Abstract
Purpose of review
The aim of this review is to focus specifically on the indications, evolution of technique, and results of surgical ablation for atrial fibrillation.
Recent findings
With the introduction of the Cox-Maze IV procedure utilizing bipolar radiofrequency ablation and cryoablation, long-term studies have demonstrated a significant decrease in aortic cross-clamp times and major complications with a comparable rate of restoration of sinus rhythm. New hybrid approaches utilizing both catheter-based ablation and minimally invasive surgical approaches have been developed, but have not been standardized. Early studies have demonstrated reasonable success rates of hybrid procedures, with advantages that include confirmation of conduction block, decreased surgical morbidity, and possibly reduced morbidity. However, hybrid approaches have the disadvantage of significantly increased operative times.
Summary
The Cox-Maze IV is currently the gold standard for surgical treatment of atrial fibrillation. New hybrid approaches have potential advantages with promising early results, but a standard lesion set, improvement in operative times, and long-term results still need to be evaluated.
Keywords: atrial fibrillation, Cox-Maze, surgical ablation
INTRODUCTION
Starting in the 1980s, several procedures were developed in an effort to treat atrial fibrillation, including left atrial (LA) isolation [1], corridor operation [2], and atrial transection [3]. However, these procedures were abandoned because of their inability to reliably prevent or successfully treat atrial fibrillation in humans. It was not until 1987 that the team led by Dr James Cox devised the Maze procedure, now known as the Cox-Maze procedure (CMP), that reliably and successfully restored sinus rhythm [3]. This procedure was refined over several iterations with changes to both lesion sets and replacement of the incisional lesions with a combination of bipolar radio-frequency ablation and cryoablation (Table 1) [4]. The latest iteration of this procedure has been termed the CMP IV. This review aims to discuss the indications for the surgical ablation of atrial fibrillation, the technique and results of the CMP IV, and the early experiences of the hybrid procedure.
Table 1.
Summary of Cox-Maze procedures
| Procedure | Year first used | Still in use | Modification from previous iteration | Limitations of procedure |
|---|---|---|---|---|
| Cox-Maze I (Cut-and-sew) | 1987 | No (32 total patients) | NA | Inability to produce appropriate sinus tachycardia |
| Postoperative LA dysfunction | ||||
|
| ||||
| Cox-Maze II (Cut-and-sew) | 1987 | No (12 total patients) | LA: transverse atriotomy across the dome of the left atrium moved posteriorly | Prolonged intraatrial conduction |
| RA: elimination of SVC→RA lesion | Have to completely transect SVC to gain LA exposure | |||
|
| ||||
| Cox-Maze III (Cut-and-sew) | 1988 | Yes | RA: placement of septal incision posterior to the orifice of the SVC | Prolonged cardiopulmonary bypass times and technical difficulty |
|
| ||||
| Cox Maze IV (Bipolar RF ablation and cryoablation) | 2002 | Yes | Combination of bipolar RF ablation and cryoablation | Continued need for cardiopulmonary bypass |
| LA: box lesion around posterior left atrium | ||||
LA, left atrial; RA, right atrial; RF, radiofrequency; SVC, superior vena cava.
Adapted from [4].
INDICATIONS FOR SURGICAL ABLATION OF ATRIAL FIBRILLATION
In 2012, a task force, which included the Society of Thoracic Surgeons, the American College of Cardiology, the Heart Rhythm Society, the European Heart Rhythm Association, and the European Cardiac Arrhythmia Society, released the updated consensus indications for surgical ablation that were divided into two separate categories: those patients undergoing a concomitant cardiac surgery and those who are not undergoing a concomitant cardiac surgery [5]. For those undergoing other cardiac surgical procedures, all patients with symptomatic atrial fibrillation should be considered for surgical ablation, regardless of whether antiarrhythmic medications have been started. However, stand-alone surgical ablation is generally indicated after patients have failed medical therapy and either have failed one or more catheter ablations or prefer surgical therapy [5]. With the advent of hybrid (combined catheter and surgical) approaches to ablation, the indications for stand-alone therapy may expand [6,7].
