Abstract
Neuroinflammation is implicated in impairments in neuronal function and cognition that arise with aging, trauma, and/or disease. Therefore, understanding the underlying basis of the effect of immune system activation on neural function could lead to therapies for treating cognitive decline. Although neuroinflammation is widely thought to preferentially impair hippocampus-dependent memory, data on the effects of cytokines on cognition are mixed. One possible explanation for these inconsistent results is that cytokines may disrupt specific neural processes underlying some forms of memory but not others. In an earlier study, we tested the effect of systemic administration of bacterial lipopolysaccharide (LPS) on retrieval of hippocampus-dependent context memory and neural circuit function in CA3 and CA1 (Czerniawski and Guzowski, 2014). Paralleling impairment in context discrimination memory, we observed changes in neural circuit function consistent with disrupted pattern separation function. In the current study we tested the hypothesis that acute neuroinflammation selectively disrupts memory retrieval in tasks requiring hippocampal pattern separation processes. Male Sprague-Dawley rats given LPS systemically prior to testing exhibited intact performance in tasks that do not require hippocampal pattern separation processes: novel object recognition and spatial memory in the water maze. By contrast, memory retrieval in a task thought to require hippocampal pattern separation, context-object discrimination, was strongly impaired in LPS-treated rats in the absence of any gross effects on exploratory activity or motivation. These data show that LPS administration does not impair memory retrieval in all hippocampus-dependent tasks, and support the hypothesis that acute neuroinflammation impairs context discrimination memory via disruption of pattern separation processes in hippocampus.
Keywords: neuroinflammation, memory retrieval, lipopolysaccharide, water maze, context discrimination, novel object recognition, hippocampus
1. Introduction
Cytokines, signaling molecules that mediate the immune response and are beneficial at basal or low levels, can produce sickness behaviors and impair cognition at pathophysiological levels (Dantzer et al., 2008; Yirmiya and Goshen, 2011). There is evidence of cognitive impairment in humans with a variety of disorders that result in elevated cytokine levels, including multiple sclerosis, Alzheimer's disease, AIDS-related dementia, cancer, and patients undergoing chemotherapy (Kaul et al., 2001; Huijbregts et al., 2004; Meyers et al., 2005; Ahles and Saykin, 2007; Guerreiro et al., 2007).
During an inflammatory response, microglia become activated, resulting in the release of cytokines, including interleukin-1 (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), in the brain (Hanisch, 2002). These pro-inflammatory cytokines have been demonstrated to directly affect neuronal function, including long-term potentiation (LTP), glutamate release, AMPA receptor trafficking, and activation of cell-signaling pathways (O'Connor and Coogan, 1999; Albensi and Mattson, 2000; D'Arcangelo et al., 2000; Tancredi et al., 2000; Vereker et al., 2000; Beattie et al., 2002; Lynch et al., 2004). Because these processes affect synaptic plasticity and neurotransmission, it is apparent that cytokines may impact neuronal processes pertinent to cognition.
There is a high density of cytokine receptors in the hippocampus, particularly the dentate gyrus (DG) (Lechan et al., 1990; Schöbitz et al., 1992), indicating that the hippocampus may be particularly vulnerable during neuroinflammation. Indeed, using animal models, researchers have observed that administration of cytokines or other immunogenic stimuli, including the bacterial endotoxin lipopolysaccharide (LPS) can disrupt hippocampus-dependent learning and memory processes (Oitzl et al., 1993; Gibertini et al., 1995; Pugh et al., 1998; Barrientos et al., 2002). Specifically, several studies have shown that acquisition of the Morris water maze and consolidation of contextual but not cued fear conditioning, are disrupted during neuroinflammation (Gibertini et al., 1995; Pugh et al., 1998; Arai et al., 2001; Barrientos et al., 2002; Thomson and Sutherland, 2005). However, there have been mixed results across studies regarding the effect of neuroinflammation on the water maze, as well as observations that cytokines do not impair, and can even facilitate, learning and memory (Cunningham and Sanderson, 2008; Yirmiya and Goshen, 2011), making it difficult to ascertain the precise impact of neuroinflammation on cognition.
Importantly, patients with neuroimmune disorders have reported difficulty with memory retrieval (Thornton et al., 2002; Woods et al., 2007), which can be just as detrimental to daily function as encoding or consolidation deficits. Despite this, however, research to date has focused primarily on memory acquisition and consolidation processes. In a recent study, we examined the effect of acute neuroinflammation induced by systemic LPS injection on retrieval of a simple contextual fear task or a context discrimination fear task (Czerniawski and Guzowski, 2014). Although both tasks are hippocampus-dependent, LPS only impaired retrieval of context discrimination memory. In addition, analysis of neural circuit activity provided evidence that LPS-mediated neuroinflammation impaired pattern separation processes in CA3 and CA1. The behavioral and neural circuit data from this study are consistent with the hypothesis that acute neuroinflammation preferentially disrupts pattern separation functions necessary for context discrimination. In the present study we tested this working hypothesis by examining the effect of systemic LPS administration on retrieval of three additional tasks that vary with respect to hippocampal information processing: the spatial water maze task, context-object discrimination (COD), and novel object recognition (NOR). The water maze is a hippocampus-dependent task that tests navigation and spatial memory, while COD is a hippocampus-dependent task that tests context discrimination (Morris et al., 1982; Aggleton and Brown, 1999; Mumby et al., 2002; Barker and Warburton, 2011). NOR, although similar to COD in that it involves incidental encoding, does not typically require the hippocampus (Barker and Warburton, 2011). Of these three tasks, COD is the only one thought to require hippocampal pattern separation, and, accordingly, is the only task predicted to be impaired by LPS treatment.
