Abstract
Currently, polymer-based prefillable syringes are being promoted to the pharmaceutical market because they provide an increased break resistance relative to traditionally used glass syringes. Despite this significant advantage, the possibility that barrel material can affect the oligomeric state of the protein drug exists. The present study was designed to compare the effect of different syringe materials and silicone oil lubrication on the protein aggregation. The stability of a recombinant fusion protein, abatacept (Orencia), and a fully human recombinant immunoglobulin G1, adalimumab (Humira), was assessed in silicone oil-free (SOF) and silicone oil-lubricated 1-mL glass syringes and polymer-based syringes in accelerated stress study. Samples were subjected to agitation stress, and soluble aggregate levels were evaluated by size-exclusion chromatography and verified with analytical ultracentrifugation. In accordance with current regulatory expectations, the amounts of subvisible particles resulting from agitation stress were estimated using resonant mass measurement and dynamic flow-imaging analyses. The amount of aggregated protein and particle counts were similar between unlubricated polymer-based and glass syringes. The most significant protein loss was observed for lubricated glass syringes. These results suggest that newly developed SOF polymer-based syringes are capable of providing biopharmaceuticals with enhanced physical stability upon shipping and handling. © 2014 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:527–535, 2015
Keywords: protein aggregation, prefilled syringe, physical stability, silicone oil, biopharmaceuticals characterization, subvisible particles, HPLC (high-performance/pressure liquid chromatography), analytical ultracentrifugation, UV/Vis spectroscopy, imaging methods
Introduction
Pharmaceutical companies are increasingly using prefillable syringes as an alternative to traditional vial packaging for the delivery of injectable drug products. Significant advantages of prefillable syringe systems over vial packaging include a greater patient safety by reducing the risk of needle exposure to pathogens when drawing solution from the vial, improved dosing accuracy, and convenience for patient in terms of product storage, administration, and disposal.1 The majority of prefillable syringe systems available on the market are made from glass. Glass syringes have a long history of use, and have proven their performance and reliability among healthcare providers. Disadvantages associated with application of glass-made syringe system include breakage, potential surface reactivity, possibility of delaminated glass contamination, and requirement of silicone oil lubrication. To overcome these problems, in recent years, polymer syringes mainly manufactured from cyclic olefin polymer (COP) have been successfully developed and are being promoted to the pharmaceutical market. Polymer-based syringes have significant advantages of increased break resistance, decreased surface reactivity, and compatibility with the broad range of pH. In addition, by using a novel proprietary coating technique for plunger stopper, polymer-based silicone oil-free (SOF) prefillable syringe system has been developed.2 The potential disadvantages of polymer-based syringes compared with glass syringes include higher susceptibility to scratches, fair chemical resistance against strong acids and alkalis, higher gas permeability, and relatively short usage history. It should be mentioned though that polymeric materials used to manufacture syringes utilized in the present study have been extensively tested and have been demonstrated to be safe and biocompatible materials for medical packaging application.3
For the therapeutic proteins, it is critically important that the protein oligomeric state remains unchanged, as aggregates and particles that can be composed of a protein alone or protein adsorbed onto foreign material are suspected to invoke immunogenicity.4–9 During shipping and handling, liquid pharmaceutical formulations are exposed to agitation that induces aggregation and particle formation through protein interaction with the interphases, such as air–solution and vial/syringe surface–solution interphases.10,11 The factors which affect the adsorption of protein to interfaces include protein properties, interface properties, and solution conditions. In the present study, to elucidate independent and combined effects of syringe barrel material, silicone oil lubrication, and different formulation conditions on aggregation and/or particulate formation, we placed two different pharmaceutical proteins, abatacept and adalimumab, formulated at two different pH, into a 1.0-mL SOF and silicone oil-lubricated glass and polymer-based syringes and subjected them to agitation stress to reproduce conditions similar to those experienced by the drug products during shipping. The resulting changes in the soluble aggregates level were evaluated by size-exclusion chromatography (SEC). Because of the potential inaccuracies in amounts of aggregates determined by SEC related to nonspecific column adsorption,12 SEC results were verified with analytical ultracentrifugation sedimentation velocity (AUC-SV), which has been demonstrated as the most appropriate orthogonal method to SEC.13,14 In accordance with the current regulatory expectations for the analysis of subvisible particles,15 resonant mass measurements (RMM) and dynamic flow-imaging analyses were also performed to analyze particles in the range of a few hundred nanometers to approximately hundred micrometers. The evaluated aggregates and particles amounts serve as a guideline to understanding the effects of different syringe materials in the presence or absence of silicone oil lubrication on the stability of the pharmaceutical proteins.
Materials and Methods
Materials
Silicone oil-free 1-mL polymer-based syringes (PLAJEX™) with long staked needle (27G), henceforth referred to as “polymer-SOF,” were provided by Terumo Company (Tokyo, Japan).2 PLAJEX™ syringe is a recently developed prefillable syringe made from COP with a novel butyl rubber plunger stopper coated using proprietary coating technique. Silicone oil-lubricated PLAJEX™ syringes with an uncoated plunger stopper, henceforth referred to as “polymer-so+,” were also provided by Terumo Company. SOF 1-mL borosilicate glass syringe barrels (conformed to the ISO 11040-4 standard), henceforth referred to as “glass-SOF,” and silicone oil-lubricated 1-mL glass syringes barrels (conformed to the ISO 11040-4 standard), henceforth referred to as “glass-so+,” were provided by TOP Company (Tokyo, Japan). Glass syringes were used with stainless steel needles and uncoated plunger stopper.
