Abstract
Mutations in the splicing factor SF3B1 are found in several cancer types and have been associated with various splicing defects. Using transcriptome sequencing data from chronic lymphocytic leukemia, breast cancer and uveal melanoma tumor samples, we show that hundreds of cryptic 3’ splice sites (3’SSs) are used in cancers with SF3B1 mutations. We define the necessary sequence context for the observed cryptic 3’ SSs and propose that cryptic 3’SS selection is a result of SF3B1 mutations causing a shift in the sterically protected region downstream of the branch point. While most cryptic 3’SSs are present at low frequency (<10%) relative to nearby canonical 3’SSs, we identified ten genes that preferred out-of-frame cryptic 3’SSs. We show that cancers with mutations in the SF3B1 HEAT 5-9 repeats use cryptic 3’SSs downstream of the branch point and provide both a mechanistic model consistent with published experimental data and affected targets that will guide further research into the oncogenic effects of SF3B1 mutation.
Author Summary
A key goal of cancer genomics studies is to identify genes that are recurrently mutated at a rate above background and likely contribute to cancer development. Many such recurrently mutated genes have been identified over the last few years, but we often do not know the underlying mechanisms by which they contribute to cancer growth. Unexpectedly, several genes in the spliceosome, the collection of RNAs and proteins that remove introns from transcribed RNAs, are recurrently mutated in different cancers. Here, we have examined mutations in the splicing factor SF3B1, a key component of the spliceosome, and identified a global splicing defect present in different cancers with SF3B1 mutations by comparing the expression of splice junctions using generalized linear models. While prior studies have reported a limited number of aberrant splicing events in SF3B1-mutated cancers, we have established that SF3B1 mutations are associated with usage of hundreds of atypical splice sites at the 3’ end of the intron. We have identified nucleotide sequence requirements for these cryptic splice sites that are consistent with a proposed mechanistic model. These findings greatly expand our understanding of the effect of SF3B1 mutations on splicing and provide new targets for determining the oncogenic effect of SF3B1 mutations.
Introduction
One of the biggest surprises to emerge from the growing catalog of somatic mutations in various cancer types is the recurrent mutation of genes encoding the RNA spliceosome [1]. Recurrent mutations in the highly conserved HEAT 5–9 repeats of splicing factor 3B subunit 1 (SF3B1) have been reported in myelodysplastic syndrome, chronic lymphocytic leukemia (CLL), breast cancer (BRCA), uveal melanoma (UM), and pancreatic cancer [2–7]. SF3B1 mutation is associated with poor prognosis in CLL but improved prognosis in myelodysplasia and UM [2,7–9]. Prior studies have shown that mutated SF3B1 CLL samples have differential exon inclusion and use some cryptic 3’ splice sites (3’SSs) relative to wild-type SF3B1 CLL samples [5,6,8,10,11]. However, it is unknown whether SF3B1 mutation is associated with the same 3’SS selection defects in different cancers. The mechanism underlying the cryptic 3’SS selection and the functional consequences thereof remain unresolved as well.
SF3B1 is a core part of the U2-small nuclear ribonucleoprotein (U2-snRNP) complex and stabilizes the binding of the U2-snRNP to the branch point (BP), a degenerate sequence motif usually located 21–34 bp upstream of the 3’SS [12,13]. SF3B1 also interacts with other spliceosomal proteins such as U2AF2, which binds the polypyrimidine tract (PPT) downstream of the BP [2,14,15]. The binding of the U2-snRNP and other spliceosome proteins around the BP prevents 3’SS selection in a ~12–18 bp region directly downstream of the BP due to steric hindrance [16,17]. Inherited cis-acting splicing mutations beyond this ~12–18 bp region downstream of the BP that result in the use of cryptic 3’SSs have been shown to occur in Mendelian disease genes [18]. Additionally, a competitive region exists ~12 bp downstream from the first 3’SS after the protected region where AG dinucleotides can compete to be used as 3’SSs based on sequence characteristics such as the PPT length, distance from the BP, nucleotide preceding the AG dinucleotide, and other features [17].
The role of SF3B1 and the U2-snRNP in recognizing and binding the BP and the localization of mutations to HEAT 5–9 repeats suggest that SF3B1 mutations are dominant drivers that may alter 3’SS selection [6]. To test this, we examined splice site usage in transcriptome data from SF3B1 mutant and SF3B1 wild-type CLL, UM and BRCA cases. We identified 619 cryptic 3’SSs used more frequently in SF3B1 mutants and clustered 10–30 bp upstream of canonical 3’SSs. The majority of these cryptic 3’SSs were observed in all three tumor types despite the divergent clinical implications of SF3B1 mutation. Our analysis of tumors with SF3B1 mutations shows that cryptic 3’SS selection occurs only in samples with missense mutations at ~10 amino acid hotspots in the fifth to ninth HEAT repeats. We analyzed the organization of splicing motifs around the cryptic 3’SSs and found that only introns with an AG dinucleotide at the boundary of the sterically protected region downstream of the BP but >10 bp upstream of the canonical 3’SS are susceptible to cryptic 3’SS selection in SF3B1 mutants. We assessed the functional impact of SF3B1 mutation and found that the cryptic 3’SSs are typically used at low frequency in the SF3B1 mutants (<10% relative to the canonical splice site) and are sometimes present in the SF3B1 wild-types but at an even lower frequency (<0.5% relative to the canonical splice site). However, we identified 10 candidate genes, some previously implicated in tumorigenesis, for which there is a high amount of out-of-frame cryptic splice site usage that may affect the function of these genes.
Results
Cryptic 3’ splice sites 10–30 bp upstream of canonical 3’ splice sites are used in SF3B1 mutants
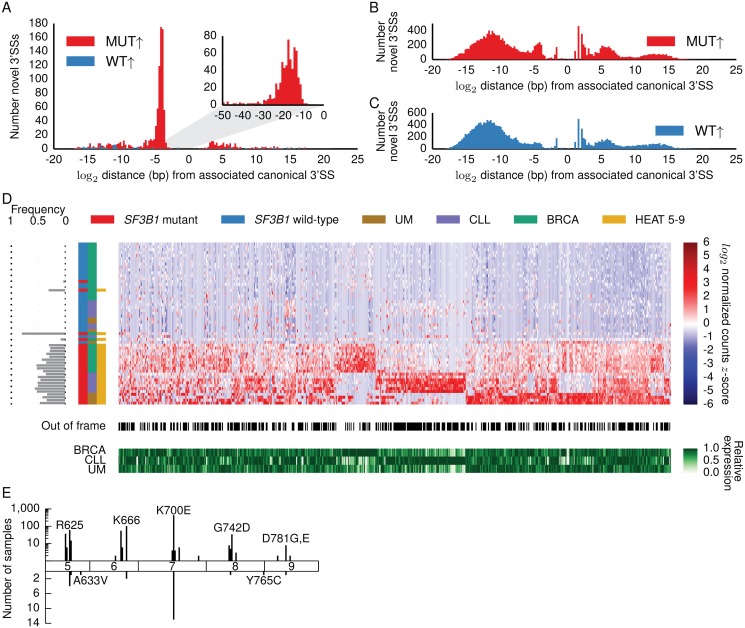
We used RNA-sequencing data from SF3B1 mutated and SF3B1 wild-type chronic lymphocytic leukemia (CLL; seven mutant, nine wild-type), breast cancer (BRCA; 14 mutant, 18 wild-type), and uveal melanoma (UM; four mutant, four wild-type) samples (S1 Fig., S1 File) to test 219,476 splice junctions present in the Gencode v14 gene annotation [19] along with 87,941 novel splice junctions (not annotated in Gencode) for differential usage by comparing junction-spanning reads using a generalized linear model as implemented in DEXSeq [20]. A splice junction is considered differentially used between mutant and wild-type samples if the expression level of that junction differs significantly after accounting for overall expression differences of the corresponding gene locus. All tested junctions were covered by at least 20 reads summed over all cancer samples in a given analysis, shared a 5’ splice site and/or 3’SS with a Gencode splice junction, and had a known splice site motif. We identified 1,749 junctions that were significantly differentially used between the SF3B1 mutant and SF3B1 wild-type samples across the three tumor types including 1,330 novel junctions, of which 1,117 are novel 3’SSs (BH-adjusted p < 0.1, S2 File). These 1,749 significant junctions were highly enriched for novel splice junctions compared to annotated junctions (Fisher exact, p < 10-200) and the novel junctions were enriched for novel 3’SSs (Fisher exact, p < 10-200) showing that SF3B1 mutations result in the usage of a large number of novel 3’SSs. These 1,749 significant junctions include 61 of 79 splice sites recently reported as specific to CLL cases with SF3B1 mutations [11] supporting the specificity of our approach while demonstrating an increased sensitivity that has allowed us to identify many more cryptic 3’SSs than previously reported. We plotted the distance between each significant novel 3’SS and its associated canonical 3’SS (defined as the nearest Gencode 3’SS that shared the same 5’ splice site—see Methods). Of the 1,117 significant novel 3’SSs, 619 were proximal cryptic 3’SSs clustered 10–30 bp upstream of their associated canonical 3’SSs while the remaining 498 cryptic 3’SSs were widely distributed (herein referred to as distal cryptic 3’SSs) (Fig. 1A, S3 File). All of the 619 proximal cryptic 3’SSs were used more often in the SF3B1 mutant samples compared to the wild-type samples and 58% were out-of-frame relative to the nearby canonical 3’SSs, suggesting that these are not canonical 3’SSs missing from Gencode. 417 of the 498 distal cryptic 3’SSs were also used more highly in the SF3B1 mutants (S4 File). The distribution of the 1,117 significant novel 3’SSs is different from that of novel 3’SSs whose usage did not differ significantly between the SF3B1 mutants and wild-types (Fig. 1B,C), further demonstrating that the usage of proximal cryptic 3’SSs is a property of SF3B1 mutants. Examining each tumor type individually, we observed the same enrichment of cryptic 3’SSs 10–30 bp upstream of canonical splice sites (S2 Fig.). Given these observations, SF3B1’s role in binding the BP, and the organization of the BP and splicing motifs in the last 30 bp of the intron [12], we focused our initial analyses on the 619 proximal cryptic 3’SSs.
