Abstract
Objective
Chromium treatment has been shown to improve mood, appetite, and glucose regulation in various psychiatric and medical patient populations. The authors propose that chromium may be useful in the treatment of binge eating disorder (BED).
Method
Twenty-four overweight adults with BED were enrolled in a 6-month double-blind placebo-controlled trial and randomly assigned to receive either 1000mcg chromium/day (“high dose”; n=8) or 600mcg chromium/day (“moderate dose”; n=9) as chromium picolinate or placebo (n=7). Mixed linear regression models were used to estimate mean change in binge frequency and related psychopathology, weight, symptoms of depression, and fasting glucose.
Results
Fasting glucose was significantly reduced in both chromium groups compared to the placebo group; similarly, numerically, but not significantly, greater reductions in binge frequency, weight, and symptoms of depression were observed in those treated with chromium versus placebo, although statistical power was limited in this pilot trial. For fasting glucose, the findings suggest a dose response with larger effects in the high dose compared to moderate dose group.
Conclusion
These initial findings support further larger trials to determine chromium’s efficacy in maintaining normal glucose regulation, reducing binge eating and related psychopathology, promoting modest weight loss, and reducing symptoms of depression in individuals with BED. Studies designed to link the clinical effects of chromium with changes in underlying insulin, serotonin, and dopamine pathways may be especially informative. If efficacious, chromium supplementation may provide a useful, low-cost alternative to or augmentation strategy for selective serotonin reuptake inhibitors, which have partial efficacy in BED. ClinicalTrials.gov NCT00904306.
Keywords: binge eating disorder, chromium, depression, glucose, weight
INTRODUCTION
Binge eating is defined as the consumption of an unusually large amount of food coupled with a feeling of loss of control over eating. Binge eating disorder (BED), the most common eating disorder, is characterized by recurrent episodes of binge eating in the absence of regular inappropriate compensatory behaviors [1]. Approximately 4% of Americans suffer with BED in their lifetime [2], and binge eating is increasingly being recognized in youth, as well [3, 4] BED confers additional serious psychiatric and medical risks including depression, obesity, and metabolic syndrome [5, 6]. The impending inclusion of BED as a distinct diagnostic category in DSM-5 [7] substantiates its significant mental health relevance and portends an increasing demand for research to improve treatment and clinical outcomes [7, 8]. Current pharmacological and psychological therapies for BED have merit but are inadequate in achieving binge eating abstinence as well as metabolic stabilization. Furthermore, our understanding of BED treatment is limited because of the short treatment and follow-up duration in most studies and because of high drop-out rates in prior clinical studies, often due to the side-effects of these and similar types of medications [9, 10]. Thus, there is a critical need for novel interventions for BED that are sustainable, lead to abstinence from binge eating, promote effective weight regulation in patients who are metabolically at risk, and have less severe side effect profiles.
Chromium is an essential mineral found in foods such as whole grain cereals and bread, lean meats, cheeses, and some spices. Chromium directly enhances insulin [11, 12] and serotonergic (5HT) [13, 14] activity and may also have downstream effects on dopaminergic (DA) signaling – all three neurotransmitters share a common protein kinase pathway involved in the central control of food intake and energy homeostasis [15-17], and 5HT and DA receptors are linked via functional heterocomplexes [18, 19]. In human studies, results of dietary chromium supplementation for treating symptoms related to binge eating have been mixed. In some studies, chromium supplementation has been found to improve glucose regulation [20] and attenuate weight gain [21, 22]; to improve appetite and mood dysregulation in depressed patients [23-26]; to reduce food intake, hunger, and fat cravings in overweight women who crave carbohydrates [27], and to improve cognitive inhibition in adults with mild cognitive impairment.[28] Food cravings [29-32] and negative affect [33, 34] have been identified as instrumental triggers for binge eating, and high disinhibition is common among individuals who binge eat [35, 36] and is associated with poor treatment outcome.[37] On the other hand, other studies have failed to find any effect of chromium supplementation on glucose metabolism or body weight [38, 39]. Across studies, the treatment protocol has varied, and the optimal dosage level and treatment duration for efficacy remain unknown. In the present 6-month placebo-controlled pilot study, we evaluated the effect of high- and moderate-dose chromium supplementation on binge eating and related psychopathology, weight, symptoms of depression, and plasma glucose concentration in overweight individuals with BED. In conducting this pilot study we sought to 1) determine feasibility and acceptability of the intervention, 2) assess side effects, and 3) test the preliminary hypotheses that high dose chromium supplementation would be associated with greater reductions in binge eating, fasting glucose, weight, and symptoms of depression than placebo.
METHOD
Participants
Forty-three participants recruited from the community by electronic and printed advertisements underwent preliminary screening and diagnostic interview to assess eligibility. Eligible participants currently met DSM-IV criteria for BED and reported no current suicidal or homicidal intent or other psychiatric condition that required acute intervention. The exclusion criteria were: 1) body mass index (BMI) <25 (underweight or normal weight) or >45 (severely obese); 2) age <18 or >60 years; 3) pregnant, planning on becoming pregnant during the study period, or lactating; 4) current chromium use; 5) current use of insulin or other medications to control glucose metabolism; 6) current use of medications known to significantly influence appetite or weight (i.e., over-the-counter appetite suppressants that contain phentermine or sibutramine, atypical antipsychotic agents with high weight gain liability [i.e., olanzapine, risperidone], prednisone, etc.; 7) fasting glucose level > 126 mg/dL; and 8) creatinine level > 1.0 for women or > 1.2 for men. To increase ecological validity of our study, we sought to include participants on current stable antidepressant therapy. However, scant data were available regarding potential drug-chromium interactions [40]. After considering the available data, the University of North Carolina (UNC) Biomedical Institutional Review Board approved the study with the additional exclusion criterion of “current psychotropic medication use other than stable monotherapy involving citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, or sertraline.” After complete description of the study to the subjects, written informed consent was obtained.
