Abstract
Objective:
In 2011, Malawi implemented Option B+ (B+), lifelong antiretroviral therapy (ART) for pregnant and breastfeeding women. We aimed to describe changes in service uptake and outcomes along the antenatal prevention of mother-to-child transmission (PMTCT) cascade post-B+ implementation.
Design:
Pre/post study using routinely collected program data from 2 large Lilongwe-based health centers.
Methods:
We compared the testing of HIV-infected pregnant women at antenatal care, enrollment into PMTCT services, receipt of ART, and 6-month ART outcomes pre-B+ (October 2009–March 2011) and post-B+ (October 2011–March 2013).
Results:
A total of 13,926 (pre) and 14,532 (post) women presented to antenatal care. Post-B+, a smaller proportion were HIV-tested (99.3% vs. 87.7% post-B+; P < 0.0001). There were 1654 (pre) and 1535 (post) HIV-infected women identified, with a larger proportion already known to be HIV-infected (18.1% vs. 41.2% post-B+; P < 0.0001) and on ART post-B+ (18.7% vs. 30.2% post-B+; P < 0.0001). A significantly greater proportion enrolled into the PMTCT program (68.3% vs. 92.6% post-B+; P < 0.0001) and was retained through delivery post-B+ (51.1% vs. 65% post-B+; P < 0.0001). Among those not on ART at enrollment, there was no change in the proportion newly initiating ART/antiretrovirals (79% vs. 81.9% post-B+; P = 0.11), although median days to initiation of ART decreased [48 days (19, 130) vs. 0 days (0, 15.5) post-B+; P < 0.0001]. Among those newly initiating ART, a smaller proportion was alive on ART 6 months after initiation (89.3% vs. 78.8% post-B+; P = 0.0004).
Conclusions:
Although several improvements in PMTCT program performance were noted with implementation of B+, challenges remain at several critical steps along the cascade requiring innovative solutions to ensure an AIDS-free generation.
Key Words: HIV, PMTCT, Option B+, retention, Malawi, Africa
INTRODUCTION
In 2012, an estimated 260,000 children in low- and middle-income countries were newly infected with HIV; the majority of these infections occurred in sub-Saharan Africa.1 With antiretroviral medications for prevention of mother-to-child transmission of HIV (PMTCT), the majority of these infections were preventable. However, PMTCT programs have been plagued by suboptimal uptake and loss to follow-up (LTFU) along the PMTCT cascade.2–5 The “PMTCT cascade” identifies the sequence of prevention and treatment measures delivered to HIV-infected women and their infants including maternal HIV testing, and for infected mothers, CD4+ cell count (CD4) testing, dispensing of maternal and infant antiretrovirals (ARVs), diagnostic testing of infants, and follow-up throughout breastfeeding.6–8 To significantly reduce the number of new infant HIV infections, uptake and retention at each step of the PMTCT cascade needs to be greater than 90%.7
In 2011, the Malawian Ministry of Health (MOH) implemented a novel approach to improve coverage and uptake of PMTCT, coined Option B+ (B+). B+ offers all HIV-infected pregnant and breastfeeding women lifelong antiretroviral therapy (ART) with a single fixed-dose combination tablet regardless of their clinical status or CD4.9 With automatic eligibility for lifelong ART, B+ precludes the need for CD4 testing or clinical determination before initiating treatment.10,11 By reducing the number of disparate steps mothers need to negotiate, B+ simplifies the PMTCT cascade to improve PMTCT uptake and outcomes.12 In 2013, the WHO endorsed B+ as the preferred PMTCT approach in settings with high HIV prevalence, high fertility, and extended breastfeeding.13–15
Early reports from Malawi suggest a dramatic increase in pregnant and breastfeeding women initiating ART with implementation of the B+ approach.13,16 There has been an over 748% increase in antiretroviral treatment coverage (triple-drug regimen) among HIV-infected pregnant women,13 with variable LTFU by health facility (0%–58%). The overall retention rate at 12 months post-initiation is comparable with the adult ART program (77%), with the majority of losses occurring within the first 3 months.13,16 However, although providing critical information on the performance of the B+ program in Malawi, these reports are limited in that they do not track services along the entire antenatal PMTCT cascade from HIV testing at antenatal care (ANC) through delivery and lack a control group.
The Tingathe program is a HIV service program created in collaboration with the MOH that uses dedicated community health workers (CHWs) as case managers to support women to navigate the multiple steps in the PMTCT cascade.6,17 Because Tingathe started 2 years before B+ implementation, program data provide a unique opportunity to examine the impact of B+ on uptake and outcomes throughout the antenatal PMTCT cascade using a pre-B+ cohort comparison group.
METHODS
Study Design
We conducted a pre/post quasi-experimental study using routinely collected patient-level data for pregnant women enrolled in Tingathe during two 18-month periods pre- (October 2009–March 2011) and post-implementation of B+ (October 2011–March 2013). Data from April 2011 to September 2011 were not used to allow for transition to the B+ program and reduce overlap between the pre-B+ and post-B+ cohorts. Data through January 2014 were abstracted to ensure follow-up through delivery.
