Abstract
Background
To date, there is no global consensus on the definition of the severity of psoriasis. The REFlective evaLuation of psoriasis Efficacy of Treatment and Severity (REFLETS) questionnaire has recently been developed to provide a better understanding of plaque-type psoriasis severity and treatment efficacy from both patient and clinician perspectives.
Objective
This study aimed to develop and psychometrically validate the new REFLETS questionnaire to evaluate patient and clinician perceptions of plaque-type psoriasis severity and treatment efficacy.
Methods
Two similar versions of the REFLETS questionnaire were developed following a rigorous methodology for clinicians and patients, referring to ‘the psoriasis of your patient' or to ‘your psoriasis’, respectively. An observational, longitudinal, multicentre study was conducted in France with 34 dermatologists and 430 mild to severe plaque-type psoriasis patients to finalize the questionnaire and evaluate its psychometric properties.
Results
Two dimensions were defined – severity and treatment efficacy – with three subdimensions within severity (impact of psoriasis, symptoms and disease course), and two individual items on joint pain. The questionnaire was well accepted by clinicians and patients. Excellent internal consistency (Cronbach's alpha = 0.66–0.98) and test–retest reliability (intraclass correlation coefficients = 0.83–0.94) were demonstrated. REFLETS scores were moderately to highly correlated to Psoriasis Area and Severity Index (r = 0.35–0.70), Skindex-29 (r = 0.46–0.82) and DLQI scores (r = 0.36–0.82). Patients with decreased psoriasis severity and those with increased treatment efficacy, according to patient global evaluations, had lower severity and higher treatment efficacy REFLETS scores, respectively.
Conclusion
REFlective evaLuation of psoriasis Efficacy of Treatment and Severity is a promising tool for assessing plaque-type psoriasis severity and treatment efficacy from patient and clinician perspectives. It may help to improve patient and clinician communication in treatment decision making.
Introduction
Psoriasis is usually classified as mild, moderate or severe, according to the proportion of erythema, induration and desquamation of psoriasis plaques and the proportion of body surface area involved.1 The Psoriasis Area and Severity Index (PASI) is a widely used tool for measuring psoriasis severity; it assesses lesions weighted by the area of involvement.2 Other common severity measures include percentage of body surface area involvement and physician's global assessment.3,4 However, despite the existence of these standard measures, there is no global consensus on the definition of severity of psoriasis5 and the PASI has some limitations in terms of clinical relevance and interrater variability.6,7
The severity of psoriasis symptoms and difficulty finding effective treatments cause psychological distress and strongly affect patients' quality of life, especially daily activities and social life.8,9 Discrepancies are observed between clinical and patient evaluations of severity and treatment effectiveness10,11, with patients generally focusing on psychological impact of the disease.12,13 Moreover, patients with psoriasis frequently display difficulties in expressing their emotions and feelings.14,15 These issues can contribute to inappropriate treatment decisions, and patients may become dissatisfied with clinicians and therapies.16,17
The European Medicines Agency recommends using both clinical and patient assessments, including health-related quality of life measures, for overall evaluation of product efficacy in psoriasis.18 The Skindex-29 and the Dermatology Life Quality Index (DLQI) are two self-administered instruments frequently used to evaluate quality of life in patients with skin disease.19,20 However, these instruments assess the patient perspective only and have been developed for use in any skin disease. Psoriasis-specific instruments such as the Psoriasis Disability Index 21, the Impact of Psoriasis Questionnaire22, the Psoriasis Index of Quality of Life23 and the Psoriasis Quality of Life24 questionnaire also exist. However, these instruments aim to assess the impact of psoriasis on quality of life as perceived by patients only. Therefore, there is a need for a psoriasis-specific instrument that better and specifically measures perceived disease severity and treatment efficacy as rated by both patients and clinicians. This new instrument could improve patient–clinician communication and thus treatment decisions.
This article describes the development and psychometric validation of the self-administered REFlective evaLuation of psoriasis Efficacy of Treatment and Severity (REFLETS) questionnaire, an instrument allowing plaque-type psoriasis severity and treatment efficacy to be evaluated simultaneously by both patients and clinicians.
Materials and methods
The questionnaire development and the validation study were independently submitted to and approved by the Toulouse University Hospital Ethics committee. Confidentiality and anonymity were guaranteed to patients and informed consent was given by all patients before entering the study. The study was conducted in compliance with the ethical principles derived from the Declaration of Helsinki.
A scientific advisory board composed of eight French dermatologists experienced in psoriasis research and management was involved in the development and validation process.
Development of the REFLETS questionnaire
Questionnaire development followed a rigorous and standardized methodology as recommended by the US FDA for patient-reported outcome measures.25 A literature review was first conducted to document patient and clinician perceptions of psoriasis severity and to identify existing instruments and criteria used to evaluate psoriasis severity and treatment efficacy. Semi-directive exploratory interviews were then conducted face-to-face with patients and by phone with dermatologists in France. Interviews aimed to identify attributes clinicians and patients use to assess the severity of psoriasis and judge treatment success.
