocyte chemotactic protein-1; VCAM-1: vascular cell adhesion molecule-1; ICAM-1: intercellular adhesion molecule-1; HDL: high-density lipoprotein; LDL: low-density lipoprotein; HOMA1-IR: homeostatic model assessment; PWV: pulse wave velocity; RHI: reactive hyperemia index; DMARD: disease-modifying antirheumatic drug; 25(OH)D: 25-hydroxyvitamin D; IL: interleukin.
Univariate analysis revealed that serum 25(OH)D levels were inversely associated with serum IL-17 levels (log-transformed) in the whole cohort (β = −0.83, p = 0.04) and in the vitamin D-insufficient group (β = −0.63, p = 0.004). This inverse association persisted in both the whole cohort and the vitamin D-insufficient group after adjustment for season, and remained significant in both groups in multivariate analysis after adjustment for known confounders, including age, BMI, sex, and race/ethnicity (β = −0.74, p = 0.04; β = −0.53, p = 0.02, respectively).
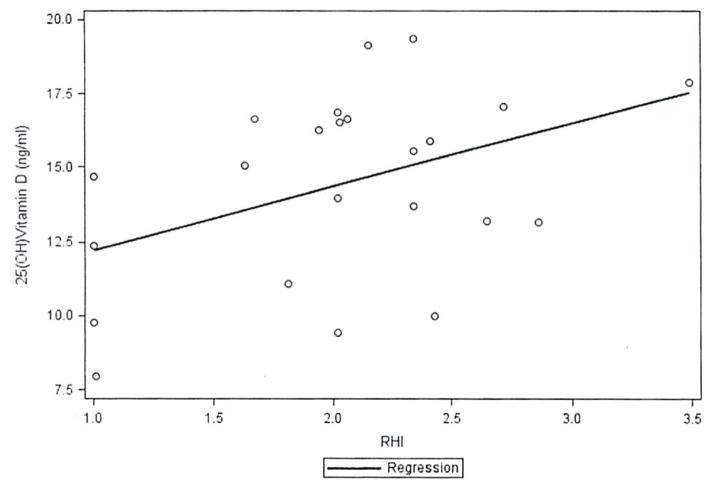
In the vitamin D-deficient group (25(OH)D < 20 ng/ml; mean 14.4 ± 3.4 ng/ml), univariate analysis showed that serum 25(OH)D levels were positively associated with microvascular endothelial function, as assessed by RHI (β = 2.1, p = 0.04), and with serum triglycerides (β = 0.03, p = 0.03). The positive association of 25(OH)D levels with microvascular endothelial function persisted in this group in multivariate analysis after adjustment for known confounders, including age, BMI, sex, and race/ethnicity (β = 2.7, p = 0.04; Figure 1). Serum IL-17 levels did not show a significant association with microvascular endothelial function, as measured by RHI, in this group (p = 0.08). Serum 25(OH)D levels were not associated with other CV risk factors, large artery compliance (by PWV), microvascular endothelial function (by RHI), or disease activity in the whole cohort or the vitamin D-insufficient group. In the vitamin D-deficient group, serum 25(OH)D levels were not associated with serum IL-17. large artery compliance (by PWV), other CV risk factors, or disease activity, although the mean values of IL-17 in this group were higher than in the whole cohort and the vitamin D-insufficient group (Table 2).
Figure 1.
Relationship between 25-hydroxyvitamin D (25(OH)D) levels and microvascular function by reactive hyperemia index (RHI).
DISCUSSION
In this cross-sectional study, we found a significant inverse association between serum vitamin D levels and IL-17 in patients with RA. Further, our study revealed a significant association between serum vitamin D levels and microvascular endothelial function in patients with RA who were vitamin D-deficient.
There is an emerging body of literature on the effects of vitamin D on CV risk in RA. One recent study showed an association between serum 25(OH)D levels and cardiometabolic risk factors such as serum high-density lipoprotein cholesterol, E-selectin, and soluble ICAM in an RA cohort15. Another study revealed an association between serum 25(OH)D levels and traditional CV risk factors such as serum low-density lipoprotein cholesterol, triglycerides. and the metabolic syndrome in patients with RA16. Goshayeshi, et al recently showed that vitamin D was protective against the metabolic syndrome in RA17. Our study suggests that vitamin D may influence nontraditional CV risk factors such as IL-17 and microvascular endothelial function in RA.
IL-17, an inflammatory cytokine derived from Th17, natural killer, natural killer T cells, and other innate immune cells, has generated significant interest in RA. IL-17, acting in synergy with TNF-α and IL-1, orchestrates diverse immune functions critical not only for pannus formation and bone and cartilage destruction in RA, but also for endothelial damage18. As we previously showed, IL-17 may modulate CV risk in RA through its effects on vascular function, primarily large-vessel arterial compliance and endothelium-dependent microvascular function6.