The objective was to compare service uptake, and antenatal outcomes throughout the PMTCT cascade from the identification of HIV-infected pregnant women at ANC to infant delivery pre- and post-B+ implementation. Comparisons included changes in antenatal HIV testing, enrollment into the PMTCT program, maternal receipt of ARVs/ART, time to ART initiation, and antenatal outcomes (maternal death, fetal demise, transferred out, withdrawal from PMTCT services after enrollment, LTFU, delivery), and ART outcomes 6 months after ART initiation among women newly initiating ART.
Study Setting and Patient Population
Program data were available for Area 25 and Kawale, 2 large urban health centers in Lilongwe, Malawi where the Tingathe program operated. The combined estimated population is 310,000 people, with 15,000 deliveries per year, and an adult HIV prevalence of 12%.18 HIV testing at ANC is performed by routine opt-out testing as per Malawi MOH HIV guidelines and over 96% of pregnant women attend at least 1 antenatal visit.10,19,20,21
PMTCT Services Available at Program Sites Pre-B+ and Post-B+ Implementation
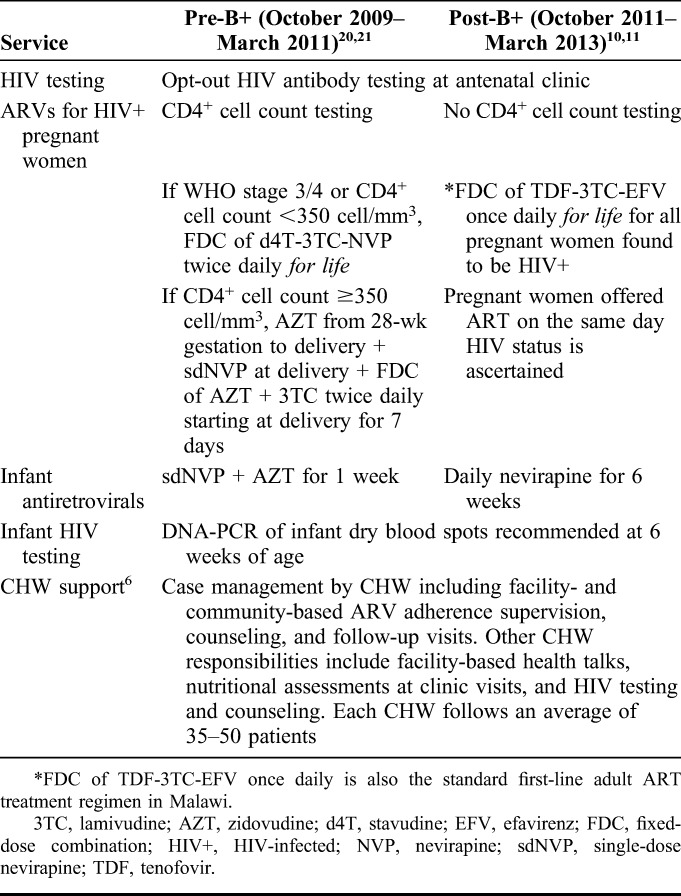
Table 1 describes PMTCT services routinely available at the program sites pre-B+ and post-B+. All PMTCT clinical care was provided in accordance with the Malawi MOH and WHO guidelines.10,11,20,21 All women identified as HIV-infected at ANC, including those already on ART, were enrolled into the PMTCT program by verbal consent. CHW support was provided by the Tingathe PMTCT program and has been previously described in detail.6 Briefly, a Tingathe CHW is assigned to each HIV-infected pregnant woman on enrollment into the PMTCT program. The CHW supports the woman to engage in longitudinal care throughout the full PMTCT cascade, from HIV testing throughout pregnancy and breastfeeding until confirmation of infant infection status. Post-B+, CHWs were trained on the new national protocols10 (Table 1) to align counseling and support services with the B+ policy of ART for all HIV+ pregnant women. Other than these adjustments, Tingathe service activities were unchanged.
TABLE 1.
PMTCT Services Routinely and Freely Available at Program Sites Pre-B+ and Post-B+ Implementation
Only HIV+ pregnant women enrolled in the Tingathe program antenatally were included in the analysis. Women were considered lost-to-follow-up13,16 if they did not return to care for more than 60 days and could not be traced.
Statistical Analysis
Antenatal PMTCT service uptake (HIV testing, enrollment into the PMTCT program, and initiation of ART/ARVs for PMTCT), and antenatal outcomes (fetal demise, maternal death before delivery, transferred out, withdrawal from PMTCT services after enrollment, LTFU, and recorded delivery) were compared between patients enrolled pre-B+ and post-B+. Wilcoxon rank-sum test and χ2 test/Fisher's exact test were used for continuous variables and categorical variables, respectively. Kaplan–Meier curves were generated to present the survival function for time from enrollment to the initiation of ART pre-B+ and post-B+. Differences between Kaplan–Meier curves were tested using log-rank test. To account for potential bias on antenatal outcomes because of the increased proportion already on ART at program enrollment post-B+, comparisons of antenatal outcomes were also conducted after excluding those already on ART at enrollment. Univariate analysis was conducted to compare 6-month ART outcomes (alive on ART, stopped ART, died, LTFU) between women newly initiated on ART at enrollment into the PMTCT program pre-B+ and post-B+.