Fig 1. Proximal cryptic 3’SSs used significantly more often in cancers with SF3B1 hotspot mutations.
log2 distance in base pairs from associated canonical 3’SSs to (A) 1,117 significantly differentially used novel 3’SSs, (B) 16,673 novel 3’SSs with canonical intron motifs (GT/AG) used more highly in the mutants but not significant, and (C) 18,660 novel 3’SSs with canonical intron motifs (GT/AG) used more highly in the wild-types but not significant. Zero represents the position of the canonical 3’SS. Negative and positive distances indicate that the cryptic 3’SS is respectively upstream or downstream from the canonical 3’SS. Inset in (A) shows base-by-base binning from zero to 50 base pairs upstream of canonical 3’SS. Red and blue histograms represent junctions with significantly higher usage in SF3B1 mutants or SF3B1 wild-type samples, respectively. (D) Upper red and blue heatmap shows for each sample the log2 library-normalized count z-score for 619 cryptic 3’SSs used significantly more often in the SF3B1 mutants and located 10–30 bp upstream of canonical 3’SSs (DEXSeq, BH-adjusted p < 0.1). Grey bars at left indicate frequency of SF3B1 mutant allele in RNA-seq data. Colorbars indicate SF3B1 mutation status, cancer type, and whether the SF3B1 mutation is located in the HEAT 5–9 repeats. Black and white colorbar indicates whether novel 3’SSs are out-of-frame (black) relative to canonical 3’SSs. Bottom green heatmap shows relative expression levels for the genes containing each cryptic 3’SS. We calculated the average expression of each gene in each cancer type and normalized by the maximum expression for each gene so that the maximum value in each column is one (see Methods). Cryptic 3’SSs not observed in all cancer types tend to have differing gene expression levels between cancers. (E) Locations and frequency of SF3B1 mutations in HEAT repeats 5–9. Mutations observed more than once in COSMIC (upper axis) cluster in ~10 amino acid hotspots in each HEAT repeat; most frequent mutation in each hotspot is labeled. Bottom axis shows locations and frequency of mutations in our study. BRCA samples with A663V and Y765C mutations do not show evidence for cryptic 3’SS selection.
Cryptic 3’SS selection is limited to tumors with mutations in HEAT repeat hotspots
We clustered all samples based on the read coverage of the 619 proximal cryptic 3’SSs and found that four SF3B1-mutated BRCA samples did not cluster with the other mutants (Fig. 1D). The SF3B1 mutation for one of these BRCA samples was a nonsense mutation not located in the HEAT 5–9 repeats while another sample had a subclonal (8.4%) HEAT 5–9 mutation with attenuated cryptic 3’SS selection (S3 Fig.). The other two samples had mutations in the HEAT 5–9 repeats but outside of the apparent ~10 amino acid mutational hotspots (Fig. 1E). We observed cryptic 3’SS selection in a TCGA lung adenocarcinoma sample with a hotspot mutation but not in lung cancer samples with SF3B1 mutations outside of the five hotspots (S4 Fig.). These results show that cryptic 3’SS selection only occurs in tumors carrying mutations in one of the five ~10 amino acid hotspots in the HEAT 5–9 repeats and is not limited to cancers in which SF3B1 is recurrently mutated.
Cryptic 3’SSs are shared across different cancer types
The majority of the 619 proximal cryptic 3’SSs were used in SF3B1-mutated samples in all three cancer types suggesting that the mechanism of cryptic 3’SS selection in SF3B1-mutated tumors is the same between different cancers (Fig. 1D). Some cryptic 3’SSs were not used in one or two of the cancer types due to lower expression of the corresponding genes in those cancers. Differences in cryptic 3’SS usage due to varying gene expression may contribute to the divergent prognostic implications of SF3B1 mutation in various cancers [2,7].
To characterize the roles of the genes affected by cryptic 3’SS usage, we performed a gene set enrichment analysis for the 912 genes that contained the 619 proximal and 417 distal cryptic 3’SSs used significantly more often in the SF3B1 mutant samples (S5 File). The gene set with the second smallest p-value consists of genes up-regulated in chronic myelogenous leukemia and the seventh gene set contains genes up-regulated in aggressive uveal melanoma samples (GSEA [21], q < 10-35). These results may reflect the fact that we are more likely to identify cryptic 3’SSs in genes that are highly expressed which may bias such a gene set enrichment analysis. Nonetheless, several gene sets with potential importance for cancer development are enriched such as genes positively correlated with BRCA1, ATM, and CHEK2 expression across normal tissues (GSEA, q < 10-28).