Dermatologists working in hospital, private practice or both were eligible to participate in the study if managing at least two patients a month with mild to severe plaque-type psoriasis, and if prescribing local, systemic or biological treatments, or phototherapy. Patients were eligible if aged above 18, diagnosed with mild to severe plaque-type psoriasis, and treated with local, traditional systemic, biological or phototherapy treatment. Patients with pustular or guttate psoriasis were excluded.
Qualitative analysis of transcripts of interviews was conducted according to the grounded theory26,27 and involved classifying subjects' quotes into concepts to create the conceptual content of the questionnaire using ATLAS.ti software (Version 6.0; GmbH, Berlin, Germany).28 The most relevant concepts related to severity of psoriasis and treatment efficacy were discussed and selected with the scientific advisory board. Items were generated for each concept using patients' words. Two test versions of the questionnaire were developed, one to be completed by clinicians, one by patients.
Comprehension tests were carried out following an iterative process with new clinicians and patients recruited using the same criteria as for exploratory interviews. Clinicians and patients completed the questionnaire and answered questions about its content, structure, item relevance and comprehension. The questionnaire was revised accordingly.
Finalization and psychometric validation of the REFLETS questionnaire
Study design and population
The validation study was an observational, prospective, longitudinal, multicentre study conducted with dermatologists in France. Each clinician recruited 10–15 patients using the same criteria as for the development interviews, but also including treatment-naive patients. The study included for each patient an inclusion visit, a first follow-up 1–3 months after inclusion, either as visit to the clinician or by mail, and a second follow-up a week later by mail. At inclusion, clinicians classified their patient as mild, moderate or severe; as no global consensus on the definition of severity of psoriasis exists, no specific definition was given to the clinicians. Clinicians answered according to their own experience. Then they collected patient socio-demographic and clinical characteristics, completed the REFLETS questionnaire, the PASI2 and two global evaluation scales to evaluate psoriasis severity (from 1: mild psoriasis to 4: very severe psoriasis) and treatment efficacy (from 1: treatment failure to 4: very effective). If patients visited again their clinicians within 3-months of inclusion visit, clinicians again completed the REFLETS questionnaire and the PASI. At each time point, patients completed the REFLETS questionnaire and evaluated psoriasis severity and treatment efficacy using global evaluation scales. Additionally, at inclusion and follow-up 1, patients completed the Skindex-2919 and the DLQI,20 two dermatology-specific instruments measuring quality of life of patients with skin disease. The Skindex-29 comprises three dimensions, emotions, symptoms and functioning, scored from 0 (no impact) to 100 (large impact on quality of life). The DLQI contains six dimensions: symptoms and feelings, daily activities, leisure, work and school, personal relationships and treatment. Dimension and total scores range from 0 (no effect) to 30 (the largest effect on patient's life).
Statistical analyses and psychometric properties
Quality of completion of the REFLETS questionnaire was first evaluated. The structure of the questionnaire was defined at inclusion using principal component analysis (PCA) with PROMAX rotation and confirmed at follow-up 1 using a multitrait analysis based on Pearson correlation coefficients. The relationship of the REFLETS scores with the PASI, patient and clinician global evaluations of psoriasis severity, Skindex-29 scores and DLQI scores was explored at inclusion to evaluate clinical and concurrent validity of the REFLETS questionnaire. The hypothesis is that REFLETS scores are higher when psoriasis severity is lower, and that REFLETS scores correlate moderately to highly with scores from other questionnaires measuring similar concepts, and poorly with scores from other questionnaires measuring non-related concepts. Cronbach's alphas were calculated for each score at inclusion to evaluate internal consistency reliability (extent to which individual items are consistent with each other in the same dimension and reflect a single underlying concept); a value of 0.70 or greater is considered satisfactory.29 The test–retest reliability of patient REFLETS scores (i.e. the intrapatient variability) was assessed at one week interval (between follow-ups 1 and 2) using intraclass correlation coefficients (ICC). Values of 0.70 or greater are considered satisfactory.29 The ability of REFLETS scores to detect changes in health status over time (i.e. responsiveness to changes)30 was assessed by comparing changes in REFLETS scores between (1) improved, stable and worsened groups of patients defined with patient global evaluations of severity or treatment efficacy, and (2) responders (defined by a decrease in PASI score ≥50% from inclusion to follow-up 1) and non-responders.31 Effect sizes (ES) were calculated within each group of patients to interpret changes in REFLETS scores as low, moderate or important with ES around 0.20, 0.50 or 0.80, respectively.32
The threshold for statistical significance was fixed at 5%. Statistical analyses were performed using SAS software for Windows (Version 9.2; SAS Institute, Inc., Cary, NC, USA).
Results
Development of the REFLETS questionnaire
Twenty clinicians and 20 patients (mean age 46 years) were first interviewed. As classified by clinicians, four patients had mild plaque-type psoriasis, ten moderate and six severe. Most patients received topical treatments (n = 17); some patients also received a systemic treatment (n = 7), a biological treatment (n = 7) or phototherapy (n = 6).