A P-value < 0.05 was considered statistically significant. SAS software version 9.3 (SAS Institute, Cary, NC) was used for all analyses. All data were de-identified before analysis.
Ethics Statement
Ethics approval was obtained from the National Health Sciences Research Committee of Malawi and the Baylor College of Medicine Institutional Review Board.
RESULTS
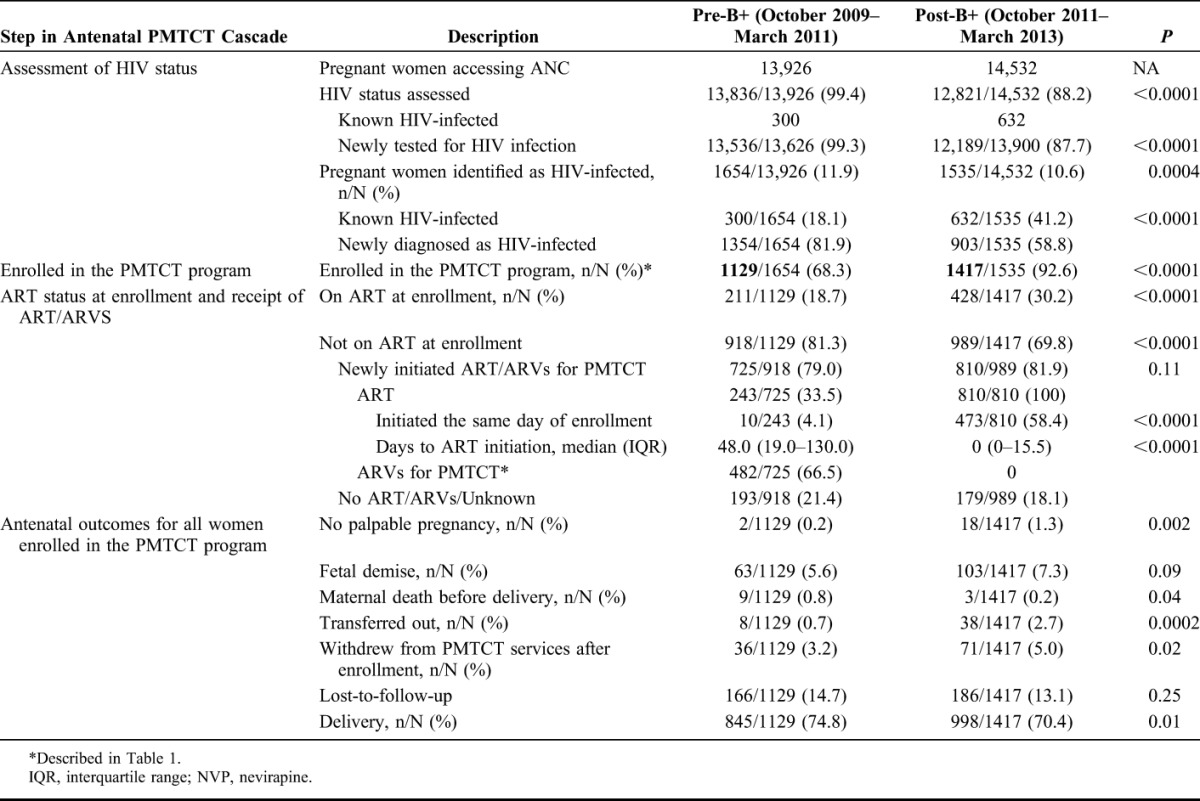
Table 2 describes changes in service uptake and antenatal outcomes pre-B+ and post-B+ implementation at critical points along the PMTCT cascade from identification at ANC through recorded birth.
TABLE 2.
Changes in Service Uptake and Outcomes Along the Antenatal PMTCT Cascade Pre-B+ and Post-B+ Implementation
Identification of HIV-Infected Women and Enrollment into PMTCT Care
Of 13,926 women accessing ANC pre-B+, 99.4% had their HIV status assessed as compared with only 88.2% of 14,532 women in the post-B+ period. There were steep drops in percentage tested during 2 discrete time periods post-B+: May 2012 to June 2012 (67.1% tested) and January to February 2013 (26.6%) (Fig. 1). Of the 1654 (pre) and 1535 (post) women identified as HIV-infected, a substantially greater proportion was already known to be HIV-infected in the postperiod (18.1% pre vs. 41.2% post; P < 0.0001).
FIGURE 1.