2. Materials and Methods
2.1. Subjects
Eighty-nine male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 250-275 g at the time of arrival served as subjects. All animals were individually housed in a temperature-controlled vivarium maintained on a 12 hr light/ dark cycle. All subjects had access to food and water ad libitum throughout the duration of the experiment and were handled 2 min/day for 5 days before to start of the experiment. On each day prior to training all animals were transported to a holding room and allowed to sit for 2 hours undisturbed. All procedures complied with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
2.2. Apparatus
The water maze (Coulbourn Instruments, Allentown, PA) consisted of a blue circular pool (174 cm diameter and 97 cm high) filled with water (22-24 °C). An escape platform (15 cm diameter, 33 cm high) was placed in one of the quadrants (“Northeast”), 2.5 cm below water surface.
Two distinct environments in adjacent rooms were used for both COD and NOR. Environment A was an open box (60 × 60 cm) with 30 cm high walls. The box had Plexiglas walls with black paper attached on the outside, with white diagonal stripes on one of the walls. Clear Plexiglas covered a natural wood floor which was divided into nine squares with green tape. Environment B was a black cylinder (70 cm in diameter) with a height of 39 cm with a black floor. There were different visual cues in the different testing rooms.
The objects used were ceramic fish and frog toothbrush holders and open glass cubes. All the objects were ~ 11cm in height, 11-12 cm width and placed 12 cm from the wall with 15 cm between the pair of objects. All objects were too heavy to be displaced by the rats. The environments and objects were cleaned thoroughly between subjects with 10% ethyl alcohol for environment A or 0.01% acetic acid solution for environment B. Cameras mounted above each environment were used to record the training and testing sessions.
2.3. Behavior
2.3.1. Spatial water maze training and testing
For training, each rat was placed in the water at one of the eight starting positions in a random order and was given 60 s to reach the platform. If the rat failed to locate the platform after 60 s, it was carefully guided to the platform and placed on it for 10 s. The rat was then taken out of the platform and allowed to rest in a holding chamber for 20 s. This was followed by another training trial. The latency to find the platform was measured for each trial. The rats were trained 5 trials each day, for 4 consecutive days. On the fifth day, the test for platform location consisted of a single probe trial, during which the platform was removed. The time spent in each quadrant of the maze and a zone (8% of the total tank area) around the target was measured. Immediately following the probe trial, the platform was placed in the quadrant opposite from the original location (“southwest”) for reversal learning. Each rat was placed on the platform in the new location for 10 s and immediately proceeded to training as before, and the latencies to reach the platform were measured. All data were collected and processed by Watermaze software (Actimetrics; Coulbourn Instruments), which includes the video equipment and a computer equipped with an analysis-management system.
2.3.2. NOR and COD training and testing
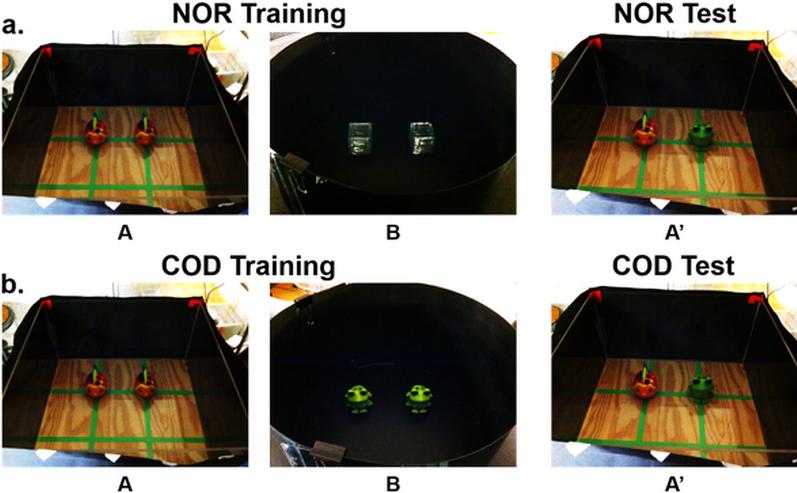
For both NOR and COD, subjects were placed into environments A and B for 5 min each for 2 days and allowed to freely explore. Subjects were returned to their home cage for a 20 min interval between these exploration sessions. The order of context presentation was counterbalanced between subjects and across days. There were pairs of different identical objects in each of the contexts. For NOR, these objects were ceramic fish in environment A and glass cubes in environment B (Figure 1a). For COD, these objects were ceramic fish in environment A and ceramic frogs in environment B (Figure 1b). The amount of time each subject explored each object (defined as nose pointed towards object within 2 cm of object) was collected using Limelight2 program (Actimetrics; Coulbourn Instruments).
Fig 1. Experimental Paradigm for NOR and COD.
Subjects were placed into contexts A and B and allowed to freely explore for 5 min each, with 20 min in between context presentations, for two consecutive days. On the third day, subjects were injected with SAL or LPS and tested 6 hr later in A’ for 5 min. a) For NOR, testing consisted replacing one of the objects in A with a novel object. b) For COD, testing consisted of replacing one of the objects in A with one of the objects previously experienced in context B.
The test session took place on Day 3 and consisted of 5 min in environment A’, which was A with one of the objects replaced by a different object. For NOR, one of the objects in A (fish) was replaced with a novel object (frog). For COD, one of the objects in A (fish) was replaced with one of the previously experienced objects in B (frog). Therefore, all subjects experienced the same exact test session, with the only difference being whether the frog object was novel or out-of-context.