Study Design
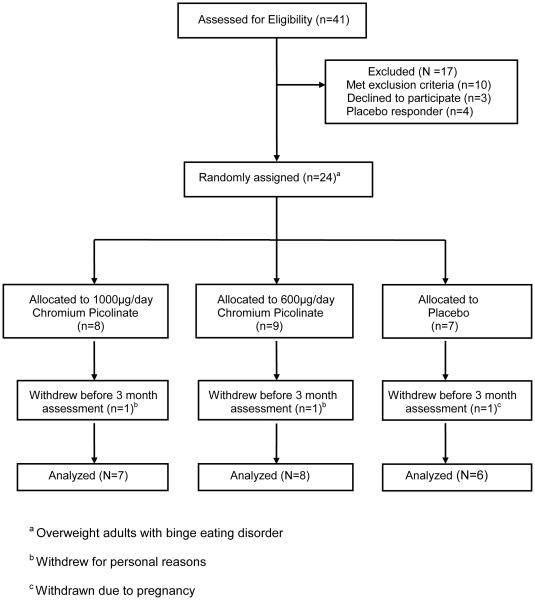
Twenty-eight (of the 41 screened) eligible participants underwent a 1-month placebo run-in, after which four participants were excluded as “placebo responders” based on previously established criteria [41] (see CONSORT Diagram, Figure 1). The remaining 24 participants were randomized in a double-blind manner to one of three treatment arms: high dose (1000mcg chromium (Cr)/day) as chromium picolinate (CrPic), moderate dose (600mcg Cr/day) as CrPic, or placebo. The chromium doses were chosen in light of prior studies in non-BED populations. This literature strongly suggested that low doses of chromium (< 400 mcg/day) could be sufficient for upregulating insulin binding and improving fasting glucose [42], moderate doses (600 mcg/day) could be sufficient to reduce depressive symptoms and cravings [23, 24], but 1000 mcg/day would be necessary to prevent weight gain [21, 43]. Because 1) avoidance of weight gain is such a critical factor in compliance and retention in overweight individuals seeking BED treatment, and 2) there is a theoretical concern of prolonged high dose exposure, establishing the minimum effective dose of chromium for binge abstinence and stabilization of metabolic parameters is an important long-term objective of our research. This study was our first step toward this long-term goal.
Figure 1.
CONSORT Diagram
After randomization, participants completed four major study visits (pre-treatment, 3-month mid-treatment, 6-month post-treatment, and 3-month follow-up), with intervening monthly visits to dispense medication and monitor symptoms. Prior to each visit, participants fasted overnight, then presented to the UNC Clinical and Translational Research Center where they completed questionnaires, an oral glucose tolerance test, and body composition measurement. Of the 24 randomized participants, 19 completed all study visits, three withdrew prior to completing the 3-month assessment, and two withdrew prior to completing the 6-month assessment. Four of those that withdrew early did so for personal reasons; one was withdrawn due to pregnancy. One of the ‘completers’ was on a stable SSRI regimen and experienced no adverse or otherwise notable effects.
Measures
Psychological Variables. BED diagnosis was determined using the Structured Clinical Interview for DSM Disorders (SCID-I/P, with Psychotic Screen) [44] and confirmed with the Eating Disorders Interview [45]. Treatment-related changes in binge eating frequency (“Over the past four weeks [28 days], have there been any times when you have felt that you have eaten what other people would regard as an unusually large amount of food given the circumstances?” “If yes, how many such episodes have you had over the past 4 weeks?”) and other aspects of disordered eating behavior were assessed using the Eating Disorders Examination – Questionnaire (EDE-Q) [46], which yields a global score and four subscale scores (restraint, eating concerns, weight concerns, and shape concerns), each with a range of 0 to 6. Symptoms of depression were assessed with the 16-item Quick Inventory of Depressive Symptomatology (Self-Report; QIDS-SR16) [47].
Biomarker Variables. Weight and height were measured using a digital scale with stadiometer. At McLendon Clinical Laboratories (UNC Hospitals), plasma glucose was assayed using a Vitros 5,1 FS Chemistry System (Ortho Clincical Diagnostics) and glycated hemoglobin (HbA1c) was assayed using a Hitachi 912 Chemistry Analyzer.
Compliance. Participants were asked to return all unused pills at each monthly study visit. For this pilot study we were interested in tracking this measure to assist in planning future studies; we did not withdraw any participants based on compliance.
Side Effects and Adverse Events. At each monthly visit, participants completed a symptom checklist and brief interview to evaluate side effects and related concerns. Participants rated the severity of 15 symptoms using a 4-point scale (1=absent, 2=slight, 3=moderate, 4=severe) and to describe any distressing or uncomfortable physical or mental changes over the past 4 weeks. The UNC School of Medicine Data Safety Monitoring Board monitored participant safety.
Data Analysis
Descriptive statistics included percent and count frequencies for categorical variables and means and standard deviations for continuous variables. Treatment group (high dose, moderate dose, placebo) differences at baseline were evaluated using the Kruskal-Wallis statistic for continuous variables and a chi-square statistic (df) for categorical variables, using the Benjamini-Hochberg false discovery rate (FDR) procedure to adjust p-values.