SURGICAL TECHNIQUE: COX-MAZE IV PROCEDURE
To simplify the CMP, our group at the Washington University has replaced the majority of the cut-and-sew lesions that comprised the CMP III with a combination of bipolar radiofrequency ablation and cryoablation and have termed the revision the CMP IV. This procedure can be performed either through a sternotomy or through a less invasive right minithoracotomy (RMT). Intrathoracic access is obtained and both pulmonary veins are bluntly dissected after the initiation of normothermic cardiopulmonary bypass. If a patient is in atrial fibrillation at the time of the surgery and no clot exists, they are electrically cardioverted and started on intravenous amiodarone. Pacing thresholds are measured from each pulmonary vein. A cuff of atrial tissue surrounding the right pulmonary and the left pulmonary veins is isolated using bipolar clamps (Fig. 1a) [8]. Isolation is confirmed by documenting exit block from all pulmonary veins when performed through a sternotomy or from the right pulmonary veins only when performed through an RMT.
FIGURE 1.
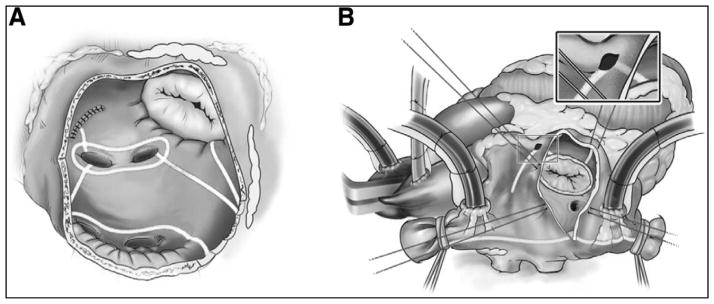
Cox-Maze IV lesion set. (a) Left atrial lesion set: radiofrequency ablation lines consist of bilateral pulmonary vein isolation, pulmonary vein roof and floor connecting lesions, lesion from LSPV and amputated atrial appendage, and lesion from inferior atriotomy to mitral valve annulus. (b) Right atrial lesion set: this lesion set consists of an ablation line along the SVC and IVC, ablation along the RA free wall with line to tricuspid valve annulus [8]. IVC, inferior vena cava; LSPV, left superior pulmonary vein; RA, right atrial; SVC, superior vena cava. Adapted from [8].
The patient is cooled to 34°C and lesions from the right atrial (RA) lesion set are performed on the beating heart (Fig. 1b) [9]. A small purse-string suture is placed at the base of the RA appendage that is wide enough to accommodate one jaw of the radiofrequency ablation clamp. An ablation lesion is created along the free wall of the RA through the purse-string. A vertical atriotomy is made extending from the intraatrial septum up toward the atrioventricular groove near the free margin of the heart and should be at least 2 cm from the free wall ablation. From the inferior aspect of the incision, the radio-frequency clamp is used to create ablation lines running up the superior vena cava (SVC) and down toward the inferior vena cava (IVC). A linear cryoprobe is used to create an endocardial ablation on the tricuspid annulus at the two o’clock position. Cryoablation is ideal to complete ablations over annular tissues because it preserves the fibrous skeleton of the heart, therefore maintaining valve competency. All cryoablations are performed at −60°C for 3 min. The cryoprobe is placed through the previously placed purse-string suture and an endocardial ablation is performed down to the ten o’clock position on the tricuspid valve. In patients with an RMT, the approach differs by replacing the atriotomy with two additional purse-string sutures: one just above the intraatrial septum midway between the SVC and IVC and one just adjacent to the atrioventricular groove.
Next, the LA lesion set is performed (Fig. 1a) [8]. The aorta is cross-clamped and the heart is arrested with cardioplegia. The LA appendage (LAA) is amputated. Through the amputated appendage, the bipolar clamp is used to create a connecting lesion into one of the left pulmonary veins. The coronary sinus is marked with methylene blue between the left and the right coronary circulations. A standard left atriotomy is performed with the option to extend superiorly onto the dome of the LA and inferiorly around the right inferior pulmonary vein, if needed. In patients whose exposure is via an RMT, the LAA is oversewn from the endocardial surface following the atriotomy. The posterior LA is then isolated using the radiofrequency ablation clamp both inferiorly and superiorly to connect the atriotomy to the previously made radiofrequency ablation lesion encircling the left pulmonary veins. From the inferior portion of the atriotomy, the radiofrequency ablation clamp is used to create an ablation line across the floor of the LA toward the mitral annulus. This ablation should cross the coronary sinus in the space between the right coronary artery and the circumflex artery. This space is usually adjacent to the P2 cusp of the posterior leaflet of the mitral valve in the majority of patients who have right dominant circulation. The atrioventricular groove, which contains thicker tissue, also lies in this area, so cryoablation is used to bridge the 1–2 cm gap from the end of the radiofrequency ablation line to the mitral valve annulus. To complete the LA lesion set, the coronary sinus is ablated in line with the isthmus lesion by performing cryoablation from the epicardial surface.
