Abstract
Background
Understanding the current epidemiology of malaria and the relationship between intervention coverage, transmission intensity, and burden of disease is important to guide control activities. We aimed to determine the prevalence of anemia, parasitemia, and serological responses to P. falciparum antigens, and factors associated with these indicators, in three different epidemiological settings in Uganda.
Methods and Findings
In 2012, cross-sectional surveys were conducted in 200 randomly selected households from each of three sites: Walukuba, Jinja district (peri-urban); Kihihi, Kanungu district (rural); and Nagongera, Tororo district (rural) with corresponding estimates of annual entomologic inoculation rates (aEIR) of 3.8, 26.6, and 125.0, respectively. Of 2737 participants, laboratory testing was done in 2227 (81.4%), including measurement of hemoglobin, parasitemia using microscopy, and serological responses to P. falciparum apical membrane antigen 1 (AMA-1) and merozoite surface protein 1, 19 kilodalton fragment (MSP-119). Analysis of laboratory results was restricted to 1949 (87.5%) participants aged ≤ 40 years. Prevalence of anemia (hemoglobin < 11.0 g/dL) was significantly higher in Walukuba (18.9%) and Nagongera (17.4%) than in Kihihi (13.1%), and was strongly associated with decreasing age for those ≤ 5 years at all sites. Parasite prevalence was significantly higher in Nagongera (48.3%) than in Walukuba (12.2%) and Kihihi (12.8%), and significantly increased with age to 11 years, and then significantly decreased at all sites. Seropositivity to AMA-1 was 53.3% in Walukuba, 63.0% in Kihihi, and 83.7% in Nagongera and was associated with increasing age at all sites. AMA-1 seroconversion rates strongly correlated with transmission intensity, while serological responses to MSP-119 did not.
Conclusion
Anemia was predominant in young children and parasitemia peaked by 11 years across 3 sites with varied transmission intensity. Serological responses to AMA-1 appeared to best reflect transmission intensity, and may be a more accurate indicator for malaria surveillance than anemia or parasitemia.
Introduction
Malaria remains an important public health problem in sub-Saharan Africa, and is responsible for over 10% of the overall disease burden [1]. In the past decade, increased donor financing and widespread scale-up of malaria control measures, including distribution of long-lasting insecticide-treated bed nets (LLINs), indoor residual spraying (IRS) with insecticides, intermittent presumptive treatment in pregnancy (IPTp), and prompt and effective treatment with highly efficacious artemisinin-based combination therapies (ACTs), have substantially reduced the malaria burden in several countries [2–7]. However, these gains have not been consistent across Africa [8], and malaria-associated morbidity and mortality remains high in some countries, including Uganda [9–11].
In 2012, Uganda was ranked fourth highest in number of malaria cases reported globally, and sixth in number of malaria-associated deaths [12]. Although the epidemiology of malaria varies widely, some of the highest levels of malaria transmission in the world have been recorded in Uganda, and much of the population lives in high transmission areas [11]. Uganda has made mixed progress towards implementing malaria control interventions in the past decade. Ownership of at least one insecticide treated net (ITN) per household increased substantially from 16% in 2006 to 60% in 2011, but national IRS coverage remains low (6–7%). In 2011, the proportion of pregnant women receiving two or more doses of intermittent preventive treatment (IPTp) was only 25%, while the proportion of febrile children treated with artemether-lumefantrine (AL), the first-line recommended treatment for uncomplicated malaria, was 44%, up from 23% in 2006 [13,14]. Data documenting the impact of scaling-up of control interventions on indicators of malaria burden in Uganda are limited. Only IRS has been associated with a reduction in malaria morbidity [15], and this benefit has been transient in areas where IRS has not been sustained [16]. Some evidence suggests that despite modest improvements in coverage of malaria control interventions, the burden of malaria may be increasing in certain areas of Uganda [17].
Monitoring and evaluation of malaria control activities relies heavily on large, nationally representative cross-sectional surveys. In Uganda, these include Demographic Health Surveys (DHS) which have been conducted every 5 years since 2001 and a single Malaria Indicator Survey (MIS) conducted in 2009. Although all these surveys include estimates of coverage of malaria control interventions, indicators of malaria burden are limited to anemia testing and, only in the 2009 MIS, estimates of parasitemia. The annual entomological inoculation rate (aEIR) is generally considered the ‘gold-standard’ measure of malaria endemicity and transmission intensity, but is highly labour-intensive and rarely measured [18]. In areas of stable malaria transmission, anemia has been used to monitor the impact of control interventions [19–21]. Parasite prevalence in children aged 2–10 years provides an indirect measure of transmission intensity across a range of malaria endemicities and is the most frequently measured malariometric index [22,23]. More recently, serological responses to malaria antigens have gained momentum as indicators of malaria transmission and infection risk, as seroprevalence reflects cumulative malaria exposure [24]. Recent studies have capitalized on the relationship between P. falciparum exposure and antibody responses to show that it is possible to estimate transmission intensity in a community by calculating seroconversion rates using age specific prevalence of specific antimalarial antibodies, including those to apical membrane antigen 1 (AMA-1) and merozoite surface protein 1, 19 kilodalton fragment (MSP-119) [25–27].
To better understand the current epidemiology of malaria in Uganda, cross-sectional surveys to estimate coverage levels of control interventions and key malaria indicators, including anemia, parasitemia, and serological responses to AMA-1 and MSP-119 were conducted in three sub-counties with varying levels of transmission intensity based on concurrently measured estimates of aEIR.
Methods
Study design
This was a cross-sectional community survey conducted in three different epidemiological settings in Uganda. A total of 200 households were recruited in each site, using a list of households randomly generated from a census database. The surveys consisted of a household questionnaire and biomarker survey. Finger-prick blood samples, obtained from all children under fifteen years of age and from a random selection of age stratified adults, were collected for measurement of hemoglobin, thick and thin blood smear, rapid diagnostic test (RDT) for malaria, and to save on filter paper. The protocol for this trial is available as supporting information (S1 Protocol).
Ethics statement
Ethical approval was obtained from the Makerere University School of Medicine Research and Ethics Committee (REC REF 2011–203), the Uganda National Council for Science and Technology (HS 1074), the London School of Hygiene & Tropical Medicine Ethics Committee (Reference 6012), and the University of California, San Francisco Committee on Human Research (Reference 027911). For all households fulfilling the selection criteria, written consent to participate in the study was sought. Written informed consent was obtained from the next of kin, caretakers, or guardians on behalf of the children enrolled in the study. Furthermore, written assent was obtained from children aged 8 years and above to participate in the study.
Study sites
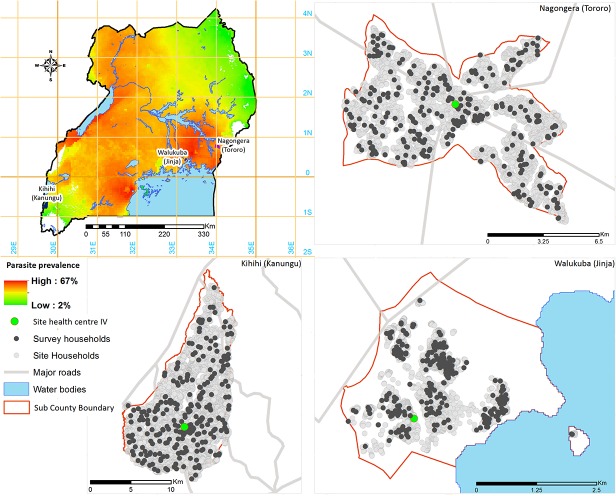
Cross-sectional surveys were conducted between January and June 2012 in three sub-counties purposively chosen to represent varied malaria transmission settings in Uganda: Walukuba, Jinja district; Kihihi, Kanungu district; and Nagongera, Tororo district (Fig. 1). In each sub-county, entomological surveys were conducted from October 2011 – September 2012 using monthly human landing catches to generate estimates of aEIR as previously described [28]. Malaria transmission at all three sites was perennial, with 2 annual peaks following the rainy seasons. Walukuba is a peri-urban area near Lake Victoria in the south-central part of the country with an estimated aEIR of 3.8 infectious bites per person per year. Kihihi is a rural area in the south-western part of the country, with an estimated aEIR of 26.6. Nagongera is a rural area in the south-eastern part of the country near the Kenyan border, with an estimated aEIR of 125.0. Key malaria control interventions in all three districts include use of LLINs, malaria case management with ACTs, and IPTp with sulfadoxine-pyrimethamine. A single round of IRS using lambda-cyhalothrin was conducted in Kanungu district in 2007 and a mass, community-based campaign to distribute free ITNs was conducted in Tororo district in January 2011.