2.4. Drugs
For all experiments, rats received an i.p. injection of either lipopolysaccharide (LPS Ecoli 026:B6; Sigma; Lot no. 037K4106) at 167 μg/kg (100,000 endotoxin units / mg) or sterile saline (SAL, 0.9% NaCl). Systemic LPS administration is a widely used model for inducing neuroinflammation, as it results in the elevation of brain cytokine levels and microglial activation (Gabellec et al., 1995; Nguyen et al., 1998; Qin et al., 2007; Henry et al., 2008). This specific dose of LPS was chosen because it is within the range of doses (100 μg/kg - 250 μg/kg) previously used in other studies examining the effect of i.p. LPS injections in rats on learning and has been shown to inhibit LTP, but not impact exploratory behavior (Pugh et al., 1998; Shaw et al., 2001, 2005; Hennigan et al., 2007; Bassi et al., 2012). Furthermore, we have previously assessed the time course of proinflammatory cytokine gene expression in the brain using this dose of LPS and observed elevated levels of IL-1β, IL-6, and TNF-α in dorsal hippocampus 6 h after i.p. injection, with a return to baseline levels 9 h post-injection (Czerniawski and Guzowski, 2014). Therefore, in the present study rats were injected with LPS (167 μg/kg) or SAL 6 h prior to testing. For all experiments, rats were assigned to receive SAL or LPS based on their performance during training for all experiments so that the mean behavioral measures for each group were equivalent prior to the pre-testing injections.
2.5. Statistical Analyses
Statistical analysis (t-test or ANOVA) of behavioral data was performed using commercial software (SigmaStat). When appropriate, Holm-Sidak post hoc tests were used for multiple comparisons.
3. RESULTS
3.1. Systemic LPS administration does not impair spatial memory retrieval or reversal learning in the water maze
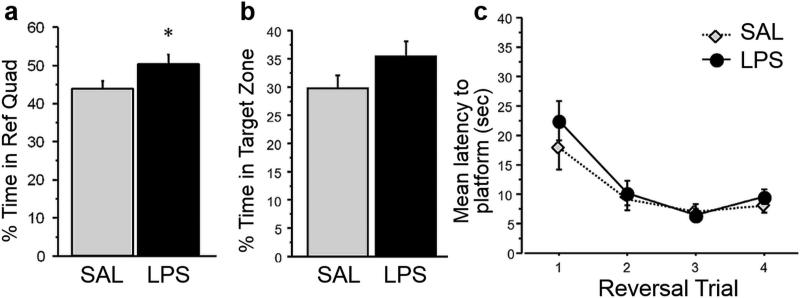
After 4 days of training to the platform in the “northeast” quadrant and reaching an asymptotic performance, each rat was administered either LPS (n = 22) or SAL (n=22) on the fifth day. Six hours later, each rat was placed in water maze without the platform for 60 s. The amount time that the rats spent in the target quadrant and zone around the platform were measured as an index of memory for platform location. There was a significant difference in time spent in quadrant platform during testing (t (42) = 2.09, p = 0.043; Figure 2a). Specifically, LPS-treated rats spent slightly more time in the target quadrant (Mean = 50.85±2.35%) compared to those injected with saline (Mean = 44.33±2.06%). The percent of time spent in a zone around the platform (8% of the tank area) did not differ between the groups (t (42) = 1.58, p = 0.12; Figure 2b). Immediately following the probe test, the platform was placed in the quadrant opposite from the original location, and four reversal training trials were given. The latencies to reach the platform in the new location were measured for each trial, and the groups did not differ from each other (t (42) = 0.894, p = 0.376; Figure 2c).
Fig 2. Water maze testing.
Percent of time (±SEM) spent in a) the reference quadrant or b) target zone during the probe test was not impaired following systemic LPS administration. c) There was no difference in the mean latency to the platform (±SEM) between LPS and SAL-treated subjects during reversal learning. *p < 0.05.
3.2. Systemic LPS administration during testing does not impair NOR
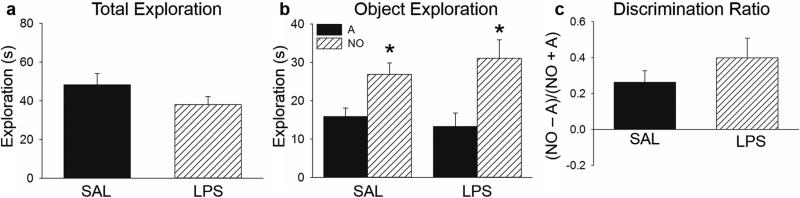
After 2 d of training, subjects were assigned to receive an i.p. injection of SAL (n = 7) or LPS (n = 6) 6 hr prior to testing. During NOR testing all subjects were placed into A’, which consisted of context A with one of the 2 objects replaced with a completely novel object, and allowed to freely explore for 5 min. The mean (±SEM) time spent exploring both objects is depicted in Figure 3a. There was no difference between saline and LPS subjects in total time spent exploring objects (t (11) = 1.397, p = 0.190), indicating that LPS did not impair locomotor activity or the motivation to approach and explore the objects.
Fig 3. NOR Testing.
a) LPS and SAL-treated subjects did not differ in total time (±SEM) spent exploring objects during test. b) Both LPS and SAL subjects spent more time exploring the novel object (NO) than the familiar object (A). c) There was no difference in the discrimination ratio (±SEM) between LPS and SAL-treated subjects. *p < 0.05.
The NOR task exploits the fact that rodents have an innate preference to explore novelty. Thus, rats that remember previously experienced objects typically spend more time exploring the novel object compared to the familiar object during test. Consistent with this, a two-way ANOVA revealed that rats spent more time exploring the novel object compared to the familiar object (F (1,25) = 17.786, p < 0.001, Figure 3b). However, there was not a significant difference between SAL or LPS subjects (F (1,25) = 0.055, p = 0.816), nor was there an interaction between injection condition or object exploration (F (1,25) = 0.987, p = 0.331). Therefore, all subjects exhibited a preference for the novel object, regardless of whether they received administration of LPS or saline before testing. In addition, we calculated a discrimination ratio [(time spent exploring novel object – time spent exploring familiar object) / (time spent exploring novel object + time spent exploring familiar object)] for each subject, with 1 being maximum time spent exploring the novel object and -1 maximum time spent exploring the familiar object (Figure 3c). Both groups had a positive mean discrimination ratio, with no significant difference between LPS and saline subjects (t (11) = 1.105, p = 0.293). Collectively the data indicate that acute inflammation during testing did not impair NOR.