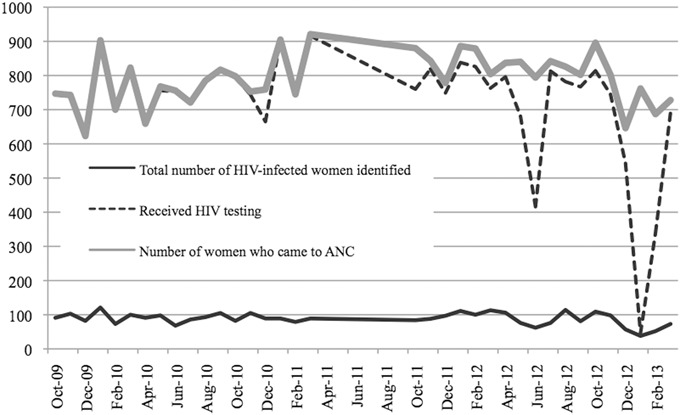
HIV testing at antenatal clinic pre-B+ and post-B+ implementation. Graph demonstrating percentage of women who received HIV testing and the total number of HIV-infected women identified pre-B+ and post-B+ (October 2011–March 2013).
There was a significant increase in the proportion of identified women enrolled into the PMTCT program post-B+ (68.3% pre vs. 92.6% post; P < 0.0001). Even excluding those known to be HIV-infected at the time of enrollment, the difference in the proportion enrolled remained significant (61% vs. 86.7% post-B+; P < 0.0001). Among enrolled women, a larger proportion was on ART at the time of enrollment, post-B+. Those enrolled post-B+ were older [median age (IQR): 26.9 (23.5–30.9) pre-B+ vs. 27.8 (23.8–31.5) post-B+; P = 0.02], and a greater proportion were enrolled during the first and second trimesters of pregnancy (57.9% pre-B+ vs. 67.8% post-B+; IQR). On enrollment, more women post-B+ reported disclosing their HIV status to their partners (24.6% pre-B+ vs. 40.0% post-B+). Analysis performed after excluding women already on ART demonstrated that baseline differences remained significant except for maternal median age [26.3 (23.1–30) years pre-B+ vs. 27 (23.1–30.6) years post-B+; P = 0.17].
Uptake and Antenatal Outcomes Among Women Enrolled into PMTCT
Including those already on ART at enrollment, there was a modest increase in the proportion of enrolled women receiving any ARVs for PMTCT (82.9) pre-B+ and (87.4) post-B+, P = 0.002. However, excluding women already on ART at enrollment, there was no change in the proportion of enrolled women newly receiving any ARVs for PMTCT pre-B+ (zidovudine prophylaxis or ART) and post-B+ (ART); 79% vs. 81.9%; P = 0.11, respectively; however, among the 243 (pre-B+) and 810 (post-B+) women newly initiating ART, time to ART initiation was significantly shorter, with 58.4% of women starting on the day of enrollment post-B+ (Table 2, Fig. 2). Before B+ implementation, 21.4% did not initiate ART/ARVs, and after B+ implementation, 18.1% of women did not initiate ART after enrollment.
FIGURE 2.
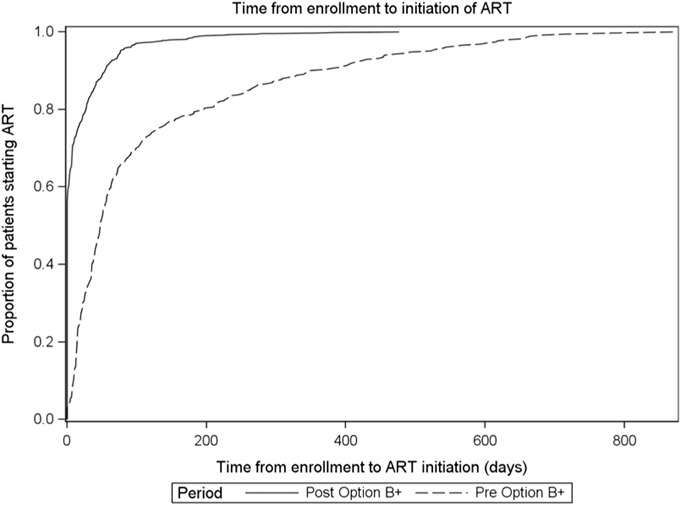
Time from enrollment at ANC to initiation of ART, pre-B+ and post-B+ implementation.
Antenatal outcomes before delivery among all women enrolled are provided in Table 2. The proportion of women enrolled who were reported as LTFU was unchanged pre-B+ and post-B+ (14.7% pre-B+ vs. 13.1% post-B+; P = 0.25). However, post-B+, a higher proportion of women withdrew from PMTCT services (3.2% pre-B+ vs. 5% post-B+; P = 0.02) or transferred out (0.7% pre-B+ vs. 2.7% post-B+; P = 0.0002). The proportion of women with fetal demise was 5.6% pre-B+ vs. 7.3% post-B+, but the difference did not reach statistical significance (P = 0.09). In the post-B+ period, a significantly smaller proportion of women were reported to have died before delivery (0.8% pre-B+ vs. 0.2% post-B+; P = 0.04), but smaller proportion reported a delivery [845 (74.8%) pre-B+ vs. 998 (70.4%) post-B+, P = 0.01].