Cryptic 3’SSs are located ~13–17 bp downstream of the branch point
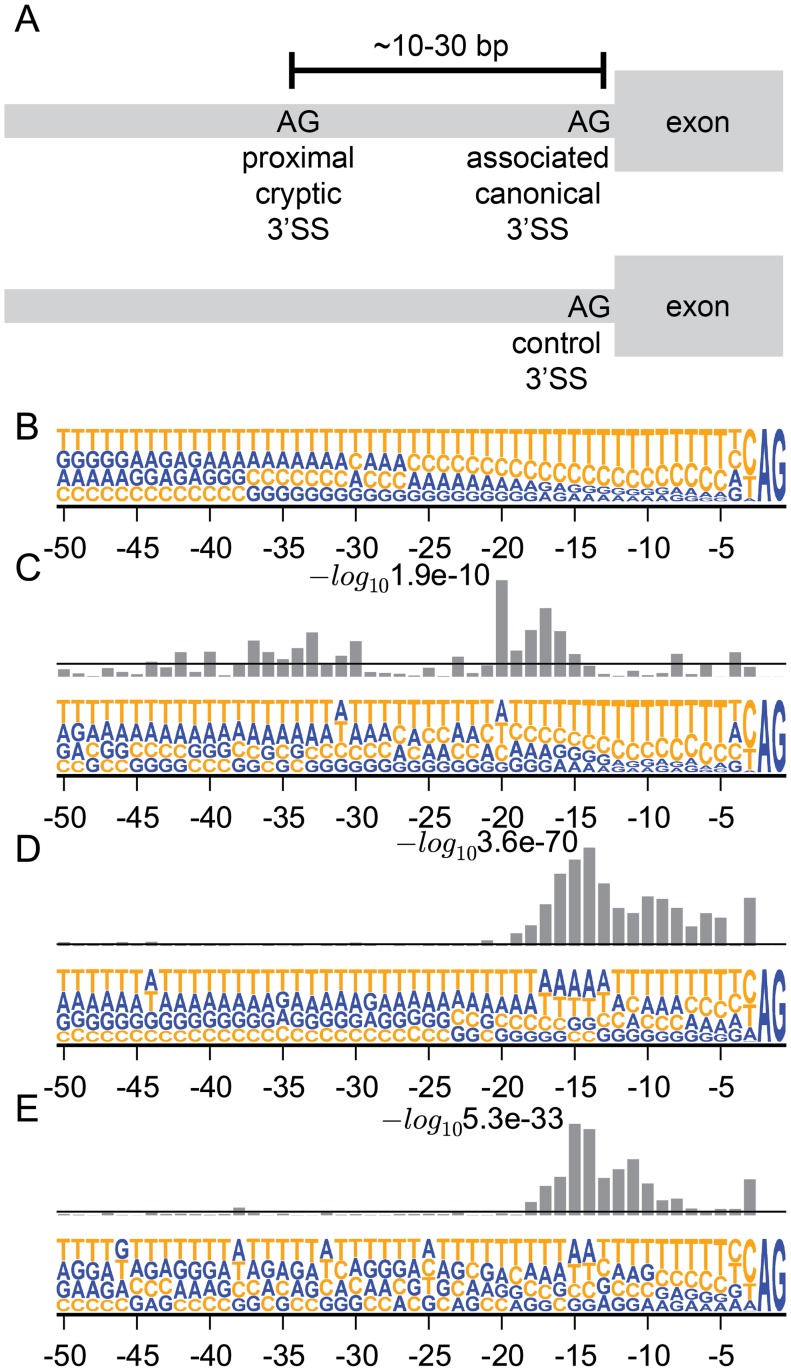
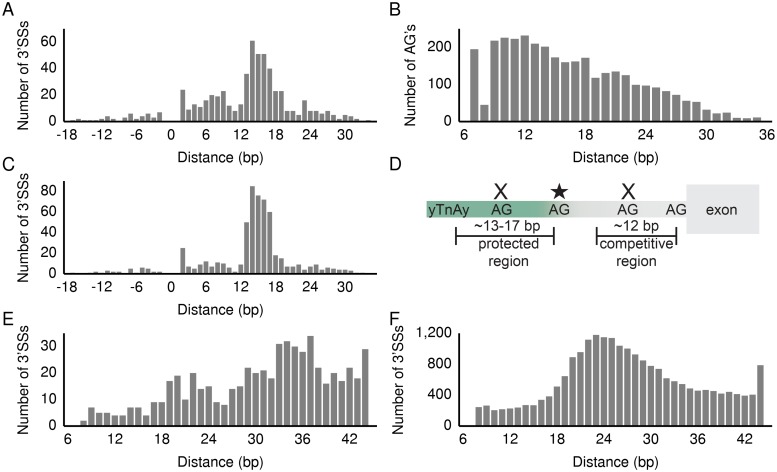
We characterized the sequence features of the 619 proximal cryptic 3’SSs and their associated canonical 3’SSs to gain further insights into the mechanism of cryptic 3’SS selection (Fig. 2A). We chose 23,066 control 3’SSs (see Methods) and plotted the nucleotide frequency [22] for the last 50 bp of the introns for all control, associated canonical, and cryptic 3’SSs as well as the enrichment of adenines relative to the control introns. The control introns have a typical nucleotide composition with a 4–24 bp PPT preceding the 3’SS (Fig. 2B) [13]. The associated canonical 3’SS introns are enriched for adenines ~15–20 bp upstream of the 3’SS since the proximal cryptic 3’SSs are located in this region (Fig. 2C). However, the introns for proximal (Fig. 2D) and distal (Fig. 2E) cryptic 3’SSs have a strong enrichment of adenines concentrated ~15 bp upstream of the splice sites. These results suggest that the increased usage of the 619 proximal and 417 distal cryptic 3’SSs in the SF3B1 mutants may result from the same mechanism. The human BP motif is highly degenerate except for a largely invariant adenine [13] leading us to suspect that the adenine signal upstream of the cryptic 3’SSs is caused by the associated canonical 3’SSs’ BP adenines. We used SVM_BP [23] to predict BPs for the associated canonical 3’SSs and calculated the distance from the highest scoring predicted BPs to the cryptic splice sites. We found that AG dinucleotides that serve as cryptic 3’SSs are enriched ~13–17 bp downstream from the predicted BP (Fig. 3A) relative to random AG dinucleotides present in control 3’SS introns (Fig. 3B, p < 10-7, Mann Whitney U). For cryptic 3’SSs not located 13–17 bp downstream from the highest scoring BP in Fig. 3A, we calculated the distance from the second highest scoring BP to the cryptic 3’SSs and found that overall, the majority of the cryptic 3’SSs were located 13–17 bp from either the highest or second highest scoring BP (Fig. 3C).
Fig 2. 3’ intron nucleotide composition for control, associated canonical, and cryptic 3’SSs.
(A) We identified 23,066 control 3’SSs whose junctions had a mean coverage greater than 100 reads over all CLL, BRCA, and UM samples to compare to the cryptic and associated canonical 3’SSs. Nucleotide frequency for the last 50 bp of the intron for (B) 23,066 control 3’SSs; (C) 613 associated canonical 3’SSs; (D) 619 proximal cryptic 3’SSs; and (E) 417 distal cryptic 3’SSs. Bar plots above each nucleotide composition plot are log10 p-values from Fisher exact tests for enrichment of adenines at each position relative to control 3’SSs. Horizontal line marks significance level of p = 0.05. (-log10 0.05 ≈ 1.3). The p-value box plots have different scales in (C), (D), and (E); the smallest p-values for each panel are labeled.
Fig 3. Location of predicted branch point relative to cryptic and canonical 3’SSs and model of cryptic 3’SS selection.
(A) Distance from highest scoring BP predicted for associated canonical 3’SSs to the corresponding proximal cryptic 3’SSs. A negative distance indicates that the cryptic 3’SS is upstream of the BP predicted for the canonical 3’SS. The small spike at 2 bp indicates that in a few cases the adenine in the cryptic 3’SS is predicted to be the BP adenine for the canonical 3’SS. (B) Distance from highest scoring BP predicted for control 3’SSs to downstream intronic AG dinucleotides that are not annotated as 3’SSs. (C) Distance from either highest or second highest scoring BP predicted for canonical 3’SSs to their associated cryptic 3’SSs (see Methods). (D) Model for proximal cryptic 3’SS selection in SF3B1 mutants. yTnAy is the human BP motif. AG dinucleotides located at the edge of the sterically protected region can be used as 3’SSs in SF3B1 mutants (star). AG dinucleotides located in the protected or competitive regions (X’s) are respectively sterically hindered from being selected as 3’SSs or out-competed by the canonical 3’SS. Distance from predicted BP to 3’SS for (E) associated canonical 3’SSs and (F) control 3’SSs (see Methods) is significantly different (p < 10-23, Mann Whitney U).
Proposed mechanism of cryptic 3’SS selection
3’SSs are typically not located within ~12–18 bp downstream of the BP because the proteins bound to the BP sterically hinder AG dinucleotides in this region and prevent them from being used as 3’SSs [16]. Our results suggest that AG dinucleotides serving as cryptic 3’SSs in SF3B1 mutants are located at the end of this sterically protected region downstream of the BP (Fig. 3D). Additionally, during the splicing reaction, the spliceosome searches ~12 bp downstream from the first 3’SS after the BP for any other 3’SSs and chooses the strongest 3’SS based on sequence features [16]. The lack of cryptic 3’SSs in the last 10 bp of the intron (Fig. 1A) indicates that cryptic 3’SSs used in SF3B1 mutants are located far enough upstream of the associated canonical 3’SSs to avoid competition for splicing. We observed that the distance between associated canonical 3’SSs and their predicted BPs is significantly greater than the distance between control 3’SSs and their BPs such that the cryptic 3’SSs at the edge of the protected region do not compete with the canonical 3’SS for splicing (p < 10-23, Mann Whitney U, Fig. 3E,F). We also predicted BP’s for the 619 proximal and 417 distal cryptic 3’SSs (as opposed to above where we predicted BP’s for the canonical 3’SSs associated with the 619 proximal 3’SSs) and found that the majority of these cryptic 3’SSs were 13–17 bp downstream of their predicted BP’s (S5 Fig.) providing further evidence that most cryptic 3’SSs (both proximal and distal) associated with SF3B1 mutations are located at the edge of the sterically protected region.