Mixed effects linear regression models were used to estimate a mean change over the 6-month active treatment period in binge frequency (episodes in the past 28 days), weight, symptoms of depression, and fasting glucose. Each regression model produced an estimate of average change in outcome for a one month change in time. To estimate the change in outcome over time by treatment group and any relevant group differences, time (month) and treatment group functioned as covariates including an interaction between the two variables. The model fit process included use of the AIC statistic and visual examination of residuals for homogeneity. A mixed effects model with a random intercept was used to account for individual variation in the outcome at baseline. In secondary analyses, a similar process was followed for the EDEQ total (global) score and four subscale scores (restraint, and eating, shape, and weight concerns). Given the exploratory nature of this pilot study, we did not adjust the p value for significance (alpha = 0.05, 2-tailed) for multiple comparisons; however, we limited pairwise comparisons to high dose vs. placebo and moderate dose vs. placebo. This approach allows for broader consideration of effects for future study, but also inflates type I error. Further, guided by influence diagnostics (Cook’s D) for binge frequency and weight as well as post-hoc evaluation of pill count compliance, we also conducted sensitivity analyses after excluding one participant from the high dose group who was deemed an “outlier”. Specifically, this individual failed to return unused pills for 3 months of the 6-month intervention and her Cook’s D values for regression analyses with binge eating (0.51) and weight (0.49) as outcomes were 2-fold greater than those of any other participant.
Statistical analyses were done with SAS/STAT software, Version 9.2 of the SAS System for Windows XP.[48] Graphics were handled in the ggplot2 package in R software.[49]
RESULTS
Characteristics of the Sample
Table 1 summarizes the demographic and clinical characteristics of the randomized sample (n=24), which was comprised mostly of women (83.3%) who self-identified as white (87.5%), were obese [mean (SD) BMI = 34.2 (5.4)], and had a mean (SD) age of 36.6 (10.6) years. The duration of illness among participants ranged from 1 to 45 years (mean = 16.0, median = 10.5). Overall, participants were normoglycemic [mean (SD) fasting plasma glucose = 90.3 (9.4) mg/dL] and, as a group, they exhibited a mild level of depression [mean (SD) QIDS-SR16 = 6.8 (3.8). There were no statistically significant differences between treatment groups on any of these baseline measures.
Table I.
Demographic and Clinical Characteristics of the Randomized Sample at Baseline
| Measure | High Dose CrPic (n=8) |
Moderate Dose CrPic (n=9) |
Placebo (n=7) |
Test Statistica | df | pb | |||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||||
| Female | 6 | 75 | 7 | 78 | 7 | 100 | 2.00 | 2 | 0.93 |
| White | 8 | 100 | 7 | 78 | 6 | 86 | 2.96 | 4 | 0.93 |
| Mean | SD | Mean | SD | Mean | SD | ||||
| Age (years) | 41.4 | 8.5 | 35.1 | 12.4 | 37.9 | 10.8 | 1.33 | 2 | 0.93 |
| Weight (kg) | 99.9 | 21.8 | 96.3 | 26.8 | 100.0 | 16.1 | 0.53 | 2 | 0.93 |
| Body mass index (kg/m2) | 34.9 | 4.1 | 33.5 | 7.0 | 34.3 | 5.4 | 0.15 | 2 | 0.93 |
| Fasting plasma glucose (mg/dL) | 90.6 | 10.0 | 92.8 | 13.8 | 91.4 | 8.4 | 0.03 | 2 | 0.99 |
| Glycated hemoglobin (HbA1c, %) | 5.4 | 0.3 | 5.7 | 0.5 | 5.4 | 0.3 | 0.79 | 2 | 0.99 |
| QIDS-SR16 | 7.1 | 5.4 | 8.1 | 3.0 | 5.7 | 3.0 | 1.77 | 2 | 0.99 |
| Binge episodes (past 28 days) | 31.0 | 24.8 | 12.8 | 3.6 | 16.7 | 9.5 | 6.67 | 2 | 0.29 |
| EDEQ-global | 3.3 | 1.3 | 3.2 | 0.9 | 3.4 | 0.7 | 0.08 | 2 | 0.99 |
| EDEQ-eating concern | 3.0 | 1.9 | 3.0 | 1.0 | 3.2 | 1.7 | 0.03 | 2 | 0.99 |
| EDEQ-shape concern | 4.6 | 1.4 | 4.3 | 1.1 | 4.5 | 0.8 | 0.56 | 2 | 0.99 |
| EDEQ-weight concern | 4.2 | 1.5 | 3.9 | 0.9 | 4.0 | 1.3 | 1.20 | 2 | 0.99 |
| EDEQ-restraint | 1.4 (1.5) | 1.9 (1.4) | 1.8 (1.4) | 1.21 | 2 | 0.99 | |||
The test statistic is a Kruskal-Wallis statistic for continuous variables and a chi-square statistic (df) for categorical variables.
All p-values adjusted with Benjamini-Hochberg false discovery rate (FDR) procedure.
Psychological Outcomes
The results of the regression analyses for binge frequency are depicted in Figure 2 (top row). Binge frequency declined over time, with the largest magnitude reduction observed in the high dose group; yet no significant differences were found in pairwise comparisons between either the high dose group [–1.65 (0.76) binges/month] or the moderate dose group [–0.93 (0.70) binges/month] versus the placebo group [–0.97 (0.78) binges/month]. Exclusion of the “outlier” increased the magnitude of the estimate in the high dose group [–2.36 (0.72) binges/month], but the between-group differences remained non-significant.
Figure 2.
Fitted regression lines depicting change in binge eating frequency (top row), symptoms of depression (second row), weight (third row), and fasting glucose (bottom row) during 6-month treatment with high or moderate dose chromium picolinate or placebo.
The results of the regression analyses for the EDE-Q are presented in Table 2. The rate of decline in eating, shape, and weight concerns was significantly greater in the high dose group compared to the placebo group. In addition, the rate of decline in weight concerns was greater in the moderate dose group compared to the placebo group. Despite these subscale score differences, pairwise comparisons for the global EDEQ between the high dose group [–0.21 (0.07)] or the moderate dose group [–0.13 (0.07)] versus the placebo group [–0.04 (0.07)] were not significant. All of these effects remained unchanged in the sensitivity analyses.