The attributes used by patients and clinicians to assess psoriasis severity and treatment efficacy included symptoms (itching, desquamation and pain); disease-specific characteristics (surface, location, chronic condition); treatment-related aspects (efficacy, rapidity and satisfaction); and impact of the disease and its treatment on patient's quality of life (social, physical and psychological aspects, professional and everyday life and couple life) (Fig.1). Based on qualitative analysis, the conceptual content of the questionnaire was defined (Fig.1), and items were generated to create the test versions of the questionnaire for both clinicians and patients. In the clinician version, items referred to ‘the psoriasis of your patient'; in the patient version, items referred to ‘your psoriasis’.
Figure 1.
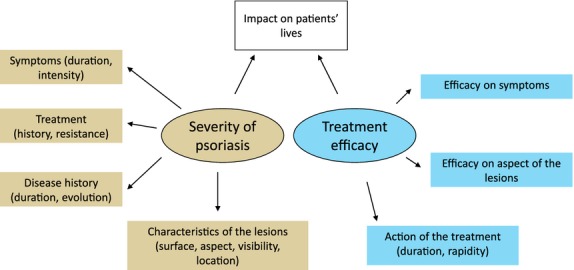
Conceptual content of the REFLETS questionnaire.
Twenty new patients (mean age 45 years) with mild (n = 6), moderate (n = 7) or severe plaque-type psoriasis (n = 7) and 15 new clinicians participated in comprehension tests. Most patients received topical treatments (n = 18), half received a systemic treatment and less than a quarter received biological treatment (n = 4) or phototherapy (n = 3). Comprehension testing showed that the questionnaire was well accepted by patients and by dermatologists. Minor reformulations based on patient and/or clinician suggestions were implemented to improve understanding.
The resulting pilot versions both contained 32 items organized into two sections: severity of psoriasis (20 items) and efficacy of treatment (12 items).
Finalization and psychometric validation of the REFLETS questionnaire
Study population and completion data
Thirty-four clinicians recruited 441 patients; 430 (97%) met all selection criteria and completed at least one item of the REFLETS questionnaire at inclusion (inclusion population). Eighty-five per cent of patients completed the REFLETS questionnaire at follow-up 1 (n = 374) and 70% at follow-up 2 (n = 310). Patient characteristics at inclusion are described in Table1.
Table 1.
Patient characteristics at inclusion in the validation study
| Mild (N = 132) | Moderate (N = 166) | Severe (N = 132) | Inclusion population (N = 430) | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 48.0 (14.6) | 46.7 (13.6) | 45.1 (14.9) | 46.6 (14.3) |
| Min–Max | 18.0–82.0 | 19.0–81.0 | 19.0–83.0 | 18.0–83.0 |
| Gender (%) | ||||
| Males | 59.1 | 58.4 | 64.4 | 60.5 |
| Time since diagnosis (years) | ||||
| Mean (SD) | 18.6 (14.8) | 17.2 (12.5) | 18.1 (12.2) | 17.9 (13.1) |
| Min–Max | 0.0–74.0 | 0.0–52.0 | 0.0–54.0 | 0.0–74.0 |
| First psoriasis episode (%) | ||||
| Yes | 5.3 | 3.0 | 4.5 | 4.2 |
| Current psoriasis flare (%) | ||||
| Yes | 40.9 | 76.5 | 74.2 | 64.9 |
| Psoriatic arthritis (%) | ||||
| Yes | 21.2 | 19.9 | 32.6 | 24.2 |
| No treatment (%) | 3.0 | 7.8 | 4.5 | 5.3 |
| Topical treatment (alone) (%) | 32.6 | 19.9 | 21.2 | 24.2 |
| Topical + phototherapies (%) | 1.5 | 7.8 | 4.6 | 4.9 |
| Topical + systemic (%) | 5.3 | 13.3 | 12.9 | 10.7 |
| Topical + biotherapies (%) | 9.1 | 6.0 | 15.9 | 10.0 |
| Topical + others (%) | – | 0.6 | – | 0.2 |
| Topical + phototherapies + systemic (%) | – | – | 0.8 | 0.2 |
| Topical + phototherapies + others (%) | – | 0.6 | – | 0.2 |
| Topical + systemic + biotherapies (%) | – | – | 0.8 | 0.2 |
| Phototherapies (alone) (%) | 0.0 | 3.0 | 3.8 | 2.3 |
| Phototherapies + systemic (%) | – | 0.6 | – | 0.2 |
| Phototherapies + others (%) | – | 0.6 | – | 0.2 |
| Systemic medications (alone) (%) | 10.6 | 17.5 | 8.3 | 12.6 |
| Systemic + biotherapies (%) | 2.3 | 0.6 | 0.8 | 1.2 |
| Systemic + others (%) | 0.8 | – | – | 0.2 |
| Biotherapies (alone) (%) | 32.6 | 20.5 | 23.5 | 25.1 |
| Others (alone) (%) | 2.3 | 1.2 | 3.0 | 2.1 |
At each visit, the mean number of missing items was lower than one of 20 for severity items and lower than one of 12 for efficacy items, for both clinicians and patients. More than 90% of clinicians and patients completed all severity items at each visit. Treatment efficacy items were only completed for patients being treated for their psoriasis. For those patients, more than 90% of patients and clinicians completed all treatment efficacy items at each visit.
