Abstract
The new balloon-expandable Edwards SAPIEN 3 THV has significant design improvements requiring adjustments in the implantation technique as compared to the previous generation SAPIEN XT. Basically, the new valve requires less oversizing due to the outer skirt, which, if positioned underneath the annulus, can reduce the occurrence and severity of paravalvular leak (PVL). As with any transcatheter vale, a thorough assessment of the device-landing-zone, the surrounding structures, and the distribution of calcifications is of vast importance. Once the SAPIEN 3 valve is positioned with the initial orientation of the middle balloon marker at the level of the leaflet hinge points, the outer skirt will remain under the annulus, despite the foreshortening of the lower inflow portion of the valve. If there is an incomplete apposition, the outer skirt can conform to the anatomy, close the gaps, and reduce the risk of PVL. When calcifications are located on the edges of the annulus, PVL is common with the SAPIEN XT THV but dramatically reduced with the SAPIEN 3 THV. If the calcification extends from the annulus into the entire LVOT, there is always an incomplete apposition of the either valve frame; however, the resulting PVL is reduced by the outer skirt of the SAPIEN 3. In 165 consecutive SAPIEN 3 patients, 89.7% (n = 145) had none or a trace PVL and there were no patients with moderate or severe PVL.
The new generation SAPIEN 3 valve allows more challenging anatomies to be treated, requires less oversizing, and can reduce PVL.
Introduction
The rapid evolution of transcatheter aortic valve implantation (TAVI) from an innovative procedure to an established, alternative treatment for patients with severe aortic stenosis was followed by significant efforts to further improve clinical outcomes. A special emphasis was put on minimizing procedure complications through improved patient selection, refined procedures, and enhanced device designs.1 The Edwards transcatheter heart valve (THV) technology was primarily focused on device iterations addressing paravalvular leak (PVL) and vascular access complications. The Edwards SAPIEN 3 THV is the newest generation balloon expandable valve. It received the CE mark approval on January 27, 2014 and is commercially available in the EU.
The New SAPIEN 3 Design
Significant design improvements have been incorporated in the newest generation Edwards SAPIEN 3 THV when compared to the earlier generation SAPIEN XT THV. Lower profile delivery systems were introduced to enable less traumatic implantation via the transfemoral, transapical, and transaortic access routes. More importantly, these changes were aimed to facilitate percutaneous access and closure even in patients with smaller iliofemoral arteries. The Commander Delivery System (transfemoral) has an ultra-low profile and is compatible with the 14 French eSheath for the 23 and 26 mm valve and the 16 French eSheath for the 29 mm valve. A dual articulation of the distal end with an angulation of >180° facilitates a correct coaxial positioning even in challenging anatomies. A new handle allows for fine controlling and final adjustments of the height of the valve. The Certitude Delivery System (transapical) is compatible with the low profile 18 French Certitude sheath for the 23 and 26 mm valve and the 21 French Certitude sheath for the 29 mm valve. It has an integrated pusher to streamline the procedure and an articulation feature to facilitate the coaxial positioning. Since major vascular complications are inversely correlated with reduced delivery profile,2,3 this design change is anticipated to translate into a significant clinical benefit. However, the most important changes are related to the SAPIEN 3 frame and the new “outer skirt.” The SAPIEN 3 frame is designed to enhance structural performance maintaining circularity, yet allowing for low profile in the crimped state. The SAPIEN 3 is currently available in the same 3 sizes (23, 26, and 29 mm) as the SAPIEN XT but the height of the frame is taller in both the expanded and crimped state as compared to the SAPIEN XT (Table1). The frame consists of 5 rungs and 12 cells, where the upper cells are larger, allowing for postprocedure coronary access, in cases of high positioning (Fig. 1). The large angles between the struts and the interwoven rows of cells allow for distribution of the leaflet material along the longer height when the valve is crimped, while maintaining radial strength when the valve is fully deployed. The difference in the cell size at the inflow and outflow portion of the frame has an impact on the foreshortening. While the foreshortening of the SAPIEN XT valve is homogeneous, it is more pronounced in the lower 2/3 than in the upper 1/3 of the SAPIEN 3 frame (Fig. 1). In contrast to the SAPIEN XT, where foreshortening is similar for all sizes, the SAPIEN 3 foreshortening varies between 6.5 mm in the 23 mm size and 8.5 mm in the 29 mm size (Table1). In order to obtain an accurate final position of the SAPIEN 3 valve, it is critically important to observe the relationship between the frame and the native anatomy during the deployment, the differential foreshortening along the frame height. The SAPIEN 3 outer skirt is another important design feature intended to fill the gaps between the prosthesis and the native anatomy, thus minimizing the risk of PVL. In addition, the height of the inner skirt was increased in comparison to the SAPIEN XT to provide a larger landing zone. This supports further the decreased risk of PVL.