Abatacept (Orencia, molecular mass 92 kDa, pI = 4.5–5.516), a recombinant fusion protein consisting of the extracellular domain of human cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) linked to a modified Fc portion of human immunoglobulin G1 (IgG1), was purchased as a lyophilized powder for intravenous infusion from Ono Pharmaceutical Company, Ltd. (Osaka, Japan) at a stock concentration of 250 mg. Adalimumab (Humira, molecular mass 148 kDa, pI = 9.01 as calculated using the SEDNTERP software), a recombinant fully human IgG1 monoclonal antibody, was purchased from Eisai Company, Ltd. (Tokyo, Japan). Gibco phosphate-buffered saline (PBS), pH 7.4 (10×), was purchased from Life Technologies. Acetic acid was purchased from Wako Pure Chemical Industries (Osaka, Japan).
Methods
Accelerated Stress
To elucidate the effect of formulation conditions on the levels of aggregation/particles formation, two different buffers with different pH were used in the present study. In our previous studies of adalimumab stability against aggregation using a number of formulations with different pH, we have detected that agitation for 96 h induced noticeable aggregation in the formulations with pH 4.0, 5.0, and 7.0 as indicated by an increase in turbidity. Because of concerns that pH 4.0, which is lower than that of stock formulation (pH 5.0) can result in the unfolding of the protein, pH 5.0 and pH 7.4 were selected. For abatacept, the same buffer conditions were chosen: pH 7.4 that is similar to that of stock formulation (pH 7.2–7.8) and pH 5.0 that is close to abatacept pI value.
A vial containing 250 mg of lyophilized abatacept was reconstituted with 10 mL of water for injections according to the manufacturer's instructions. First, sucrose was removed by extensive dialysis against Millipore water for 24 h at 4°C with two water changes. The obtained solution was further dialyzed into 1× PBS, pH 7.4, or 10 mM acetate buffer, pH 5.0 using slide-a-lyzer dialysis cassettes (MWCO 10,000 Da; Thermo Fisher Scientific Inc., Waltham, MA) with two buffer exchanges over 24 h at 4°C. The adalimumab stock solution was buffer-exchanged to 1× PBS, pH 7.4 trough gel filtration chromatography using the AKTAprime plus HPLC system with HiLoad™ 16/60 Superdex™ 200 prep grade column (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). The level of polysorbate 80 in the resulting solution was below quantification limit of 0.001% as was confirmed using modified Dragendorff reagent. A portion of the resulting solution was further dialyzed to 10 mM acetate buffer at pH 5.0 using dialysis cassettes. Protein concentration was determined using UV absorption and no significant protein loss was confirmed.
The buffer-exchanged protein solutions were diluted to1 mg/mL by a respective buffer and filtered through a 0.22-μm polyvinylidene fluoride syringe filter (Millipore, Billerica, MA). One milliliter of each protein solution formulated at either 1× PBS or 10 mM acetate buffer was placed into 1-mL syringes, leaving a headspace of 5 mm. Four different types of syringes were used (three syringes of each type): (1) polymer-SOF; (2) glass-SOF; (3) polymer-so+; and (4) glass-so+. To reproduce the conditions experienced during shipping of therapeutic proteins, syringes were subjected to shaking at 500 rounds/min in a desktop orbital shaker Mix-VR (TAITEC Company Ltd., Saitama, Japan) at 4°C for 1 week. The samples before stress treatment were used as controls.
Absorbance and Transmission Measurements
Absorbance at 280 nm (A280) and transmission at 350 nm (T350) of the samples were acquired using DU-500 UV/Visible spectrophotometer (Beckman Coulter Inc., Brea, CA) and a 2-mL disposable cuvette with a cell length of 1 cm (Eppendorf, Hamburg, Germany). Samples recovered from syringes were centrifuged for 30 min at 15,600g, and percentage of insoluble large aggregates was determined from the difference of A280 before and after centrifugation, taken as a percentage of the A280 present before centrifugation.
High-Performance SEC
The samples were analyzed using the Alliance 1100 HPLC system (Waters, Milford, MA), with simultaneous UV absorbance detection at 215 and 280 nm with a TSK gel G3000SWXL column (Tosoh Bioscience, Tokyo, Japan) and 1× PBS pH 7.4 as the mobile phase. Twenty microliter aliquot was injected into the HPLC system. Flow rate was 0.5 mL/min, and elution time was set at 30 min. High protein mass recovery (>98%) was confirmed for all studied formulations, with the exception of glass-so+, for which the recovery was about 85%.
Analytical Ultracentrifugation Sedimentation Velocity
The sedimentation velocity experiments were conducted using ProteomeLab XL-I analytical ultracentrifuge (Beckman Coulter) equipped with a 4-hole An60 Ti rotor. The experiments were performed at 42,000 rpm at 20°C using Beckman Coulter 12-mm double-sector charcoal-filled epon centerpieces and sapphire windows. Cells were aligned using an alignment tool (Spin Analytical, Inc., Berwick, ME). Data were acquired using an absorbance optical system at wavelengths of 280 nm (abatacept) or 292 nm (adalimumab). The scans were collected as fast as possible between 6.0 and 7.2 cm from the axis of rotation with the radial increment of 30 μm. Data were analyzed using the C(s) method implemented in SEDFIT (version 14.1).17 The partial specific volumes of abatacept and adalimumab were obtained by using the program SEDNTERP 1.09 and were 0.7306 and 0.7282 cm3/g, respectively. The buffers viscosity and density were also calculated using SEDTERP and were 1.018 cP and 1.0052 g/mL for PBS, and 1.004 cP and 0.9987 g/mL for acetate buffer.