Our results suggest that the mechanism of cryptic 3’SS selection in SF3B1 mutants is not altered BP recognition because a more varied distribution of distances from the cryptic 3’SS to the canonical 3’SS BP would be expected if BP recognition was altered. Studying the role of cryptic 3’SS in inherited Mendelian disease genes, Královicová et al. 2005 used splicing reporters with cryptic 3’SSs located in the PPT and found that moving the cryptic 3’SS into the ~12–18 bp sterically protected region reduced or eliminated cryptic 3’SS selection. On the other hand, moving an AG dinucleotide out of the sterically protected region allowed for its selection as a cryptic 3’SS [18]. These published experimental results and the rigid distance between the BP and the cryptic 3’SSs observed in our study are consistent with a model of altered 3’SS selection in SF3B1 mutants due to a change in the size of the sterically hindered region downstream of the BP.
To test whether the sequences requirements defined here are sufficient for cryptic 3’SS usage, we identified 11,302 introns whose canonical 3’SSs passed our coverage cutoff of 20 reads summed over all samples and had potential cryptic 3’SSs (intronic AG dinucleotides that were 10–30 bp upstream of an annotated 3’SS and 13–17 bp downstream of the highest-scoring predicted BP). For 900 of these introns, the potential cryptic 3’SSs also passed the coverage cutoff, of which 310 were used significantly more often in the SF3B1 mutants. This analysis demonstrates that not every potential cryptic 3’SS is differentially used in the mutants, so the sequence requirements described here appear to be necessary for cryptic 3’SS usage but not sufficient.
Cryptic 3’SSs are used infrequently relative to canonical 3’SSs
Although the cryptic splice sites described here are used significantly more often in the SF3B1 mutants, the biological effects are likely dependent on the proportion of transcripts that use the cryptic 3’SSs relative to the canonical 3’SSs. We therefore calculated the percent spliced in (PSI) for the proximal cryptic 3’SSs relative to their associated canonical 3’SSs in the CLL samples since they have a higher sequencing depth than the other tumor samples (S1 Fig.) that allows for more accurate quantification of splicing and because the distribution of well-characterized low- and high-risk CLL prognostic factors was similar between the SF3B1 mutated and wild-type samples (Fig. 4A). To calculate PSI for the 325 proximal cryptic 3’SSs used significantly more often in the SF3B1 mutants from the CLL-only analysis (S6–S7 Files), we divided the number of reads that span the cryptic 3’SS by the number of reads that span both the cryptic 3’SS and its associated canonical 3’SS. We observed that some cryptic 3’SSs are used exclusively in SF3B1 mutants while others are also used in SF3B1 wild-type samples but at a lower frequency relative to the mutants (Fig. 4A). 67% of the cryptic 3’SSs were included in <10% of transcripts compared to their associated canonical 3’SS. These results suggest that the cryptic splice sites are either included rarely even in the SF3B1 mutants or that transcripts with cryptic splice sites are subject to a higher rate of nonsense-mediated decay (NMD). To investigate the potential role of NMD, we identified differentially expressed genes between the SF3B1 mutant and wild-type samples in a joint analysis of all three cancers and performed a gene set enrichment analysis. We found that genes in the “Reactome NMD enhanced by the exon junction complex” set were enriched (GSEA [21], q < 10-28) among the 272 differentially expressed genes (DESeq2, BH-adjusted p < 0.1, S8–S9 Files) suggesting that NMD may be different between the SF3B1 mutants and wild-types. 33 of the 582 genes that contained the 619 proximal cryptic 3’SSs were differentially expressed with the expression of 29/33 of these genes lower in the SF3B1 mutants. Genes containing a proximal cryptic 3’SSs were more likely to be differentially expressed (Fisher exact, p < 10-8) and more likely to have lower expression in SF3B1 mutants (Fisher exact, p = 0.0009). These results suggest that cryptic 3’SS selection may affect gene expression for a subset of genes. However, the observation that in-frame cryptic 3’SSs likely not subject to NMD and out-of-frame cryptic 3’SSs potentially subject to NMD are included at similar rates relative to their associated canonical 3’SSs (Fig. 4A) suggests that most genes’ expression are not affected by cryptic 3’SS selection and most cryptic 3’SSs are observed at a low frequency because they are spliced in infrequently compared to their associated canonical 3’SSs.
Fig 4. Percent spliced in for cryptic 3’ splice sites in CLL analysis.
(A) Heatmap shows the percent spliced in (PSI) values for cryptic 3’SS relative to the canonical 3’SS in CLL SF3B1 mutated or wild-type samples for 325 proximal cryptic 3’SSs used significantly more often in the CLL mutants (DEXSeq, BH-adjusted p < 0.1). SF3B1 mutation presence and the status of prognostic factors IGHV and ZAP70 are shown in left colorbars. Black and white colorbar indicates whether novel 3’SSs are out-of-frame (black) relative to canonical 3’SSs. In-frame and out-of-frame cryptic 3’SSs are used at similar rates relative to their associated canonical 3’SSs. (B) Beeswarm plots indicating the PSI values for the cryptic 3’SS relative to the associated canonical 3’SS in ten genes with high levels of cryptic 3’SS inclusion in CLL SF3B1 mutants (M) compared to wild-type (W) samples. No reads were observed spanning the cryptic YIF1A junction in any wild-type CLL samples. The number in the upper corner of each plot is the distance in base pairs from the highest or second-highest scoring BP predicted for the associated canonical 3’SS to the cryptic 3’SS.
To identify cryptic 3’SSs with relatively high PSI values in the SF3B1 mutant versus wild-type samples, we searched for cryptic 3’SSs that were 1) used more than 50% of the time in the CLL SF3B1 mutants; 2) used less than 20% of the time in wild-type samples; and 3) had an average coverage of at least 30 junction-spanning reads in the mutant samples. Despite the generally low PSI values for the 325 cryptic 3’SSs from the CLL-only analysis, we identified four genes previously implicated in cancer (TTI1 [24–26], MAP3K7 [27–29], FXYD5 [30], PFDN5 [31]) and six others (YIF1A, ORAI2, ZNF91, ZNF548, RP11–1280I22.1, RP11–532F12.5) with out-of-frame cryptic 3’SSs that were consistently preferred to the associated canonical 3’SS in the CLL SF3B1 mutant samples (Fig. 4B). Ferreira et al. identified the junctions in ORAI2, ZNF91, and TTI1 in CLL SF3B1 mutants as well [11]. Nine of the ten junctions were significant in our BRCA-only analysis and showed high differences in relative inclusion (S6 Fig., S10–S11 Files). These genes are not differentially expressed between the CLL SF3B1 mutant and wild-type samples (S12 File) but the frequent inclusion of out-of-frame cryptic 3’SSs may affect their biological function.
Discussion
Here we have shown that a consequence of SF3B1 mutations in different cancer types is genome-wide selection of hundreds of cryptic 3’SSs. We have shown the cryptic 3’SSs have specific sequence requirements; AG dinucleotides used as cryptic 3’SSs in SF3B1 mutants are located at the end of the sterically protected region ~13–17 bp downstream of the BP but are >10 bp upstream of nearby canonical 3’SSs allowing them to avoid competition for splicing. These sequence requirements limit the introns susceptible to cryptic 3’SS selection to those where the BP is located farther from the 3’SS than the typical ~24 bp. While these requirements appear necessary for cryptic 3’SS usage, they are not sufficient, as we did not detect cryptic 3’SS usage in all introns with AG dinucleotides that satisfy these requirements. Characteristics such as RNA conformation, RNA binding protein sites, BP prediction inaccuracies, cryptic or downstream canonical 3’SS strength, gene/transcript expression, sequencing depth, or other factors may also play a role in determining whether cryptic 3’SSs are used and detected by RNA sequencing.