TABLE II.
Monthly Rate of Change in Eating Disorder Examination–Questionnaire Scores
| Measure | High Dose CrPic (n=7) |
Moderate Dose CrPic (n=8) |
Placebo (n=6) |
High Dose vs. Placebo |
Moderate Dose vs. Placebo |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Mean | SD | Mean | SD | Mean | SD | t | df | p | t | df | p | |
| Global score | −0.21 | 0.07 | −0.13 | 0.07 | −0.04 | 0.07 | −1.72 | 31 | 0.10 | −0/95 | 31 | 0.36 |
| Eating concerns | −0.29 | 0.08 | −0.11 | 0.08 | −0.02 | 0.08 | −2.23 | 37 | 0.04 | −0.78 | 37 | 0.44 |
| Shape concerns | −0.24 | 0.08 | −0.16 | 0.07 | −0.01 | 0.08 | −2.08 | 37 | 0.05 | −1.45 | 37 | 0.15 |
| Weight concerns | −0.20 | 0.07 | −0.18 | 0.06 | 0.06 | 0.07 | −2.67 | 37 | 0.02 | −2.48 | 37 | 0.02 |
| Restraint | −0.13 | 0.10 | −0.01 | 0.09 | −0.06 | 0.10 | −0.55 | 37 | 0.58 | 0.37 | 37 | 0.71 |
Figure 2 (second row) depicts the results of the regression analyses for symptoms of depression. Symptoms of depression declined over time in the high dose [–0.30 (0.21)], moderate dose [–0.41 (0.19)], and placebo [–0.03 (0.21)] groups but the pairwise differences were not statistically significant. The results of the sensitivity analysis were not substantially different [i.e., –0.30 (0.22) in the high dose group]. For the single participant on stable SSRI therapy, symptoms of depression remained low throughout the study (QIDS-SR16 ≤ 3).
Biomarker Outcomes
The results of the regression analyses for weight are depicted in Figure 2 (third row). During the 6-month treatment period, 8 of 14 (57%) participants in the CrPic groups lost weight whereas all seven participants in the placebo group gained weight; however, no significant differences were found in any pairwise comparisons between either the high dose group [0.19 (0.25) kg/month] or the moderate dose group [–0.13 (0.23) kg/month] versus the placebo group [0.55 (0.25) kg/month]. In the sensitivity analysis (after excluding the “outlier”), the estimated weight change was –0.23 (0.21) kg/month in the high dose group and –0.13 (0.18) kg/month in the moderate dose group, and in pairwise comparisons both the high dose (t=–2.72, df=35, p<0.02) and low dose group (t=–2.59, df=35, p<0.02) differed significantly from the placebo group.
Figure 2 (bottom row) depicts the results of the regression analyses for glucose. The estimated mean (standard error) change in fasting glucose (mg/dL/month) was –1.08 (0.80), –0.67 (0.74), and 2.53 (0.80) for the high dose, moderate dose, and placebo groups, respectively. In pairwise comparisons, both the high dose (t=–3.19, df=35, p<0.01) and moderate dose (t=–2.93, df=35, p<0.01) groups differed significantly from the placebo group. The results of the sensitivity analysis were not substantially different [i.e., fasting glucose –1.10 (0.90) mg/dL/month in the high dose group]. Some participants experienced clinically meaningful changes in fasting glucose: among study completers, the change at 6 months ranged from −32 mg/dL (high dose group) to 53 mg/dL (placebo group).
In the overall sample, mean (SD) HbA1c was essentially unchanged at 6 months [5.6 (0.4)] compared to baseline [5.6 (0.4)]. At study entry, nine participants exhibited above normal HbA1c (range 5.7% to 6.7%, mean = 5.9%); two were randomized to receive high dose chromium (mean HbA1c = 5.8%), five to moderate dose chromium (mean HbA1c = 6.0%) and two to placebo (mean HbA1c = 5.8%). At 6 months, mean HbA1c decreased 0.4% and 0.3% in the high and moderate dose groups, respectively, and increased 0.4% in the placebo group. Six of the chromium-treated participants experienced a reduction in fasting glucose (range –32 mg/dL to 3 mg/dL), whereas fasting glucose increased in both placebo-treated subjects (3 mg/dL, 53 m/dL). Notably, the largest numeric reduction in fasting glucose (–32 mg/dL) occurred in a pre-diabetic participant assigned to high dose chromium and the largest numeric increase in fasting glucose (53 mg/dL) occurred in a pre-diabetic participant assigned to placebo.
Adverse Outcomes and Side Effects
A total of eight adverse events were recorded. All eight events consisted of elevated HbA1c level above normal; these occurred in five different participants in the placebo group. In general, side effects were low: headache was reported most frequently prior to treatment (n=2, n=4, n=3 in the high dose, moderate dose, and placebo group, respectively) and tended to decrease in the chromium-treated groups and increase in the placebo group at end-of-treatment (n=1, n=2, n=4, respectively). (See Supplemental Table for complete data).
DISCUSSION
Results of this pilot study suggest that further study of dietary chromium supplementation is warranted as the direction of effects indicates it may help curb binge eating and related psychopathology, promote modest weight loss and reductions in symptoms of depression, and maintain normal glucose regulation in overweight adults with BED. For fasting glucose, the findings suggest a dose response with the magnitude of glucose reduction being larger in the high dose CrPic group compared to moderate dose CrPic group. Similarly, in the sensitivity analyses, binge eating and weight were reduced to a greater extent in the high dose compared to the moderate dose group. Until these preliminary findings can be replicated in a larger, adequately powered study, they should be interpreted with considerable caution.