Resonant Mass Measurement
An Affinity Biosensors Archimedes system (Malvern Instruments Limited, Malvern, Worcestershire, United Kingdom) was equipped with a Hi-Q Micro Sensor and controlled by ParticleLab software. The sensor was flushed for 60 s with purified water before the analysis. Subsequently, possible impurities in the system were removed by two “sneeze” operations and the system was flushed again for 60 s with purified water. The sample solution was then loaded for 15 s. Before the analysis, the limit of detection (LOD) was determined in automatic LOD mode. Samples were analyzed for 300 s.
Microflow Imaging
Subvisible (micron sized) protein particles were counted using a microflow imaging (MFI) digital particle analyzer 4200 (Brightwell Technologies Inc., Ottawa, Canada). Prior to each analysis, the system was primed with sufficient amount of Millipore water to obtain a particle-free baseline. Stressed samples at 1 mg/mL were diluted, if necessary, 2–10× times with the respective buffer, and volumes of 0.5 mL were analyzed at a flow rate of 0.1 mL/min. The Protein Simple MFI View Analysis Suite (MVAS) version 1.3 (Particle sizing Systems Japan Co., Ltd., Tokyo, Japan) was used for data analysis.
FlowCAM
Subvisible particles for the abatacept PBS formulation were imaged using bench-top FlowCAM instrument (Fluid Imaging Technologies, Scarborough, ME). Sample volumes of 0.2 mL were analyzed at a flow rate of 0.08 mL/min. Flash duration was set to 35.50 ms, and Camera Gain was set to 0. VisualSpreadsheet software version 3.4.8 (DKSH Japan K.K., Tokyo, Japan) was used for data analysis.
Results and Discussion
Quantification of Protein Aggregates
Results of percentage transmission measurements are summarized in Table1. Agitation stress caused decrease in transmission at 350 nm in all samples, indicating the presence of large aggregates. For either of the protein formulation, samples prefilled in SOF glass or polymer syringes had similar relatively high percent transmission values, whereas samples prefilled in silicone-lubricated syringe types had much lowervalues.
Table 1.
Results of Percentage Transmission Measurements
| Abatacept |
Adalimumab |
|||
|---|---|---|---|---|
| Sample | PBS | Acetate Buffer | PBS | Acetate Buffer |
| Control | 99.77 ± 0.23 | 99.54 ± 0.23 | 98.63 ± 0.23 | 98.63 ± 0.23 |
| Polymer-SOF | 97.72 ± 0.45 | 98.40 ± 0.23 | 98.40 ± 0.23 | 98.40 ± 0.00 |
| Glass-SOF | 99.08 ± 0.23 | 97.72 ± 0.45 | 97.05 ± 0.00 | 97.72 ± 0.23 |
| Polymer-so+ | 72.44 ± 0.17 | 39.36 ± 0.18 | 95.94 ± 0.22 | 93.97 ± 0.22 |
| Glass-so+ | 39.45 ± 0.18 | 14.72 ± 0.10 | 68.08 ± 0.47 | 60.26 ± 0.14 |
Data are shown as the average of three measurements ± standard deviation.
For each type of syringe, amounts of insoluble aggregates and soluble species were estimated (Table2). The amounts of insoluble aggregates were estimated by subtracting the amount of protein in supernatant after centrifugation from total sample amount before stress testing. In agreement with percentage transmission measurements, amounts of insoluble aggregates detected in SOF syringes were similar between glass and polymer syringes irrespective of the sample and were only slightly higher than those in control samples. Surface of borosilicate glass is hydrophilic, whereas the COP plastic surface is rather hydrophobic, suggesting that the difference in surface hydrophobicity is insignificant for the aggregates formation. In agreement with previous studies, the increased loss of monomer was detected in silicone oil-lubricated compared with SOF syringes.18 Because of the established silicone oil sensitivity,19 abatacept samples prefilled in silicone oil-lubricated syringes produced a substantial amount of insoluble aggregates. The effect of silicone oil on insoluble aggregates formation was more pronounced in case of silicone oil-lubricated glass syringes compared with silicone oil-lubricated polymer syringes, with the exception of adalimumab formulated in acetate buffer, for which no significant differences in insoluble aggregates levels were observed between glass-so+ and polymer-so+syringes.
Table 2.
Aggregates Quantification Results by SEC
| Soluble Species (%)b |
||||
|---|---|---|---|---|
| Sample | Insoluble Aggregates (%)a | Monomer | Dimer | Higher-Order Aggregates |
| Abatacept | ||||
| PBS | ||||
| Control | <1 | 98.5 ± 0.1 | 1.5 ± 0.1 | − |
| Polymer-SOF | 2 | 98.4 ± 0.0 | 1.6 ± 0.0 | − |
| Glass-SOF | 2 | 98.4 ± 0.1 | 1.6 ± 0.1 | − |
| Polymer-so+ | 5 | 97.5 ± 0.3 | 1.6 ± 0.1 | 0.9 ± 0.1 |
| Glass-so+ | 7 | 91.4 ± 0.0 | 3.8 ± 0.0 | 4.8 ± 0.0 |
| Acetate Buffer | ||||
| Control | 4 | 93.2 ± 0.0 | 6.8 ± 0.0 | − |
| Polymer-SOF | 4 | 88.5 ± 0.0 | 11.5 ± 0.0 | − |
| Glass-SOF | 4 | 90.9 ± 0.0 | 9.1 ± 0.0 | − |
| Polymer-so+ | 14 | 89.3 ± 0.1 | 10.7 ± 0.1 | − |
| Glass-so+ | 19 | 92.6 ± 0.0 | 7.4 ± 0.1 | − |
| Adalimumab | ||||
| PBS | ||||
| Control | <1 | 100.0 ± 0.0 | − | − |
| Polymer-SOF | <1 | 100.0 ± 0.0 | − | − |
| Glass-SOF | <1 | 99.9 ± 0.1 | 0.1 ± 0.1 | − |
| Polymer-so+ | <1 | 99.9 ± 0.1 | 0.1 ± 0.1 | − |
| Glass-so+ | 15 | 99.1 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Acetate Buffer | ||||
| Control | <1 | 100.0 ± 0.0 | − | − |
| Polymer-SOF | <1 | 100.0 ± 0.0 | − | − |
| Glass-SOF | <1 | 100.0 ± 0.0 | − | − |
| Polymer-so+ | <1 | 100.0 ± 0.0 | − | − |
| Glass-so+ | <1 | 99.3 ± 0.1 | 0.4 ± 0.0 | 0.3 ± 0.1 |
Stressed samples were centrifuged for 30 min at 15,600g, and percent insoluble aggregation was determined from the difference of A280 before and after centrifugation, taken as a percentage of the A280 present before centrifugation.