Finalization of the questionnaire
Based on iterative PCA, multitrait analysis and discussion with the scientific advisory board, three items were removed from the pilot version (disease duration, difficulty finding effective treatment and sustained benefits after end of treatment). The REFLETS final structure comprises 29 items divided into two dimensions: psoriasis severity and treatment efficacy (Table2).33 The psoriasis severity dimension included three subdimensions (disease course, symptoms and impact of psoriasis). Two items on joint pain and treatment efficacy on joint pain were retained as single items within the two dimensions. For each dimension, sub-dimension and single item, scores were calculated from 0 to 100; higher scores indicating more severe psoriasis or more effective treatment. The final structure of the questionnaire was confirmed at follow-up 1.
Table 2.
Final structure of the REFLETS questionnaire and scores at inclusion, N = 430 patients and 34 clinicians
| Dimensions | Scores | Item content | No. of items | Score at inclusion, mean (SD) |
|
|---|---|---|---|---|---|
| Patients | Clinicians | ||||
| Psoriasis severity | Total psoriasis severity: | 18 | 41.0 (21.4) | 38.5 (20.1) | |
|
Self-rated global severity; rashes: frequency, duration | 3 | 63.0 (22.3) | 62.6 (22.0) | |
|
Itching, desquamation, pain on plaques; characteristics of lesions: surface, aspect, visibility | 6 | 47.1 (25.7) | 39.2 (22.3) | |
|
Embarrassment with others, mood, physical impact, professional impact, daily activities, sleep, isolation, having a relationship | 9 | 29.7 (23.2) | 29.2 (22.6) | |
| Joint pain | Joint pain | 1 | 22.8 (31.3) | 14.0 (26.4) | |
| Treatment efficacy | Total treatment efficacy | Efficacy on symptoms: itching, desquamation, pain on plaques; Efficacy on plaque aspect: global aspect, surface; Rapidity of action; Efficacy on quality of life; Satisfaction with treatment | 9 | 64.9 (27.0) | 63.5 (28.8) |
| Efficacy on joint pain | Efficacy on joint pain | 1 | 53.8 (37.3) | 51.1 (40.9) | |
Scores are presented as mean (SD); treatment efficacy and efficacy on joint pain concerned only patients who were treated (N = 407).
Psychometric validation
REFlective evaLuation of psoriasis Efficacy of Treatment and Severity scores were moderately correlated to the PASI score. All correlations were higher for the clinician version (r = 0.47–0.70) than for the patient version (r = 0.35–0.54). For both versions, the highest correlations between REFLETS and PASI scores were seen for the REFLETS symptoms score (0.54 and 0.70, patient and clinician versions, respectively) and the REFLETS total severity score (0.52 and 0.67, respectively). REFLETS scores were also moderately to highly correlated to Skindex-29 (r = 0.46–0.82) and DLQI scores (r = 0.36–0.82) for both versions; however, unlike correlations with the PASI score, correlations with these measures were lower for the clinician than for the patient version. Only the joint pain and treatment efficacy on joint pain scores were poorly correlated to the PASI, Skindex-29 and DLQI scores. Overall, concurrent validity results showed moderate to high correlations between REFLETS scores and clinician PASI score, and patient Skindex-29 and DLQI scores when measuring similar concepts. These results suggest that REFLETS scores provide additional information not covered by the other standard measures.
Both REFLETS versions demonstrated good clinical validity (Table3 for the patient version of the REFLETS questionnaire). There was a statistically significant link between patient REFLETS scores and the global evaluation of severity by clinicians; when clinicians reported increased severity, patient REFLETS scores indicated increased severity and decreased treatment efficacy. Similar results were found when comparing patient REFLETS scores with the severity and treatment efficacy global evaluation scales completed by patients. Similar results were found with the REFLETS clinician version (data not shown).
Table 3.