Table 1.
SAPIEN 3 and SAPIEN 3 Valve Parameters
| 23 mm |
26 mm |
29 mm |
||||
|---|---|---|---|---|---|---|
| SAPIEN XT | SAPIEN 3 | SAPIEN XT | SAPIEN 3 | SAPIEN XT | SAPIEN 3 | |
| Annulus size range (mm) | 18–22 | 18–22 | 21–25 | 21–25 | 24–27 | 24–28 |
| Crimped height (mm) | 17 | 24.5 | 20 | 27 | 22 | 31 |
| Expanded height (mm) | 14 | 18 | 17 | 20 | 19 | 22.5 |
| Fore-shortening (mm) | 3 | 6.5 | 3 | 7 | 3 | 8.5 |
| Internal fabric skirt height (mm) | 6.7 | 9.3 | 8.7 | 10.2 | 11.6 | 11.6 |
| External skirt height (mm) | 6.6 | 7.0 | 8.1 | |||
Figure 1.
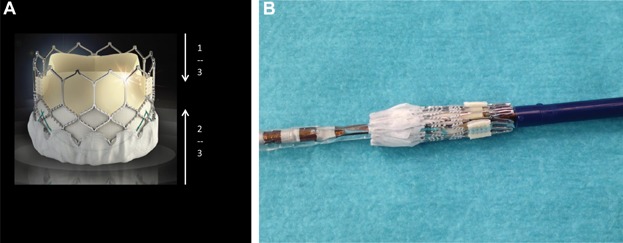
(A) SAPIEN 3 frame design with large upper cells and small lower cells leading to more pronounce foreshortening in the lower 2/3 of the frame. (B) A crimped SAPIEN 3 valve.
The Device Landing-Zone
During the patient selection for either SAPIEN XT or SAPIEN 3, a thorough assessment of the device-landing-zone and surrounding structures using 3D imaging is of major importance. The device-landing-zone is subdivided into a supraannular portion, annular plane, and sub-annular portion4 which consists of the proximal left ventricular outflow tract (LVOT) (Fig. 2). A thorough understanding of the SAPIEN 3 design in relation to the landing zone, and the severity and distribution of calcification, will help to reduce the risk of PVL or other procedural complications, such as annular rupture, coronary obstruction, or conduction disturbances requiring new pacemaker (PM) implantation. In the first 165 patients implanted with the commercially available SAPIEN 3 valve at our center, we have not seen any coronary occlusions and the rate of pace makers was low (12.9%). However, it is important to emphasize that we usually implant patients with ≥10 mm distance between the annulus and the origin of the coronary arteries.
Figure 2.
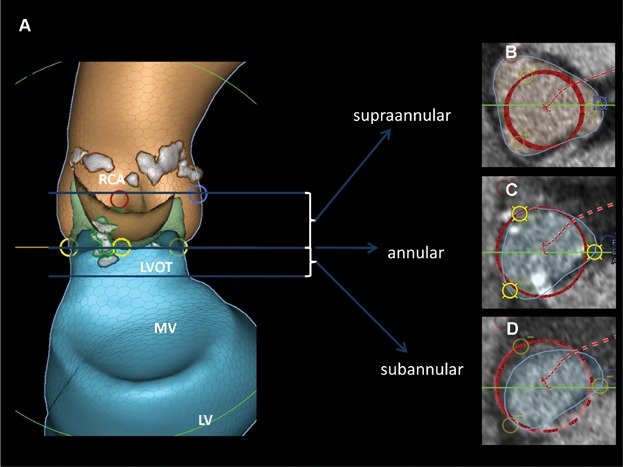
Preimplant analysis. (A) LCA and blue circle, origin of the left coronary artery; RCA and red circle, origin of the right coronary artery; yellow circle, nadir of the cups; LVOT, left ventricular outflow tract; MV, mitral valve region; LV, left ventricle; grey area, calcifications. (B–D) Blue circle, cross sectional area of region of interest; red circle, valve dummy in the region of interest.