Data are shown as the average of three measurements ± standard deviation.
Size-exclusion chromatography analysis was performed to determine the fraction of soluble aggregates remaining after insoluble aggregates were removed by centrifugation at 15,600g for 30 min. For abatacept samples formulated in PBS, the monomer fraction and aggregates levels were similar for polymer-SOF and glass-SOF samples and were close to those in the control sample. The fraction of monomer remaining in the solution was considerably less in all abatacept samplesformulated in acetate buffer compared with PBS samples, consistent with greater amounts of insoluble aggregates in acetate buffer. Polymer-SOF, glass-SOF, and polymer-so+ adalimumab samples contained only monomers. In contrast, small amounts of dimers and oligomers were detected by SEC for silicone oil-lubricated glass syringe samples. Overall, similar to insoluble aggregates, soluble aggregates levels detected in silicone oil-lubricated glass syringes were higher than those in silicone oil-lubricated polymer or SOF syringes, with the exception of glass-so+ abatacept sample formulated in acetate buffer. For this sample, the recovery mass was estimated to be approximately 82%, thus suggesting substantial adsorption of the aggregated protein to silica matrix of the SECcolumn.20
To verify the performance of SEC analysis, aggregates were also quantified by AUC-SV (Fig. 1). In general, there was a reasonable agreement between SEC and AUC-SV results regarding detected oligomeric species and their respective amounts as reported previously.14 For the abatacept control, polymer-SOF, and glass-SOF samples formulated in PBS, the estimates of dimeric fractions obtained from AUC-SV (Fig. 1a) were slightly lower than those obtained by SEC (Table2). It is assumed that these differences originate from reversibility of agitation-induced soluble aggregates: SEC analysis was performed immediately following the completion of agitation, whereas AUC-SV analysis was conducted on samples that have been stored for a week at 4°C after agitation completion. In case of abatacept samples formulated in acetate buffer, levels of aggregation determined by SEC using PBS as a mobile phase were significantly lower than levels measured by AUC-SV performed in acetate buffer (Suppl. Fig. S1). The differences in SEC and AUC-SV results can be attributed to the sample dilution during the SEC analysis, which often causes the dissociation of noncovalent aggregates. In agreement with the results of previous studies, the levels of aggregates were slightly higher when measured by AUC-SV compared with the SEC estimates.13,21 The possibility also exists that the differences in the percentages of aggregates detected by SEC and AUC-SV could be the consequence of change in noncovalent aggregates distribution owing to the mobile phase composition (PBS), which is different from the sample formulation (acetate buffer).22 In addition, discrepancy between SEC and AUC-SV estimates can be a result of nonspecific interaction of aggregates with the column caused by the combined effects of the mobile and stationary phases, which leads to aggregates filtration in the column, thus compromising the accuracy of aggregates quantification.23 In fact, protein mass recovery for polymer-so+ and glass-so+ abatacept samples formulated in acetate buffer was slightly lower than that for PBSformulations.
Figure 1.
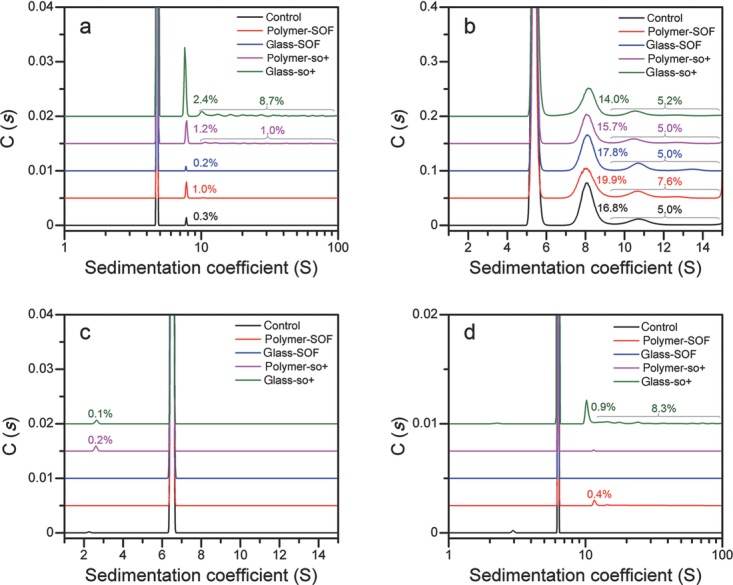
Aggregates quantification results by AUC-SV for the abatacept PBS formulation (a), abatacept acetate buffer formulation (b), adalimumab PBS formulation (c), and adalimumab acetate buffer formulation (d).