Examining differential splice junction usage allowed us to identify many more cryptic 3’SSs than previous studies while still identifying 61 of 79 cryptic 3’SSs recently reported for CLL SF3B1 mutants using a method based on relative inclusion [5,6,8,10,11]. When examining the three cancer types in our study individually, the number of cryptic 3’SSs identified was highly dependent on the sequencing depth of the samples (S1–S2 Figs., S2 File). Additionally, examining cryptic 3’SSs expressed higher in the SF3B1 mutants but not significantly (Fig. 1B) shows a modest enrichment of novel 3’SSs 10–30 bp upstream of canonical 3’SSs. These observations suggest that deeper sequencing will continue to reveal proximal cryptic 3’SSs in SF3B1 mutants that are used very infrequently or are present in lowly expressed genes.
Selection of cryptic 3’SSs in the region downstream of the BP has been reported for some inherited diseases including those resulting from disrupted tumor suppressor genes such as ATM, NF1, and TP53 [18]. Using a curated a list of aberrant splice sites associated with different diseases from the literature, Královicová et al. 2005 found that in cases where cryptic 3’SS selection was not caused by mutation of the 3’YAG consensus sequence, cryptic 3’SSs were often located ~19 bp upstream of associated canonical 3’SSs and ~11–15 bp downstream of the BP [18]. Most of the diseases considered in Královicová et al. 2005 are Mendelian diseases where a cryptic 3’SS disrupts or abolishes the function of a single disease gene. In these cases, a mutation in the PPT between the sterically protected and competitive regions has introduced a cryptic 3’SS (Fig. 3D). For cancers with SF3B1 mutations, we suspect that the size of the sterically protected region is slightly altered allowing for existing AG dinucleotides to be used as cryptic 3’SSs in hundreds of genes. It is also possible SF3B1 mutations could cause destabilization of the U2 snRNP complex or alter interactions with U2AF2, affecting the ability to recognize the canonical 3’SS and leading to cryptic 3’SS selection. However, the rigid distance (~13–17 bp) from the predicted BPs to the cryptic 3’SSs for most of the cryptic 3’SSs is most consistent with a change in the size of the sterically protected region downstream of the branch point.
We found that cryptic 3’SS selection is limited to tumors with mutations in the five ~10 amino acid hotspots in the SF3B1 HEAT 5–9 repeats and that these mutations are associated with cryptic 3’SS selection across different cancer types and even in cancers in which SF3B1 is not recurrently mutated. 58% of these cryptic 3’SSs are out-of-frame relative to nearby canonical 3’SSs, but the biological impact of these cryptic 3’SSs is likely a function of how frequently they are used relative to the nearby canonical 3’SSs. We found that while the cryptic 3’SSs are used more often in the SF3B1 mutated samples compared to wild-type samples, they are used relatively infrequently (<10%) compared to nearby canonical 3’SSs. While the differentially expressed genes between the SF3B1 mutated and wild-type samples are enriched for genes in the NMD pathway, even in-frame cryptic 3’SSs are used at a low frequency indicating that the associated canonical 3’SS is mostly preferred to the cryptic 3’SS even in SF3B1 mutants. Nonetheless, we identified ten genes, including four with known roles in cancer, which had a high frequency of cryptic splice site usage relative to the nearby canonical splice site. Further studies are required to determine whether low-frequency cryptic 3’SS selection in hundreds of genes, high-frequency cryptic 3’SS selection in a small group of genes, and/or other splicing alterations drive the oncogenic effect of SF3B1 mutation.
Methods
Sample selection
Ethics statement. For the chronic lymphocytic leukemia (CLL) samples, the UCSD IRB approved the study and all subjects gave informed consent (Project #080918). Refer to the informed consent for The Cancer Genome Atlas and Harbour et al. for consent information for other cancer samples [7].
CLL. Seven SF3B1-mutated CLL cases and nine SF3B1 wild-type CLL cases were identified from the CLL Consortium database. The mutations were originally characterized by PCR and verified in the RNA-sequencing data [9]. Sample dates were chosen on average 95 days prior to treatment and at least 287 days after prior treatment to select samples with high tumor cell count. Samples were chosen to have relatively similar numbers of IGHV mutated/unmutated and ZAP-70 positive/negative samples (Fig. 4).
BRCA, LUAD, and LUSC. SF3B1 mutant samples were identified using the Broad GDAC TCGA analysis (http://gdac.broadinstitute.org/runs/analyses__2013_02_22/) in TCGA tumor types with no publication restrictions. Samples with SF3B1 mutations outside of Gencode version 14 exons were excluded. We excluded any cancer types with less than four SF3B1 mutants or for which paired-end RNA-sequencing data was not available leaving breast cancer (BRCA), lung adenocarcinoma (LUAD), and lung squamous cell carcinoma (LUSC). We chose 1.25 as many SF3B1 wild-type controls as mutated samples for each cancer type randomly from samples without mutations in SF3B1 or other splicing factors. RNA sequencing data was downloaded from CGHub [32].
UM. Uveal melanoma samples were downloaded from the Short Read Archive (SRA062359) [7]. As reported in Furney et al., four uveal melanoma samples had SF3B1 mutations in codon 625 and four had wild-type copies of SF3B1 [33].
Library preparation and sequencing for CLL samples
RNA was extracted from peripheral blood mononucleocytes from seven SF3B1-mutated CLL cases and nine SF3B1 wild-type cases per the manufacturer’s specifications using Qiagen RNeasy mini-spin columns, and RIN scores determined using an Agilent Bioanalyzer. RNA was polyA selected and processed using SMART cDNA synthesis (Clontech) to prepare sequencing libraries. Samples were sequenced on Illumina HiSeq2000 instruments generating an average of 239 million paired 75 bp reads per sample (S1 Fig.).
Adapter trimming
Sequencing adapters and poly-A/T tails were trimmed for CLL samples only using cutadapt version 1.1 (-m 20—n 10—b AAGCAGTGGTATCAACGCAGAGTACTTTTTTTTTTT—b AAGCAGTGGTATCAACGCAGAGTACGCGGG—b AAGCAGTGGTATCAACGCAGAGT—b TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTT—b AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA) [34]. Read pairs where or one of both reads were of length less than 20 were removed.
Read alignment
RNA-seq reads were aligned to the human genome (hg19) using STAR 2.3.0e (—alignSJDBoverhangMin 1—seedSearchStartLmax 12—alignSplicedMateMapLminOverLmate 0.08—outFilterScoreMinOverLread 0.08—outFilterMatchNminOverLread 0.08—outFilterMultimapNmax 100—outFilterIntronMotifs RemoveNoncanonicalUnannotated—outSJfilterOverhangMin 6 6 6 6) and a splice junction database consisting of junctions from Gencode, UCSC knownGene, AceView, lincRNAs, and H-Inv [19,35–39]. Duplicate read pairs were removed prior to alignment by comparing the sequences of all read pairs and keeping only one read pair per set of read pairs with identical sequences.
Splice junction read coverage
Splice junction read coverages were obtained from the SJ.out.tab output file from STAR.
Novel splice junction identification
Novel splice junctions were defined as those junctions identified by STAR not present in Gencode version 14 that (i) were covered by at least 20 reads summed over all cancer samples in a given analysis, (ii) shared a 5’ splice site and/or 3’SS with a Gencode junction, and (iii) had one of the following motifs: GU/AG, CU/AC, GC/AG, CU/GC, AU/AC, GU/AU. Novel junctions were calculated separately for each analysis.
Splice junction usage
Known and novel junctions that had a coverage of at least 20 reads over all samples, used a known intron motif, and contained a known Gencode 5’ splice site or 3’SS were aggregated by gene and tested for differential usage using DEXSeq’s testForDEUTRT function (v1.8.0, R v3.0.3) [20]. Splice junctions used in more than one Gencode gene were removed. When multiple cancer types were analyzed, we provided cancer type as a covariate to DEXSeq. Raw p-values were adjusted for multiple hypothesis testing using the Benjamini Hochberg procedure. To examine the impact of the coverage cutoff of 20 reads summed over all samples on our results, we increased the cutoff to 50, 75, and 100 reads summed over all samples and found that 42%, 32%, and 24% of the significant novel 3’SSs remained at each of these cutoffs. The enrichment for proximal cryptic 3’SS remained at all cutoffs, so we used the 20 read cutoff to maximize sensitivity.
Identification of associated canonical 3’SSs for cryptic 3’SSs
Associated canonical 3’SSs were identified for novel/cryptic 3’SSs as follows. First, all Gencode splice sites that shared a 5’ splice site with the novel 3’SS were identified. Then, the closest Gencode 3’SS from these splice sites that was downstream of the cryptic 3’SS was chosen as the associated canonical 3’SS for that cryptic 3’SS. If there was no Gencode 3’SS downstream of the cryptic 3’SS, the closest Gencode 3’SS upstream of the cryptic 3’SS was chosen as the associated canonical 3’SS.