3.3. Systemic LPS administration during testing impairs COD
The paradigm for COD was the same as described above for NOR. The only notable differences were that 1) different objects were used in context B during training and 2) one of the objects in A was replaced with one of the objects previously experienced in B during test in A’. It was, however, the same exact object used during NOR testing but rather than being completely novel during testing as it was in NOR, it was a familiar object that was out-of-context during the COD test. Subjects were injected with SAL (n = 12) or LPS (n = 10) in the same manner as described above. Similar to NOR, there was no difference between saline and LPS subjects in total time spent exploring objects (t (20) = 0.391, p = 0.700; Figure 4a), suggesting that LPS did not impair locomotor activity or the motivation to approach and explore the objects.
Fig 4. COD Testing.
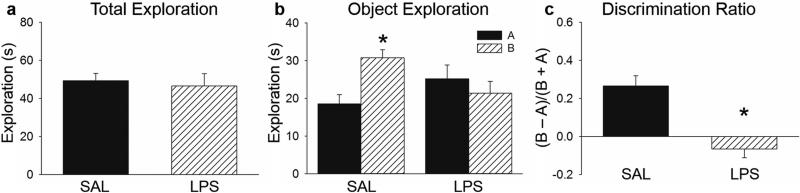
a) LPS and SAL-treated subjects did not differ in total time (±SEM) spent exploring objects during test. b) SAL subjects spent more time exploring the out-of-context (B) compared to the in-context object (A) during testing but LPS-treated rats did not. c) LPS-treated subjects had a significantly lower discrimination ratio (±SEM) than control subjects during COD testing. *p < 0.05.
We predicted that control subjects would spend more time exploring the out-of-context object (B) than the familiar object (A), but that LPS subjects would not. A twoway ANOVA did not reveal a significant difference between condition (SAL vs. LPS) (F (1,43) = 0.245, p = 0.624) or object exploration (A vs. B) (F (1,43) = 2.213, p = 0.145), but did reveal a significant interaction between condition and object exploration (F (1,43) = 8.087, p = 0.007, Figure 4b). While saline subjects spent significantly more time exploring the B object compared to A, LPS subjects did not exhibit a preference in exploring either object (Holm Sidak, p < 0.05). Discrimination ratios also significantly differed between groups (t (20) = 4.585, p < 0.001), with a discrimination index near 0 for LPS subjects, indicating no discrimination between the in-context and out-of-context objects (Figure 4c). Therefore, administration of LPS prior to testing eliminated the ability to recognize a previously experienced object, which was out of context. Together these experiments demonstrate that acute neuroinflammation impaired COD (where the object was familiar but out-of-context) but not NOR (where the object was completely novel).
4. Discussion
We have demonstrated that systemic LPS administration disrupted memory retrieval in COD, but not NOR or the water maze. Therefore, while LPS-treated rats were able to remember the location of the hidden platform in the water maze and could distinguish a novel object from a familiar one, they were deficient in determining that a previously experienced object was in a different context. Thus, our data indicate that acute neuroinflammation does not impair memory retrieval in all hippocampus-dependent tasks, but rather that it may specifically disrupt tasks that require context discrimination.
The fact that we did not observe impairment in the water maze at first appears at odds with the prevalent notion that this task is reliably disrupted during inflammatory conditions. However, as reviewed by Cunningham and Sanderson (2008), the literature on the effect of immune activation on acquisition in the water maze is far from clear. Most previous studies have interpreted longer latencies to find the platform as a cognitive deficit (Oitzl et al., 1993; Gibertini et al., 1995; Arai et al., 2001; Song and Horrobin, 2004), yet latency may not be the best measure because longer latencies could be attributed to a performance deficit due to sickness behaviors (Sparkman et al., 2005a). Instead, the probe test may more accurately measure whether an animal has successfully used spatial cues to learn and remember the location of the hidden platform. Interestingly, several studies examining the effect of neuroinflammation on acquisition of water maze reported no impairment during the probe trial (Arai et al., 2001; Sparkman et al., 2005a; Sparkman et al., 2005b; Thomson and Sutherland, 2006). Yet it is important to note that these studies all varied with respect to the type of immunogenic stimuli, duration, and route of administration as well species used, making it difficult to compare across studies. Using the same dose and timepoint of LPS injection that impairs memory retrieval in COD and context discrimination conditioning (Czerniawski and Guzowski, 2014), we did not observe an impairment in retrieval of spatial reference memory following systemic LPS administration. In fact, LPS-treated rats spent significantly more time in the reference quadrant than control subjects. However, this was a very subtle effect size and there were no differences between groups in the percent of time spent in the target zone during the probe test or during reversal leaning, suggesting that LPS did not produce a global enhancement in spatial memory retrieval. Nonetheless, our data clearly demonstrate that spatial memory retrieval, as determined by both the probe test and reversal learning, was not impaired during acute neuroinflammation.
In addition to the water maze, another commonly used behavioral test for assessing the impact of cytokines in cognition is contextual fear conditioning (CFC). Although others have reported that consolidation of CFC is disrupted following LPS administration (Pugh et al., 1998; Thomson and Sutherland, 2005), we have recently observed that acute neuroinflammation dramatically impairs memory retrieval in context discrimination conditioning (CDC) but not in a standard CFC paradigm (Czerniawski and Guzowski, 2014). This discrepancy in the effect of LPS administration on the consolidation vs. retrieval of CFC may be explained by the various effects pathophysiological levels of cytokines can have on neural processes required for consolidation, including impaired LTP and BDNF downregulation (O'Connor and Coogan, 1999; Vereker et al., 2000; Barrientos et al., 2004; Lynch et al., 2004; Kranjac et al., 2012). Thus, the acquisition and consolidation of various types of memories may be differentially affected compared to retrieval processes. Nonetheless our data indicate that 1) there are differences in the impact of cytokines on different stages of memory processing and 2) context discrimination memory retrieval may be particularly sensitive to disruption due to acute neuroinflammation.