BED increases the risk of developing metabolic disturbances associated with obesity, cardiovascular disease, and type-2 diabetes [6]. During the present 6-month study, fasting glucose and weight increased significantly in the placebo-treated group (median change ~6.5 mg/dL and ~7 pounds). Notably, these biological health risk markers worsened in the placebo-treated group despite a modest reduction in binge frequency (~6 fewer per month at the end of treatment). At these rates, without intervention, a non-obese individual with good glucose control could transition to an obese, diabetic state in a relatively short period of time. Nearly 38% of our randomized sample had pre-diabetes; but, one must wonder, given the lengthy average duration of illness for our participants, why were more of them not in a pre-diabetic state or worse? None, with the exception of one participant on stable SSRI therapy, were undergoing treatment for BED prior to enrolling. One could speculate that our study attracted participants at a time when their binge eating symptoms had escalated and motivated them to seek treatment. As such, we may have captured our assessments during a critical window of time when binge eating was exerting its greatest acute impact on glucose control. Alternatively, one could surmise a threshold effect, such that our assessments were conducted during a window of time when the cumulative impact of binge eating over many years had finally begun to overwhelm homeostatic glucose mechanisms. Future studies with larger sample size could address this question directly by assessing the relation between illness duration and glucose response to CrPic treatment. Meanwhile, we note that in the present pilot study HbA1c normalized in 86% (6 of 7) of those with pre-diabetes who received chromium compared with 0% (0 of 2) of those with pre-diabetes who received placebo. Thus, our preliminary findings suggest that, independent of its impact on binge eating per se, CrPic supplementation warrants further study as an approach for forestalling the serious long-term metabolic consequences associated with BED, especially among those at greater risk.
Based on its known insulin sensitizing actions, chromium has been investigated as an alternative treatment in type-2 diabetes and other insulin-resistant conditions, but results have been mixed [20, 22, 50-54] and routine prescription of chromium for individuals with diabetes remains highly controversial [55]. Insulin sensitivity is thought to account for ~40% of the variance in the clinical response to chromium [56], but factors that account for the remaining variance are largely unknown. Neuroimaging studies demonstrate abnormalities in brain reward, emotion/attention, and sympathetic nervous system pathways [for review, [57]] and, more specifically, in 5HT and DA activity [58-60] in individuals who binge eat. Missing from the literature are studies designed to capitalize on chromium’s “triple threat” capacity to co-regulate insulin, serotonin, and dopamine by incorporating neuroimaging and biomarker methods to compare the effect of chromium supplementation in groups of individuals with differing biological risk profiles (i.e., insulin resistance vs. insulin resistance plus symptoms of depression vs. insulin resistance plus symptoms of depression plus binge eating). These types of comparative studies are feasible and justified given the high comorbidity of binge eating, depression, and diabetes [5, 61-65]; they would help build a more refined understanding of the chromium response phenotype and, ultimately, increase the utility of chromium supplementation as an individualized approach to treating the complex patient who presents with a constellation of eating, metabolic, and mood disturbances.
The major limitations of this study are the small sample size, the limited ethnic diversity of the sample, and the lack of a biological indicator of compliance such as serum or urinary chromium level. These limitations are particularly relevant when considering the results of our sensitivity analyses. The “outlier” participant, who we identified through statistical procedures and examination of pill count data, had considerable influence on the magnitude of effects for binge frequency and weight observed in the high dose group. This individual differed from the other participants in several ways: she presented with the highest overall binge frequency (80 binges in the past 28 days), was highly placebo-responsive yet continued to report a high binge frequency (30 binges in the past 28 days after the placebo run-in phase), maintained the highest binge frequency throughout treatment (40 binges in the past 28 days at end of treatment), gained 7.3kgs during the placebo run-in and a total of 21.8kgs by the end of treatment, and failed to return unused pills during 3 of the 6 treatment months. One might surmise two plausible explanations for this participant’s excessive weight gain and persistent high binge frequency: 1) non-compliance or 2) there is a binge eating frequency threshold above which high dose CrPic is ineffective. Although we cannot rule out either explanation, it is noteworthy that the participant with the next highest binge severity at study entry (60 binges in the past 28 days) was also randomized to the high dose CrPic group; this individual lost 9.7kgs and achieved abstinence (zero binges in the past 28 days) at end of treatment. A third theoretical factor could be chromium overdose; however, based on the publically available clinical trial and safety data [66], the abuse potential of chromium is low, making this explanation less likely. Future studies that include larger groups representing a range of pre-treatment binge severity and that use biological indicators of compliance will help clarify these issues.
These data encourage further examination of CrPic in the treatment of BED. Adequately-powered studies are needed to determine the efficacy of CrPic. Within these larger efficacy studies, it will be essential to identify the behavioral and biological mechanisms of action of CrPic in BED and to refine the CrPic response phenotype to maximize its efficacy within an individualized medicine approach. Future studies might explore CrPic efficacy in related disorders such as bulimia nervosa and night eating syndrome as well as address the long-term use of CrPic as a strategy for relapse prevention and/or for prevention in subthreshold binge eating. Given its capacity to influence serotonergic function, in particular, CrPic augmentation in partial responders to antidepressant therapies may be worthwhile. Once efficacy is established, then logical next steps may include studies of the comparative effectiveness of CrPic versus existing therapies for reducing core BED psychopathology and behavior.
Supplementary Material
ACKNOWLEDGMENTS
For their contributions to this project, the authors wish to thank the UNC Department of Psychiatry Eating Disorders Program clinical core for conducting clinical interviews to determine participants’ eating disorder status (T32MH076694); Michelle Scotton-Franklin, MSN, APRN, PMHNP-BC, FNP-BC, for conducting physical examinations to determine study eligibility; Dr. Monica Algars for her critique and editing of the manuscript draft; the UNC Nutrition Obesity Research Center’s Diet, Physical Activity and Body Comp Core (DK56350) and the UNC Clinical and Translational Research Center (UL1RR025747) for providing clinical research space and personnel for data collection; and Nutrition21, Inc. for providing chromium picolinate (Chromax®) and placebo.