SURGICAL RESULTS: COX-MAZE IV PROCEDURE
The CMP has long been considered the gold standard in surgical ablation and continues to be the procedure with the single highest success rate for terminating atrial tachyarrhythmias (ATAs) [9]. Our group prospectively followed 100 patients who received a lone CMP IV procedure from 2002 to 2010. Follow-up was scheduled at 3, 6, and 12 month intervals and annually thereafter. The majority of patients were evaluated with either 24 h Holter monitoring or more prolonged monitoring. Patients were considered to have late recurrence if they had any episode of atrial fibrillation, atrial flutter, or atrial tachycardia longer than 30 s. The mean follow-up time was 17±10 months. One-third of patients had paroxysmal atrial fibrillation (31%), whereas the rest had either persistent atrial fibrillation (pAF; 6%) or longstanding persistent (LSP; 63%) atrial fibrillation. This study showed that, at both 12 and 24 months, 90% of patients were free from ATAs, whereas 82% were free from ATAs and off antiarrhythmic medications [10]. In a separate study [11], this same group was then retrospectively compared with 112 patients who had a lone CMP III procedure from 1992 to 2002. It should be noted that the CMP III group determined recurrence using the criteria of symptomatic atrial fibrillation, which likely overestimated the procedural success. This comparison showed no difference in freedom from atrial fibrillation off antiarrhythmic medications between the CMP III group and the CMP IV group (83 vs. 82%) [11].
The CMP IV procedure has also been shown to be advantageous in the perioperative setting when compared with the CMP III. Mean aortic cross-clamp times were significantly decreased with a lone CMP IV compared with a lone CMP III (41 ± 13 vs. 93 ± 34 min) [9,10]. Mean concomitant cross-clamp times have also been shown to be shorter when comparing the CMP IV with the CMP III (93 ± 29 vs. 122 ± 37 min) [9,12]. In addition, the CMP IV does have a significantly lower major complication rate compared with the CMP III. In our previous series comparing the CMP III with the CMP IV, major complication was defined as reoperation for bleeding, early stroke, renal failure, mediastinitis, and a need for intra-aortic balloon pump (10 vs. 1%) [11]. There were no differences in 3-month pacemaker implantation rate or 30-day mortality rate when comparing both versions of the procedure: they were 7–8 and 1–2%, respectively [11].
The original CMP IV employed in 2005 did not fully isolate the posterior LA. Only a single connecting lesion existed between the left pulmonary and the right pulmonary veins. There was no superior connecting lesion. This was done because there was initially concern that complete electrical exclusion of the entire posterior LA might have a detrimental effect on LA function. This has since been disproven in our research laboratory. Cardiac MRI showed that LA function was maintained with the addition of a box lesion [13]. In fact, studies from our group comparing 78 patients with a true box lesion set with 22 patients without one showed a marked increase in freedom from atrial fibrillation off antiarrhythmic medications (85 vs. 47%) at greater than 1-year follow-up [10]. Because of this, all CMP IV at our institution now utilize the full box lesion to isolate the entire posterior LA.
Risk factors for recurrence at 1 year include failure to perform a box lesion, increasing LA size, and early ATAs [14]. The utility of the box lesion in improving freedom from recurrent ATAs is in agreement with the data from the electrophysiology laboratory, which have shown that a wide area circumferential ablation, involving a large portion of the posterior LA, is more effective than pulmonary vein isolation alone [5,15,16]. Increased LA size has been shown by multiple groups to be a significant risk factor for recurrence, with a probability of recurrence exceeding 50% once the LA approaches 8 cm [8,17–19]. Finally, early ATAs have previously been shown by our group to be a risk factor for recurrence [8]. It is likely that early ATAs are a marker of more advanced atrial fibrosis.