Fig 1. Map of study sites in Uganda.
Enumeration and mapping of households
In 2011, all households and other key features in the study areas were enumerated and mapped to generate a sampling frame for the surveys. A household was defined as any single permanent or semi-permanent dwelling structure acting as the primary residence for a person or group of people that generally cook and eat together. Household locations were mapped using hand-held eTrex global positioning system (GPS) receivers (Garmin Ltd., Olathe, KS) and readings were taken from the door of the household, if possible, or from a point that was most representative of the household.
Recruitment and enrolment
We enrolled 200 households from each site using a list randomly generated from the household enumeration database using computer-generated random numbers. Study personnel conducted door-to-door recruitment to identify those households with at least one adult household resident in sequential order according to the recruitment list. Residents not home during the initial contact were re-visited on at least three other occasions before eliminating them from the sample selection process.
When a household was identified, study personnel briefly described the purpose of the study to the head of the household or any adult household member present at home in the appropriate language. Households were included if: 1) an adult aged 18 years or older was the head of the household, and 2) the head of the household agreed to provide informed consent. Households were excluded if: 1) no adult resident was at home on more than 3 visits, and 2) the household was vacant. For all households fulfilling the selection criteria, written consent to participate in the study was sought.
Study procedures
Upon enrolment, a household questionnaire, based on the Malaria Indicator Survey questionnaire developed by the Roll Back Malaria Monitoring and Evaluation Reference Group [29], was administered to heads of households or their designate. The questionnaire was used to obtain information on all household members, including age, gender, and relationship to the head of the household. It was also used to collect information on ownership and use of bed nets, and proxy indicators of wealth including ownership of assets and land, household construction, and food security. Finger-prick blood samples were obtained from all children under fifteen years of age, and from one randomly selected household member within the following age categories: 15–24 years, 25–34 years, 35–44 years, 45–54 years, and > 55 years. Blood samples were used for measurement of hemoglobin, thick and thin blood smear, and storage on filter paper.
Laboratory evaluations
Thick and thin blood smears were stained with 2% Giemsa for 30 minutes. Thick smears were evaluated for the presence of parasitemia (asexual forms only) and gametocytes. Parasite densities were calculated by counting the number of asexual parasites per 200 leukocytes (or per 500, if the count was less than 10 parasites or gametocytes per 200 leukocytes), assuming a leukocyte count of 8,000/ul. A smear was considered negative after reviewing 100 high-powered fields. All positive thick blood smears had corresponding thin smears reviewed for species identification. For quality control, all slides were read by a second microscopist and a third reviewer was used to settle any discrepant readings. Hemoglobin concentration was assessed using a battery-operated portable hemoglobinometer (HemoCue Ltd., Angelholm, Sweden) and estimated to an accuracy of 1 g/dL.
Blood spots were collected onto filter papers (Whatman 3MM; Whatman, Maidstone, UK), allowed to dry overnight and stored at -20°C with dessicant for serology studies. The recombinant proteins P. falciparum AMA-1 (3D7) and MSP-119 (Wellcome genotype) were used as antigens in indirect ELISA as previously described [30]. Briefly, antigens were coated on plates at the concentration of 0.5 mg/mL in coating buffer and incubated at 4oC overnight. The plates were washed in PBST, and blocked with 1% (w/v) skimmed milk solution for three hours. After washing, samples were added in duplicate at a final dilution of 1:1,000 to each plate together with a pool of hyper immune serum and the plates were incubated overnight at 4oC. The plates were washed and 50 μl of HRP-conjugated rabbit anti human IgG (DAKO, #P0214) were added into each well and incubated for three hours. After a further series of washes, substrate solution (OPD, Sigma #P8287, in PBS) was added and the reaction was allowed to develop for 15–20 min before addition of stopping solution (2M H2SO4). The optical density was read using an ELISA reader at 450 nm.
Data management and statistical analysis
Data were collected using hand-held computers which were programmed to include range checks, structure checks and internal consistency checks. All statistical analyses were carried out using Stata version 12 (STATA Corporation, College Station, TX). Malaria indicators of interest were; 1) anemia defined as hemoglobin less than 11 g/dl, 2) parasitemia defined on the basis of expert microscopy results as presence of any asexual parasites on thick blood smear, 3) AMA-1 antibody seropositivity and, 4) MSP-119 antibody seropositivity. Raw optical density (OD) measurements were averaged and normalized against the positive control samples on each plate. The cut-off for seropositivity was calculated, as previously described with a separate cut-off value generated for each antigen [27]. Seroconversion rates were estimated by fitting a simple reversible catalytic model to seroprevalence data for each antigen [24]. Analyses of malaria indicators were limited to study participants aged 40 years or younger due to the relatively limited number of observations, particularly in one site (Walukuba), and concerns about lack of precision of analyses including participants above 40 years of age. Comparisons between the sites for categorical variables were made using the Chi-squared or Fisher’s exact test and for continuous variables using the t-test. Associations between risk factors of interest and binary malaria indicators were made using multivariate generalized estimating equations stratified by study site with adjustment for clustering of study participants within the same household. In these models the relationships between age and malaria indicators were fitted using linear splines. A household wealth index was created using principal component analysis of data based on household’s dwelling unit ownership of various durable goods, land, and household food security and then categorized into tertiles [31]. Graphical presentation of the relationships between age and both the prevalence of anemia and parasitemia were made using lowess smoothing.
Results
Participant characteristics and coverage of control interventions
At total of 200 households were surveyed from each sub-county, including 2737 study participants “Table 1”. The age and gender of participants were similar across the three sites. The majority of households in Nagongera, and over half in Walukuba and Kihihi, owned at least one ITN. However, the proportion of households with at least one ITN per 2 residents was much lower at the 3 sites. Only 5 households in Walukuba reported receiving IRS in the prior 12 months. The proportion of mothers who received ≥ 2 doses of SP for IPTp during their last pregnancy ranged from 35.3% in Walukuba to 52.8% in Kihihi. The use of ACTs for antimalarial treatment was high (≥ 75%) at all sites. A total of 2227 (81.4%) participants underwent laboratory testing, and of these, 1949 (87.5%) aged ≤ 40 years were included in the analysis of laboratory results “Table 2”.