Relationship between REFLETS scores and patient groups defined through external parameters at inclusion – patient version
| Disease course | Symptoms | Joint pain | Impact of psoriasis | Total severity | Treatment efficacy | Efficacy on joint pain | |
|---|---|---|---|---|---|---|---|
| Severity of psoriasis (clinician's judgement) | |||||||
| Mild (N = 132) | 49.6 (21.8) | 28.7 (21.6) | 21.0 (29.4) | 15.6 (16.1) | 25.6 (15.9) | 79.7 (19.9) | 60.3 (35.6) |
| Moderate (N = 166) | 65.3 (19.2) | 50.7 (19.5) | 19.1 (29.0) | 31.8 (21.6) | 43.6 (17.6) | 58.2 (24.3) | 52.4 (35.9) |
| Severe (N = 132) | 73.5 (19.7) | 60.9 (26.0) | 29.1 (35.1) | 41.3 (23.9) | 53.3 (21.4) | 57.9 (30.5) | 48.5 (40.0) |
| P < 0.001 for all scores, except joint pain (P = 0.020) and efficacy on joint pain (P = 0.225) | |||||||
| Severity of psoriasis (global scale - patient) | |||||||
| Mild (N = 45) | 38.0 (19.9) | 21.0 (17.2) | 16.3 (29.0) | 8.6 (10.9) | 17.6 (12.6) | 85.1 (16.1) | 72.5 (29.4) |
| Moderate (N = 127) | 55.2 (18.8) | 38.8 (16.7) | 19.7 (28.7) | 20.9 (14.5) | 32.5 (12.5) | 64.4 (22.3) | 41.1 (32.0) |
| Severe (N = 192) | 69.2 (18.0) | 52.3 (22.9) | 21.8 (30.7) | 34.6 (21.5) | 46.3 (18.4) | 60.4 (27.4) | 58.5 (38.5) |
| Very severe (N = 64) | 76.8 (22.6) | 65.0 (32.9) | 36.0 (36.9) | 46.7 (29.8) | 57.8 (27.0) | 64.4 (33.7) | 52.4 (40.7) |
| P < 0.001 for all scores, except joint pain (P = 0.002) and efficacy on joint pain (P = 0.012) | |||||||
| Treatment efficacy (global scale – patient) | |||||||
| Not at all (N = 24) | 79.6 (16.6) | 72.3 (22.2) | 40.6 (37.5) | 55.1 (22.0) | 65.0 (17.8) | 11.9 (14.4) | 5.6 (13.0) |
| Not very (N = 107) | 72.1 (17.3) | 60.0 (19.2) | 21.2 (32.4) | 40.6 (21.4) | 52.2 (17.5) | 39.0 (15.9) | 27.8 (27.0) |
| Effective (N = 181) | 59.8 (20.5) | 40.4 (21.1) | 22.8 (30.7) | 25.0 (19.6) | 36.0 (17.2) | 72.7 (14.7) | 59.5 (34.9) |
| Very effective (N = 79) | 49.9 (24.8) | 28.9 (26.8) | 20.5 (31.4) | 16.0 (19.7) | 25.9 (20.5) | 93.7 (6.1) | 80.8 (26.0) |
| P < 0.001 for all scores, except joint pain (P = 0.051) | |||||||
Scores are presented as mean (SD); treatment efficacy and efficacy on joint pain concerned only patients who were treated.
For both REFLETS versions, the internal consistency reliability was confirmed for all scores (Cronbach's alphas coefficients ranging from 0.86 to 0.98) except for the ‘disease course’ sub-dimension (0.66 and 0.67 for patient and clinician versions, respectively). The test–retest (intrapatient variability) of patient REFLETS scores between the two follow-ups was confirmed for all scores (ICCs ranging from 0.83 to 0.94).
The patient version of the REFLETS questionnaire was responsive to change, with the exception of joint pain and efficacy on joint pain scores (Fig.2). Patients reporting global improvement in psoriasis severity also had improved REFLETS severity scores (Fig.2a). Similarly, patients reporting greater global treatment efficacy also had better treatment efficacy REFLETS scores (Fig.2b). Responders on PASI score from inclusion to follow-up 1 had higher improvements on severity and treatment efficacy REFLETS scores than non-responders (Fig.2c). Similar results were observed for the clinician version of the REFLETS questionnaire (data not shown).
Figure 2.
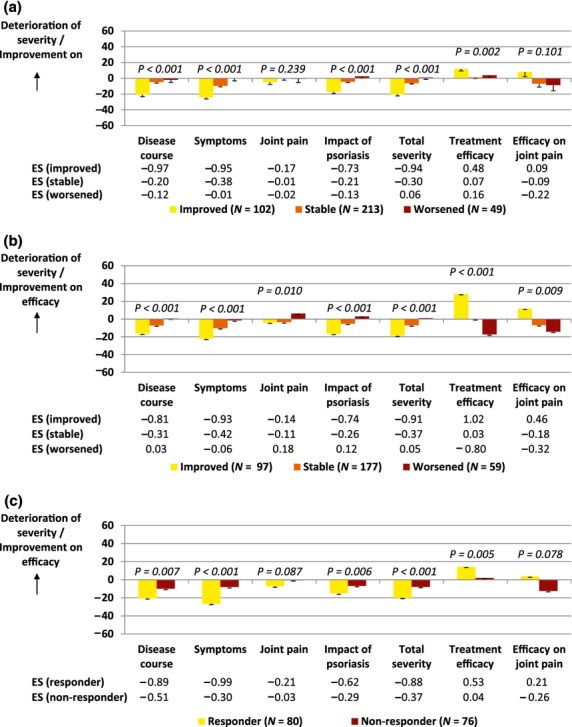
Responsiveness of REFLETS scores for the patient version. According to (a) changes in patient global evaluation of psoriasis severity (improved, stable or worsened patients), (b) changes in patient global evaluation of treatment efficacy and (c) a responder threshold based on a decrease in PASI score (responders or non-responders).