Few groups have evaluated the long-term outcomes of the CMP IV procedure when performed through an RMT. Early results are promising when compared with the CMP IV performed through a sternotomy. Our group has shown that freedom from atrial fibrillation off antiarrhythmic drugs (AADs) was not significantly different between the two groups at 3, 6, 12, and 24 months [20]. Furthermore, the patients who underwent RMT had fewer complications, decreased ICU stays, and decreased median length of hospital stays when compared with those patients who underwent sternotomy [21▪▪]. These promising results demonstrate that the CMP IV performed through an RMT approach is as effective as sternotomy in the treatment of atrial fibrillation.
Other lesion sets, including isolated pulmonary vein isolation (PVI), have been evaluated. The atrial fibrillation catheter ablation versus surgical ablation treatment trial, which was a two-center, randomized prospective clinical trial, included 124 patients and compared catheter-based ablation with thoracoscopic PVI in patients with AAD-refractory atrial fibrillation and either LA dilatation and hypertension or failed prior catheter ablation [22]. The study [22] demonstrated that the 12-month freedom from ATAs and AADs was 37% for the catheter ablation group and 66% for the PVI group (P = 0.0022). Therefore, although results with surgical PVI were not as good as a full biatrial Cox-Maze lesion set, they were superior to catheter-based ablation in patients with unfavorable atrial substrates and more complex disease. This study also demonstrated that, in patients who received surgical ablation, freedom from ATAs was higher in those with paroxysmal atrial fibrillation compared with those with pAF (69 vs. 56%).
HYBRID APPROACH
Recently, hybrid approaches employing both catheter ablation and surgical ablation have been utilized in an attempt to provide high procedural success rates without the associated shortcomings of either technique. Advantages of the hybrid approach are as follows. First, the ability to confirm conduction block. Second, the ability to close identified gaps that might result in a long-term recurrence. Third, minimization of surgical injury to structures not easily reached. Fourth, elimination of risk to the phrenic nerve and esophagus because of the surgeons’ ability to protect these structures. Fifth, reduced risk of tamponade because of an open pericardium. Finally, reduced risk of embolism from catheter ablations, as fewer ablations are used.
The disadvantages of hybrid approaches include their long operative times when compared with catheter or surgical ablation alone and their higher complication rates in some centers. The biggest problem for hybrid procedures has been the lack of good ablation technology to create the connecting lesions. On the beating heart, the only devices that have been able to achieve reliable transmural lesions have been the bipolar clamps. No unidirectional device has been able to uniformly create transmural lesions that have likely affected procedural success. At this time, there is no standardized approach to this procedure, with multiple groups performing a combination of lesions summarized in Table 2 [23].
Table 2.
Operative approach and lesion sets for published hybrid ablation series
| Authors | # Pts in hybrid group | Surgical chest access | Single-stage | Surgical ablation technology | Lesions performeda | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAA Exclusion/excision | PVI | Box | GP | LOM | SVC/IVC/BCL | MIL | CTI | ALAL | |||||
| Muneretto et al. [24] | 36 | R-sided VATS | N | Monopolar RF probe | N | Y | Y | Yc | N | N/N/N | N | Y (21) | Yd |
| Krul et al. [6] | 31 | Bilateral VATS | Y | Bipolar RF clamp/pen | Y | Y | Yb (±13) | Y (30) | Y | N/N/N | Y (13) | N | N |
| Mahapatra et al. [7] | 15 | Bilateral VATS | N | Bipolar RF clamp/pen | Y (14) | Y | N | Y | Y | Y/N/N | Y | Y | Y |
| La Meir et al. [25] | 19 | R-sided VATS | Y | Monopolar RF probe | N | Y | Y | Y | N | N/N/N | Y (3) | Y (2) | N |
| La Meir et al. [29] | 35 | Bilateral VATS | Y | Bipolar RF clamp/pen | Y (15) | Y | Y (31) | Y | N | Y (8)/Y (3)/Y (10) | Y (7) | Y (3) | N |
| Pison et al. [26] | 26 | Bilateral VATS | Y | Bipolar RF clamp/pen | Y (7) | Y | Y (22) | N | N | Y (7)/Y (3)/Y (9) | Y (3) | Y (3) | N |
ALAL, additional left atrial lesions; BCL, bilateral caval connecting line; Box, box lesion set; CTI, cavotricuspid isthmus; GP, ganglionated plexus; IVC, inferior vena cava circumferential lesion; LAA, left atrial appendage; LOM, ligament of Marshall; MIL, mitral isthmus lesion; N, no; Pts, patients; PVI, pulmonary vein isolation; R, right [22]; RF, radiofrequency; SVC, superior vena cava circumferential lesion; VATS, video-assisted thoracoscopic surgery; Y, yes.