Sizing and PVL
PVL originates from the incomplete apposition of the valve stent against the native annulus or LVOT. This occurs when (1) the implanted valve is too small in relation to the size of the annulus (undersizing or underdeployment); or (2) the aortic annulus has a pronounced ellipsoidal shape so that the entire circumference of the deployed valve cannot uniformly contact the device-landing-zone; or (3) thick and irregular calcification has infiltrated the area of the aortic annulus or LVOT so that complete and consistent contact of the valve frame is impeded. Oversizing of a THV can certainly minimize the risk of significant PVL; however, excessive oversizing may increase the risk of complications.4–6 An uniform sizing algorithm for both valve platforms is not available, but in general, the SAPIEN 3 needs to be less oversized than the SAPIEN XT valve. The 23 mm SAPIEN 3 valve is indicated for valve areas ranging from 338 to 430 mm2 measured by CT; the 26 mm valve—for areas between 430 and 546 mm2, and the 29 mm valve—for areas between 540 and 680 mm2. For most SAPIEN 3 implantations, over sizing of −5% to 10% can be considered as appropriate (Table2). In same case, the SAPIEN 3 can be oversized to 10–20%. This is typically needed for annulus areas, which fall in the lower bound of the recommended area range for each specific SAPIEN 3 size: 338–360 mm2 for the 23 mm valve; 430–460 mm2 for the 26 mm valve, and 540–580 mm2 for the 29 mm valve. Correct valve sizing is critically important for both the SAPIEN 3 and the SAPIEN XT, and the valve-specific sizing recommendations should be followed. However, calcification-patterns have to be individually assessed and considered. The expansion of the valve depends on the patient's anatomy of the valvar complex and structure and the different frame properties of SAPIEN XT and SAPIEN 3. Balloon volume is sometimes adjusted and maybe considered when making sizing decisions. The speed of the balloon filling does not impact on sizing but slow inflation is recommended since it supports delivery system stability and procedure predictability. PVL occurs rarely after implantation of a SAPIEN XT or SAPIEN 3, when calcification is limited to the aortic valve leaflets and the correct valve size is chosen in relation to the anatomy of the valvular complex. When calcification is located on the edges of the annulus or protrudes into the LVOT, the valve frame might be held away from these areas causing PVL immediately after the implantation of a SAPIEN XT (Fig. 3). A balloon postdilation using more balloon-volume can be used to reduce the degree of PVL; however, this may increase the risk of annular rupture.4 The SAPIEN 3 has clear advantages under these circumstances. Once the valve is properly positioned with the initial orientation of the middle balloon marker at the level of the native leaflet hinge points, the outer skirt will be located under the annulus in all valve sizes, despite the foreshortening of the lower inflow portion of the valve (Fig. 4). If there is an incomplete apposition, the outer skirt can conform to the native anatomy, close the gaps, and reduce the risk of PVL. If the calcification extends from the annulus into the entire LVOT (Fig. 5), an incomplete apposition of the valve frame will always occur. Despite this, the PVL, although not eliminated, will be considerably reduced by the outer skirt of the SAPIEN 3 valve.
Table 2.