Table 1. Characteristics of study sites, households, and participants.
| Category | Characteristic | Study Site | ||
|---|---|---|---|---|
| Walukuba | Kihihi | Nagongera | ||
| Transmission intensity | aEIR a using human landing catches (95% CI) | 3.8 (0–11.4) | 26.6 (7.6–49.4) | 125 (72.2–183) |
| Sample size | Number of household | 200 | 200 | 200 |
| Number of study participants | 797 | 948 | 992 | |
| Demographics of survey participants | Female gender, n (%) | 399 (50.1%) | 499 (52.6%) | 537 (54.1%) |
| Median age in years (IQR) | 18 (6–28) | 15 (6–30) | 14 (6–32) | |
| Coverage of malaria control interventions | Households with at least 1 ITN, n (%) | 115 (57.5%) | 102 (51.0%) | 157 (78.5%) |
| Households with at least 1 ITN per 2 residents, n (%) | 57 (28.5%) | 34 (17.0%) | 71 (35.5%) | |
| Households reporting IRS in the last 12 months, n (%) | 5 (2.5%) | 0 | 0 | |
| IPTp with ≥ 2 doses of SP during last pregnancy b , n/N (%) | 30/85 (35.3%) | 48/91 (52.8%) | 31/78 (39.7%) | |
| Use of ACT if received recent antimalarial treatment c , n/N (%) | 24/32 (75.0%) | 32/39 (82.1%) | 49/63 (77.8%) | |
aAnnual entomological inoculation rate (measured Oct 2011 – Sept 2012)
bAmong women who reported giving birth in the previous 4 years
cAmong children under the age of 5 years with reported fever in the previous 2 weeks
Table 2. Estimates of malaria indicators.
| Category | Characteristic | Study Site | ||
|---|---|---|---|---|
| Walukuba | Kihihi | Nagongera | ||
| Sample size | Number of participants selected for laboratory testing | 631 | 788 | 808 |
| Number of participants selected for laboratory testing ≤ 40 years of age | 582 | 682 | 685 | |
| Anemia testing | Mean hemoglobin g/dL (SD) | 12.6 (2.0) | 12.8 (1.8) | 12.6 (1.9) |
| Mean hemoglobin g/dL children < 5 years (SD) | 11.3 (1.6) | 11.7 (1.5) | 10.9 (1.6) | |
| Hemoglobin < 11 g/dL, n (%) | 110 (18.9%) | 89 (13.1%) | 119 (17.4%) | |
| Hemoglobin < 11 g/dL children < 5 years, n/N (%) | 63/155 (40.7%) | 54/183 (29.5%) | 78/158 (49.4%) | |
| Hemoglobin < 8 g/dL, n (%) | 7 (1.2%) | 4 (0.6%) | 3 (0.4%) | |
| Blood smear results | Positive for any asexual parasitemia, n (%) | 71 (12.2%) | 87 (12.8%) | 331 (48.3%) |
| Positive for any asexual parasitemia children 2–10 years, n/N (%) | 38/233 (16.3%) | 50/280 (17.9%) | 189/317 (59.6%) | |
| Geometric mean parasite density/μL | 438 | 881 | 950 | |
| P. falciparum infection a , n (%) | 67 (94.4%) | 85 (97.7%) | 326 (98.5%) | |
| Positive for any gametocytes, n (%) | 15 (2.6%) | 13 (1.9%) | 102 (14.9%) | |
| Seroprevalence b | AMA-1 seropositive, n/N (%) | 309/580 (53.3%) | 428/679 (63.0%) | 565/675 (83.7%) |
| Median AMA-1 antibody titer (IQR) | 87 (19–452) | 209 (32–913) | 709 (182–1887) | |
| AMA-1 seroconversion rate (95% CI) | 0.10 (0.09–0.12) | 0.17 (0.14–0.19) | 0.42 (0.36–0.49) | |
| MSP1–19 seropositive, n/N (%) | 173/544 (31.8%) | 344/678 (50.7%) | 310/679 (45.7%) | |
| Median MSP1–19 antibody titer (IQR) | 66 (40–170) | 101 (40–539) | 94 (41–458) | |
| MSP1–19 seroconversion rate (95% CI) | 0.036 (0.031–0.043) | 0.089 (0.078–0.100) | 0.064 (0.057–0.073) | |
aOnly including those positive for asexual parasites
b No serology results obtain in 16 subjects for AMA-1 and 48 subjects for MSP1–19
Anemia
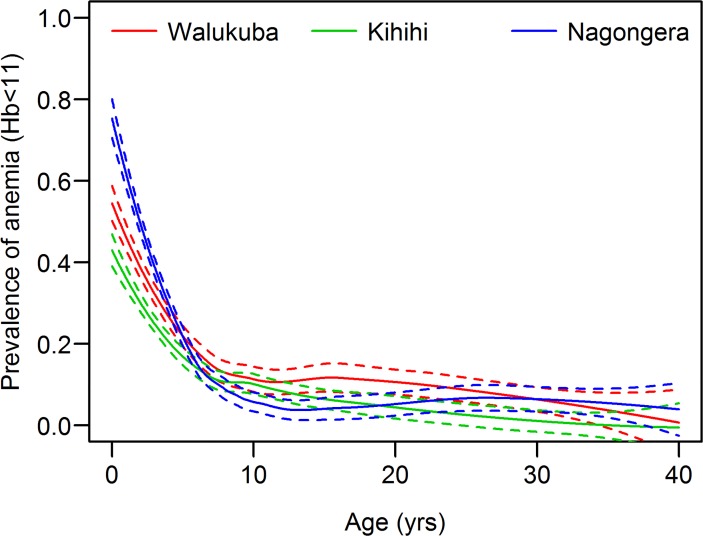
Mean hemoglobin levels were generally high (> 12.0 g/dL) and severe anemia (hemoglobin < 8g/dL) was uncommon at all sites “Table 2”. The prevalence of anemia (hemoglobin < 11g/dL) was highest in Walukuba, the site with the lowest EIR “Table 2”. In children under-five, prevalence of anemia was significantly lower in Kihihi (29.5%), than in Walukuba (40.7%, p = 0.03) and Nagongera (49.4%, p<0.001). The probability of anemia was highest in children under-five, decreasing sharply until approximately 10 years of age at all 3 sites (Fig. 2). Considering all sites combined, the prevalence of anemia was 35.7% in those ≤ 5 years of age and 8.0% in those 6–40 years of age (p<0.001). In the adjusted analysis, the prevalence of anemia was most strongly associated with age “Table 3”. For children ≤ 5 years of age, the odds of anemia decreased greatly with increasing age at all the sites (ORs ranging from 0.47 to 0.70 per 1 year increase in age). For participants aged 6–40 years there was a more gradual decrease in the prevalence of anemia in Walukuba (OR 0.96 per 1 year increase) and Kihihi (OR 0.91 per 1 year increase in age). The association between anemia and other factors varied by site; in Kihihi and Nagongera, participants with parasitemia were significantly more likely to be anemic, but ITN use and other factors were not significantly associated. In Walukuba, participants who slept under an ITN the previous night were significantly less likely to be anemic.
Fig 2. Prevalence of anemia (hemoglobin < 11.0 g/dL) by age, in three different epidemiological settings in Uganda.