The quantitative results of AUC-SV were similar to those obtained using SEC for both adalimumab formulations, with the exception of sample formulated in acetate buffer and prefilled in glass-so+ syringe, for which the significant fraction of oligomers was overlooked in SEC analysis. Overall, for both protein formulations, the loss of monomer for samples in silicone oil-lubricated syringes is clearly indicated by both SEC and AUC-SV.
Quantification of Protein Particles
The results of particle counting obtained by RMM are shown in Figure 2. On the basis of different buoyancy, protein particles and silicone oil droplets were discriminated and counted separately.24 The experiments were performed using microsensor, and the results of particle counting are shown in the range of 0.5–2.0 μm. PBS-formulated abatacept samples showed similar content of protein particles in polymer and glass SOF syringes, which were lower compared with samples in silicone oil-lubricated syringes (Fig. 2a). In the abatacept acetate buffer formulation, particle counts were similar between silicone oil-lubricated and SOF syringes in the range 0.5–1.0 μm, however, a substantial amount of larger particles was observed in the silicone oil-lubricated syringes with the highest level of protein particles detected in glass-so+ syringes (Fig. 2b). Particle counts were similar in control, polymer-SOF, and glass-SOF syringes for both adalimumab formulations (Figs. 2c and 2d). In contrast, greater amounts of particles were detected for silicone oil-lubricated syringes, and for adalimumab formulated in acetate buffer, particles counts were higher in glass-so+ sample than in other syringe types.
Figure 2.
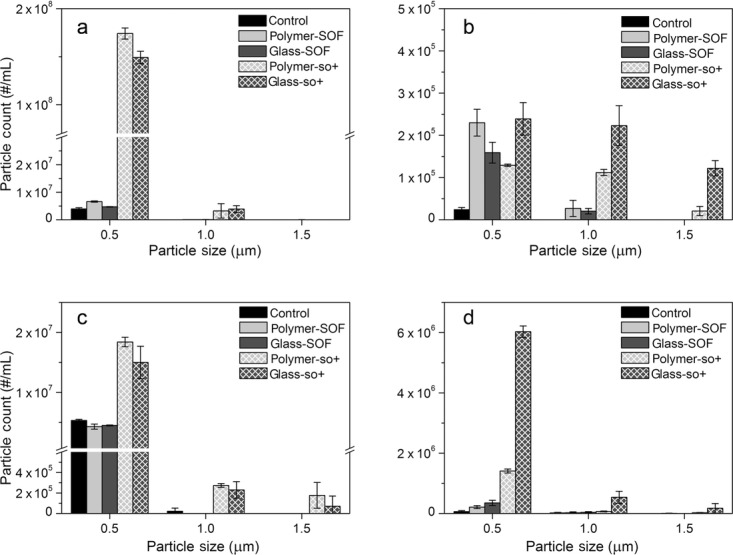
Results of particle counts by RMM for the abatacept PBS formulation (a), abatacept acetate buffer formulation (b), adalimumab PBS formulation (c), and adalimumab acetate buffer formulation (d).
Concentrations of protein particles determined by MFI are shown in Figure 3 and total particle counts are summarized in Suppl. Tables S1 and S2. For each protein formulation, the algorithm that allows discriminating silicone oil droplets from total particle counts was developed. Samples from SOF syringe filled with the protein formulation and silicone oil-lubricated syringe filled with the respective formulation buffer were subjected to MFI analysis to derive respective representative profiles of protein particles and silicone oil droplets. We found that circularity is an appropriate parameter that can be used to discriminate between protein particles and silicone oil droplets. For each detected particle, the average circularity value and its 95% confidence interval was obtained in the size range from 1 to 10 μm. The mean values of the confidence intervals for protein particles and silicone oil droplets were used as a cutoff value (Suppl. Table S3). In the size range above 10 μm, the recorded number of silicone oil droplet counts was inappropriate for this approach. Therefore, to discriminate protein particles from silicone oil droplets in the size range above 10 μm, “Find Similar” option of MVAS 1.3 software was utilized.
Figure 3.
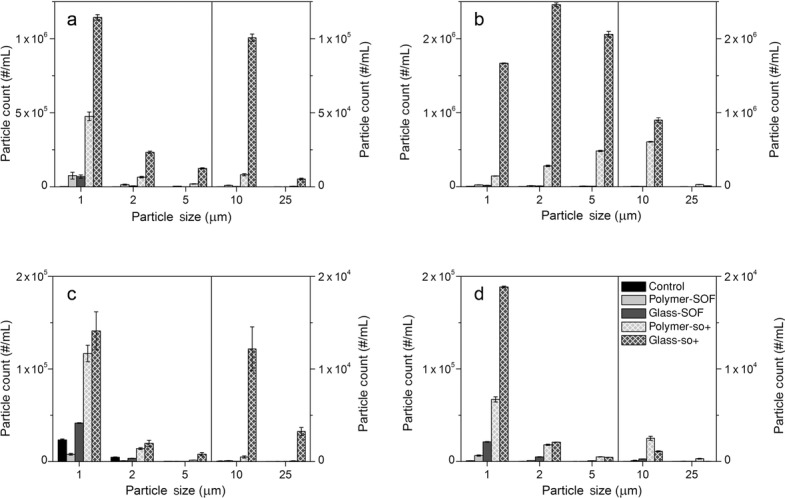
Concentrations of protein particles determined by MFI for the abatacept PBS formulation (a), abatacept acetate buffer formulation (b), adalimumab PBS formulation (c), and adalimumab acetate buffer formulation (d).