Sizing of the SAPIEN 3 Valve in Regards to the 3D Annular Area by CT as Recommended in the Edwards SAPIEN 3 Information for Use
| 3D Annular Area (mm2) | % Annular Area Over or Under (−) Nominal by 3D
CT |
||
|---|---|---|---|
| 23 mm | 26 mm | 29 mm | |
| 314 | 29.3 | ||
| 320 | 26.9 | ||
| 330 | 23.0 | ||
| 338 | 20.1 | ||
| 346 | 17.3 | ||
| 350 | 16.0 | ||
| 360 | 12.8 | ||
| 370 | 9.7 | ||
| 380 | 6.8 | ||
| 390 | 4.1 | ||
| 400 | 1.5 | 29.8 | |
| 410 | −1.0 | 26.6 | |
| 415 | −2.2 | 25.1 | |
| 420 | −3.3 | 23.6 | |
| 430 | −5.6 | 20.7 | |
| 440 | −7.7 | 18.0 | |
| 450 | −9.8 | 15.3 | |
| 452 | 14.8 | ||
| 460 | 12.8 | ||
| 470 | 10.4 | ||
| 480 | 8.1 | ||
| 490 | 5.9 | ||
| 500 | 3.8 | 29.8 | |
| 510 | 1.8 | 27.3 | |
| 520 | −0.2 | 24.8 | |
| 530 | −2.1 | 22.5 | |
| 540 | −3.9 | 20.2 | |
| 546 | −4.9 | 18.9 | |
| 550 | −5.6 | 18.0 | |
| 560 | −7.3 | 15.9 | |
| 570 | −8.9 | 13.9 | |
| 580 | 11.9 | ||
| 590 | 10.0 | ||
| 600 | 8.2 | ||
| 610 | 6.4 | ||
| 615 | 5.5 | ||
| 620 | 4.7 | ||
| 630 | 3.0 | ||
| 640 | 1.4 | ||
| 650 | −0.2 | ||
| 660 | −1.7 | ||
| 670 | −3.1 | ||
| 680 | −4.6 | ||
| 683 | −5.0 | ||
| 690 | −5.9 | ||
| 700 | −7.3 | ||
| 710 | −8.6 | ||
| 720 | −9.9 | ||
Figure 3.
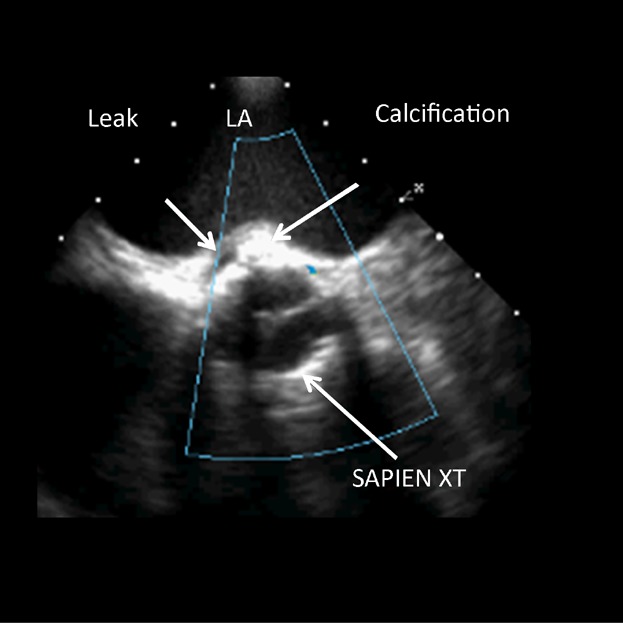
Calcification at the height of the annulus behind the SAPIEN XT frame. LA, left atrium.
Figure 4.
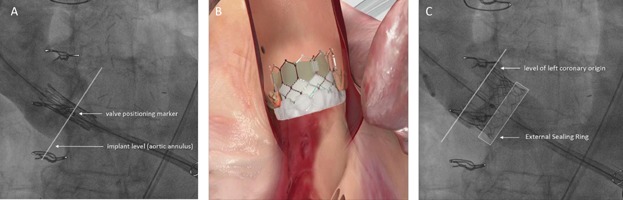
SAPIEN 3 implantation. (A) Correct position before deployment with middle balloon marker at the leaflet hinge points. (B, C) Outer skirt located in LVOT.
Figure 5.