Gene set enrichment for genes with cryptic 3’SS usage
We performed a gene set enrichment analysis using GSEA [21] for the genes that contained cryptic 3’SSs by combining the genes that contained the 619 proximal (S3 File) and the 417 distal cryptic 3’SSs (S4 File).
Identification of control 3’SSs
We identified 23,066 control 3’SSs by choosing splice sites that are annotated in Gencode, whose average coverage over BRCA, CLL, and UM samples is greater than 100, and whose 5' splice site does not have any novel 3'SSs. We characterized intronic AG dinucleotides for these control junctions by analyzing the intronic sequence downstream of the predicted branch points minus the last 10 bp of the intron since alternative 3’SSs can be located in the last 10 bp of the intron.
Hierarchical clustering
All heatmap rows and columns were clustered using scipy.cluster.hierarchy.linkage with either the “complete” or “single” distance metric.
SF3B1 mutant allele frequency
Mutant allele frequency was determined by calculating per-base coverages using unique properly paired reads with samtools mpileup for the SF3B1 locus and counting the number of reads supporting either the reference or alternate alleles.
Gene expression
Reads that were not contained within Gencode v14 exons in the STAR genomic alignment were discarded. The remaining reads were re-aligned to the Gencode v14 transcriptome using Bowtie2 (v2.1.0,-t-k 400-X 400—no-mixed—no-discordant) and transcript expression was estimated using eXpress (v1.3.0,—max-indel-size 20) [40,41]. Gene expression was estimated by summing together the effective counts or FPKM values for all transcripts contained in a gene.
Relative average expression of genes with cryptic 3’SSs
For the green heatmap in Fig. 1D, the average expression (FPKM) of each gene containing a cryptic 3’SS was determined for each cancer type. The average expression values were then normalized for each gene by dividing by the largest average expression of the three cancers for that gene. Therefore each column in the green heatmap in Fig. 1D has one value of 1.0 while the other two values are between 0.0 and 1.0 and represent the expression of the gene in that cancer relative to the maximum.
Definition of HEAT repeats
HEAT repeat locations were defined according to the definition of HEAT repeats in Wang et al. 1998 [15].
COSMIC SF3B1 mutations
COSMIC v66 complete export was downloaded and the number of mutations at each location in the SF3B1 heat domains 5–9 was plotted for locations with at least two observed mutations in COSMIC [42].
Nucleotide frequency plots
Nucleotide frequency plots were constructed using WebLogo (unit_name = ’probability’) [22]. Adenine enrichment was calculated by counting the number of adenines and non-adenines at each intron position for a given splice site class and comparing to the number of adenines and non-adenines in control 3’SSs using a Fisher exact test.
Branch point identification
SVM_BP was used to predict branch points [23]. The SVM_BP code was altered to allow for branch points eight bp from the 3’SS by setting mindist3ss = 8 in svm_getfeat.py (see https://github.com/cdeboever3/svm-bpfinder). SVM_BP was run with options “Hsap 50.” When multiple branch points were predicted for one 3’SS, we chose the branch point with the highest sequence score (bp_scr). In some instances, there was more than one cryptic 3’SS associated with a canonical 3’SS, so we randomly chose only one of these cryptic splice sites for further analysis. For Fig. 3C, we plotted the distance from highest scoring BP predicted for canonical 3’SSs to their associated cryptic 3’SSs as in Fig. 3A. However, the distances for cryptic 3’SSs located less than 13 bp or more than 17 bp from the BP in Fig. 3A were replaced with the distance from the second highest scoring BP. S5C–S5D Fig. were created similarly.
Differential gene expression
Gene expression was estimated as described above. We summed the effective counts from eXpress for all transcripts from each gene to obtain effective read counts for each gene. We provided these read counts to DESeq2 (v1.2.10, R v3.0.3) and tested for differential gene expression using nbinomWaldTest using cancer type as a covariate for the analysis with different cancers [43]. We only tested genes where the sum of effective read counts over all samples was greater than 100. p-values were adjusted using the Benjamini-Hochberg procedure. Gene set enrichment analysis was performed using GSEA [21].
Percent spliced in for cryptic 3’SSs relative to associated canonical 3’SSs
Percent spliced in (PSI) values for cryptic 3’SSs relative to canonical 3’SSs were calculated by dividing the number of reads that span the cryptic 3’SS (c) by the number of reads that span the cryptic 3’SS plus the number of reads that span the canonical 3’SS (a), , for each sample. The ten 3’SSs with high PSI values in CLL were identified by identifying cryptic 3’SSs whose median PSI was greater than 50% in the CLL SF3B1 mutants but less than 20% in the wild-type samples and whose average coverage was at least 30 junction-spanning reads in the CLL mutant samples. These junctions were also chosen to be out-of-frame although the cryptic 3’SS in ORAI2 is located in the 5’ untranslated region.
Code, data, and reproducibility
We have made the code and intermediate data files needed to replicate this study available on Github (https://github.com/cdeboever3/deboever-sf3b1-2015) and Figshare (http://dx.doi.org/10.6084/m9.figshare.1120663). Instructions are provided in the Github repository for reproducing our figures, tables, and statistical analyses. Sequencing data is available through dbGaP (phs000767).
Supporting Information
We sequenced the transcriptomes of peripheral blood mononucleocytes from seven SF3B1-mutated chronic lymphocytic leukemia (CLL) cases and nine SF3B1 wild-type cases. We also obtained data from breast cancer (BRCA; 14 mutant, 18 wild-type), lung squamous cell carcinoma (LUSC; four mutant, five wild-type) and lung adenocarcinoma (LUAD; seven mutant, nine wild-type) samples from the TCGA and uveal melanoma (UM; four mutant, four wild-type) samples from Harbour et al. 2013.
(TIF)
log2 distance in base pairs from 280, 1,476, and 86 significantly differentially used novel 3’SSs (S2 File) to their associated canonical 3’SSs in (A) BRCA, (B) CLL, and (C) UM analyses respectively. Novel 3’SSs were associated with canonical 3’SSs only if they shared the same 5’ splice site. Zero represents the position of the canonical 3’SS. Negative and positive distances indicate that the cryptic 3’SS is respectively upstream or downstream from the canonical 3’SS. Inset shows base-by-base binning from zero to 50 base pairs upstream of canonical 3’SS. Red and blue histograms represent junctions with significantly higher usage in SF3B1 mutants or SF3B1 wild-type samples respectively. The number of cryptic 3’SS identified varied with the overall sequencing depth of the different data sets.
(TIF)
Heatmap shows for each BRCA sample the log2 library-normalized count z-score for 192 proximal cryptic 3’SSs used significantly more often in the SF3B1 mutants and located 10–30 bp upstream of canonical 3’SSs (S2 File). SF3B1 mutants are labeled with the observed missense or nonsense (*) mutation as well as the frequency of the mutant allele in the RNA-sequencing data. Attenuated cryptic 3’SS selection is visible for the K700E mutant with only 8.4% allele frequency. A633V and Y765C mutants do not show evidence for cryptic 3’SS selection. Black and white colorbar indicates whether novel 3’SSs are out-of-frame (black) relative to canonical 3’SSs.
(TIF)
Heatmap shows for each sample the log2 library-normalized count z-score for the 578 proximal cryptic 3’SSs used significantly more often in the SF3B1 mutants in the CLL, BRCA, UM, LUAD, and LUSC joint analysis (S2 File). Grey bars indicate frequency of SF3B1 mutant allele in RNA-seq data. Colorbars indicate SF3B1 mutation status, cancer type, and whether the SF3B1 mutation is located in the HEAT 5–9 repeats. Black and white colorbar indicates whether novel 3’SSs are out-of-frame (black) relative to canonical 3’SSs.
(TIF)
Distance from 3’SS to highest scoring predicted branch point (BP). We were able to predict BPs for (A) 584 of 619 proximal cryptic 3’SSs and (B) 405 of 417 distal cryptic 3’SSs (as opposed to predicting the BPs for the associated canonical 3’SSs as in Fig. 3). Distance from either highest or second highest scoring predicted BP to (C) proximal cryptic 3’SSs and (D) distal cryptic 3’SSs. Cryptic 3’SSs that are used more often in SF3B1 mutants have BPs located ~13–17 bp upstream regardless of whether they are 10–30 bp upstream of canonical 3’SSs.