Vitamin D may have immunomodulatory effects on Th17 cells. The active, hormonal form of vitamin D, 1,25 dihyroxy-vitamin D (1,25(OH)2D3), acts synergistically with all-transretinoic acid to inhibit the development of human Th17 cells from both naive and memory CD4+ T cells, and decreases IL-17 mRNA expression in memory CD4+ T cells19. In addition, 1,25(OH)2D3 directly inhibits Th17 polarization in vitro in patients with early RA who are treatment-naive20.
Although such in vitro studies suggest that vitamin D may influence Th17 development and polarization, our study is the first, to our knowledge, to demonstrate that serum 25(OH)D levels are associated with serum IL-17 levels in patients with established RA. After controlling for confounding variables, we found low serum 25(OH)D levels were associated with increased serum levels of IL-17. Interestingly, 1,25(OH)2D3 induces the expression of the proapoptotic transcription factors CCAAT enhancer-binding protein-beta (C/EBPβ), and C/EBP homologous protein (CHOP) in many cell types including Th17 cells and macrophages. Stimulation of Th17 cells with 1,25(OH)2D3 induces the expression of CHOP in these cells21. CHOP overexpression in developing Th17 cells, by promoting apoptosis. can suppress IL-17 synthesis. We speculate that RA patients with low serum 25(OH)D and consequently low 1,25(OH)2D3 have reduced CHOP expression in Th17 cells, and subsequent increased IL-17 synthesis.
In patients who were vitamin D-deficient, lower serum levels were associated with decreased microvascular RHI. As we reported recently, IL-17 is a key determinant of vascular function in RA6. Therefore, these effects of vitamin D on microvascular endothelial function may be mediated through IL-17. Our finding of a lack of association between serum 25(OH)D levels and IL-17, and serum IL-17 and microvascular function, in the vitamin D-deficient group (although with higher mean values of IL-17) may be reflective of the small sample size of this group. Alternatively, other mechanisms may operate; previous studies demonstrated that the protective effects of vitamin D on endothelial function may be due to a decrease in oxidative stress or an increase in expression of vascular endothelial growth factor22,23,24. Based on our findings, we postulate that there may be a threshold biological effect of vitamin D on endothelial function, with levels < 20 ng/ml affecting microvascular function, but levels above this threshold not improving endothelial function.
Other factors may be at play here as well. Determinants of arterial stiffness and macrovascular function as measured by PWV may be quite different from those of microvascular function as measured by RHI. The results of studies in this regard are quite conflicting. Some investigators have shown that traditional CV risk factors influence macrovascular function and arterial stiffness, while others have demonstrated that ongoing inflammation affects microvascular function25,26. However, a recent study showed that RA-related inflammation had no effects on microvascular or macrovascular function, while traditional CV risk factors affected both27. We hypothesize that vitamin D, by modulating the inflammatory milieu, may preferentially affect microvascular function.
A recent study showed no beneficial effect of vitamin D supplementation on arterial stiffness and endothelial function in postmenopausal women (without RA) with low vitamin D levels (defined in that study as serum 25(OH)D < 30 ng/ml)28. However, it should be pointed out that the definitions of vitamin D deficiency and measure of endothelial function used in that study were different (flow-mediated vasodilatation of the brachial artery), the subjects were healthy postmenopausal women, and IL-17 was not included among the CV risk factors analyzed. We hypothesize that the chronic inflammatory milieu in RA and the role of IL-17 in determining vascular function in RA may be unique features contributing to the effects of vitamin D on CV risk in this disease.
We acknowledge the limitations of our study. The cross-sectional design precluded inclusion of a control group and assumption of a cause-effect relationship between vitamin D and IL-17 and vascular function in RA. Although we controlled for age, race, sex, BMI, and season in our analysis, we could not quantify sunlight exposure, an important potential confounder. Our vitamin D-deficient subset was relatively small, and the association seen between vitamin D and microvascular endothelial function in this group needs to be confirmed in larger cohorts, especially whether such an effect is mediated by IL-17. The cohort studied was predominantly white, limiting the generalizability of our results to other racial groups, particularly African Americans, whose vitamin D levels are lower29.
There is emerging evidence supporting an association between vitamin D and CV risk in RA15,16,17. We have shown that vitamin D may influence the Th17 response and endothelial function in RA. These findings remain hypothesis-generating, and further studies with larger cohorts are needed to replicate these findings and investigate underlying mechanisms. Effective correction of vitamin D deficiency may have beneficial effects on IL-17-driven inflammation and vascular function in RA.
Acknowledgments
Supported by the US National Institutes of Health through PHS grant RO1 HL086553 (MJK), Rheumatic Disease Core Center P30 AR48335 (PR), and UL1RR024986 (University of Michigan CTSA). Also supported in part by the Michigan Diabetes Research and Training Center, University of Michigan.