Table 3. Factors associated with anemia (Hb < 11 g/dL).
| Risk factor | Category | Walukuba | Kihihi | Nagongera | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hb < 11g/dL | Adjusted OR a (95% CI) | p-value | Hb < 11g/dL | Adjusted OR a (95% CI) | p-value | Hb < 11g/dL | Adjusted OR a (95% CI) | p-value | ||
| Age | ≤ 5 years | 71/183 (38.8%) | 0.66 (0.58–0.76) b | <0.001 | 56/211 (26.5%) | 0.70 (0.59–0.84) b | <0.001 | 82/191 (42.9%) | 0.47 (0.40–0.56) b | <0.001 |
| 6–40 years | 39/399 (9.8%) | 0.96 (0.93–0.99) b | 0.004 | 33/470 (7.0%) | 0.91 (0.87–0.95) b | <0.001 | 37/494 (7.5%) | 0.97 (0.93–1.01) b | 0.23 | |
| Gender | Male | 43/259 (16.6%) | 1.0 (reference) | - | 42/305 (13.8%) | 1.0 (reference) | - | 54/290 (18.6%) | 1.0 (reference) | - |
| Female | 67/323 (20.7%) | 1.50 (0.99–2.27) | 0.05 | 47/376 (12.5%) | 1.12 (0.70–1.79) | 0.64 | 65/395 (16.5%) | 0.90 (0.60–1.33) | 0.59 | |
| Slept under a LLTIN the prior evening | No | 70/356 (19.7%) | 1.0 (reference) | - | 58/480 (12.1%) | 1.0 (reference) | - | 45/335 (13.4%) | 1.0 (reference) | - |
| Yes | 40/226 (17.7%) | 0.55 (0.31–0.97) | 0.04 | 31/201 (15.4%) | 1.01 (0.60–1.70) | 0.98 | 74/350 (21.1%) | 1.45 (0.89–2.36) | 0.14 | |
| Wealth index tertiles | Lowest | 36/200 (18.0%) | 1.0 (reference) | - | 36/232 (15.5%) | 1.0 (reference) | - | 53/230 (23.0%) | 1.0 (reference) | - |
| Middle | 38/192 (19.8%) | 1.28 (0.72–2.27) | 0.39 | 27/223 (12.1%) | 0.77 (0.39–1.51) | 0.45 | 31/232 (13.4%) | 0.63 (0.34–1.16) | 0.14 | |
| Highest | 36/190 (19.0%) | 1.31 (0.71–2.42) | 0.39 | 26/226 (11.5%) | 0.80 (0.42–1.53) | 0.50 | 35/223 (15.7%) | 0.97 (0.54–1.73) | 0.92 | |
| Results of blood slide | Negative | 95/511 (18.6%) | 1.0 (reference) | - | 70/594 (11.8%) | 1.0 (reference) | - | 46/354 (13.0%) | 1.0 (reference) | - |
| Positive | 15/71 (21.1%) | 1.59 (0.79–3.17) | 0.19 | 19/87 (21.8%) | 2.46 (1.45–4.19) | 0.001 | 73/331 (22.1%) | 3.05 (1.92–4.86) | <0.001 | |
a Odds ratio controlling for repeated measures in the same household
b Odds ratio expressed per 1 year increase in age
Parasitemia
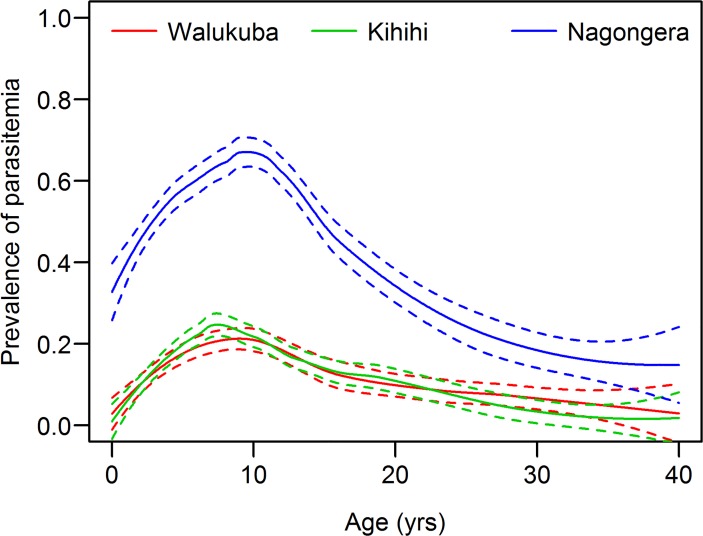
The prevalence of asexual parasitemia was significantly higher in Nagongera than the other two sites (p<0.001 for both comparisons), but was comparable in Walukuba and Kihihi, despite the difference in aEIR in the two sites “Table 2”. A similar pattern was seen for participants of all ages and children aged 2–10 years. Parasite prevalence increased with age, peaking by 11 years, and declining thereafter in all sites (Fig. 3). In the adjusted analysis, age was the only factor significantly associated with parasite prevalence “Table 4”. Although the parasite prevalence was substantially higher in Nagongera compared to the other two sites across all ages, the relationship between age and parasitemia was remarkably similar across all three sites. Increasing age was significantly associated with an increased odds of parasitemia up to 11 years of age (ORs ranging from 1.07 to 1.13 per 1 year increase in age) and then from 12–40 years increasing age was significantly associated with a decreased odds of parasitemia (ORs ranging from 0.89 to 0.92 per 1 year increase in age). Other factors, including gender, ITN use, and socioeconomic status were not significantly associated with parasitemia.
Fig 3. Prevalence of parasitemia (blood slide positive) by age, in three different epidemiological settings in Uganda.
Table 4. Factors associated with asexual parasitemia.
| Risk factor | Category | Walukuba | Kihihi | Nagongera | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parasite prevalence | Adjusted OR a (95% CI) | p-value | Parasite prevalence | Adjusted OR a (95% CI) | p-value | Parasite prevalence | Adjusted OR a (95% CI) | p-value | ||
| Age | ≤ 11 years | 46/314 (14.7%) | 1.13 (1.04–1.21) b | 0.002 | 58/362 (16.0%) | 1.10 (1.02–1.18) b | 0.009 | 235/411 (57.2%) | 1.07 (1.01–1.13) b | 0.03 |
| 12–40 years | 25/268 (9.3%) | 0.92 (0.87–0.97) b | 0.001 | 29/320 (9.1%) | 0.89 (0.85–0.94) b | <0.001 | 96/274 (35.0%) | 0.90 (0.87–0.93) b | <0.001 | |
| Gender | Male | 38/259 (14.7%) | 1.0 (reference) | - | 44/305 (14.4%) | 1.0 (reference) | - | 153/290 (52.8%) | 1.0 (reference) | - |
| Female | 33/323 (10.2%) | 0.65 (0.39–1.09) | 0.10 | 43/377 (11.4%) | 0.85 (0.53–1.37) | 0.50 | 178/395 (45.1%) | 0.99 (0.73–1.35) | 0.96 | |
| Slept under a LLTIN the prior evening | No | 40/356 (11.2%) | 1.0 (reference) | - | 68/481 (14.1%) | 1.0 (reference) | - | 183/335 (53.6%) | 1.0 (reference) | - |
| Yes | 31/226 (13.7%) | 1.52 (0.85–2.72) | 0.15 | 19/201 (9.5%) | 0.74 (0.36–1.54) | 0.42 | 148/350 (42.3%) | 0.80 (0.53–1.19) | 0.27 | |
| Wealth index tertiles | Lowest | 24/200 (12.0%) | 1.0 (reference) | - | 22/232 (9.5%) | 1.0 (reference) | - | 102/230 (44.4%) | 1.0 (reference) | - |
| Middle | 28/192 (14.6%) | 1.10 (0.60–2.03) | 0.76 | 34/223 (15.3%) | 1.42 (0.71–2.83) | 0.32 | 128/232 (55.2%) | 1.48 (0.94–2.33) | 0.09 | |
| Highest | 19/190 (10.0%) | 0.60 (0.28–1.25) | 0.17 | 31/227 (13.7%) | 1.12 (0.53–2.38) | 0.77 | 101/223 (45.3%) | 0.89 (0.54–1.48) | 0.66 | |