(TIF)
Beeswarm plots showing the PSI values for the cryptic 3’SS relative to the associated canonical 3’SS in nine of ten genes with high levels of cryptic 3’SS inclusion in CLL SF3B1 mutants (M) compared to wild-type (W) samples that were also expressed in the BRCA samples. The number in the upper corner of each plot is the distance in base pairs from the highest or second-highest scoring BP predicted for the associated canonical 3’SS to the cryptic 3’SS.
(TIF)
SF3B1 mutated samples have columns for frequency of SF3B1 mutation in RNA-seq data, mutation type, codon change and whether the mutation is in the HEAT 5–9 repeats. These columns are empty for SF3B1 wild-type tumor samples.
(TSV)
DEXSeq was used to test for differential splice junction usage in a joint analysis of the CLL, BRCA, and UM samples as well as individually for each cancer type. “Novel” indicates that the junction is not annotated in Gencode. Proximal indicates that a novel 3’SS is 10–30 bp upstream of a canonical Gencode 3’SS.
(TSV)
Location of 5’ splice sites and 3’SSs are one-based coordinates that denote the start and end of the intron. The columns COSMIC, TSgene, and ncg denote whether the gene is present in COSMIC, TSGene, or the Network of Cancer Genes respectively.
(TSV)
Location of 5’ splice sites and 3’SSs are one-based coordinates that denote the start and end of the intron. The columns COSMIC, TSgene, and ncg denote whether the gene is present in COSMIC, TSGene, or the Network of Cancer Genes respectively.
(TSV)
(XLS)
Location of 5’ splice sites and 3’SSs are one-based coordinates that denote the start and end of the intron. The columns COSMIC, TSgene, and ncg denote whether the gene is present in COSMIC, TSGene, or the Network of Cancer Genes respectively.
(TSV)
Note that there are only 324 values because one canonical 3’SS was filtered due to low coverage so a PSI value could not be calculated.
(TSV)
(TSV)
(XLS)
Location of 5’ splice sites and 3’SSs are one-based coordinates that denote the start and end of the intron. The columns COSMIC, TSgene, and ncg denote whether the gene is present in COSMIC, TSGene, or the Network of Cancer Genes respectively.
(TSV)
Note that there are only 191 values because one canonical 3’SS was filtered due to low coverage so a PSI value could not be calculated.
(TSV)
(TSV)
Acknowledgments
The authors are grateful to the Chronic Lymphocytic Leukemia Research Consortium for providing the CLL samples and Marco A. Marra, Richard A. Moore, Joanne E. Johnson, Andrew J. Mungall and the Transcriptome Consortium at the Canada’s Michael Smith Genome Sciences Centre for performing transcriptome sequencing for the CLL samples.
Data Availability
We have made the code and intermediate data files needed to replicate this study available on Github (https://github.com/cdeboever3/deboever-sf3b1-2015) and Figshare (http://dx.doi.org/10.6084/m9.figshare.1120663). Instructions are provided in the Github repository for reproducing our figures, tables, and statistical analyses. Sequencing data is available through dbGaP (phs000767). Interested researchers must be approved for access by dbGaP to preserve patient anonymity.
Funding Statement
CD is supported in part by the University of California, San Diego, Genetics Training Program through an institutional training grant from the National Institute of General Medical Sciences (T32GM008666) and the California Institute for Regenerative Medicine (CIRM) Interdisciplinary Stem Cell Training Program at UCSD II (TG2-01154). PJS was supported by the UCSD Cancer Center Training Grant in Drug Development – the Cancer Therapeutics Training Program (T32CA121938). This study was supported by the National Cancer Institute (1R21CA152613), the National Institutes of Health CLL Research Consortium grant (PO1CA81534), and the CIRM Highly Active Anti-Leukemia Stem Cell Therapy (HALT) team grant (DR1-01430). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Watson IR, Takahashi K, Futreal PA, Chin L (2013) Emerging patterns of somatic mutations in cancer. Nature reviews Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wan Y, Wu CJ (2013) SF3B1 mutations in chronic lymphocytic leukemia. Blood 121: 4627–4634. 10.1182/blood-2013-02-427641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, et al. (2012) Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491: 399–405. 10.1038/nature11547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin M, Masshofer L, Temming P, Rahmann S, Metz C, et al. (2013) Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nature genetics 45: 933–U296. 10.1038/ng.2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, et al. (2011) Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 478: 64–69. 10.1038/nature10496 [DOI] [PubMed] [Google Scholar]
- 6. Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, et al. (2011) Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. The New England journal of medicine 365: 1384–1395. 10.1056/NEJMoa1103283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harbour JW, Roberson EDO, Anbunathan H, Onken MD, Worley LA, et al. (2013) Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nature genetics 45: 133–135. 10.1038/ng.2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quesada V, Conde L, Villamor N, Ordonez GR, Jares P, et al. (2012) Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nature genetics 44: 47–52. 10.1038/ng.1032 [DOI] [PubMed] [Google Scholar]
- 9. Schwaederle M, Ghia E, Rassenti LZ, Obara M, Dell'Aquila ML, et al. (2013) Subclonal evolution involving SF3B1 mutations in chronic lymphocytic leukemia. Leukemia 27: 1214–1217. 10.1038/leu.2013.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang L, Lawrence MS, Wan Y, Stojanov P, Sougnez C, et al. (2011) SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. The New England journal of medicine 365: 2497–2506. 10.1056/NEJMoa1109016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferreira PG, Jares P, Rico D, Gomez-Lopez G, Martinez-Trillos A, et al. (2013) Transcriptome characterization by RNA sequencing identifies a major molecular and clinical subdivision in chronic lymphocytic leukemia. Genome Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Padgett RA (2012) New connections between splicing and human disease. Trends in Genetics 28: 147–154. 10.1016/j.tig.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao KP, Masuda A, Matsuura T, Ohno K (2008) Human branch point consensus sequence is yUnAy. Nucleic Acids Research 36: 2257–2267. 10.1093/nar/gkn073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gozani O, Potashkin J, Reed R (1998) A potential role for U2AF-SAP 155 interactions in recruiting U2 snRNP to the branch site. Molecular and Cellular Biology 18: 4752–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang C, Chua K, Seghezzi W, Lees E, Gozani O, et al. (1998) Phosphorylation of spliceosomal protein SAP 155 coupled with splicing catalysis. Genes & development 12: 1409–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith CWJ, Chu TT, Nadalginard B (1993) Scanning and Competition between Ags Are Involved in 3' Splice-Site Selection in Mammalian Introns. Molecular and Cellular Biology 13: 4939–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chua K, Reed R (2001) An upstream AG determines whether a downstream AG is selected during catalytic step II of splicing. Molecular and Cellular Biology 21: 1509–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kralovicova J, Christensen MB, Vorechovsky I (2005) Biased exon/intron distribution of cryptic and de novo 3' splice sites. Nucleic Acids Res 33: 4882–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, et al. (2012) GENCODE: The reference human genome annotation for The ENCODE Project. Genome Research 22: 1760–1774. 10.1101/gr.135350.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anders S, Reyes A, Huber W (2012) Detecting differential usage of exons from RNA-seq data. Genome Research 22: 2008–2017. 10.1101/gr.133744.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, et al. (2005) Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America 102: 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crooks GE, Hon G, Chandonia JM, Brenner SE (2004) WebLogo: A sequence logo generator. Genome Research 14: 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corvelo A, Hallegger M, Smith CWJ, Eyras E (2010) Genome-Wide Association between Branch Point Properties and Alternative Splicing. Plos Computational Biology 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernandez-Saiz V, Targosz BS, Lemeer S, Eichner R, Langer C, et al. (2013) SCFFbxo9 and CK2 direct the cellular response to growth factor withdrawal via Tel2/Tti1 degradation and promote survival in multiple myeloma. Nature Cell Biology 15: 72–U164. [DOI] [PubMed] [Google Scholar]
- 25. Hurov KE, Cotta-Ramusino C, Elledge SJ (2010) A genetic screen identifies the Triple T complex required for DNA damage signaling and ATM and ATR stability. Genes & development 24: 1939–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaizuka T, Hara T, Oshiro N, Kikkawa U, Yonezawa K, et al. (2010) Tti1 and Tel2 are critical factors in mammalian target of rapamycin complex assembly. The Journal of biological chemistry 285: 20109–20116. 10.1074/jbc.M110.121699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hofer-Warbinek R, Schmid JA, Stehlik C, Binder BR, Lipp J, et al. (2000) Activation of NF-kappa B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. The Journal of biological chemistry 275: 22064–22068. [DOI] [PubMed] [Google Scholar]
- 28. Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T (2000) BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. Journal of Biological Chemistry 275: 17647–17652. [DOI] [PubMed] [Google Scholar]
- 29. Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, et al. (1999) XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. The EMBO journal 18: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nam JS, Hirohashi S, Wakefield LM (2007) Dysadherin: A new, player in cancer progression. Cancer letters 255: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujioka Y, Taira T, Maeda Y, Tanaka S, Nishihara H, et al. (2001) MM-1, a c-Myc-binding protein, is a candidate for a tumor suppressor in leukemia/lymphoma and tongue cancer. J Biol Chem 276: 45137–45144. [DOI] [PubMed] [Google Scholar]
- 32. Wilks C, Cline MS, Weiler E, Diehkans M, Craft B, et al. (2014) The Cancer Genomics Hub (CGHub): overcoming cancer through the power of torrential data. Database (Oxford) 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furney SJ, Pedersen M, Gentien D, Dumont AG, Rapinat A, et al. (2013) SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer discovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads.