In order to further test if memory retrieval processes requiring context discrimination are specifically impaired during acute neuroinflammation, we trained rats in either COD, which is hippocampus-dependent, or NOR, which is hippocampus-independent (Aggleton and Brown, 1999; Mumby et al., 2002; Barker and Warburton, 2011). It is important to note that the only difference in the test for NOR and COD was whether one of the objects in environment A was replaced by an object previously experienced in environment B (COD) or a novel object (NOR). In this regard, we were able to specifically test whether acute neuroinflammation affects recognizing a familiar object vs. retrieving a context-object association. Consistent with previous reports, systemic LPS administration 6 h prior to testing did not affect NOR (Hauss-Wegrzyniak et al., 2000; Belarbi and Rosi, 2013). However, there have also been reports that acute LPS administration, whether i.p. or i.c.v, can impair NOR (Hennigan et al., 2007; Miwa et al., 2011). It is important to note that LPS was administered prior to training in both these studies whereas in the present study it was administered prior to testing. Hennigan et al. (2007) also observed impairment in the expression of LTP in the dentate gyrus (DG) of the hippocampus, indicating that plasticity processes critical for consolidation were disrupted. Therefore it is likely that disrupting plasticity and signaling pathways during (or immediately following) training may impair consolidation but that disrupting these processes before testing do not affect retrieval. Here we demonstrate that an acute administration of LPS prior to testing did not affect the ability to discriminate between a novel and previously experienced object.
The fact that LPS administration did not impair retrieval of NOR is important for a number of reasons. First, it shows that this dose of LPS did not affect the ability of rats to perceive or recognize the objects. Second, the inherent drive to explore novelty was intact following immune challenge. Lastly, LPS administration did not diminish motivation to explore the contexts or objects. This finding in particular is important because LPS has been shown to induce lethargy and reduce motivation which could present a confound when using exploratory behavior to assess cognitive processes (Dantzer, 2001; Henry et al., 2008). Importantly, there was no difference in total time spent exploring objects in NOR or COD between saline and LPS-treated animals in the present study. Moreover, LPS-treated rats did not exhibit any overt sickness behaviors such as lethargy or diarrhea in these experiments. Therefore, the impairment we observed in COD is likely not due to effects of LPS on exploratory preference, motivation, or locomotor ability, but on the cognitive demands of the task itself.
To our knowledge, we are the first to examine the effect of neuroinflammation on COD. Due to the literature providing support that LPS disrupts hippocampus-dependent forms of learning (Pugh et al., 1998; Gilbertini et al. 1996; Shaw et al., 2001; Barrientos et al., 2002) and that COD, but not NOR, depends on the integrity of the hippocampus (Aggleton & Dix, 1999; Mumby et al., 2012; Barker & Warburton, 2011), we predicted that LPS would produce an impairment in COD. Although both objects during test were equally familiar, control subjects preferentially explored the out-of-context compared to the in-context object. LPS-treated rats, however, did not differ in time spent exploring either object. The fact that systemic LPS administration disrupted COD but not NOR suggests that systemic LPS administration impaired the ability to retrieve the association between an object and a specific context, but not the ability to recognize an object itself.
It is important to note that all rats in the present study were single-housed, which can lead to a greater susceptibility to stress effects than group-housing (Liu et al., 2013). Different stressors have been shown to interact with the immune response to LPS, leading to altered cytokine production in the brain (Goujon et al., 1995; Johnson et al., 2002). In one recent study, single-housed mice exhibited greater levels of cytokine production and impairment in the water maze following influenza infection compared to mice who experienced an enriched environment that included group-housing (Jurgens and Johnson, 2012). However, in the present study we did not observe any LPS-induced deficits in the water maze, suggesting there was not a debilitating stress-immune interaction due to single-housing. Additionally, while COD and NOR had virtually the same experiment design and task demands of one another, systemic LPS administration only produced a deficit in COD but not NOR, which has previously been shown to be impaired by different stressors (Baker and Kim, 2002; Eagle et al., 2013). Therefore, since we did not observe any LPS-induced memory retrieval deficits in NOR or the water maze, it is unlikely that stress due to single-housing exacerbated the effects of systemic LPS administration.
The data from the present study, as well as other recent work from our laboratory and others (Field et al., 2012; Griffin et al., 2013; Czerniawski and Guzowski, 2014), suggest that acute neuroinflammation does not impair memory retrieval in all hippocampus-dependent tasks, but rather in tasks that specifically require context discrimination (Table 1). Context discrimination is thought to require a certain type of neural computation, namely pattern separation, a process by which two similar input patterns are made more orthogonal (dissimilar) as output patterns (Guzowski et al., 2004; Yassa and Stark, 2011). The DG, as well as CA3 and CA1, are all capable of performing pattern separation, and there is evidence that this process facilitates context discrimination (Vazdarjanova and Guzowski, 2004; Leutgeb et al., 2007; McHugh et al., 2007; Nakashiba et al., 2012). In a recent study (Spanswick and Sutherland, 2010), rats with a reduction in granule cells in dentate gyrus due to adrenalectomy could discriminate between a novel and familiar object but not an out-of-context and in-context object. As DG-CA3 circuits in particular are important for pattern separation and context discrimination (McHugh et al., 2007; Nakashiba et al., 2012), the deficit in COD in the present study may stem from disruption in pattern separation processes within the hippocampus which are unnecessary for retrieval in the water maze or NOR.
Table 1.