(n) is specified when not all patients received a particular ablation line.
13 patients had an additional superior pulmonary vein connecting line and an unspecified subset of those also had an inferior pulmonary vein connecting line.
GP was presumably taken with epicardial fat removal prior to ablation with the unipolar probe. Removal was not confirmed.
When firing foci were identified within the pulmonary veins, an additional pulmonary vein antral line was performed even in the presence of an effective box lesion [24].
Additional lines were also made to target complex atrial fractionated electrograms [6].
Adapted from [23].
The procedure can be performed in either single-stage or two-stage fashion. A sequential two-stage procedure has been described, claiming that it is more cost effective to identify those patients that have atrial fibrillation recurrence after a surgical procedure and treat these patients with a subsequent percutaneous endocardial ablation [7,24]. Others have elected a single-stage procedure that combines both the surgical epicardial and catheter endocardial ablations and benefits the patient by avoiding a second procedure and hospitalization [6,25,26]. Our group started with a single-stage approach but went to a two-stage operation because of a high complication rate.
The surgical portion of the procedure is usually performed through either a right-sided or bilateral video-assisted thoracoscopy or even a transdiaphragmatic approach using pericardioscopy [27▪▪]. Most groups perform epicardial pulmonary vein isolation by way of a radiofrequency clamp in addition to various other lesions (Table 2) [23]. It is important with the mitral isthmus line that the lesion be completed both endocardially and epicardially because epicardial ablation alone is not trans-mural and leaves patients susceptible to the development of atrial flutter. The LAA excision/exclusion is typically performed with a stapler or clip and is based on factors such as the CHADS2 score [28]. Our group advocates LAA exclusion/excision in all patients as a means to decrease stroke and allow for discontinuation of warfarin.
The endocardial portion is performed through a femoral vein approach and a transseptal sheath is placed for an access to the left heart. Entrance and exit block are confirmed from both endocardial and epicardial pacing attempts from the pulmonary veins, and some centers attempt to induce atrial fibrillation by pacing from the coronary sinus at the shortest cycle length that produces 1 : 1 atrial capture. For cases in which atrial fibrillation was induced, additional linear lines are created and ablation gaps are identified and closed endocardially. Furthermore, some groups perform an endocardial ablation line at the cavotricuspid isthmus for patients in RA flutter.
The hybrid approach, thus, by far has yielded good early results, despite long operative times even with a focus on patients with pAF or LSP atrial fibrillation who have failed previous ablation [7,10]. La Meir et al. [29] compared outcomes at 1 year between patients undergoing the hybrid procedure (n = 35) and patients undergoing minimally invasive PVI and box ablation with ganglionic plexi ablation ± circumferential SVC, IVC ablation, or LAA exclusion (n = 28). They found a trend toward a greater freedom from ATAs more than 30 s off AADs in hybrid patients compared with the minimally invasive surgical group [91 (32/35) vs. 82% (23/28), P = 0.07] [29]. This difference was more profound, however, when comparing LSP patients. In this case, freedom from ATAs off AADs was significant [82 (9/11) vs. 44% (4/9), P = 0.001]. However, it is difficult to draw any conclusions in this very small study with limited follow-up.
Other groups have compared the hybrid approach to patients with previously failed catheter ablations. Mahapatra et al. [7] compared the hybrid approach in 15 patients with LSP or pAF who had failed previous catheter ablation and compared this group with 30 matched patients who underwent repeat catheter ablation. Freedom from ATAs and AADs was significantly improved by performing the hybrid procedure [87 (13/15) vs. 53% (16/30), P = 0.04, mean follow-up of 20.7 ± 4.5 months]. Because of these reports, a multicenter trial, the Dual Epicardial Endocardial Persistent Atrial Fibrillation trial, is underway and is scheduled to finish by December 2015 with a goal of evaluating the safety and efficacy of the hybrid approach.