Categorization of REFLETS scores
A similar categorization of the total severity REFLETS score was obtained for the patient version (Fig.3) and clinician version (data not shown). Between 45 and 50% of mild patients and less than 10% of moderate and severe patients as classified by clinicians' judgment at inclusion had REFLETS total severity scores between 0 and 20. A score between 20 and 50 was reported by 50–55% of mild, 60% of moderate and 40% of severe patients. A score between 50 and 100 was reported by less than 10% of mild, 30% of moderate and 60% of severe patients.
Figure 3.
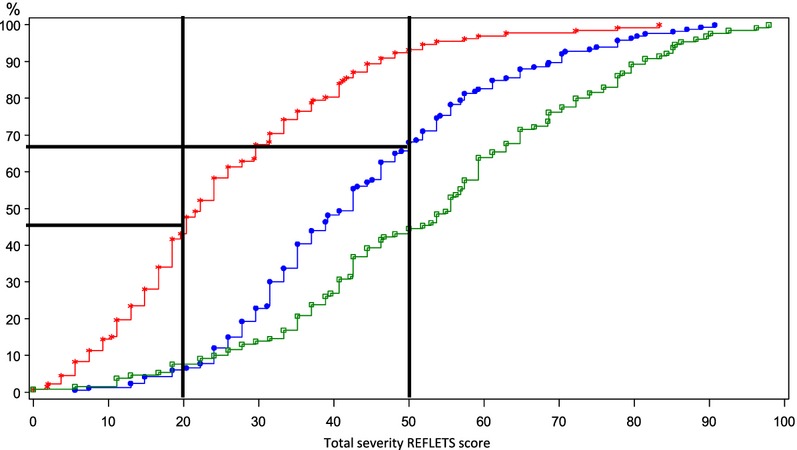
Categorization of the total severity REFLETS score for the patient version. Mild patients (N = 132) are represented in red, moderate patients (N = 166) in blue and severe patients (N = 130) in green.
Discussion
The need to evaluate the impact of psoriasis on patient quality of life when evaluating psoriasis severity is acknowledged in the literature.3,11 Interestingly, during our exploratory interviews, the impact of psoriasis and its treatment on patient's quality of life was identified as a criterion to evaluate disease severity by both patients and clinicians. Given the limitations of standard questionnaires used in clinical practice to evaluate psoriasis severity,6,7,11,34 and the lack of consensual definition of psoriasis severity, our study aimed to develop and validate a new instrument that assesses both patient and clinician perceptions of psoriasis severity and treatment efficacy.
The finalized REFLETS questionnaire consists of 29 items distributed into two dimensions (severity and treatment efficacy). Patient and clinician versions are identical but refer to ‘your psoriasis’ and ‘the psoriasis of your patient’, respectively. The attributes used by patients and clinicians to assess psoriasis severity and treatment efficacy were strikingly similar. The finalization and psychometric validation of the questionnaire confirmed the two dimensions defined during development, and the similar structure of the patient and clinician versions.
The REFLETS questionnaire was well understood and accepted by patients and clinicians in the validation study. The quality of completion was very good, with more than 90% of clinicians and patients completing all items, regardless of the severity of psoriasis of the patients included (31% mild, 38% moderate, 31% severe). Altogether, this suggests that REFLETS is appropriate for evaluation of all stages of psoriasis severity in clinical practice.
Almost one-fourth of the validation study population reported experiencing psoriatic arthritis. This is somewhat higher than what has been generally reported in the US35 or Europe,36 but in agreement with a recent review concluding that psoriatic arthritis may affect up to 24% of psoriasis patients.37 Additionally, it is also possible that the group of patients with mild psoriasis included patients with severe cases that had regressed to mild cases at the time of inclusion, explaining the surprisingly high number of patients with psoriatic arthritis in the mild group. This possibility is suggested by the fact that almost 33% of patients in the mild group had been treated with biotherapies, which are usually indicated for moderate to severe cases unresponsive to other treatments, or for those who have experienced harmful side-effects from other treatments.38
The REFLETS questionnaire was developed and validated in a French population. To enable its use in future international studies, the questionnaire should be translated and linguistically validated in other languages, including English, and the validation study should be replicated to confirm results in those populations.
The psychometric validation of the REFLETS questionnaire demonstrated that it is valid, reliable and responsive to change. REFLETS enables discrimination between psoriasis patients based on disease severity and treatment efficacy. Concurrent validity results between REFLETS scores and Skindex-29, DLQI and PASI scores suggest that REFLETS scores provide additional information that is not covered by standard measures such as the PASI, the DLQI or the Skindex-29. Excellent internal consistency was demonstrated for both patient and clinician versions of REFLETS. Excellent test–retest reliability was shown for the patient version. Finally, REFLETS was shown to be responsive to changes between inclusion and the one to 3-month follow-up evaluation, according to severity and treatment efficacy global evaluation scales and changes in PASI score.