Effects of acute neuroinflammation on memory retrieval.
| Task | Hippocampus-dependent? | Behavioral Measure | SAL (mean±SEM) | LPS (mean±SEM) | p-value | Retrieval Impairment? |
|---|---|---|---|---|---|---|
|
Morris Water Maze: Probe Test |
YES | % time in target quadrant |
44.33 ± 2.06 |
50.85 ± 2.35 |
< 0.05 | NO |
|
Novel Object Recognition |
NO | Discrimination Ratio |
0.263 ± 0.064 |
0.399 ± 0.110 |
0.293 | NO |
|
Context-Object Discrimination |
YES | Discrimination Ratio |
0.267 ± 0.053 |
−0.065 ± 0.047 |
< 0.001 | YES |
|
Context Discrimination Conditioninga |
YES | Discrimination Ratio |
0.816 ± .096 |
−0.060 ± 0.129 |
< 0.001 | YES |
|
Context Fear Conditioninga |
YES | Freezing (%) |
66.29 ± 7.28 |
59.06 ± 6.18 |
0.455 | NO |
Findings from our laboratory demonstrating that systemic LPS impairs memory retrieval for hippocampus-dependent tasks that require context discrimination, but not necessarily for all hippocampus-dependent tasks.
Data from (Czerniawski and Guzowski, 2014).
Cognitive processes that require pattern separation may be vulnerable to impairment during neuroinflammation due to the high density of cytokine receptors in DG and, to a lesser extent, CA3 (Lechan et al., 1990; Schöbitz et al., 1992). Supporting this notion, we observed that acute neuroinflammation disrupted pattern separation at the neural circuit activity level in CA3 and CA1 of LPS-treated rats that showed strong retrieval deficits of context discrimination at the behavioral level (Czerniawski and Guzowski, 2014). Additionally, reducing adult neurogenesis in DG, in the absence of explicitly inducing neuroinflammation, impairs orthogonalization of similar (but not dissimilar) contexts in CA3 (Niibori et al., 2012). This suggests that decreased or altered granule cell activity, which may occur via cytokine effects during neuroinflammation, is responsible for the disruption in pattern separation processes in the hippocampus, thus resulting in impaired context discrimination during retrieval. Notably, LPS-treated rats have elevated IL-1β and impaired in vivo LTP in perforant path-granule cell synapses (Vereker et al., 2000; Lynch et al., 2004). Further supporting a strong role for IL-1-mediated signaling in the neuroinflammation induced deficits in context discrimination, IL-1β can inhibit NMDA receptor-mediated synaptic transmission in DG (Coogan and O'Connor, 1997; Coogan et al., 1999) and disruption of NMDA receptor function in DG is sufficient to impair context discrimination (McHugh et al., 2007; Eadie et al., 2012).
Collectively these data suggest that the high density of cytokine receptors in DG may make memory functions requiring the DG, such as context discrimination, particularly vulnerable to the deleterious effects of neuroinflammation. Altered activity in DG could then impact processing in CA3 via altered mossy fiber input, and subsequently CA1 functioning via Schaffer collateral input from CA3. Therefore, retrieval of some hippocampus-dependent tasks that do not require DG based computations (e.g., pattern separation), such as the standard water maze or CFC tasks, are intact during neuroinflammation while hippocampus-dependent tasks that require DG information processing, such as CDC and COD, are disrupted. In conclusion, the present findings underscore the importance of assessing the impact of neuroinflammation on memory retrieval requiring specific neural processes and not whole brain regions, given that it is now established that structures, such as the hippocampus, can perform multiple, often independent neural processes (Moser, 2011; Nakashiba et al., 2012).
Elevated cytokine production is ubiquitous in patients with numerous conditions including multiple sclerosis, Alzheimer's disease, Parkinson's disease, schizophrenia, AIDS-related dementia, traumatic brain injury, depressive disorders, cancer, chemotherapy, and even normal aging (Blum-Degena et al., 1995; Kaul et al., 2001; Yaffe et al., 2003; Imamura et al., 2005; Meyers et al., 2005; Drzyzga et al., 2006; Raison et al., 2006; Weisman et al., 2006; Ahles and Saykin, 2007; Guerreiro et al., 2007). Importantly, cognitive deficits are associated with many of these conditions (Kaul et al., 2001; Thornton et al., 2002; Yaffe et al., 2003; Huijbregts et al., 2004; Meyers et al., 2005; Huijbregts et al., 2006; Woods et al., 2007; Ahles et al., 2008). Therefore, understanding the dynamic interaction between cytokines and neurons and how they affect cognitive processes has strong clinical implications. Our data suggesting that acute neuroinflammation disrupts retrieval of certain types of memories, supported by specific neural processes, can potentially help guide the development of diagnostic tests as well as putative treatments for human patients.
Highlights.