For the abatacept PBS formulation, particle concentrations were similar for SOF polymer and glass syringe samples. Considerably larger amounts of particles were detected for silicone oil-lubricated syringes samples. The amount of large particles was greater in glass-so+ than in any other syringe type. In the abatacept acetate buffer formulation, similar particle counts were obtained for SOF syringes, and the highest counts were obtained for the glass-so+ samples. In agreement with MFI results for the abatacept, adalimumab formulations showed similar particles concentrations for SOF syringes samples and higher levels of particles were detected for silicone oil-lubricated samples.
The loss of native protein upon particles formation is essentially undetectable as cumulative mass of the protein in particles only comprises a few micrograms.11 Nonetheless,recent findings on aggregation behavior of keratinocyte growth factor 2 during agitation have made it evident that particle counting is much more sensitive measure of aggregation than loss of soluble protein.25 As the RMM results (Fig. 2) and MFI results (Fig. 3) did not demonstrate significant differences in the particles counts for samples in SOF syringes, in the absence of silicone oil similar particle levels can be expected in glass and polymersyringes.
Representative images of the protein particles detected by FlowCAM in the abatacept PBS formulation in each different type of syringe are shown in Figure 4 and Suppl. Figure S2. The images obtained for protein particles from SOF syringes are visually similar to each other, indicating the presence of particles with sizes smaller than 60 μm. Images recorded for polymer-so+ sample showed considerably larger particles than in SOF samples. Even larger particles (with the largest particle size of 130 μm), which morphologically appeared as elongated fibrillar particles were observed in glass-so+ sample.
Figure 4.
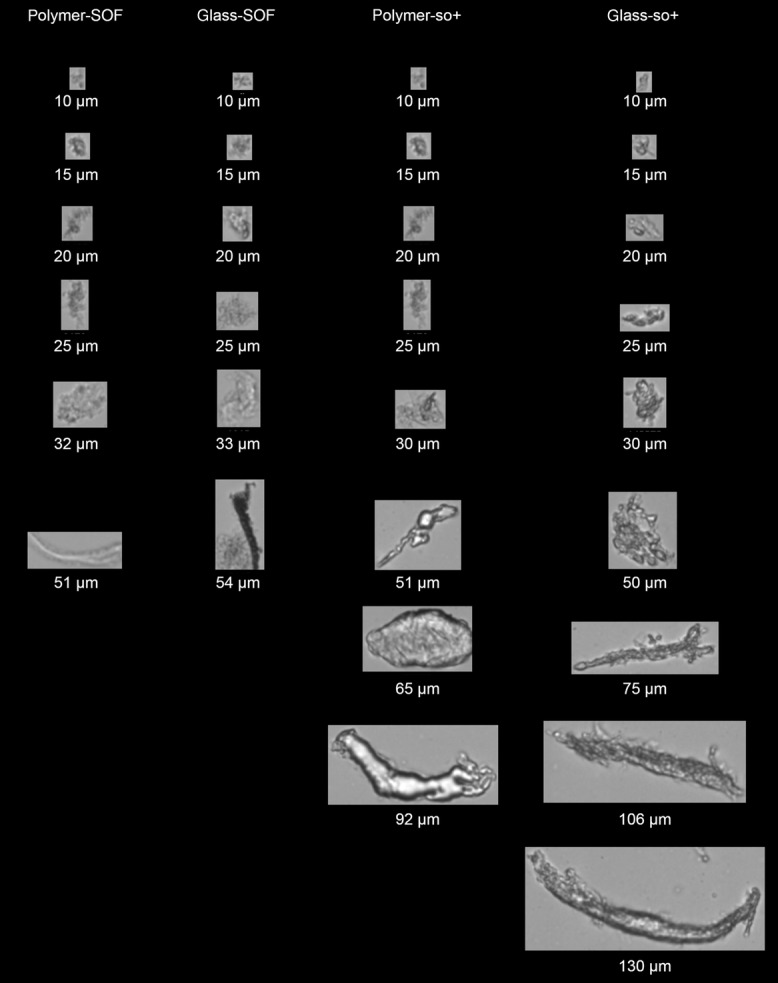
Representative FlowCAM images of protein particles obtained for the abatacept PBS formulation.
One of the major concerns associated with the usage of prefilled syringes is undesirable protein aggregation/particle formation because of protein ability to adsorb to all types of surfaces/interfaces. Layers of adsorbed protein form films that are suspected to serve as a major source of protein particles upon rupture.26 In the present study, the adsorption of protein to the unlubricated glass surface could be attributed to charge–charge effects, whereas in the case of unlubricated polymer, protein could be adsorbed to the syringe surface mainly via hydrophobic interactions. Agitation of protein solutions in the presence of COP beads with no headspace did not induce aggregate formation (data not shown). Thus, the disruption of the layers of adsorbed protein followed by the release of the torn film pieces into bulk solution phase can be attributed to the air bubble in the headspace as suggested previously.11 An increase in the particle counts upon agitation was detected in all samples suggesting that the air bubble is a prerequisite for the formation of protein particles.
Microflow imaging results indicated that at least with the proteins and syringes used in the present study, no significant differences in particle levels were observed between SOF glass and polymer-based syringes.
Particle counts in samples from silicone oil-lubricated syringes were higher than those from SOF syringes, as confirmed by both RMM and MFI results. This finding suggests that silicone oil lubrication increases adsorption of the protein to the surface, and/or accelerates disruption of the formed layer, which leads to the increased particle formation. During agitation, the dissociation of adsorbed protein from the surface was caused by the air bubble, and after agitation completion the layers of adsorbed protein were removed when the solution was pushed through the syringe barrel with the plunger to collect samples for the further analyses. In case of silicone oil-lubricated syringes, the particle counts were higher in glass-so+ than in polymer-so+ syringes, indicating the stronger adsorption capacity of proteins used in this study to the silicone oil-lubricated glass surface rather than polymer-based surface.