While robust psychometric properties were demonstrated for multi-item dimensions of REFLETS, results were more limited for the two single-item scores related to joint pain. This was not surprising, as only a small proportion of patients with psoriasis experience psoriatic arthritis. It may have been enlightening to evaluate these two single-item scores specifically with patients experiencing psoriatic arthritis. Despite that these two items concern only a sub-group of patients and that evaluating psychometric properties on single-item scores often results in limited conclusions, they were retained in the questionnaire because both patients and clinicians considered joint pain an essential factor in psoriasis severity and treatment efficacy evaluation.
Altogether, these results support the ability of the REFLETS questionnaire to assess both severity and treatment efficacy in psoriasis, from both patient and clinician perspectives, making it unique among evaluation measures currently available and a promising tool for use in clinical practice. It may help foster better patient–clinician communication and thus improve clinical decision making and patient management.
Acknowledgments
We are extremely grateful to the patients who contributed to this study and we thank all participating investigators (list provided as supplementary information). Juliette Meunier (Mapi) provided statistical support, and Jérémy Lambert (Mapi) provided medical writing support (both funded by AbbVie).
Conflicts of interest
HB, MB, PB, PJ, DJ, MM, JPO and CP have served as consultants for AbbVie Laboratories and received research funding from AbbVie Laboratories. As part of the scientific advisory board, they were involved at each milestone of the development and validation process. BA, HG and ARC are employees of Mapi, which was funded by AbbVie to conduct the development and validation of the instrument, perform the statistical analysis, and interpret the results. ET is employed by AbbVie and may own AbbVie stock. AbbVie participated in the interpretation of data, review and approval of the manuscript.
Funding sources
The design, study conduct and financial support for the study were provided by AbbVie Laboratories.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
List of investigators who participated in the validation study.
References
- 1.Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64(Suppl. 2):ii30–ii36. doi: 10.1136/ard.2004.031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica. 1978;157:238–244. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 3.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(Suppl. 2):ii65–ii68. doi: 10.1136/ard.2004.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Kerkhof PC, Kragballe K, Austad J, et al. Psoriasis: severity assessment in clinical practice. Conclusions from workshop discussions and a prospective multicentre survey of psoriasis severity. Eur J Dermatol. 2006;16:167–171. [PubMed] [Google Scholar]
- 5.Raychaudhuri SK, Maverakis E, Raychaudhuri SP. Diagnosis and classification of psoriasis. Autoimmun Rev. 2014;13:490–495. doi: 10.1016/j.autrev.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Gourraud PA, Le GC, Puzenat E, Aubin F, Ortonne JP, Paul CF. Why statistics matter: limited inter-rater agreement prevents using the psoriasis area and severity index as a unique determinant of therapeutic decision in psoriasis. J Invest Dermatol. 2012;132:2171–2175. doi: 10.1038/jid.2012.124. [DOI] [PubMed] [Google Scholar]
- 7.Spuls PI, Lecluse LL, Poulsen ML, Bos JD, Stern RS, Nijsten T. How good are clinical severity and outcome measures for psoriasis?: quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933–943. doi: 10.1038/jid.2009.391. [DOI] [PubMed] [Google Scholar]
- 8.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280–284. [PubMed] [Google Scholar]
- 9.Sampogna F, Tabolli S, Abeni D. Living with psoriasis: prevalence of shame, anger, worry, and problems in daily activities and social life. Acta Derm Venereol. 2012;92:299–303. doi: 10.2340/00015555-1273. [DOI] [PubMed] [Google Scholar]
- 10.Ersser SJ, Surridge H, Wiles A. What criteria do patients use when judging the effectiveness of psoriasis management? J Eval Clin Pract. 2002;8:367–376. doi: 10.1046/j.1365-2753.2002.00372.x. [DOI] [PubMed] [Google Scholar]
- 11.Naldi L. Scoring and monitoring the severity of psoriasis. What is the preferred method? What is the ideal method? Is PASI passe? facts and controversies. Clin Dermatol. 2010;28:67–72. doi: 10.1016/j.clindermatol.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Kirby B, Richards HL, Woo P, Hindle E, Main CJ, Griffiths CE. Physical and psychologic measures are necessary to assess overall psoriasis severity. J Am Acad Dermatol. 2001;45:72–76. doi: 10.1067/mjd.2001.114592. [DOI] [PubMed] [Google Scholar]
- 13.Perrott SB, Murray AH, Lowe J, Mathieson CM. The psychosocial impact of psoriasis: physical severity, quality of life, and stigmatization. Physiol Behav. 2000;70:567–571. doi: 10.1016/s0031-9384(00)00290-0. [DOI] [PubMed] [Google Scholar]