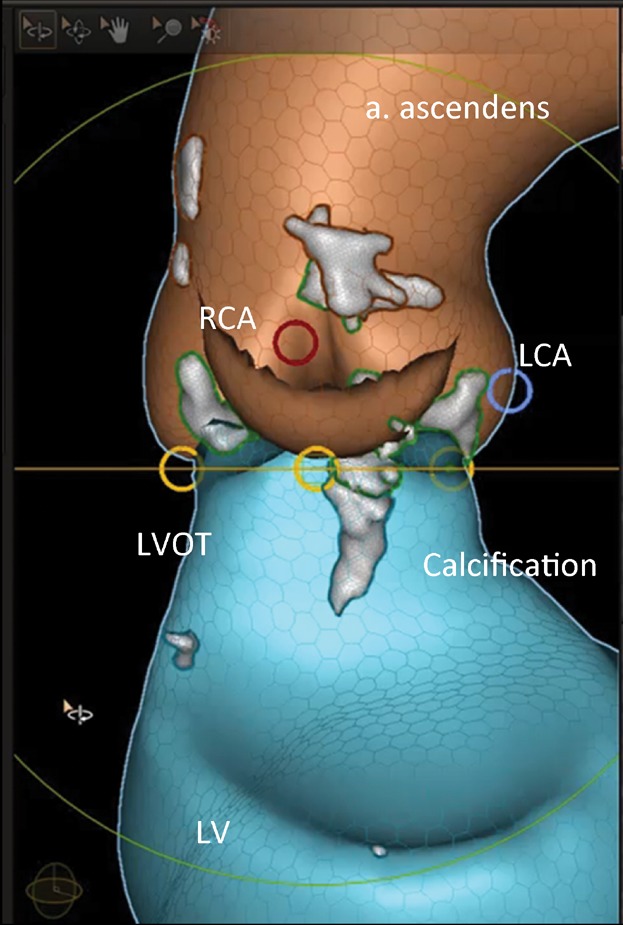
Calcification in the LVOT. LCA and blue circle, origin of the left coronary artery; RCA and red circle, origin of the right coronary artery; yellow circle, nadir of the cups; LVOT, left ventricular outflow tract; LV, left ventricle; grey area, calcifications.
In the first 165 patients with usual comorbidity (Table3), which were implanted with the commercially available SAPIEN 3 valve at our center, we have seen no evidence of PVL in 123 patients (74.5%), trace PVL in 25 patients (15.2%), and mild PVL in 17 patients (10.3%). There were no patients with moderate or severe PVL. We were able to achieve these results with only 28 patients requiring postdilation (17.0%). Balloon postdilation is reported in literature from rates between 5% and 41%7,8 and is commonly used to better expand the stent of the transcatheter valve and reduce the degree of PVL. However, postdilation is an independent predictor of cerebrovascular events,9 and may be also associated with complications such as conduction disturbances, annulus rupture, or coronary occlusion.5,7 Therefore, the reduced need for postdilation due to the low incidence of moderate or severe PVL is a significant clinical benefit of the SAPIEN 3 valve.
Table 3.
Patient Demographics and Baseline Parameters
| Baseline Characteristics | Values |
|---|---|
| Age (years; mean ± SD) | 81.6 ± 5.1 |
| Female gender (%) | 42.4 |
| Ejection fraction (mean ± SD) (%) | 56.2 ± 12.7 |
| Peripheral arterial disease (%) | 17.0 |
| Coronary artery disease (%) | 60.1 |
| Carotid stenosis >50% (%) | 21.8 |
| Prior cardiac surgery (%) | 17.0 |
| Neurological dysfunction (%) | 3.0 |
| Pulmonary hypertension >60 mmHg (%) | 20.1 |
| Recent myocardial infarction <90 days (%) | 7.3 |
| COPD (%) | 13.9 |
| Mitral valve lesion or defect (>II°, %) | 11.5 |
| Serum creatinine >200 µmol/L (%) | 4.8 |
| Diabetes mellitus (IDDM/NIDDDM) (%) | 19.4 |
| Critical preoperative state (%) | 1.2 |
| Emergency operation (%) | 0.6 |
| Log. EuroSCORE I (mean ± SD) | 18.7 ± 12.5 |
COPD, chronic obstructive pulmonary disease; SD, standard deviation; IDDM, insulin dependent diabetes mellitus; NIDDM, noninsulin dependent diabetes mellitus.