This study was supported by an independent Young Investigator award to Dr. Brownley from the Brain & Behavior Research Foundation (formerly NARSAD). The sponsor of the study had no role in the study design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
References
- [1].American Psychiatric Association . Diagnostic and Statistical Manual for Psychiatric Disorders: Fourth Edition. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- [2].Hudson JI, , Hiripi E, Pope HG, Jr., Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–58. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tanofsky-Kraff M, Goossens L, Eddy KT, Ringham R, Goldschmidt A, Yanovski SZ, et al. A multisite investigation of binge eating behaviors in children and adolescents. J Consult Clin Psychol. 2007;75(6):901–13. doi: 10.1037/0022-006X.75.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tanofsky-Kraff M, Yanovski SZ, Wilfley DE, Marmarosh C, Morgan CM, Yanovski JA. Eating-disordered behaviors, body fat, and psychopathology in overweight and normal-weight children. J Consult Clin Psychol. 2004;72(1):53–61. doi: 10.1037/0022-006X.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reichborn-Kjennerud T, Bulik C, Sullivan P, Tambs K, Harris J. Psychiatric and medical symptoms in binge eating in the absence of compensatory behaviors. Obes Res. 2004;12:1445–54. doi: 10.1038/oby.2004.181. [DOI] [PubMed] [Google Scholar]
- [6].Hudson JI, Lalonde JK, Coit CE, Tsuang MT, McElroy SL, Crow SJ, et al. Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge-eating disorder. Am J Clin Nutr. 2010;91(6):1568–73. doi: 10.3945/ajcn.2010.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].American Psychiatric Association DSM-5 Proposed Diagnostic Criteria for Binge Eating Disorder 2010. [cited 2012 October 23] Available from: http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=372.
- [8].Wilfley DE, Bishop ME, Wilson GT, Agras WS. Classification of eating disorders: toward DSM-V. Int J Eat Disord. 2007;40(Suppl 3):S123–9. doi: 10.1002/eat.20436. [DOI] [PubMed] [Google Scholar]
- [9].Brownley KA, Berkman ND, Sedway JA, Lohr KN, Bulik CM. Binge eating disorder treatment: a systematic review of randomized controlled trials. Int J Eat Disord. 2007;40(4):337–48. doi: 10.1002/eat.20370. [DOI] [PubMed] [Google Scholar]
- [10].Berkman ND, Bulik CM, Brownley KA, Lohr KN, Sedway JA, Rooks A, et al. Management of eating disorders. Evid Rep Technol Assess (Full Rep) 2006;135:1–166. [PMC free article] [PubMed] [Google Scholar]
- [11].Davis CM, Vincent JB. Chromium oligopeptide activates insulin receptor tyrosine kinase activity. Biochemistry. 1997;36(15):4382–5. doi: 10.1021/bi963154t. [DOI] [PubMed] [Google Scholar]
- [12].McCarty MF. Enhancing central and peripheral insulin activity as a strategy for the treatment of endogenous depression--an adjuvant role for chromium picolinate? Med Hypotheses. 1994;43(4):247–52. doi: 10.1016/0306-9877(94)90075-2. [DOI] [PubMed] [Google Scholar]
- [13].Attenburrow MJ, Odontiadis J, Murray BJ, Cowen PJ, Franklin M. Chromium treatment decreases the sensitivity of 5-HT2A receptors. Psychopharmacology (Berl) 2002;159(4):432–36. doi: 10.1007/s00213-001-0960-7. [DOI] [PubMed] [Google Scholar]
- [14].Piotrowska A, Mlyniec K, Siwek A, Dybala M, Opoka W, Poleszak E, et al. Antidepressant-like effect of chromium chloride in the mouse forced swim test: involvement of glutamatergic and serotonergic receptors. Pharmacol Rep. 2008;60(6):991–5. [PubMed] [Google Scholar]
- [15].Daws LC, Avison MJ, Robertson SD, Niswender KD, Galli A, Saunders C. Insulin signaling and addiction. Neuropharmacology. 2011;61(7):1123–8. doi: 10.1016/j.neuropharm.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Papazoglou I, Berthou F, Vicaire N, Rouch C, Markaki EM, Bailbe D, et al. Hypothalamic serotonin-insulin signaling cross-talk and alterations in a type 2 diabetic model. Mol Cell Endocrinol. 2012;350(1):136–44. doi: 10.1016/j.mce.2011.12.007. [DOI] [PubMed] [Google Scholar]
- [17].Gerozissis K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur J Pharmacol. 2008;585(1):38–49. doi: 10.1016/j.ejphar.2008.01.050. [DOI] [PubMed] [Google Scholar]
- [18].Albizu L, Holloway T, Gonzalez-Maeso J, Sealfon SC. Functional crosstalk and heteromerization of serotonin 5-HT2A and dopamine D2 receptors. Neuropharmacology. 2011;61(4):770–7. doi: 10.1016/j.neuropharm.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].de Bartolomeis A, Buonaguro EF, Iasevoli F. Serotonin-glutamate and serotonin-dopamine reciprocal interactions as putative molecular targets for novel antipsychotic treatments: from receptor heterodimers to postsynaptic scaffolding and effector proteins. Psychopharmacology (Berl) 2013;225(1):1–19. doi: 10.1007/s00213-012-2921-8. [DOI] [PubMed] [Google Scholar]
- [20].Albarracin CA, Fuqua BC, Evans JL, Goldfine ID. Chromium picolinate and biotin combination improves glucose metabolism in treated, uncontrolled overweight to obese patients with type 2 diabetes. Diabetes Metab Res Rev. 2008;24(1):41–51. doi: 10.1002/dmrr.755. [DOI] [PubMed] [Google Scholar]