After excluding those already on ART at enrollment, we continued to see significant differences between the groups (no palpable pregnancy: 0.2% pre-B+ vs. 1.3% post-B+, P = 0.007; fetal demise: 5.1% pre-B+ vs. 6.2% post-B+, P = 0.32; transferred out: 0.8% pre-B+ vs. 2.4% post-B+, P = 0.004; withdrawal from PMTCT services after enrollment: 3.8% pre-B+ vs. 6.1% post-B+, P = 0.005; LTFU: 16.1% pre-B+ vs. 15.2% post-B+, P = 0.57; delivery: 73.1% pre-B+ vs. 68.1% post-B+, P = 0.02). The change in maternal death before delivery, although still lower post-B+, was no longer significant (0.9% pre-B+ vs. 0.2% post-B+; P = 0.06).
Summary of Outcomes for All Women Identified Through Entire Antenatal Cascade
Figure 3A summarizes uptake of services at critical points in the antenatal PMTCT cascade from HIV testing through delivery. Notably, pre-B+, the largest drop-off occurred at enrollment into the PMTCT program. Post-B+, despite significant improvement in enrollment, there were ongoing incremental losses at each step along the cascade.
FIGURE 3.
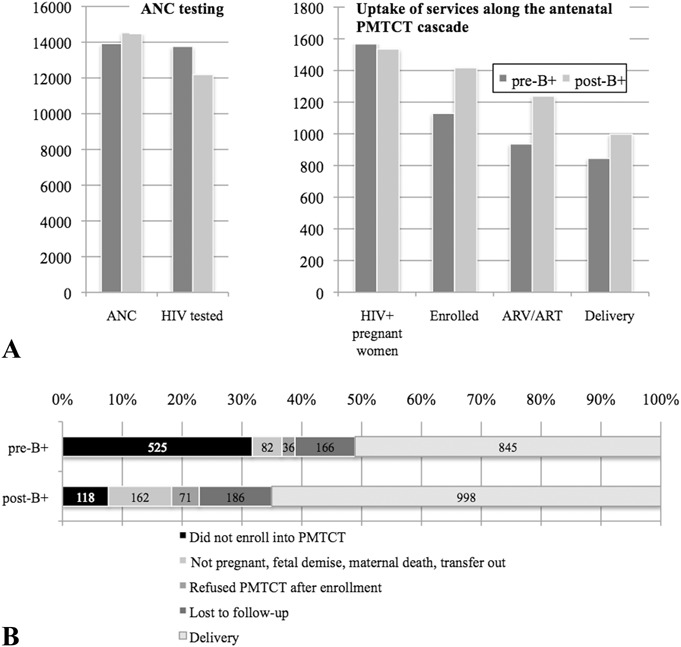
A, Uptake of services along the antenatal PMTCT cascade, pre-B+ and post-B+ implementation. B, Cumulative maternal outcomes of all women identified at ANC to delivery, pre-B+ (n = 1645) and post-B+ (n = 1535) implementation.
Figure 3B illustrates the proportional contribution of each outcome pre-B+ and post-B+. The figure highlights the significant increase in enrollment of HIV-infected women into the PMTCT program post-B+, and the resulting increased proportional contribution of reported deliveries to overall maternal outcomes post-B+. Overall, although LTFU among all women enrolled did not change (14.7% vs. 13.1%; P = 0.25), because of the significant increase in enrollment into the PMTCT program post-B+, proportion of all HIV-infected women retained through the antenatal cascade was greater post-B+ [845/1654 (51.1%) pre-B+ vs. 998/1535 (65%) post-B+; P < 0.0001].
Six Months' Outcomes Among Women Newly Initiating ART
In addition to the antenatal outcomes for all women enrolled reported above, we examined ART outcomes 6 months after ART initiation for the 984 women who newly initiated ART after enrollment into the PMTCT program (225 pre-B+ and 759 post-B+). Those known to have transferred out or were receiving ART at outside health facilities [18 (pre) and 51 (post) patients], were excluded. At 6 months after ART initiation, 89.3% pre-B+ vs. 78.8% post-B+ were retained alive on ART (P < 0.0004); 5.8% vs. 11.2% were lost-to-follow-up (P = 0.02); 2.2% vs. 8.2% (P = 0.002) stopped ART; and 2.7% vs. 1.5% had unknown outcomes, 3 women enrolled post-B+ were known to have died (P = 1.00).
DISCUSSION
This study is the first to use patient-level data to examine service uptake and outcomes across the antenatal PMTCT cascade from identification of maternal HIV infection through delivery in Malawi after the implementation of the B+ approach.
Suboptimal HIV Testing Post-B+
Our first notable finding was that the proportion of women presenting to ANC whose HIV status was ascertained declined post-B+ from 99% to 84%.