We tested if acute neuroinflammation impairs retrieval of different memory tasks
Systemic LPS administration was used to induce acute neuroinflammation in brain
Systemic LPS did not affect novel object recognition or spatial memory tasks
Systemic LPS impaired context-object discrimination memory
Acute neuroinflammation affects specific forms of hippocampus-dependent memories
Acknowledgements
This work was supported by NIH R01 MH08930 (JFG) with additional support from NIH Training in the Neurobiology of Aging Grant AG00096 (JC). The authors thank Terra White for helpful comments on an earlier version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal–anterior thalamic axis. BEHAVIORAL AND BRAIN SCIENCES. 1999;22:425–489. [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast cancer research and treatment. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Arai K, Matsuki N, Ikegaya Y, Nishiyama N. Deterioration of Spatial Learning Performances in Lipopolysaccharide-Treated Mice. The Japanese Journal of Pharmacology. 2001;87:195–201. doi: 10.1254/jjp.87.195. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of Stress and Hippocampal NMDA Receptor Antagonism on Recognition Memory in Rats. Learning & Memory. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GRI, Warburton EC. When Is the Hippocampus Involved in Recognition Memory? The Journal of Neuroscience. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. Journal of neuroimmunology. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Bassi GS, Kanashiro A, Santin FM, de Souza GE, Nobre MJ, Coimbra NC. Lipopolysaccharide-induced sickness behaviour evaluated in different models of anxiety and innate fear in rats. Basic Clin Pharmacol Toxicol. 2012;110:359–369. doi: 10.1111/j.1742-7843.2011.00824.x. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Belarbi K, Rosi S. Modulation of adult-born neurons in the inflamed hippocampus. Frontiers in cellular neuroscience. 2013;7:145. doi: 10.3389/fncel.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum-Degena D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1β and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neuroscience Letters. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Coogan A, O'Connor JJ. Inhibition of NMDA receptor-mediated synaptic transmission in the rat dentate gyrus in vitro by IL-1β. NeuroReport. 1997;8:2107–2110. doi: 10.1097/00001756-199707070-00004. [DOI] [PubMed] [Google Scholar]
- Coogan AN, O'Neill LAJ, O'Connor JJ. The p38 mitogen-activated protein kinase inhibitor SB203580 antagonizes the inhibitory effects of interleukin-1β on long-term potentiation in the rat dentate gyrus in vitro. Neuroscience. 1999;93:57–69. doi: 10.1016/s0306-4522(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Sanderson DJ. Malaise in the water maze: Untangling the effects of LPS and IL-1β on learning and memory. Brain, Behavior, and Immunity. 2008;22:1117–1127. doi: 10.1016/j.bbi.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Guzowski JF. Acute Neuroinflammation Impairs Context Discrimination Memory and Disrupts Pattern Separation Processes in Hippocampus. The Journal of Neuroscience. 2014;34:12470–12480. doi: 10.1523/JNEUROSCI.0542-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcangelo G, Tancredi V, Onofri F, D'Antuono M, Giovedi S, Benfenati F. Interleukin-6 inhibits neurotransmitter release and the spread of excitation in the rat cerebral cortex. The European journal of neuroscience. 2000;12:1241–1252. doi: 10.1046/j.1460-9568.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Annals of the New York Academy of Sciences. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzyzga Ł , Obuchowicz E, Marcinowska A, Herman ZS. Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain, Behavior, and Immunity. 2006;20:532–545. doi: 10.1016/j.bbi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Cushman J, Kannangara TS, Fanselow MS, Christie BR. NMDA receptor hypofunction in the dentate gyrus and impaired context discrimination in adult Fmr1 knockout mice. Hippocampus. 2012;22:241–254. doi: 10.1002/hipo.20890. [DOI] [PubMed] [Google Scholar]
- Eagle AL, Fitzpatrick CJ, Perrine SA. Single prolonged stress impairs social and object novelty recognition in rats. Behavioural Brain Research. 2013;256:591–597. doi: 10.1016/j.bbr.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field RH, Gossen A, Cunningham C. Prior pathology in the basal forebrain cholinergic system predisposes to inflammation-induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium. J Neurosci. 2012;32:6288–6294. doi: 10.1523/JNEUROSCI.4673-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabellec M-M, Griffais R, Fillion G, Haour F. Expression of interleukin 1α, interleukin 1β and interleukin 1 receptor antagonist mRNA in mouse brain: regulation by bacterial lipopolysaccharide (LPS) treatment. Molecular Brain Research. 1995;31:122–130. doi: 10.1016/0169-328x(95)00042-q. [DOI] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial Learning Impairment in Mice Infected with Legionella pneumophila or Administered Exogenous Interleukin-1-β. Brain, Behavior, and Immunity. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Goujon E, Parnet P, Laye S, Combe C, Kelley KW, Dantzer R. Stress Downregulates Lipopolysaccharide-Induced Expression of Proinflammatory Cytokines in the Spleen, Pituitary, and Brain of Mice. Brain, Behavior, and Immunity. 1995;9:292–303. doi: 10.1006/brbi.1995.1028. [DOI] [PubMed] [Google Scholar]
- Griffin EW, Skelly DT, Murray CL, Cunningham C. Cyclooxygenase-1-dependent prostaglandins mediate susceptibility to systemic inflammation-induced acute cognitive dysfunction. J Neurosci. 2013;33:15248–15258. doi: 10.1523/JNEUROSCI.6361-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro RJ, Santana I, Bras JM, Santiago B, Paiva A, Oliveira C. Peripheral inflammatory cytokines as biomarkers in Alzheimer's disease and mild cognitive impairment. Neurodegener Dis. 2007;4:406–412. doi: 10.1159/000107700. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hanisch U-K. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vannucchi MG, Wenk GL. Behavioral and ultrastructural changes induced by chronic neuroinflammation in young rats. Brain Research. 2000;859:157–166. doi: 10.1016/s0006-8993(00)01999-5. [DOI] [PubMed] [Google Scholar]