- [21].Martin J, Wang ZQ, Zhang XH, Wachtel D, Volaufova J, Matthews DE, et al. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with Type 2 diabetes. Diabetes Care. 2006;29:1826–32. doi: 10.2337/dc06-0254. [DOI] [PubMed] [Google Scholar]
- [22].Lydic ML, McNurland M, Bembo S, Mitchell L, Komaroff E, Gelato M. Chromium picolinate improves insulin sensitivity in obese subjects with polycystic ovary syndrome. Fertil Steril. 2006;86:243–46. doi: 10.1016/j.fertnstert.2005.11.069. [DOI] [PubMed] [Google Scholar]
- [23].Davidson JRT, Abraham K, Connor KM, McLeod MN. Effectiveness of chromium in atypical depression: A placebo-controlled trial. Biol Psychiatry. 2003;53(3):261–64. doi: 10.1016/s0006-3223(02)01500-7. [DOI] [PubMed] [Google Scholar]
- [24].Docherty JP, Sack DA, Roffman M, Finch M, Komorowski JR. A double-blind, placebo-controlled, exploratory trial of chromium picolinate in atypical depression: Effect on carbohydrate craving. J Psychiatr Pract. 2005;11:302–14. doi: 10.1097/00131746-200509000-00004. [DOI] [PubMed] [Google Scholar]
- [25].McLeod MN, Gaynes BN, Golden RN. Chromium potentiation of antidepressant pharmacotherapy for dysthymic disorder in 5 patients. J Clin Psychiatry. 1999;60(4):237–40. doi: 10.4088/jcp.v60n0406. [DOI] [PubMed] [Google Scholar]
- [26].McLeod MN, Golden RN. Chromium treatment of depression. Int J Neuropsychopharmacol. 2000;3(4):311–14. doi: 10.1017/S146114570000208X. [DOI] [PubMed] [Google Scholar]
- [27].Anton SD, Morrison CD, Cefalu WT, Martin CK, Coulon S, Geiselman P, et al. Effects of chromium picolinate on food intake and satiety. Diabetes Technol Ther. 2008;10(5):405–12. doi: 10.1089/dia.2007.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Krikorian R, Eliassen JC, Boespflug EL, Nash TA, Shidler MD. Improved cognitive-cerebral function in older adults with chromium supplementation. Nutr Neurosci. 2010;13(3):116–22. doi: 10.1179/147683010X12611460764084. [DOI] [PubMed] [Google Scholar]
- [29].Greeno C, Wing R, Shiffman S. Binge antecedents in obese women with and without binge eating disorder. Journal of Consulting and Clinical Psychology. 2000;68:95–102. [PubMed] [Google Scholar]
- [30].van der Ster Wallin G, Norring C, Holmgren S. Binge eating versus nonpurged eating in bulimics: Is there a carbohydrate craving after all? Acta Psychiatr Scand. 1994;89(6):376–81. doi: 10.1111/j.1600-0447.1994.tb01532.x. [DOI] [PubMed] [Google Scholar]
- [31].Gendall KA, Joyce PR, Sullivan PF, Bulik CM. Food cravers: characteristics of those who binge. Int J Eat Disord. 1998;23(4):353–60. doi: 10.1002/(sici)1098-108x(199805)23:4<353::aid-eat2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [32].Vanderlinden J, Dalle Grave R, Fernandez F, Vandereycken W, Pieters G, Noorduin C. Which factors do provoke binge eating? An exploratory study in eating disorder patients. Eat Weight Disord. 2004;9(4):300–5. doi: 10.1007/BF03325086. [DOI] [PubMed] [Google Scholar]
- [33].Abraham S, Beumont P. How patients describe bulimia or binge eating. Psychosom Med. 1982;12:625. doi: 10.1017/s0033291700055732. [DOI] [PubMed] [Google Scholar]
- [34].Grilo CM, Shiffman S, Carter-Campbell JT. Binge eating antecedents in normal-weight nonpurging females: Is there consistency? Int J Eat Disord. 1994;16(3):239–49. doi: 10.1002/1098-108x(199411)16:3<239::aid-eat2260160304>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- [35].Yanovski SZ. The chicken or the egg: binge eating disorder and dietary restraint. Appetite. 1995;24(3):258. doi: 10.1016/s0195-6663(95)99811-x. [DOI] [PubMed] [Google Scholar]
- [36].de Zwaan M, Mitchell JE, Seim HC, Specker SM, Pyle RL, Raymond NC, et al. Eating related and general psychopathology in obese females with binge eating disorder. Int J Eat Disord. 1994;15(1):43–52. doi: 10.1002/1098-108x(199401)15:1<43::aid-eat2260150106>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [37].Downe KA, Goldfein JA, Devlin MJ. Restraint, hunger, and disinhibition following treatment for binge-eating disorder. Int J Eat Disord. 2009;42(6):498–504. doi: 10.1002/eat.20639. [DOI] [PubMed] [Google Scholar]
- [38].Iqbal N, Cardillo S, Volger S, Bloedon LT, Anderson RA, Boston R, et al. Chromium picolinate does not improve key features of metabolic syndrome in obese nondiabetic adults. Metab Syndr Relat Disord. 2009;7(2):143–50. doi: 10.1089/met.2008.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yazaki Y, Faridi Z, Ma Y, Ali A, Northrup V, Njike VY, et al. A pilot study of chromium picolinate for weight loss. J Altern Complement Med. 2010;16(3):291–9. doi: 10.1089/acm.2009.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McLeod MN. Lifting depression : the chromium connection. Basic Health Publications; Laguna Beach, CA: 2005. [Google Scholar]
- [41].Appolinario JC, Bacaltchuk J, Sichieri R, Claudino AM, Godoy-Matos A, Morgan C, et al. A randomized, doubld-blind, placebo-controlled study of sibutramine in the treatment of binge-eating disorder. Arch Gen Psychiatry. 2003;60:1109–16. doi: 10.1001/archpsyc.60.11.1109. [DOI] [PubMed] [Google Scholar]