a Odds ratio controlling for repeated measures in the same household
b Odds ratio expressed per 1 year increase in age
AMA-1 antibodies
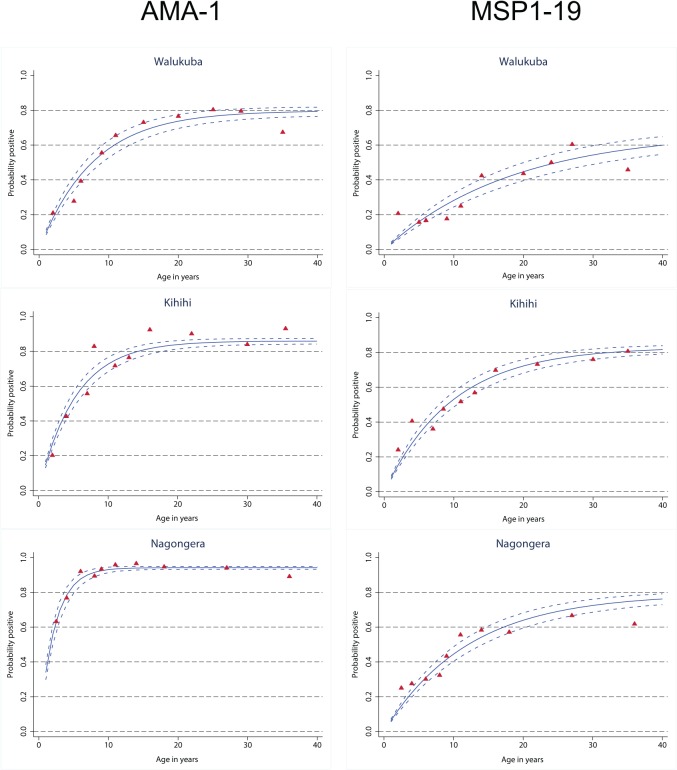
The antibody response to AMA-1, whether evaluated as seroprevalence, titer, or seroconversion rate, increased with increasing transmission intensity “Table 2”. Seroprevalences to AMA-1 were 53.3% in Walukuba, 63.0% in Kihihi and 83.7% in Nagongera (p<0.001 for all comparisons). AMA-1 seroconversion rates were 0.10, 0.17, and 0.42, which, based on prior calibration, corresponded to calculated aEIRs of 3, 9, and 121 for Walukuba, Kihihi, and Nagongera, respectively. Peak seroprevalences were proportionate to the level of transmission intensity and the age at which the peak was reached was inversely proportional to transmission intensity (Fig. 4). In the adjusted analysis, increasing age was associated with increased odds of seropositivity to AMA-1 up to 10 years of age at all three sites with the strength of this association increasing with increasing transmission intensity “Table 5”. In Nagongera, seropositivity to AMA-1 peaked by 10 years of age but continued to increase significantly from 11–20 years of age in Walukuba and Kihihi. Parasitemia was strongly associated with AMA-1 seropositivity at all sites “Table 5”. In Walukuba only, gender and socioeconomic status were also significantly associated with AMA-1 seropositivity, with significantly higher odds for females, and significantly lower odds for those participants of highest socioeconomic status.
Fig 4. Seroconversion to AMA-1and MSP-119 by age, in three different epidemiological settings in Uganda.
Table 5. Factors associated with seropositivity to AMA-1.
| Risk factor | Category | Walukuba | Kihihi | Nagongera | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seropositive | Adjusted OR a (95% CI) | p-value | Seropositive | Adjusted OR a (95% CI) | p-value | Seropositive | Adjusted OR a (95% CI) | p-value | ||
| Age | ≤ 10 years | 100/299 (33.4%) | 1.25 (1.15–1.36) b | <0.001 | 137/341 (40.2%) | 1.39 (1.28–1.51) b | <0.001 | 274/366 (74.9%) | 1.47 (1.31–1.66) b | <0.001 |
| 11–20 years | 85/117 (72.7%) | 1.11 (1.03–1.20) b | 0.007 | 155/185 (83.8%) | 1.11 (1.01–1.22) b | 0.03 | 173/181 (95.6%) | 1.00 (0.86–1.16) b | 0.99 | |
| 21–40 years | 124/164 (75.6%) | 0.96 (0.91–1.02) b | 0.21 | 136/153 (88.9%) | 1.00 (0.94–1.06) b | 0.94 | 118/128 (92.2%) | 0.96 (0.87–1.05) b | 0.35 | |
| Gender | Male | 118/259 (45.6%) | 1.0 (reference) | - | 188/304 (61.8%) | 1.0 (reference) | - | 234/287 (81.5%) | 1.0 (reference) | - |
| Female | 191/321 (59.5%) | 1.86 (1.25–2.77) | 0.002 | 240/375 (64.0%) | 0.92 (0.65–1.30) | 0.64 | 331/388 (85.3%) | 1.40 (0.89–2.18) | 0.14 | |
| Slept under a LLTIN the prior evening | No | 193/354 (54.5%) | 1.0 (reference) | - | 311/481 (64.7%) | 1.0 (reference) | - | 292/330 (88.5%) | 1.0 (reference) | - |
| Yes | 116/226 (51.3%) | 1.11 (0.73–1.70) | 0.63 | 117/198 (59.1%) | 1.21 (0.73–2.00) | 0.46 | 273/345 (79.1%) | 0.74 (0.41–1.33) | 0.31 | |
| Wealth index quartiles | Lowest | 120/198 (60.6%) | 1.0 (reference) | - | 144/230 (62.6%) | 1.0 (reference) | - | 183/228 (80.3%) | 1.0 (reference) | - |
| Middle | 101/192 (52.6%) | 0.74 (0.42–1.29) | 0.29 | 139/222 (62.6%) | 0.88 (0.51–1.53) | 0.65 | 200/230 (87.0%) | 0.97 (0.50–1.91) | 0.94 | |
| Highest | 88/190 (46.3%) | 0.57 (0.33–0.97) | 0.04 | 145/227 (63.9%) | 0.76 (0.42–1.36) | 0.35 | 182/217 (83.9%) | 0.76 (0.39–1.48) | 0.42 | |
| Results of blood slide | Negative | 255/509 (50.1%) | 1.0 (reference) | - | 360/593 (60.7%) | 1.0 (reference) | - | 268/348 (77.0%) | 1.0 (reference) | - |
| Positive | 54/71 (76.1%) | 3.88 (2.03–7.43) | <0.001 | 68/86 (79.1%) | 2.73 (1.44–5.18) | 0.002 | 297/327 (90.8%) | 3.39 (1.84–6.25) | <0.001 | |
a Odds ratio controlling for repeated measures in the same household
b Odds ratio expressed per 1 year increase in age
MSP-119 antibodies
Seroprevalences of antibodies to MSP-119, median MSP-119 antibody titers, and seroconversion rates were lower than with AMA-1 and demonstrated different patterns across the three sites “Table 2”. Seropositivity to MSP-119 was highest in Kihihi, and was significantly lower in Walukuba (31.8%), than in Kihihi (50.7%, p<0.001) and Nagongera (45.7%, p<0.001). MSP-119 seroconversion rates were 0.036, 0.089, and 0.064 for Walukuba, Kihihi, and Nagongera, respectively, which corresponded to calculated aEIRs of 2 in Walukuba and 11 in Kihihi, but only 6 in Nagongera, much lower than the aEIR actually observed (125) [30]. In the adjusted analysis, there was an inconsistent relationship between age and seropositivity to MSP-119 up to 3 years of age across the 3 sites “Table 6”. From 4–20 years of age there was a consistent and significant relationship between increasing age and increasing seropositivity to MSP-119 and no significant relationship from 21–40 years of age across the 3 sites. Only in Walukuba was parasitemia significantly associated with seropositivity to MSP-119 “Table 6”.