- Hennigan A, Trotter C, Kelly AM. Lipopolysaccharide impairs long-term potentiation and recognition memory and increases p75NTR expression in the rat dentate gyrus. Brain Res. 2007;1130:158–166. doi: 10.1016/j.brainres.2006.10.066. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts SC, Kalkers NF, de Sonneville LM, de Groot V, Polman CH. Cognitive impairment and decline in different MS subtypes. Journal of the neurological sciences. 2006;245:187–194. doi: 10.1016/j.jns.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Huijbregts SCJ, Kalkers NF, de Sonneville LMJ, de Groot V, Reuling IEW, Polman CH. Differences in cognitive impairment of relapsing remitting, secondary, and primary progressive MS. Neurology. 2004;63:335–339. doi: 10.1212/01.wnl.0000129828.03714.90. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Ono K, Suzuki H, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Cytokine production of activated microglia and decrease in neurotrophic factors of neurons in the hippocampus of Lewy body disease brains. Acta Neuropathol. 2005;109:141–150. doi: 10.1007/s00401-004-0919-y. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior Stressor Exposure Sensitizes LPS-Induced Cytokine Production. Brain, Behavior, and Immunity. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Jurgens HA, Johnson RW. Environmental enrichment attenuates hippocampal neuroinflammation and improves cognitive function during influenza infection. Brain, Behavior, and Immunity. 2012;26:1006–1016. doi: 10.1016/j.bbi.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kranjac D, McLinden KA, Deodati LE, Papini MR, Chumley MJ, Boehm GW. Peripheral bacterial endotoxin administration triggers both memory consolidation and reconsolidation deficits in mice. Brain Behav Immun. 2012;26:109–121. doi: 10.1016/j.bbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Toni R, Clark BD, Cannon JG, Shaw AR, Dinarello CA, Reichlin S. Immunoreactive interleukin-1β localization in the rat forebrain. Brain Research. 1990;514:135–140. doi: 10.1016/0006-8993(90)90445-h. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu R, Tai F, Ma L, Wei B, Yang X, Zhang X, Jia R. Effects of group housing on stress induced emotional and neuroendocrine alterations. Brain Res. 2013;1502:71–80. doi: 10.1016/j.brainres.2013.01.044. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Walsh C, Delaney A, Nolan Y, Campbell VA, Lynch MA. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10 – a role for IL-1β? Journal of Neurochemistry. 2004;88:635–646. doi: 10.1046/j.1471-4159.2003.02157.x. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- Miwa M, Tsuboi M, Noguchi Y, Enokishima A, Nabeshima T, Hiramatsu M. Effects of betaine on lipopolysaccharide-induced memory impairment in mice and the involvement of GABA transporter 2. J Neuroinflammation. 2011;8:153. doi: 10.1186/1742-2094-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser EI. The multi-laned hippocampus. Nat Neurosci. 2011;14:407–408. doi: 10.1038/nn.2783. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learning & Memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1β protein in the rat. Journal of Neuroscience. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niibori Y, Yu TS, Epp JR, Akers KG, Josselyn SA, Frankland PW. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nature communications. 2012;3:1253. doi: 10.1038/ncomms2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JJ, Coogan AN. Actions of the Pro-Inflammatory Cytokine Il-1[beta] on Central Synaptic Transmission. Experimental Physiology. 1999;84:601–614. [PubMed] [Google Scholar]
- Oitzl MS, van Oers H, Schöbitz B, Ron de Kloet E. Interleukin-1β, but not interleukin-6, impairs spatial navigation learning. Brain Research. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective Effects of Peripheral Lipopolysaccharide Administration on Contextual and Auditory-Cue Fear Conditioning. Brain, Behavior, and Immunity. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong J-S, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöbitz B, Voorhuis DAM, De Kloet ER. Localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Neuroscience Letters. 1992;136:189–192. doi: 10.1016/0304-3940(92)90046-a. [DOI] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O'Mara SM. Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat dentate gyrus. Behavioural Brain Research. 2001;124:47–54. doi: 10.1016/s0166-4328(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O'Mara SM. Cyclooxygenase inhibition attenuates endotoxin-induced spatial learning deficits, but not an endotoxin-induced blockade of long-term potentiation. Brain Research. 2005;1038:231–237. doi: 10.1016/j.brainres.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Song C, Horrobin D. Omega-3 fatty acid ethyl-eicosapentaenoate, but not soybean oil, attenuates memory impairment induced by central IL-1β administration. Journal of Lipid Research. 2004;45:1112–1121. doi: 10.1194/jlr.M300526-JLR200. [DOI] [PubMed] [Google Scholar]
- Spanswick SC, Sutherland RJ. Object/context-specific memory deficits associated with loss of hippocampal granule cells after adrenalectomy in rats. Learn Mem. 2010;17:241–245. doi: 10.1101/lm.1746710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Kohman RA, Scott VJ, Boehm GW. Bacterial endotoxin-induced behavioral alterations in two variations of the Morris water maze. Physiol Behav. 2005a;86:244–251. doi: 10.1016/j.physbeh.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Martin LA, Calvert WS, Boehm GW. Effects of intraperitoneal lipopolysaccharide on Morris maze performance in year-old and 2-month-old female C57BL/6J mice. Behav Brain Res. 2005b;159:145–151. doi: 10.1016/j.bbr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D'Antuono M, Cafe C, Giovedi S, Bue MC, D'Arcangelo G, Onofri F, Benfenati F. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. Journal of neurochemistry. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- Thomson LM, Sutherland RJ. Systemic administration of lipopolysaccharide and interleukin-1beta have different effects on memory consolidation. Brain Res Bull. 2005;67:24–29. doi: 10.1016/j.brainresbull.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Thomson LM, Sutherland RJ. Interleukin-1beta induces anorexia but not spatial learning and memory deficits in the rat. Behav Brain Res. 2006;170:302–307. doi: 10.1016/j.bbr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Thornton AE, Raz N, Tucke KA. Memory in multiple sclerosis: contextual encoding deficits. Journal of the International Neuropsychological Society : JINS. 2002;8:395–409. doi: 10.1017/s1355617702813200. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24:6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- Weisman D, Hakimian E, Ho GJ. Interleukins, Inflammation, and Mechanisms of Alzheimer's Disease. In: Gerald L, editor. In: Vitamins & Hormones. Academic Press; 2006. pp. 505–530. [DOI] [PubMed] [Google Scholar]
- Woods SP, Carey CL, Moran LM, Dawson MS, Letendre SL, Grant I. Frequency and predictors of self-reported prospective memory complaints in individuals infected with HIV. Arch Clin Neuropsychol. 2007;22:187–195. doi: 10.1016/j.acn.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends in Neurosciences. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]