The results of MFI data analysis performed to discriminate protein particles from silicone oil droplets revealed that higher protein particle counts detected by MFI in glass-so+ compared with polymer-so+ were associated with higher silicone oil droplets counts in silicone oil-lubricated glass rather than in polymer syringes (Suppl. Tables S4 and S5). Similarly, the amount of insoluble aggregates was generally greater in glass-so+ compared with polymer-so+ syringes. These results suggest that a correlation exists between silicone oil levels and amounts of particles formed in solution. In the present study, the amount of silicon oil used to lubricate the inner surface of syringe was about 0.7 mg per syringe for polymer-based and1 mg per syringe for glass syringe as claimed by manufacturers. Nonetheless, it is not clear whether higher silicone oil droplets counts in glass-so+ rather than in polymer-so+ syringes were simply the consequence of larger amount of silicone oil used for syringe lubrication, or whether additional combined effects of syringe barrel material and silicone oil lubrication could have had an impact on particle formation. Further studies may be needed to evaluate the effect of different silicone oil levels applied to the different syringe barrel material.
For both polymer-so+ and glass-so+, the abatacept acetate buffer formulation showed larger amounts of particles than those detected in PBS formulation (Suppl. Table S1). Similarly, for adalimumab formulations prefilled in silicone oil-lubricated syringes, amounts of particles were larger in acetate buffer formulation compared with PBS formulation (Suppl. Table S2). Interestingly, the increased protein particles counts in acetate buffer formulation were consistent with higher amounts of silicone oil droplets detected relative to PBS formulation, again suggesting a positive correlation between the amount of silicone oil droplets and particle counts. These observations indicate that acetate buffer promotes detachment of silicone oil droplets from the inner surface of syringe, which in turn leads to increase in the number of particles. In case of the abatacept (pI = 4.5–5.5), the differences in particle counts between acetate buffer (pH 5.0) and PBS formulation (pH 7.4) were more pronounced than for the adalimumab (pI = 9.01) formulations, suggesting that formulations at pH near the isoelectric point can favor the formation of aggregates and particles.
For the adalimumab solutions prefilled in SOF syringes, no soluble aggregates were detected and particle counts were similar for different formulations. However, in the adalimumab formulations collected from glass-so+ syringes, soluble aggregates were detected and particle counts were considerably higher than in the other syringe types. This result suggests that a correlation in the amount of aggregates detected by SEC/AUC-SV and particle counts obtained by RMM/MFI can be present.
Conclusions
The effects of syringe material and silicone oil lubrication on the levels of aggregation/particles formation were investigated for the abatacept and adalimumab formulations at pH 5.0 and 7.4. In all cases, samples from SOF glass and polymer-based syringes had similar amounts of insoluble aggregates, soluble aggregates, and subvisible particles. Silicone oil lubrication caused the formation of insoluble aggregates in samples other than the adalimumab formulated in acetate buffer, and the effect was more pronounced in case of silicone oil-lubricated glass syringes compared with silicone oil-lubricated polymer syringes. For both abatacept and adalimumab formulations, the loss of monomer for samples in silicone oil-lubricated syringes was confirmed by SEC and AUC-SV. Similarly, particle counts in samples from silicone oil-lubricated syringes were higher than those from SOF syringes, as confirmed by both RMM and MFI results. For the same lubricated syringe type, particle counts were higher in acetate buffer formulations than in PBS formulations. The highest particles counts were consistently observed in silicone oil-lubricated glass syringes, which was associated with larger silicone oil amounts detected in silicone oil-lubricated glass syringes compared with polymer-based syringes. This study confirms that SOF polymer-based syringes PLAJEX™ are capable of providing biopharmaceuticals with enhanced physical stability upon shipping and handling.
Acknowledgments
We would like to thank TOP Company for providing glass syringes and TERUMO Company for providing polymer syringes. We also thank Malvern instruments, Division of Spectris Company, Ltd. for providing access to the Archimedes instrument and DKSH Japan K.K. for providing access to FlowCAM instrument and VisualSpreadsheet analysis software. In addition, we would like to thank Particle sizing Systems Japan Co., Ltd. for providing access to MVAS 1.3 software.
Supporting Information
This article contains supplementary material available from the authors upon request or via the Internet at http://onlinelibrary.wiley.com/.
References
- 1.Makwana S, Basu B, Makasana Y, Dharamsi A. Prefilled syringes: An innovation in parenteral packaging. Int J Pharm Investig. 2011;1(4):200–206. doi: 10.4103/2230-973X.93004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshino K, Nakamura K, Yamashita A, Abe Y, Iwasaki K, Kanazawa Y, Funatsu K, Yoshimoto T, Suzuki S. Functional evaluation and characterization of a newly developed silicone oil-free pre-fillable syringe system. J Pharm Sci. 2014;103(5):1520–1528. doi: 10.1002/jps.23945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeon Corporation. 2010. COPCyclo olefin polymer ZEONEX®ZEONOR®Cyclo olefin polymer ( ) for medical package applications. No. K2010–2.