Calcifications and Complications
Although rarely, ruptures can occur at any of the 3 levels of the device landing-zone (supraannular, annular, and sub-annular). The mechanism of rupture is variable.4 A supraannular rupture can occur in the presence of a flat, obliterated sinus of Valsalva in combination with a severe calcification of the valve leaflet (Fig. 6). The expansion of the valve frame during the deployment may push the localized calcification laterally into and through the flat sinus. This can be avoided when the preimplantation images are carefully reviewed to make sure there is enough space between the leaflet and the sinus to safely accommodate the nodule of calcification (Fig. 2). If there is not enough space at all, an alternative treatment strategy should be considered. Calcification located predominantly at the edge of the leaflet, most likely will shift in a cranial-lateral direction during the deployment. If the sinus is protruding in this location, then the calcification will not injure the wall of the sinus. There is no difference in the implantation techniques of the SAPIEN XT and SAPIEN 3 with regard to supraannular severe calcifications.
Figure 6.
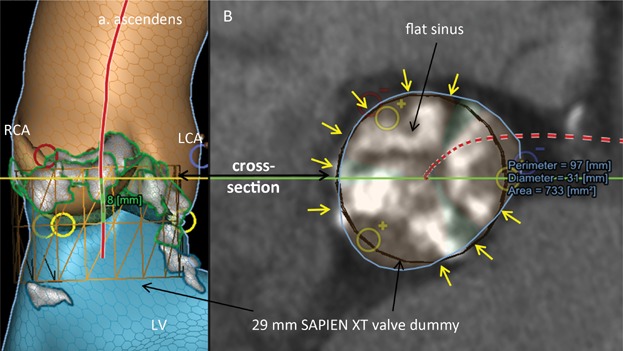
Risk of supraannular rupture. (A) LCA and blue circle, origin of the left coronary artery; RCA and red circle, origin of the right coronary artery; yellow circle, nadir of the cups; red line, centerline; LV, left ventricle; grey/white area, calcifications. (B) Blue circle, cross sectional area of region of interest 8 mm above the nadir of the cusp; yellow arrows, no space between cross sectional area and valve dummy.
Thick, circumferential calcifications located at the annulus bear the risk of annuls rupture, especially with valve over sizing, when they are getting pushed laterally. Precarious locations of calcifications are those towards the anterior ventricular wall and the interventricular septum or towards the right atrium or right ventricle (Fig. 7A–C). In contrast, calcifications towards the anterior mitral leaflet are safer, since this part of the annulus is somewhat distensible, and can better tolerate calcifications being pushed out of the way (Figs. 7D and 3). The final implantation results differ between the SAPIEN XT and SAPIEN 3 valve in regards to the type of calcifications. With both valves, an incomplete apposition at the level of the annulus may occur, but the degree of PVL would be different. The more severe PVL will be with the SAPIEN XT, and will most likely require postdilation, which may increase the risk of annular rupture if the calcification is pushed out laterally. The SAPIEN 3 has definite advantages here. In most patients, the cross sectional area at the annulus is larger than the area of the LVOT within 4 mm band under the annulus. When the SAPIEN 3 is implanted, the outer skirt lies exactly in this part of the LVOT (Fig. 4) and can reduce the PVL and avoid any further balloon postdilation associated with risk of rupture.
Figure 7.
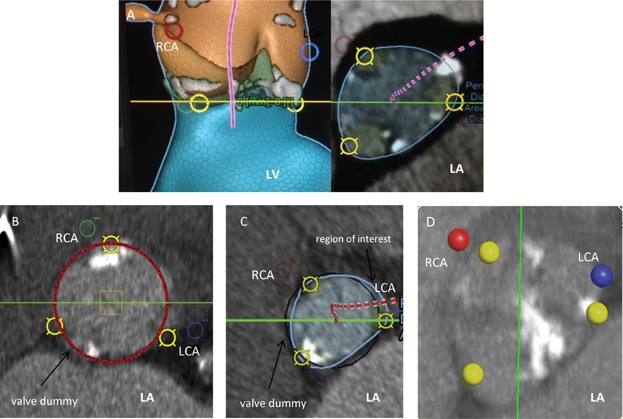
Annular calcification locations. (A) Anterior ventricular wall/interventricular septum. (B) Right ventricle. (C) Right atrium. (D) Anterior mitral leaflet region. LCA and blue circle, origin of the left coronary artery; RCA and red/green circle/point, origin of the right coronary artery; yellow circle, nadir of the cups; grey/white area, calcifications; LA, left atrium; LV, left ventricle.