CONCLUSION
The CMP IV helped solve many of the limitations of the cut-and-sew CMP III with the addition of radio-frequency ablation technology while achieving similar success rates with significantly less morbidity. The hybrid technique attempts to improve the CMP IV by eliminating cardiopulmonary bypass while combining the benefits of radiofrequency ablation and percutaneous catheter techniques. However, there is still a lack of a consensus lesion set, long operative times, and uncertain long-term efficacy. As the mechanisms for atrial fibrillation are better understood and technology improves, surgical treatments will likely become less invasive with improved success rates as procedures are tailored to patient-specific lesions.
KEY POINTS.
The Cox-Maze IV is the current gold standard in the surgical ablation of atrial fibrillation
Not isolating the entire posterior LA and all four pulmonary veins greatly increases atrial fibrillation recurrence
Hybrid procedures, while showing encouraging early results, lack lesion consensus, have prolonged operative times, and, at this time, have unproven long-term efficacy.
Acknowledgments
T32 HL007776, RO1 HL03225.
Funding provided in part by NIH T32 HL007776 and RO1 HL03225.
Footnotes
Conflicts of interest
R.J.D. is a consultant for AtriCure, and has received research and educational funding from AtriCure and Edwards. All other authors have no disclosures.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Williams JM, Ungerleider RM, Lofland GK, Cox JL. Left atrial isolation: new technique for the treatment of supraventricular arrhythmias. J Thorac Cardiovasc Surg. 1980;80:373–380. [PubMed] [Google Scholar]
- 2.Defauw JJ, Guiraudon GM, van Hemel NM, et al. Surgical therapy of paroxysmal atrial fibrillation with the ‘corridor’ operation. Ann Thorac Surg. 1992;53:564–570. doi: 10.1016/0003-4975(92)90312-r. [DOI] [PubMed] [Google Scholar]
- 3.Cox JL. The surgical treatment of atrial fibrillation. IV. Surgical technique. J Thorac Cardiovasc Surg. 1991;101:584–592. [PubMed] [Google Scholar]
- 4.Cox JL, Boineau JP, Schuessler RB, et al. Modification of the maze procedure for atrial flutter and atrial fibrillation. I. Rationale and surgical results. J Thorac Cardiovasc Surg. 1995;110:473–484. doi: 10.1016/S0022-5223(95)70244-X. [DOI] [PubMed] [Google Scholar]
- 5.Calkins H, Kuck KH, Cappato R, et al. Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Krul SP, Driessen AH, van Boven WJ, et al. Thoracoscopic video-assisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions: first results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:262–270. doi: 10.1161/CIRCEP.111.961862. [DOI] [PubMed] [Google Scholar]
- 7.Mahapatra S, LaPar DJ, Kamath S, et al. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and longstanding persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg. 2011;91:1890–1898. doi: 10.1016/j.athoracsur.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damiano RJ, Jr, Schwartz FH, Bailey MS, et al. The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg. 2011;141:113–121. doi: 10.1016/j.jtcvs.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–1828. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 10.Weimar T, Bailey MS, Watanabe Y, et al. The Cox-maze IV procedure for lone atrial fibrillation: a single center experience in 100 consecutive patients. J Interv Card Electrophysiol. 2011;31:47–54. doi: 10.1007/s10840-011-9547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weimar T, Schena S, Bailey MS, et al. The Cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol. 2012;5:8–14. doi: 10.1161/CIRCEP.111.963819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrance CP, Henn MC, Miller JR, et al. Comparison of the stand-alone Cox-Maze IV procedure to the concomitant Cox-Maze IV and mitral valve procedure for atrial fibrillation. Ann Cardiothorac Surg. 2014;3:55–61. doi: 10.3978/j.issn.2225-319X.2013.12.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voeller RK, Zierer A, Lall SC, et al. The effects of the Cox maze procedure on atrial function. J Thorac Cardiovasc Surg. 2008;136:1257–1264. doi: 10.1016/j.jtcvs.2008.