References
- 1.Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004) Am J Cardiol. 2008;102:1540–4. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 3.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. Faseb J. 2001;15:2579–85. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 4.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–7. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 5.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–31. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 6.Marder W, Khalatbari S, Myles JD, Hench R, Yalavarthi S, Lustig S, et al. Interleukin 17 as a novel predictor of vascular function in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1550–5. doi: 10.1136/ard.2010.148031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 8.Karatzis EN, Ikonomidis I, Vamvakou GD, Papaioannou TG, Protogerou AD, Andreadou I, et al. Long-term prognostic role of flow-mediated dilatation of the brachial artery after acute coronary syndromes without ST elevation. Am J Cardiol. 2006;98:1424–8. doi: 10.1016/j.amjcard.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 9.Poredos P. Endothelial dysfunction and cardiovascular disease. Pathophysiol Haemost Thromb. 2002;32:274–7. doi: 10.1159/000073580. [DOI] [PubMed] [Google Scholar]
- 10.Kerekes G, Soltesz P, Nurmohamed MT, Gonzalez-Gay MA, Turiel M, Vegh E, et al. Validated methods for assessment of subclinical atherosclerosis in rheumatology. Nat Rev Rheumatol. 2012;8:224–34. doi: 10.1038/nrrheum.2012.16. [DOI] [PubMed] [Google Scholar]
- 11.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 14.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–7. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 15.Haque UJ, Bathon JM, Giles JT. Association of vitamin D with cardiometabolic risk factors in rheumatoid arthritis. Arthritis Care Res. 2012;64:1497–504. doi: 10.1002/acr.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker JF, Mehta NN, Baker DG, Toedter G, Shults J, Von Feldt JM, et al. Vitamin D, metabolic dyslipidemia, and metabolic syndrome in rheumatoid arthritis. Am J Med. 2012;125:1036. doi: 10.1016/j.amjmed.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Goshayeshi L, Saber H, Sahebari M, Rezaieyazdi Z, Rafatpanah H, Esmaily H, et al. Association between metabolic syndrome, BMI, and serum vitamin D concentrations in rheumatoid arthritis. Clin Rheumatol. 2012;31:1197–203. doi: 10.1007/s10067-012-1995-3. [DOI] [PubMed] [Google Scholar]
- 18.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda U, Wakita D, Ohkuri T, Chamoto K, Kitamura H, Iwakura Y, et al. lα,25-Dihydroxyvitamin D3 and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunol Lett. 2010;134:7–16. doi: 10.1016/j.imlet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Colin EM, Asmawidjaja PS, van Hamburg JP, Mus AM, van Driel M, Hazes JM, et al. 1,25-Dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010;62:132–42. doi: 10.1002/art.25043. [DOI] [PubMed] [Google Scholar]
- 21.Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem. 2010;285:38751–5. doi: 10.1074/jbc.C110.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiseman H. Vitamin D is a membrane antioxidant. Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett. 1993;326:285–8. doi: 10.1016/0014-5793(93)81809-e. [DOI] [PubMed] [Google Scholar]
- 23.Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: Lessons from animal models. Arthritis Res Ther. 2005;7:29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardus A, Panizo S, Encinas M, Dolcet X, Gallego C, Aldea M, et al. 1,25-Dihydroxyvitamin D3 regulates VEGF production through a vitamin D response element in the VEGF promoter. Atherosclerosis. 2009;204:85–9. doi: 10.1016/j.atherosclerosis.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Aizer J, Karlson EW, Chibnik LB, Costenbader KH, Post D, Liang MH, et al. A controlled comparison of brachial artery flow mediated dilation (FMD) and digital pulse amplitude tonometry (PAT) in the assessment of endothelial function in systemic lupus erythematosus. Lupus. 2009;18:235–42. doi: 10.1177/0961203308096663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vita JA, Keaney JF, Jr, Larson MG, Keyes MJ, Massaro JM, Lipinska I, et al. Brachial artery vasodilator function and systemic inflammation in the Framingham Offspring Study. Circulation. 2004;110:3604–9. doi: 10.1161/01.CIR.0000148821.97162.5E. [DOI] [PubMed] [Google Scholar]
- 27.Sandoo A, Kitas GD, Carroll D, Zanten JV. The role of inflammation and cardiovascular disease risk on microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: a cross-sectional and longitudinal study. Arthritis Res Ther. 2012;14:R117. doi: 10.1186/ar3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gepner AD, Ramamurthy R, Krueger DC, Korcarz CE, Binkley N, Stein JH. A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PLoS One. 2012;7:e36617. doi: 10.1371/journal.pone.0036617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peiris AN, Bailey BA, Peiris P, Copeland RJ, Manning T. Race and vitamin D status and monitoring in male veterans. J Natl Med Assoc. 2011;103:492–7. doi: 10.1016/s0027-9684(15)30363-1. [DOI] [PubMed] [Google Scholar]