- 4.Schellekens H. Immunogenicity of therapeutic proteins: Clinical implications and future prospects. Clin Ther. 2002;24(11):1720–1740. doi: 10.1016/s0149-2918(02)80075-3. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg AS. Effects of protein aggregates: An immunologic perspective. AAPS J. 2006;8(3):E501–E507. doi: 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh SK, Afonina N, Awwad M, K Bechtold-Peters, Blue JT, Chou D, Cromwell M, Krause HJ, Mahler HC, Meyer BK, Narhi L, Nesta DP, Spitznagel T. An industry perspective on the monitoring of subvisible particles as a quality attribute for protein therapeutics. J Pharm Sci. 2010;99(8):3302–3321. doi: 10.1002/jps.22097. [DOI] [PubMed] [Google Scholar]
- 7.Fradkin AH, Carpenter JF, Randolph TW. Glass particles as an adjuvant: A model for adverse immunogenicity of therapeutic proteins. J Pharm Sci. 2011;100(11):4953–4964. doi: 10.1002/jps.22683. [DOI] [PubMed] [Google Scholar]
- 8.Barnard JG, Babcock K, Carpenter JF. Characterization and quantitation of aggregates and particles in interferon-β products: Potential links between product quality attributes and immunogenicity. J Pharm Sci. 2013;102(3):915–928. doi: 10.1002/jps.23415. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter JF, Randolph T, Jiskoot W, Crommelin DJA, Middaugh CR, Winter G, Y-X Fan, Krishner S, Verthelyi D, Kozlowski S, Clouse KA, Swann PG, Rosenberg A, Cherney B. Overlooking subvisible particles in therapeutic protein products: Gaps that may compromise product quality. J Pharm Sci. 2009;98(4):1201–1205. doi: 10.1002/jps.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maa YF, Hsu CC. Protein denaturation by combined effect of shear and air-liquid interface. Biotechnol Bioeng. 1997;54(6):503–512. doi: 10.1002/(SICI)1097-0290(19970620)54:6<503::AID-BIT1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Basu P, Krishnan S, Thirumangalathu R, Randolph TW, Carpenter JF. IgG1 aggregation and particle formation induced by silicone-water interfaces on siliconized borosilicate glass beads: A model for siliconized primary containers. J Pharm Sci. 2013;102(3):852–865. doi: 10.1002/jps.23434. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJ, Middaugh CR, Winter G. Potential inaccurate quantitation and sizing of protein aggregates by size exclusion chromatography: Essential need to use orthogonal methods to assure the quality of therapeutic protein products. J Pharm Sci. 2010;99(5):2200–2208. doi: 10.1002/jps.21989. [DOI] [PubMed] [Google Scholar]
- 13.Berkowitz SA. Role of analytical ultracentrifugation in assessing the aggregation of protein biopharmaceuticals. AAPS J. 2006;8(3):E590–E605. doi: 10.1208/aapsj080368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krayukhina E, Uchiyama S, Nojima K, Okada Y, Hamaguchi I, Fukui K. Aggregation analysis of pharmaceutical human immunoglobulin preparations using size-exclusion chromatography and analytical ultracentrifugation sedimentation velocity. J Biosci Bioeng. 2013;115(1):104–110. doi: 10.1016/j.jbiosc.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Kirshner S. Talk at workshop on protein aggregation and immunogenicity. Breckenridge, Colorado: 2012. Regulatory expectations for analysis of aggregates and particles. 12 July. [Google Scholar]
- 16. Bristol-Myers Squibb Canada. 2013. Orencia (abatacept): Product monograph. Retrieved from http://www.bmscanada.ca/static/products/en/pm_pdf/ORENCIA_EN_PM.pdf Last accessed September 17th, 2014.
- 17.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys J. 2000;78(3):1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thirumangalathu R, Krishnan S, Ricci MS, Brems DN, Randolph TW, Carpenter JF. Silicone oil- and agitation-induced aggregation of a monoclonal antibody in aqueous solution. J Pharm Sci. 2009;98(9):3167–3181. doi: 10.1002/jps.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majumdar S, Ford BM, Mar KD, Sullivan VJ, Ulrich RG, AJ D'souza. Evaluation of the effect of syringe surfaces on protein formulations. J Pharm Sci. 2011;100(7):2563–2573. doi: 10.1002/jps.22515. [DOI] [PubMed] [Google Scholar]
- 20.Yumioka R, Sato H, Tomizawa H, Yamasaki Y, Ejima D. Mobile phase containing arginine provides more reliable SEC condition for aggregation analysis. J Pharm Sci. 99(2):618–620. doi: 10.1002/jps.21857. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Andya JD, Shire SJ. A critical review of analytical ultracentrifugation and field flow fractionation methods for measuring protein aggregation. AAPS J. 2006;8(3):E580–E589. doi: 10.1208/aapsj080367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philo JS. A critical review of methods for size characterization of non-particulate protein aggregates. Curr Pharm Biotechnol. 2009;10(4):359–372. doi: 10.2174/138920109788488815. [DOI] [PubMed] [Google Scholar]
- 23.Gabrielson JP, Brader ML, Pekar AH, Mathis KB, Winter G, Carpenter JF, Randolph TW. Quantitation of aggregate levels in a recombinant humanized monoclonal antibody formulation by size-exclusion chromatography, asymmetrical flow field flow fractionation, and sedimentation velocity. J Pharm Sci. 2007;96(2):268–279. doi: 10.1002/jps.20760. [DOI] [PubMed] [Google Scholar]
- 24.Patel AR, Lau D, Liu J. Quantification and characterization of micrometer and submicrometer subvisible particles in protein therapeutics by use of a suspended microchannel resonator. Anal Chem. 2012;84(15):6833–6840. doi: 10.1021/ac300976g. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Qi W, Schwartz DK, Randolph TW, Carpenter JF. The effects of excipients on protein aggregation during agitation: An interfacial shear rheology study. J Pharm Sci. 2013;8:2460–2470. doi: 10.1002/jps.23622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudiuk S, L Cohen-Tannoudji, Huilleb S, Tribet C. Importance of the dynamics of adsorption and of a transient interfacial stress on the formation of aggregates of IgG antibodies. Soft Matter. 2012;8:2651–2661. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