- 14.Masmoudi J, Maalej I, Masmoudi A, Rached H, Rebai A, Turki H. Jaoua A [Alexithymia and psoriasis: a case-control study of 53 patients] Encephale. 2009;35:10–17. doi: 10.1016/j.encep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Richards HL, Fortune DG, Griffiths CE, Main CJ. Alexithymia in patients with psoriasis: clinical correlates and psychometric properties of the Toronto Alexithymia Scale-20. J Psychosom Res. 2005;58:89–96. doi: 10.1016/j.jpsychores.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Dubertret L, Mrowietz U, Ranki A, et al. European patient perspectives on the impact of psoriasis: the EUROPSO patient membership survey. Br J Dermatol. 2006;155:729–736. doi: 10.1111/j.1365-2133.2006.07405.x. [DOI] [PubMed] [Google Scholar]
- 17.Maza A, Richard MA, Aubin F, et al. Significant delay in the introduction of systemic treatment of moderate to severe psoriasis: a prospective multicentre observational study in outpatients from hospital dermatology departments in France. Br J Dermatol. 2012;167:643–648. doi: 10.1111/j.1365-2133.2012.10991.x. [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency, Committee For Medicinal Products For Human Use (CHMP) Guideline on clinical investigation of medicinal products indicated for the treatment of psoriasis. [WWW document]. URL http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003329.pdf (last accessed: 16 January 2014)
- 19.Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997;133:1433–1440. [PubMed] [Google Scholar]
- 20.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 21.Finlay AY, Kelly SE. Psoriasis–an index of disability. Clin Exp Dermatol. 1987;12:8–11. doi: 10.1111/j.1365-2230.1987.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 22.McKenna KE, Stern RS. The impact of psoriasis on the quality of life of patients from the 16-center PUVA follow-up cohort. J Am Acad Dermatol. 1997;36:388–394. doi: 10.1016/s0190-9622(97)80214-9. [DOI] [PubMed] [Google Scholar]
- 23.McKenna SP, Cook SA, Whalley D, et al. Development of the PSORIQoL, a psoriasis-specific measure of quality of life designed for use in clinical practice and trials. Br J Dermatol. 2003;149:323–331. doi: 10.1046/j.1365-2133.2003.05492.x. [DOI] [PubMed] [Google Scholar]
- 24.Dauden E, Herrera E, Puig L, et al. Validation of a new tool to assess health-related quality of life in psoriasis: the PSO-LIFE questionnaire. Health Qual Life Outcomes. 2012;10:56. doi: 10.1186/1477-7525-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S.Department of Health and Human Services, Food and Drug Admin-istration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. [WWW document]. URL http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf (last accessed: 16 January 2014)
- 26.Charmaz K. Grounded theory. In: Smith JA, Harre R, van Langenhove L, editors. Rethinking Methods in Psychology. London, UK: Sage; 1995. pp. 27–49. [Google Scholar]
- 27.Charmaz K. Grounded Theory in the 21st Century. 3rd edn. Thousand Oaks, CA: Sage; 2005. [Google Scholar]
- 28.Murh T. User's Manual for Atlas.ti 6.0. Berlin: Atlas.ti; 2004. [Google Scholar]
- 29.Nunnally JC, Bernstein IH. Psychometric Theory. 3rd edn. New York: McGraw-Hill Inc; 1994. [Google Scholar]
- 30.Guyatt GH, Deyo RA, Charlson M, Levine MN, Mitchell A. Responsiveness and validity in health status measurements: a clarification. J Clin Epidemiol. 1989;42:403–408. doi: 10.1016/0895-4356(89)90128-5. [DOI] [PubMed] [Google Scholar]
- 31.Finlay AY, Salek MS, Haney J. Intramuscular alefacept improves health-related quality of life in patients with chronic plaque psoriasis. Dermatology. 2003;206:307–315. doi: 10.1159/000069942. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press; 1977. [Google Scholar]
- 33.Mapi Research Trust. 2014. REFlective evaLuation of psoriasis Efficacy of Treatment and Severity (REFLETS) questionnaire. [WWW document]. URL http://www.proqolid.org/proqolid/instruments/reflective_evaluation_of_psoriasis_efficacy_of_treatment_and_severity_reflets (last accessed: 10 April 2014)
- 34.Puzenat E, Bronsard V, Prey S, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24(Suppl. 2):10–16. doi: 10.1111/j.1468-3083.2009.03562.x. [DOI] [PubMed] [Google Scholar]
- 35.Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53:573. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 36.Gisondi P, Girolomoni G, Sampogna F, Tabolli S, Abeni D. Prevalence of psoriatic arthritis and joint complaints in a large population of Italian patients hospitalised for psoriasis. Eur J Dermatol. 2005;15:279–283. [PubMed] [Google Scholar]
- 37.Prey S, Paul C, Bronsard V, et al. Assessment of risk of psoriatic arthritis in patients with plaque psoriasis: a systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24(Suppl. 2):31–35. doi: 10.1111/j.1468-3083.2009.03565.x. [DOI] [PubMed] [Google Scholar]
- 38.Rich SJ, Bello-Quintero CE. Advancements in the treatment of psoriasis: role of biologic agents. J Manag Care Pharm. 2004;10:318–325. doi: 10.18553/jmcp.2004.10.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of investigators who participated in the validation study.