The decline was attributed mainly to test kit shortages,22,23 with acute drops in testing during 2 discrete time periods. The importance of timely and reliable HIV testing to the success of B+ has been noted by the Malawi government which reported that “failure to ascertain maternal HIV status at ANC is now responsible for 54% of new infant infections in Malawi.”13 After this statement, the Malawi MOH has performed a comprehensive review of HIV Testing and Counseling (HTC) services,22,24 including test kit management, refresher training of HTC counselors, and new accountability measures such as a daily activity register.24 This experience should highlight to the other countries preparing to implement B+ that in addition to focusing on scale-up of B+ services, continued attention needs to be given to test kit management and supply chain issues.
Improvement in Enrollment into PMTCT, but Substantial Refusal to Start ART and Higher Rates of Withdrawal From the Program After Initial Enrollment
Within the Tingathe program, B+seems to have dramatically improved initial enrollment into PMTCT services (68.3%–92.6%; P < 0.0001). Part of this improvement is likely because of a larger percentage of patients entering PMTCT already on ART (18.7% pre vs. 30.2% post). However, even after excluding those known to be HIV-infected at the time of enrollment, the difference in the proportion enrolled remained significant (61% vs. 86.7% post-B+; P < 0.0001). The same day initiation of ART recommended by the B+ policy is another likely contributing factor, with 58.4% of women initiating ART on the same day as enrollment as compared with 4.1% pre-B+. Furthermore, women no longer had to wait for CD4+ results to be initiated on ART, which likely improved enrollment into PMTCT care. Finally, maturation of the PMTCT program with more community acceptance of the intervention likely also contributed. These results are consistent with the overall national experience, with the Malawi HIV program reporting an impressive 7-fold increase in antiretroviral treatment coverage among pregnant women after B+ implementation.13
Despite improved enrollment into PMTCT, there were still significant challenges before ART initiation, with over 15% of women not initiating ART post-B+. Further, there were higher rates of withdrawal from the program after initial enrollment. Women may be reluctant to start ART when there is no clear indication to start for their own health, and as others have suggested, perhaps women find PMTCT coercive25 and so initially enroll only to withdraw later. Further exploration of why women are not initiating ART and tailored interventions to address these challenges could improve uptake.
Improved Duration on ART, but Increased LTFU and Substantial Stopping of ART Among Those Initiated
In comparison with the pre-B+ period, we found that more women initiated ART and time to ART initiation was significantly shorter [48 days (19, 130) vs. 0 day (0, 15.5)]. Before B+, determination of ART eligibility required CD4+ testing that was not reliably available, resulting in delays in ART initiation. The policy change to B+ likely facilitated prompt ART initiation, thereby improving time to ART initiation and duration of coverage before delivery, which likely translates into lower MTCT risk. However, LTFU on ART increased after B+ (5.8% pre-B+ vs. 11.2% post-B+). As other studies have suggested, this drop may reflect better retention in care for women who start ART for their own health because of more advanced disease.26,27 Alternatively, with more rapid ART initiation through B+, women may have been less prepared for ART, and therefore less likely to remain in care.28–30 In addition, high LTFU, especially early dropouts, could reflect women's resistance to and lack of acceptance of ART.16
Previous studies have noted general community acceptance of B+ (89.8% in favor of universal treatment for pregnant women),31 and low rates of stopping ART (0.2%).16 However, in our study, a much higher 8.2% of women who initiated ART post-B+ reported having stopped ART by 6 months. The higher rates may reflect more candid reporting by women to CHWs, and thereby more accurate classification. It may be secondary to women not knowing their own health status or may signal experience with side effects or higher opportunity costs than being off ART. Further investigation of why women may be choosing to stop ART could help inform optimization of ART delivery.
Antenatal Outcomes
This study is the first to report on maternal antenatal outcomes among all women receiving PMTCT in Malawi post-B+. Notably, we report a decrease in maternal deaths post-B+ (0.8% pre-B+ vs. 0.2% post-B+). This decrease was of borderline significance once women already on ART at enrollment were excluded (0.9% pre-B+ vs. 0.2% post-B+; P = 0.06). As other studies have demonstrated, B+ facilitates more rapid ART initiation16 and may therefore have a favorable impact on maternal health by improving timely ART among those who need it for their own health. Furthermore, although not statistically significant, there was a trend toward increased fetal demise (5.6% pre-B+ vs. 7.3% post-B+). This may be attributable to better reporting of fetal demise because more women enrolled earlier (during the first and second trimesters) post-B+ (57.0% pre-B+ vs. 67.8% post-B+); or as other studies have suggested, increased ART use during pregnancy may be contributing32,33 (26.5% pre-B+ vs. 81.9% post-B+ received ART during pregnancy).