Table 6. Factors associated with seropositivity to MSP-119.
| Risk factor | Category | Walukuba | Kihihi | Nagongera | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seropositive | Adjusted OR a (95% CI) | p-value | Seropositive | Adjusted OR a (95% CI) | p-value | Seropositive | Adjusted OR a (95% CI) | p-value | ||
| Age | ≤ 3 years | 25/119 (21.0%) | 0.78 (0.60–1.03) b | 0.08 | 29/141 (20.6%) | 1.23 (0.94–1.62) b | 0.13 | 42/121 (34.7%) | 0.64 (0.48–0.84) b | 0.002 |
| 4–20 years | 74/272 (27.2%) | 1.12 (1.07–1.17) b | <0.001 | 197/385 (51.2%) | 1.13 (1.08–1.17) b | <0.001 | 187/429 (43.6%) | 1.12 (1.08–1.16) b | <0.001 | |
| 21–40 years | 74/153 (48.4%) | 1.00 (0.96–1.06) b | 0.88 | 118/152 (77.6%) | 1.01 (0.97–1.05) b | 0.59 | 81/129 (62.8%) | 0.98 (0.94–1.02) b | 0.33 | |
| Gender | Male | 69/241 (28.6%) | 1.0 (reference) | - | 149/304 (49.0%) | 1.0 (reference) | - | 119/288 (41.3%) | 1.0 (reference) | - |
| Female | 104/303 (34.3%) | 1.17 (0.76–1.79) | 0.49 | 195/374 (52.1%) | 0.99 (0.73–1.34) | 0.94 | 191/391 (48.9%) | 1.18 (0.83–1.68) | 0.37 | |
| Slept under a LLTIN the prior evening | No | 111/335 (33.1%) | 1.0 (reference) | - | 244/479 (50.9%) | 1.0 (reference) | - | 161/332 (48.5%) | 1.0 (reference) | - |
| Yes | 62/209 (29.7%) | 0.88 (0.57–1.37) | 0.58 | 100/199 (50.3%) | 1.09 (0.69–1.70) | 0.72 | 149/347 (42.9%) | 0.83 (0.59–1.18) | 0.30 | |
| Wealth index tertiles | Lowest | 68/183 (37.2%) | 1.0 (reference) | - | 114/230 (49.6%) | 1.0 (reference) | - | 88/229 (38.4%) | 1.0 (reference) | - |
| Middle | 55/184 (29.9%) | 0.77 (0.47–1.26) | 0.29 | 113/221 (51.1%) | 1.12 (0.67–1.86) | 0.67 | 116/231 (50.2%) | 1.64 (1.11–2.41) | 0.01 | |
| Highest | 50/177 (28.3%) | 0.77 (0.48–1.22) | 0.26 | 117/227 (51.5%) | 1.08 (0.62–1.86) | 0.79 | 106/219 (48.4%) | 1.48 (0.97–2.26) | 0.07 | |
| Results of blood slide | Negative | 146/477 (30.6%) | 1.0 (reference) | - | 294/592 (49.7%) | 1.0 (reference) | - | 172/350 (49.1%) | 1.0 (reference) | - |
| Positive | 27/67 (40.3%) | 2.11 (1.16–3.81) | 0.01 | 50/86 (58.1%) | 1.27 (0.74–2.17) | 0.39 | 138/329 (42.0%) | 0.93 (0.66–1.31) | 0.67 | |
a Odds ratio controlling for repeated measures in the same household
b Odds ratio expressed per 1 year increase in age
Discussion
In this study, we evaluated the prevalence of key malaria indicators and associated factors in three different epidemiological settings in Uganda. The prevalence of anemia was predominant in the youngest children at all sites and did not correlate well with the level of transmission intensity. Severe anemia was uncommon at all sites. The prevalence of parasitemia was highest in Nagongera, the highest transmission site, but results were similar in Walukuba and Kihihi, despite an almost ten-fold difference in aEIR. The relationship between age and parasite prevalence was similar at all three sites, increasing with age until peaking by 11 years and then decreasing with age. Seroprevalence and seroconversion rates of antibodies to AMA-1 appeared to best reflect differences in transmission intensity between the three sites. In contrast, seropositivity of antibodies to MSP-119 did not follow a consistent pattern with age, and the seroconversion rate was unexpectedly low in Nagongera.
Despite the recent increase in donor financing and focus on scaling-up of available malaria control interventions, including ITNs, IRS, IPTp, and treatment with ACTs, coverage levels remain far below targets in Uganda. Although ownership and usage of ITNs has increased substantially in Uganda in the past decade, and ITN distribution has been extended beyond the traditional target groups of children under-five and pregnant women with the recent shift in policy to universal coverage, we found that ≤ 35% of households in the 3 study sites own enough ITNs to cover the household population (one ITN per two residents). Fortunately, Uganda is currently undertaking a national ITN distribution campaign aiming to achieve universal net coverage for the whole country. The same is true for IRS, which was reinstated as a malaria control measure in Uganda in 2006, after a hiatus of about 40 years. Supported by the US President’s Malaria Initiative and other partners, Uganda’s IRS strategy has emphasized implementation in epidemic-prone areas, high-risk situations, such as camps for internally displaced persons or refugees, and more recently, high-transmission areas [32]. But resources for IRS are limited and only a few districts in Uganda benefit from this control strategy. Similarly, coverage of IPTp remains low. We found that the proportion of mothers who received ≥ 2 doses of SP for IPTp during their last pregnancy ranged from 35–53% in the 3 sites; this is below targets, but higher than the estimate of national IPTp coverage (25%) measured in the 2011 DHS survey. On a more positive note, the majority (≥ 75%) of febrile children under-five received ACTs for antimalarial treatment, consistent with results from recent national surveys, suggesting increased access and availability of ACTs in Uganda.
Although the etiology of anemia in tropical areas is multifactorial, P. falciparum malaria is commonly associated with anemia in children living in holoendemic malaria areas [21,33–35]. We found that anemia was age-dependent at the 3 sites, as reported in other studies [36]. However, the prevalence of anemia did not correlate with the transmission intensity of the sites, and in fact, was highest in Walukuba, the site with lowest transmission. Reasons for this finding are unclear although schistosomasis or hookworm infection may have contributed. There is a complex interplay between anemia, malaria parasitemia, red blood cell disorders, intestinal helminths and malnutrition, however available data seems to suggest that malaria parasitemia is the significant risk factor for anaemia [37,38]. Parasitemia was an independent predictor of anemia at the higher transmission sites of Kihihi and Nagongera, suggesting that malaria plays a role in the burden of anemia in higher transmission settings. This finding is supported by prior studies demonstrating that children living in areas with very high malaria transmission carry a markedly higher burden of anemia and febrile malaria episodes [39,40]. However, we found that severe anemia (hemoglobin < 8.0 g/dL) was uncommon, even in Nagongera, the highest transmission site. In Uganda, the prevalence of anemia, including moderate to severe anemia, in children under-five has declined steadily since 2000, despite the persisting burden of malaria in the country. Although childhood anemia remains an important metric of overall child health, it is possible that malaria may no longer be the primary contributor to anemia in Ugandan children in all settings, raising the question of whether anemia should continue to be used as a metric of malaria-specific disease burden.
Parasite prevalence, particularly in children aged 2–10 years, is a commonly used measure of malaria transmission intensity and disease burden across a range of endemicities [22,23]. However, parasite prevalence is affected by seasonal variation in transmission levels, survey timing, and peak transmission seasons, and may be best considered a short-term measure of malaria [24]. In addition, parasite prevalence has a complex relationship with age and may be influenced by host immunity and use of antimalarial drugs. We found that prevalence of asexual parasitemia was significantly higher in Nagongera, but was comparable in Walukuba and Kihihi, despite the difference in aEIR in the two sites, suggesting that parasite prevalence did not reflect the difference in transmission at the two lower intensity sites. Furthermore, we found that compared to previous surveys parasite prevalence appears to be increasing in Nagongera, decreasing in Walukuba, and is relatively unchanged in Kihihi. Measuring malaria parasite prevalence over time is challenging because malaria transmission dynamics are sensitive to climatic variability [41] and are heterogeneous over small areas [42], complicating interpretation of trends. Fluctuations in climate patterns over several years may also contribute to similar fluctuations in parasite prevalence levels (inter-annual variation) which could mask successes or lapses in malaria control efforts. Thus, although parasite prevalence is arguably the most direct measure of malaria burden, it has several limitations and may not be able to accurate classify the full spectrum of transmission intensity or track temporal changes.