- [42].Anderson RA, Polansky MM, Bryden NA, Bhathena SJ, Canary JJ. Effects of supplemental chromium on patients with symptoms of reactive hypoglycemia. Metabolism. 1987;36(4):351–5. doi: 10.1016/0026-0495(87)90206-x. [DOI] [PubMed] [Google Scholar]
- [43].Volpe SL, Huang HW, Larpadisorn K, Lesser II. Effect of chromium supplementation and exercise on body composition, resting metabolic rate and selected biochemical parameters in moderately obese women following an exercise program. J Am Coll Nutr. 2001;20(4):293–306. doi: 10.1080/07315724.2001.10719050. [DOI] [PubMed] [Google Scholar]
- [44].First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/PSY SCREEN) Biometrics Research New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- [45].Fairburn C, Cooper Z. The Eating Disorders Examination (12th Edition) In: Fairburn C, Wilson G, editors. Binge-Eating: Nature, Assessment and Treatment. Guilford Press; New York: 1993. pp. 317–60. [Google Scholar]
- [46].Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord. 1994;16(4):363–70. [PubMed] [Google Scholar]
- [47].Rush A, Trivedi M, Ibrahim H, Carmody T, Arnow B, Klein D, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- [48].SAS Institute Inc. SAS/STAT® 9.2 User's Guide. SAS Institute, Inc.; Cary, NC: 2008. [Google Scholar]
- [49].Wickham H. ggplot2: Elegant graphics for data analysis. Springer; New York: 2009. [Google Scholar]
- [50].Sharma S, Agrawal RP, Choudhary M, Jain S, Goyal S, Agarwal V. Beneficial effect of chromium supplementation on glucose, HbA(1)C and lipid variables in individuals with newly onset type-2 diabetes. J Trace Elem Med Biol. 2011 doi: 10.1016/j.jtemb.2011.03.003. [DOI] [PubMed] [Google Scholar]
- [51].Ali A, Ma Y, Reynolds J, Wise JP, Sr., Inzucchi SE, Katz DL. Chromium effects on glucose tolerance and insulin sensitivity in persons at risk for diabetes mellitus. Endocr Pract. 2011;17(1):16–25. doi: 10.4158/EP10131.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cefalu WT, Rood J, Pinsonat P, Qin J, Sereda O, Levitan L, et al. Characterization of the metabolic and physiologic response to chromium supplementation in subjects with type 2 diabetes mellitus. Metabolism. 2010;59(5):755–62. doi: 10.1016/j.metabol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30(8):2154–63. doi: 10.2337/dc06-0996. [DOI] [PubMed] [Google Scholar]
- [54].Broadhurst CL, Domenico P. Clinical studies on chromium picolinate supplementation in diabetes mellitus--a review. Diabetes Technol Ther. 2006;8(6):677–87. doi: 10.1089/dia.2006.8.677. [DOI] [PubMed] [Google Scholar]
- [55].Wang ZQ, Cefalu WT. Current concepts about chromium supplementation in type 2 diabetes and insulin resistance. Curr Diab Rep. 2010;10(2):145–51. doi: 10.1007/s11892-010-0097-3. [DOI] [PubMed] [Google Scholar]
- [56].Wang ZQ, Qin J, Martin J, Zhang XH, Sereda O, Anderson RA, et al. Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metabolism. 2007;56(12):1652–5. doi: 10.1016/j.metabol.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Frank GK, Kaye WH. Current status of functional imaging in eating disorders. Int J Eat Disord. 2012;45(6):723–36. doi: 10.1002/eat.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jimerson D, Lesem M, Kaye W, Brewerton T. Low serotonin and dopamine metabolite concentrations in cerebrospinal fluid from bulimic patients with frequent binge episodes. Arch Gen Psychiatry. 1992;49:132–38. doi: 10.1001/archpsyc.1992.01820020052007. [DOI] [PubMed] [Google Scholar]
- [59].Davis C, Levitan RD, Kaplan AS, Carter J, Reid C, Curtis C, et al. Reward sensitivity and the D2 dopamine receptor gene: A case-control study of binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):620–8. doi: 10.1016/j.pnpbp.2007.09.024. [DOI] [PubMed] [Google Scholar]
- [60].Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiology & Behavior. 2008;94(1):121–35. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Smith DE, Marcus MD, Lewis CE, Fitzgibbon M, Schreiner P. Prevalence of binge eating disorder, obesity, and depression in a biracial cohort of young adults. Ann Behav Med. 1998;20(3):227–32. doi: 10.1007/BF02884965. [DOI] [PubMed] [Google Scholar]
- [62].Yanovski S, Nelson J, Dubbert B, Spitzer R. Association of binge eating disorder and psychiatric comorbidity in obese subjects. Am J Psychiatry. 1993;150:1472–79. doi: 10.1176/ajp.150.10.1472. [DOI] [PubMed] [Google Scholar]
- [63].Allison KC, Crow SJ, Reeves RR, West DS, Foreyt JP, Dilillo VG, et al. Binge eating disorder and night eating syndrome in adults with type 2 diabetes. Obesity (Silver Spring) 2007;15(5):1287–93. doi: 10.1038/oby.2007.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Renn BN, Feliciano L, Segal DL. The bidirectional relationship of depression and diabetes: a systematic review. Clin Psychol Rev. 2011;31(8):1239–46. doi: 10.1016/j.cpr.2011.08.001. [DOI] [PubMed] [Google Scholar]
- [65].Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23(11):1165–73. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- [66].U.S. Department of Health and Human Services FDA Adverse Event Reporting System (FAERS) [cited 2013 March 11] Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.