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaynor SL, Schuessler RB, Bailey MS, et al. Surgical treatment of atrial fibrillation: predictors of late recurrence. J Thorac Cardiovasc Surg. 2005;129:104–111. doi: 10.1016/j.jtcvs.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–2360. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 16.Pappone C, Oreto G, Rosanio S, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001;104:2539–2544. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 17.Kamata J, Kawazoe K, Izumoto H, et al. Predictors of sinus rhythm restoration after Cox maze procedure concomitant with other cardiac operations. Ann Thorac Surg. 1997;64:394–398. doi: 10.1016/S0003-4975(97)00139-2. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi J, Kosakai Y, Nakano K, et al. Improved success rate of the maze procedure in mitral valve disease by new criteria for patients’ selection. Eur J Cardiothorac Surg. 1998;13:247–252. doi: 10.1016/s1010-7940(97)00328-x. [DOI] [PubMed] [Google Scholar]
- 19.Gillinov AM, Sirak J, Blackstone EH, et al. The Cox maze procedure in mitral valve disease: predictors of recurrent atrial fibrillation. J Thorac Cardiovasc Surg. 2005;130:1653–1660. doi: 10.1016/j.jtcvs.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Lee AM, Clark K, Bailey MS, et al. A minimally invasive Cox-maze procedure: operative technique and results. Innovations (Phila) 2010;5:281–286. doi: 10.1097/IMI.0b013e3181ee3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪▪.Lawrance CP, Henn MC, Miller JR, et al. A minimally invasive Cox maze IV procedure is as effective as sternotomy while decreasing major morbidity and hospital stay. J Thorac Cardiovasc Surg. 2014;148:955–962. doi: 10.1016/j.jtcvs.2014.05.064. As surgical ablation trends toward more minimally invasive techniques, it is important to document that the success of the procedure as measured by freedom from atrial fibrillation off antiarrhythmic medications is not sacrificed. This study shows that not only can a minimally invasive Cox-Maze IV achieve equivalent success rates to those performed through a sternotomy, but also it decreases morbidity and hospital stay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation. 2012;125:23–30. doi: 10.1161/CIRCULATIONAHA.111.074047. [DOI] [PubMed] [Google Scholar]
- 23.Robertson JO, Lawrance CP, Maniar HS, Damiano RJ., Jr Surgical techniques used for the treatment of atrial fibrillation. Circ J. 2013;77:1941–1951. doi: 10.1253/circj.cj-13-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muneretto C, Bisleri G, Bontempi L, et al. Successful treatment of lone persistent atrial fibrillation by means of a hybrid thoracoscopic-transcatheter approach. Innovations (Phila) 2012;7:254–258. doi: 10.1097/IMI.0b013e31826f0462. [DOI] [PubMed] [Google Scholar]
- 25.La Meir M, Gelsomino S, Lorusso R, et al. The hybrid approach for the surgical treatment of lone atrial fibrillation: one-year results employing a monopolar radiofrequency source. J Cardiothorac Surg. 2012;7:71. doi: 10.1186/1749-8090-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pison L, La Meir M, van Opstal J, et al. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2012;60:54–61. doi: 10.1016/j.jacc.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 27▪▪.Gehi AK, Mounsey JP, Pursell I, et al. Hybrid epicardial-endocardial ablation using a pericardioscopic technique for the treatment of atrial fibrillation. Heart Rhythm. 2013;10:22–28. doi: 10.1016/j.hrthm.2012.08.044. This series not only identifies an innovative approach to hybrid ablation but also highlights some of the issues of transitioning to a more minimally invasive approach, including the potential for decreased safety and efficacy. [DOI] [PubMed] [Google Scholar]
- 28.Pet M, Robertson JO, Bailey M, et al. The impact of CHADS2 score on late stroke after the Cox maze procedure. J Thorac Cardiovasc Surg. 2013;146:85–89. doi: 10.1016/j.jtcvs.2012.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Meir M, Gelsomino S, Luca F, et al. Minimally invasive surgical treatment of lone atrial fibrillation: early results of hybrid versus standard minimally invasive approach employing radiofrequency sources. Int J Cardiol. 2012;167:1469–1475. doi: 10.1016/j.ijcard.2012.04.044. [DOI] [PubMed] [Google Scholar]