Serological markers are useful adjunct measures of transmission as antibodies can persist for months or years after infection, thereby smoothing out the effects of seasonal or unstable malaria transmission [43] and represent cumulative exposure to infection. At our study sites, antibodies to AMA-1 consistently increased with increasing aEIR with a good correlation between seroconversion rates and measured aEIR. AMA-1 seropositivity increased with age, reaching a higher peak at a younger age as the level of transmission intensity increased. These results support the utility of the prevalence, magnitude and rate of acquisition of antibodies to AMA-1 as a reliable marker of the level of transmission as has been observed previously [22,30]. However, in this setting, antibody responses to MSP-119 did not follow the same pattern; antibody prevalence decreased in the first 5 years of life and the seroconversion rate was surprisingly low for the high transmission site of Nagongera. The former may be due to a combination of short-term fluctuations in transmission and high density parasite infections in some young children. Antibody responses to MSP-119 have recently been shown to be more affected by seasonal changes in transmission [44]. The unexpectedly low seroconversion rate to MSP-119 in Nagongera is consistent with observations in other hyperendemic settings in Uganda and elsewhere [45]. The reasons for this are unclear but a possible explanation may be down-regulation of the immune response at very high transmission levels.
This study was conducted in areas with varying malaria transmission, and the findings may apply in areas of similar transmission patterns. The concurrent measurement of aEIR, parasite prevalence and serology enabled us to compare malariometric indices across different endemicity settings. However, studies were conducted at only 3 sites and we could not assess the trends nor draw firm conclusions on causality because of the cross sectional design. Nonetheless, such surveys are important for tracking temporal trends of malariometric indices and could be used to measure the impact of control interventions.
In summary, we found that coverage of malaria control interventions remains below targets in these 3 sites in Uganda. Although anemia and parasite prevalence are widely used indicators of the burden of malaria, we found that they did not directly correlate with transmission intensity in our study sites. Seroprevalence of AMA-1 was a better marker of infection risk and disease burden and may be a better indicator than anemia or parasitemia for monitoring and evaluation of trends in malaria burden and the impact of malaria control activities.
Supporting Information
(DOC)
Acknowledgments
We would like to thank the study team of Winnie Nuwagaba, Ntege David, Gama Stephen, Omara Joseph, Bampinga Ronald, Bukirwa Angella, and Were Moses. We acknowledge Isaac Ssewanyana and Roma Sobieski for conducting the ELISA assays. We thank all those in the study communities for participating in the study. We acknowledge the Infectious Diseases Research Collaboration (IDRC) for administrative and technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding was provided by the National Institutes of Health as part of the International Centers of Excellence in Malaria Research (ICMER) program (U19AI089674). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World malaria report 2010, World Health Organization, (2010) Available: http://www.who.int/malaria/world_malaria_report_2010/.
- 2. Aregawi MW, Ali AS, Al-Mafazy AW, Molteni F, Katikiti S, Warsame M, et al. (2011) Reductions in malaria and anaemia case and death burden at hospitals following scale-up of malaria control in Zanzibar, 1999–2008. Malar J 10: 46 1475-2875-10-46 [pii]; 10.1186/1475-2875-10-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnes KI, Durrheim DN, Little F, Jackson A, Mehta U, Allen E, et al. (2005) Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med 2: e330 05-PLME-RA-0166R2 [pii] 10.1371/journal.pmed.0020330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ceesay SJ, Casals-Pascual C, Erskine J, Anya SE, Duah NO, Fulford AJ, et al. (2008) Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet 372: 1545–1554. 10.1016/S0140-6736(08)61654-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karema C, Aregawi MW, Rukundo A, Kabayiza A, Mulindahabi M, Fall IS, et al. (2012) Trends in malaria cases, hospital admissions and deaths following scale-up of anti-malarial interventions, 2000–2010, Rwanda. Malar J 11: 236 1475-2875-11-236 [pii] 10.1186/1475-2875-11-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Meara WP, Bejon P, Mwangi TW, Okiro EA, Peshu N, Snow RW, et al. (2008) Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet 372: 1555–1562. S0140-6736(08)61655-4 [pii] 10.1016/S0140-6736(08)61655-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Otten M, Aregawi M, Were W, Karema C, Medin A, Bekele W, et al. (2009) Initial evidence of reduction of malaria cases and deaths in Rwanda and Ethiopia due to rapid scale-up of malaria prevention and treatment. Malar J 8: 14 1475-2875-8-14 [pii] 10.1186/1475-2875-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Meara WP, Mangeni JN, Steketee R, Greenwood B (2010) Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis 10: 545–555. S1473-3099(10)70096-7 [pii] 10.1016/S1473-3099(10)70096-7 [DOI] [PubMed] [Google Scholar]
- 9. Okiro EA, Bitira D, Mbabazi G, Mpimbaza A, Alegana VA, Talisuna AO, et al. (2011) Increasing malaria hospital admissions in Uganda between 1999 and 2009. BMC Med 9: 37 1741-7015-9-37 [pii] 10.1186/1741-7015-9-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talisuna A, Adibaku S, Dorsey G, Kamya MR, Rosenthal PJ (2011) Malaria in Uganda: Challenges to control on the long road to elimination. II. The path forward. Acta Trop. S0001–706X(11)00196–3 [pii] 10.1016/j.actatropica.2011.06.013 [DOI] [PMC free article] [PubMed]
- 11.Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, et al. (2011) Malaria in Uganda: Challenges to control on the long road to elimination I. Epidemiology and current control efforts. Acta Trop. S0001–706X(11)00061–1 [pii] 10.1016/j.actatropica.2011.03.004 [DOI] [PMC free article] [PubMed]
- 12.World malaria report 2013, World Health Organization (2013) Available: http://www.who.int/entity/malaria/publications/world_malaria_report_2013/report/en/index.html.
- 13.Uganda Demographic and Health Survey 2011, available at (www.ubos.org/onlinefiles/uploads/ubos/UDHS/UDHS2011.pdf).
- 14.Uganda Demographic and Health Survey 2006, available at (dhsprogram.com/pubs/pdf/FR194/FR194.pdf).