- 35. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, et al. (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsu F, Kent WJ, Clawson H, Kuhn RM, Diekhans M, et al. (2006) The UCSC Known Genes. Bioinformatics 22: 1036–1046. [DOI] [PubMed] [Google Scholar]
- 37. Thierry-Mieg D, Thierry-Mieg J (2006) AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome biology 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, et al. (2011) Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes & development 25: 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamasaki C, Murakami K, Takeda J, Sato Y, Noda A, et al. (2010) H-InvDB in 2009: extended database and data mining resources for human genes and transcripts. Nucleic Acids Research 38: D626–D632. 10.1093/nar/gkp1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nature Methods 9: 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roberts A, Pachter L (2013) Streaming fragment assignment for real-time analysis of sequencing experiments. Nature Methods 10: 71–U99. 10.1038/nmeth.2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, et al. (2011) COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Research 39: D945–D950. 10.1093/nar/gkq929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome biology 11: R106 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
We sequenced the transcriptomes of peripheral blood mononucleocytes from seven SF3B1-mutated chronic lymphocytic leukemia (CLL) cases and nine SF3B1 wild-type cases. We also obtained data from breast cancer (BRCA; 14 mutant, 18 wild-type), lung squamous cell carcinoma (LUSC; four mutant, five wild-type) and lung adenocarcinoma (LUAD; seven mutant, nine wild-type) samples from the TCGA and uveal melanoma (UM; four mutant, four wild-type) samples from Harbour et al. 2013.
(TIF)
log2 distance in base pairs from 280, 1,476, and 86 significantly differentially used novel 3’SSs (S2 File) to their associated canonical 3’SSs in (A) BRCA, (B) CLL, and (C) UM analyses respectively. Novel 3’SSs were associated with canonical 3’SSs only if they shared the same 5’ splice site. Zero represents the position of the canonical 3’SS. Negative and positive distances indicate that the cryptic 3’SS is respectively upstream or downstream from the canonical 3’SS. Inset shows base-by-base binning from zero to 50 base pairs upstream of canonical 3’SS. Red and blue histograms represent junctions with significantly higher usage in SF3B1 mutants or SF3B1 wild-type samples respectively. The number of cryptic 3’SS identified varied with the overall sequencing depth of the different data sets.
(TIF)
Heatmap shows for each BRCA sample the log2 library-normalized count z-score for 192 proximal cryptic 3’SSs used significantly more often in the SF3B1 mutants and located 10–30 bp upstream of canonical 3’SSs (S2 File). SF3B1 mutants are labeled with the observed missense or nonsense (*) mutation as well as the frequency of the mutant allele in the RNA-sequencing data. Attenuated cryptic 3’SS selection is visible for the K700E mutant with only 8.4% allele frequency. A633V and Y765C mutants do not show evidence for cryptic 3’SS selection. Black and white colorbar indicates whether novel 3’SSs are out-of-frame (black) relative to canonical 3’SSs.
(TIF)
Heatmap shows for each sample the log2 library-normalized count z-score for the 578 proximal cryptic 3’SSs used significantly more often in the SF3B1 mutants in the CLL, BRCA, UM, LUAD, and LUSC joint analysis (S2 File). Grey bars indicate frequency of SF3B1 mutant allele in RNA-seq data. Colorbars indicate SF3B1 mutation status, cancer type, and whether the SF3B1 mutation is located in the HEAT 5–9 repeats. Black and white colorbar indicates whether novel 3’SSs are out-of-frame (black) relative to canonical 3’SSs.
(TIF)
Distance from 3’SS to highest scoring predicted branch point (BP). We were able to predict BPs for (A) 584 of 619 proximal cryptic 3’SSs and (B) 405 of 417 distal cryptic 3’SSs (as opposed to predicting the BPs for the associated canonical 3’SSs as in Fig. 3). Distance from either highest or second highest scoring predicted BP to (C) proximal cryptic 3’SSs and (D) distal cryptic 3’SSs. Cryptic 3’SSs that are used more often in SF3B1 mutants have BPs located ~13–17 bp upstream regardless of whether they are 10–30 bp upstream of canonical 3’SSs.
(TIF)
Beeswarm plots showing the PSI values for the cryptic 3’SS relative to the associated canonical 3’SS in nine of ten genes with high levels of cryptic 3’SS inclusion in CLL SF3B1 mutants (M) compared to wild-type (W) samples that were also expressed in the BRCA samples. The number in the upper corner of each plot is the distance in base pairs from the highest or second-highest scoring BP predicted for the associated canonical 3’SS to the cryptic 3’SS.
(TIF)
SF3B1 mutated samples have columns for frequency of SF3B1 mutation in RNA-seq data, mutation type, codon change and whether the mutation is in the HEAT 5–9 repeats. These columns are empty for SF3B1 wild-type tumor samples.
(TSV)
DEXSeq was used to test for differential splice junction usage in a joint analysis of the CLL, BRCA, and UM samples as well as individually for each cancer type. “Novel” indicates that the junction is not annotated in Gencode. Proximal indicates that a novel 3’SS is 10–30 bp upstream of a canonical Gencode 3’SS.
(TSV)
Location of 5’ splice sites and 3’SSs are one-based coordinates that denote the start and end of the intron. The columns COSMIC, TSgene, and ncg denote whether the gene is present in COSMIC, TSGene, or the Network of Cancer Genes respectively.
(TSV)
Location of 5’ splice sites and 3’SSs are one-based coordinates that denote the start and end of the intron. The columns COSMIC, TSgene, and ncg denote whether the gene is present in COSMIC, TSGene, or the Network of Cancer Genes respectively.
(TSV)
(XLS)
Location of 5’ splice sites and 3’SSs are one-based coordinates that denote the start and end of the intron. The columns COSMIC, TSgene, and ncg denote whether the gene is present in COSMIC, TSGene, or the Network of Cancer Genes respectively.
(TSV)
Note that there are only 324 values because one canonical 3’SS was filtered due to low coverage so a PSI value could not be calculated.
(TSV)
(TSV)
(XLS)
Location of 5’ splice sites and 3’SSs are one-based coordinates that denote the start and end of the intron. The columns COSMIC, TSgene, and ncg denote whether the gene is present in COSMIC, TSGene, or the Network of Cancer Genes respectively.
(TSV)
Note that there are only 191 values because one canonical 3’SS was filtered due to low coverage so a PSI value could not be calculated.
(TSV)
(TSV)
Data Availability Statement
We have made the code and intermediate data files needed to replicate this study available on Github (https://github.com/cdeboever3/deboever-sf3b1-2015) and Figshare (http://dx.doi.org/10.6084/m9.figshare.1120663). Instructions are provided in the Github repository for reproducing our figures, tables, and statistical analyses. Sequencing data is available through dbGaP (phs000767). Interested researchers must be approved for access by dbGaP to preserve patient anonymity.