Significance for Countries Planning to Implement B+
This close examination of the antenatal PMTCT cascade in the first country to implement B+ may have implications for other countries as they implement, transition to, or consider implementing B+. Our findings suggest that countries may expect to see their largest gains with enrollment of women into PMTCT services, improved ART coverage, and more rapid initiation of ART in pregnant women. However, such countries should also expect to need to address continued incremental losses along the PMTCT cascade, with more women refusing to start ART and choosing to stop after having been initiated. Moreover, our findings suggest that the PMTCT landscape is evolving with substantial increases in women already on ART at PMTCT enrollment. With the successful scale-up of ART globally, other countries may see similar changes in their PMTCT population, which could have important implications both for PMTCT planning and how PMTCT is measured in the future.
Limitations
There are several limitations to our study. First, because of the rapid and widespread scale-up of B+, similar to the other reports from Malawi on B+,13,16 we did not have a contemporary comparison group (control). To address this, we used a historical comparison (pre-B+) group to assess the impact of B+ throughout the antenatal cascade. Nonetheless, the pre/post study design has inherent limitations. The improvements in PMTCT uptake post B+ may represent maturity of the national ART/PMTCT and Tingathe programs, or may represent the result of other unknown contemporary influences or epidemiologic trends. However, other than training the CHWs on the new national B+ protocols,10 Tingathe service activities were unchanged. Second, outcome data were not available for the sizeable number of women who were not enrolled into the PMTCT program. Third, as mentioned above, the results are from 2 large urban health centers within a CHW supported program, and although the population is likely to be similar to those in other large urban areas, the results may not be representative of outcomes in the other parts of Malawi. Finally, although we have presented some indirect evidence on the potential impact on MTCT with more women entering PMTCT on ART and longer duration of ART during pregnancy, this study does not report on infant outcomes or MTCT rates. We are, however, ultimately interested in the impact of B+ on MTCT, and as our cohort matures, we expect to report on these critical outcomes in future studies.
Summary
In summary, this study suggests that B+'s simplified approach has resulted in several improvements in the antenatal PMTCT cascade including greater proportion of HIV-infected pregnant women enrolled into PMTCT services, increased use of ART during pregnancy, and more rapid initiation of ART. As compared with the pre-B+ period where the most notable drop-off was at PMTCT enrollment, the losses in the post-B+ period were incremental along the PMTCT cascade. Suboptimal uptake included lower proportion tested for HIV, refusal to start ART, continued losses after ART initiation because of women choosing to stop ART, and LTFU. This close examination of the cascade in the first country to implement B+ may have implications for other countries as they implement, transition to, or consider implementing B+. B+ has made significant contributions toward reshaping the PMTCT dialogue and forcing us to focus again on health outcomes and service delivery for HIV-infected women and their children. However, challenges remain and require innovative solutions to ensure an AIDS-free generation.
ACKNOWLEDGMENTS
The authors thank the Malawi Ministry of Health for their partnership in this endeavor. They also thank the doctors, nurses, community health workers, in the Tingathe program and participating Ministry of Health facilities, and the women and infants living or affected by HIV.
Footnotes
This study was made possible by the Tingathe program supported by USAID cooperative agreement number 674-A-00-10-00093-00. M.H.K. was supported by the Fogarty International Center of the National Institutes of Health (NIH) under award number K01 TW009644. Data analysis was provided by the Design and Analysis Core of the Baylor-UT Houston Center for AIDS Research, an NIH funded program numbered P30-AI36211.
Presented in part at the 21st Conference on Retrovirology and Opportunistic Infections, Poster 803, March 3–6, 2014, Boston, MA.
The authors have no conflicts of interest to disclose.
M.H.K. and S.A. contributed equally to the development of this article. M.H.K. and S.A. conceived and designed the study, were responsible for study coordination and data management, helped analyze data, interpreted findings, and wrote the article. E.J.A. reviewed the study protocol, provided guidance on the conduct of the study, helped interpret findings, and helped to write the article. T.P.G., M.H., E.Y.C., F.C., and P.N.K. reviewed the study protocol, provided guidance on the conduct of the study, and critically reviewed the article for important intellectual content. X.Y. and C.N. assisted in statistical analysis, interpretation, and article writing. All authors have read and approved the final article.