- 15. Kigozi R, Baxi SM, Gasasira A, Sserwanga A, Kakeeto S, Nasr S, et al. (2012) Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS One 7: e42857 PONE-D-12-10962 [pii]. 10.1371/journal.pone.0042857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bukirwa H, Yau V, Kigozi R, Filler S, Quick L, Lugemwa M, et al. (2009) Assessing the impact of indoor residual spraying on malaria morbidity using a sentinel site surveillance system in Western Uganda. Am J Trop Med Hyg 81: 611–614. 81/4/611 [pii] 10.4269/ajtmh.2009.09-0126 [DOI] [PubMed] [Google Scholar]
- 17. Jagannathan P, Muhindo MK, Kakuru A, Arinaitwe E, Greenhouse B, Tappero J, et al. (2012) Increasing incidence of malaria in children despite insecticide-treated bed nets and prompt anti-malarial therapy in Tororo, Uganda. Malar J 11: 435 1475-2875-11-435 [pii] 10.1186/1475-2875-11-435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burkot TR, Graves PM (1995) The value of vector-based estimates of malaria transmission. Ann Trop Med Parasitol 89: 125–134. [DOI] [PubMed] [Google Scholar]
- 19. Akhwale WS, Lum JK, Kaneko A, Eto H, Obonyo C, Bjorkman A, et al. (2004) Anemia and malaria at different altitudes in the western highlands of Kenya. Acta Trop 91: 167–175. S0001706X04000737 [pii]. 10.1016/j.actatropica.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 20. Korenromp EL, Armstrong-Schellenberg JR, Williams BG, Nahlen BL, Snow RW (2004) Impact of malaria control on childhood anaemia in Africa—a quantitative review. Trop Med Int Health 9: 1050–1065. TMI1317 [pii] 10.1111/j.1365-3156.2004.01317.x [DOI] [PubMed] [Google Scholar]
- 21. Mathanga DP, Campbell CH Jr., Vanden Eng J, Wolkon A, Bronzan RN, Malenga GJ, et al. (2010) Comparison of anaemia and parasitaemia as indicators of malaria control in household and EPI-health facility surveys in Malawi. Malar J 9: 107 1475-2875-9-107 [pii] 10.1186/1475-2875-9-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guerra CA, Hay SI, Lucioparedes LS, Gikandi PW, Tatem AJ, Noor AM, et al. (2007) Assembling a global database of malaria parasite prevalence for the Malaria Atlas Project. Malar J 6: 17 1475-2875-6-17 [pii] 10.1186/1475-2875-6-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hay SI, Snow RW (2006) The malaria Atlas Project: developing global maps of malaria risk. PLoS Med 3: e473 06-PLME-HIA-0393R2 [pii] 10.1371/journal.pmed.0030473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SL, Carneiro I, et al. (2005) Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci U S A 102: 5108–5113. 0408725102 [pii] 10.1073/pnas.0408725102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bretscher MT, Supargiyono S, Wijayanti MA, Nugraheni D, Widyastuti AN, Lobo NF, et al. (2013) Measurement of Plasmodium falciparum transmission intensity using serological cohort data from Indonesian schoolchildren. Malar J 12: 21 1475-2875-12-21 [pii] 10.1186/1475-2875-12-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Estevez PT, Satoguina J, Nwakanma DC, West S, Conway DJ, Drakeley CJ (2011) Human saliva as a source of anti-malarial antibodies to examine population exposure to Plasmodium falciparum. Malar J 10: 104 1475-2875-10-104 [pii] 10.1186/1475-2875-10-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stewart L, Gosling R, Griffin J, Gesase S, Campo J, Hashim R, et al. (2009) Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS One 4: e6083 10.1371/journal.pone.0006083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kilama M, Smith DL, Hutchinson R, Kigozi R, Yeka A, Lavoy G, et al. (2014) Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J 13: 111 1475-2875-13-111 [pii] 10.1186/1475-2875-13-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roll Back Malaria (2010) Saving Lives with Malaria Control: Counting Down to the Millenium Development Goals. Available: http://www.rbm.who.int/ProgressImpactSeries/docs/report3-en.pdf.
- 30. Corran P, Coleman P, Riley E, Drakeley C (2007) Serology: a robust indicator of malaria transmission intensity? Trends Parasitol 23: 575–582. S1471-4922(07)00277-2 [pii] 10.1016/j.pt.2007.08.023 [DOI] [PubMed] [Google Scholar]
- 31. Filmer D, Pritchett LH (2001) Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography 38: 115–132. [DOI] [PubMed] [Google Scholar]
- 32.Uganda Malaria Control Strategic Plan 2005/6–2009/10. Available: health.go.ug/mcp/index2.html.
- 33. Desai MR, Terlouw DJ, Kwena AM, Phillips-Howard PA, Kariuki SK, Wannemuehler KA, et al. (2005) Factors associated with hemoglobin concentrations in pre-school children in Western Kenya: cross-sectional studies. Am J Trop Med Hyg 72: 47–59. 72/1/47 [pii]. [PubMed] [Google Scholar]
- 34. Owusu-Agyei S, Fryauff DJ, Chandramohan D, Koram KA, Binka FN, Nkrumah FK, et al. (2002) Characteristics of severe anemia and its association with malaria in young children of the Kassena-Nankana District of northern Ghana. Am J Trop Med Hyg 67: 371–377. [DOI] [PubMed] [Google Scholar]
- 35. Reithinger R, Ngondi JM, Graves PM, Hwang J, Getachew A, Jima D (2013) Risk factors for anemia in children under 6 years of age in Ethiopia: analysis of the data from the cross-sectional Malaria IndicatorSurvey, 2007. Trans R Soc Trop Med Hyg 107: 769–776. trt096 [pii] 10.1093/trstmh/trt096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Premji Z, Hamisi Y, Shiff C, Minjas J, Lubega P, Makwaya C (1995) Anaemia and Plasmodium falciparum infections among young children in an holoendemic area, Bagamoyo, Tanzania. Acta Trop 59: 55–64. 0001706X9400079G [pii]. [DOI] [PubMed] [Google Scholar]
- 37. Green HK, Sousa-Figueiredo JC, Basanez MG, Betson M, Kabatereine NB, Fenwick A, et al. (2011) Anaemia in Ugandan preschool-aged children: the relative contribution of intestinal parasites and malaria. Parasitology 138: 1534–1545. S0031182011001016 [pii] 10.1017/S0031182011001016 [DOI] [PubMed] [Google Scholar]
- 38. Osterbauer B, Kapisi J, Bigira V, Mwangwa F, Kinara S, Kamya MR, et al. (2012) Factors associated with malaria parasitaemia, malnutrition, and anaemia among HIV-exposed and unexposed Ugandan infants: a cross-sectional survey. Malar J 11: 432 1475-2875-11-432 [pii] 10.1186/1475-2875-11-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lusingu JP, Vestergaard LS, Mmbando BP, Drakeley CJ, Jones C, Akida J, et al. (2004) Malaria morbidity and immunity among residents of villages with different Plasmodium falciparum transmission intensity in North-Eastern Tanzania. Malar J 3: 26 1475-2875-3-26 [pii]. 10.1186/1475-2875-3-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maxwell CA, Chambo W, Mwaimu M, Magogo F, Carneiro IA, Curtis CF (2003) Variation of malaria transmission and morbidity with altitude in Tanzania and with introduction of alphacypermethrin treated nets. Malar J 2: 28 1475-2875-2-28 [pii]. 10.1186/1475-2875-2-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ye Y, Madise N, Ndugwa R, Ochola S, Snow RW (2009) Fever treatment in the absence of malaria transmission in an urban informal settlement in Nairobi, Kenya. Malar J 8: 160 1475-2875-8-160 [pii] 10.1186/1475-2875-8-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bejon P, Williams TN, Liljander A, Noor AM, Wambua J, Ogada E, et al. (2010) Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. PLoS Med 7: e1000304 10.1371/journal.pmed.1000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Riley E, Wagner G, Roper C (1996) Estimating the force of malaria infection. Parasitol Today 12: 410–411. 0169475896806338 [pii]. [DOI] [PubMed] [Google Scholar]
- 44. Supargiyono S, Bretscher MT, Wijayanti MA, Sutanto I, Nugraheni D, Rozqie R, et al. (2013) Seasonal changes in the antibody responses against Plasmodium falciparum merozoite surface antigens in areas of differing malaria endemicity in Indonesia. Malar J 12: 444 1475-2875-12-444 [pii] 10.1186/1475-2875-12-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Proietti C, Pettinato DD, Kanoi BN, Ntege E, Crisanti A, Riley EM, et al. (2011) Continuing intense malaria transmission in northern Uganda. Am J Trop Med Hyg 84: 830–837. 84/5/830 [pii] 10.4269/ajtmh.2011.10-0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.