REFERENCES
- 1.UNAIDS report on the global AIDS epidemic 2013: Joint United Nations Programme on HIV/AIDS (UNAIDS) UNAIDS, Geneva, Switzerland; 2013. [Google Scholar]
- 2.Braun M, Kabue MM, McCollum ED, et al. Inadequate coordination of maternal and infant HIV services detrimentally affects early infant diagnosis outcomes in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2011;56:e122–e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson L, Grant AD, Watson-Jones D, et al. Linking women who test HIV-positive in pregnancy-related services to long-term HIV care and treatment services: a systematic review. Trop Med Int Health. 2012;17:564–580. [DOI] [PubMed] [Google Scholar]
- 4.Wettstein C, Mugglin C, Egger M, et al. Missed opportunities to prevent mother-to-child-transmission: systematic review and meta-analysis. AIDS. 2012;26:2361–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gloyd SS, Robinson Julia, Dali SergeA, et al. PMTCT Cascade Analysis in Cote D'Ivoire: Results From a National Representative Sample. Washington, DC: USAID; 2014. [Google Scholar]
- 6.Kim MH, Ahmed S, Buck WC, et al. The Tingathe programme: a pilot intervention using community health workers to create a continuum of care in the prevention of mother to child transmission of HIV (PMTCT) cascade of services in Malawi. J Int AIDS Soc. 2012;15(suppl 2):17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker PM, Mphatswe W, Rollins N. Antiretroviral drugs in the cupboard are not enough: the impact of health systems' performance on mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;56:e45–e48. [DOI] [PubMed] [Google Scholar]
- 8.Kellerman SE, Ahmed S, Feeley-Summerl T, et al. Beyond prevention of mother-to-child transmission: keeping HIV-exposed and HIV-positive children healthy and alive. AIDS. 2013;27(suppl 2):S225–S233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378:282–284. [DOI] [PubMed] [Google Scholar]
- 10.Clinical Management of HIV in Children and Adults. Malawi Integrated HIV Guidelines. Lilongwe, Malawi: Ministry of Health; 2011. [Google Scholar]
- 11.WHO PMTCT Update. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 12.Ahmed S, Kim MH, Abrams EJ. Risks and benefits of lifelong antiretroviral treatment for pregnant and breastfeeding women: a review of the evidence for the Option B+ approach. Curr Opin HIV AIDS. 2013;8:474–489. [DOI] [PubMed] [Google Scholar]
- 13.Impact of an innovative approach to prevent mother-to-child transmission of HIV–Malawi, July 2011-September 2012. MMWR Morb Mortal Wkly Rep. 2013;62:148–151. [PMC free article] [PubMed] [Google Scholar]
- 14.Price AJ, Kayange M, Zaba B, et al. Uptake of prevention of mother-to-child-transmission using Option B+ in northern rural Malawi: a retrospective cohort study. Sex Transm Infect. 2014;90:309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 16.Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (“Option B+”) in Malawi. AIDS. 2014;28:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MH, Ahmed S, Preidis GA, et al. Low rates of mother-to-child HIV transmission in a routine programmatic setting in Lilongwe, Malawi. PLoS One. 2013;8:e64979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lilongwe District Health Office. Semi-permanent Data. Lilongwe, Malawi: Lilongwe DHO; 2008. [Google Scholar]
- 19.National Statistical Office (Malawi) and ORC MACRO. Malawi demographic and Health Survey 2004. Calverton, MD: National Statistics Office and ORC MACRO; 2005. [Google Scholar]
- 20.Guidelines for the Use of Antiretroviral Therapy in Malawi. 3rd ed Lilongwe, Malawi: Ministry of Health; 2008. [Google Scholar]
- 21.Malawi Ministry of Health. Prevention of Mother to Child Transmission of HIV and Paediatric HIV Care Guidelines. 2nd ed Lilongwe, Malawi: Malawi Ministry of Health; 2008. [Google Scholar]
- 22.Task Force Report on Stock Outs of HIV Test Kits. Lilongwe, Malawi; 6November2012. [Google Scholar]
- 23.Baylor-Tingathe Program. Q2 USAID Report. 2012. Lilongwe, Malawi. [Google Scholar]
- 24.World Health Organization. Implementation of option B+ for prevention of mother-to-child transmission of HIV: the Malawi experience. Congo: World Health Organization Regional Office for Africa Brazzaville, Republic of Congo; 2014. [Google Scholar]
- 25.Hardon AVE, Bongololo-Mbera G, Cherutich P, et al. Women's views on consent, counseling and confidentiality in PMTCT: a mixed-methods study in four African countries. BMC Public Health. 2012;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-, middle and high income countries: a systematic review and meta-analysis. AIDS. 2012;26(16)2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giordano TP, Hartman C, Gifford AL, et al. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials. 2009;10:299–305. [DOI] [PubMed] [Google Scholar]
- 28.Gebrekristos HT, Mlisana KP, Karim Q. Patients’ readiness to start highly active antiretroviral treatment for HIV. BMJ. 2005;331:772–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orrell C. Antiretroviral adherence in a resource-poor setting. Curr HIV/AIDS Rep. 2005;2(4):171–176. [DOI] [PubMed] [Google Scholar]
- 30.Shaffer N, Abrams EJ, Becquet R. Option B+ for prevention of mother-to-child transmission of HIV in resource-constrained settings: great promise but some early caution. AIDS. 2014;28:599–601. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh AC, Mburu G, Garner AB, et al. Community and service provider views to inform the 2013 WHO consolidated antiretroviral guidelines: key findings and lessons learnt. AIDS. 2014;28(suppl 2):S205–S216. [DOI] [PubMed] [Google Scholar]
- 32.Townsend CL, Tookey PA, Newell ML, et al. Antiretroviral therapy in pregnancy: balancing the risk of preterm delivery with prevention of mother-to-child HIV transmission. Antivir Ther. 2010;15:775–783. [DOI] [PubMed] [Google Scholar]
- 33.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206:1695–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]