A subannular rupture may occur in presence of significant LVOT calcifications. Calcifications in the region of the free anterior wall are considered to be most hazardous, whereas those near the anterior mitral leaflet do not necessarily result in rupture (Fig. 8). It is important to keep in mind that the cross sectional area of the LVOT is usually smaller than the annulus area. If severe LVOT calcification is present the maximum over sizing should be 10% and the size of the valve should be chosen based on the dimension of the LVOT rather than the annulus. If there is a large difference between the annular and LVOT dimensions then an alternative treatment strategy should be considered. In this case, a high implantation of the SAPIEN valve can be considered which is easier with the SAPIEN XT than with the SAPIEN 3 valve. LVOT calcifications can increase the risk of PVL. While the implantation of a SAPIEN XT would be more likely associated with a substantial PVL requiring postdilation, a SAPIEN 3 implantation would most likely end up with none or mild PVL. The reason is that the outer skirt will reduce the leak if the frame is held away from the LVOT and will function as a type of padding that restricts the leak. In our series of S3 patients, PVL after implantation was substantially reduced after 3–5 minutes because of the delayed expansion of the outer skirt. Finally, in our first 801 of 1,000 implantations of the Edwards SAPIEN and SAPIEN XT THV the PM rate was 12.5%. In the first 165 SAPIEN 3 implants after CE certification the PM rate was 12.9%. This rate is similar to the PM rates reported for the SAPIEN XT despite the increased height of the frame.
Figure 8.
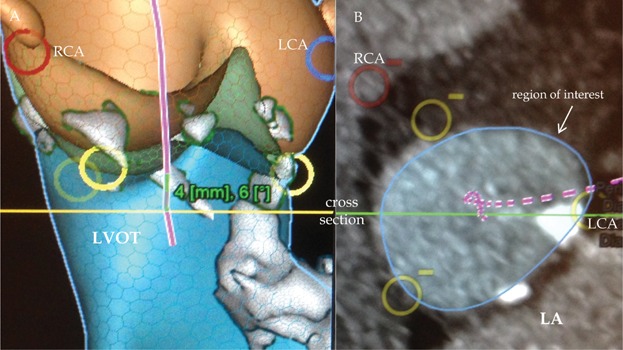
Subannular calcification. (A) LCA and blue circle, origin of the left coronary artery; RCA and red circle, origin of the right coronary artery; yellow circle, nadir of the cups; LVOT, left ventricular outflow tract; purple line, centerline; grey area, calcifications. (B) Blue circle, cross sectional area of region of interest 4 mm below the nadir of the cups; white area, calcifications; LA, left atrium.
Conclusions
The third generation SAPIEN 3 valve has an improved frame design aiming PVL reduction. Optimal implantation of the SAPIEN 3 allowing placement of the outer skirt underneath the annulus requires less oversizing, as compared to the previous balloon expandable valves, and is possible in all valve sizes when at the start of implantation the middle marker is at the level of the aortic leaflet hinge points. Significant calcifications are also less challenging for the new frame design. The SAPIEN 3 valve, undoubtedly, will demonstrate further clinical benefits in a large series of implants. Compared to the SAPIEN XT, the new generation SAPIEN 3 valve will allow more challenging anatomies to be treated.
References
- 1.Tang GH, Lansman SL, Cohen M. Transcatheter aortic valve replacement: Current developments, ongoing issues, future outlook. Cardiol Rev. 2013;21:55–76. doi: 10.1097/CRD.0b013e318283bb3d. [DOI] [PubMed] [Google Scholar]
- 2.Masson JB, Kovac J, Schuler G. Transcatheter aortic valve implantation: Review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc Interv. 2009;2:811–820. doi: 10.1016/j.jcin.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Webb JG, Chandavimol M, Thompson CR. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation. 2006;113:842–850. doi: 10.1161/CIRCULATIONAHA.105.582882. [DOI] [PubMed] [Google Scholar]
- 4.Schymik G, Heimeshoff M, Bramlage P. Ruptures of the device landing zone in patients undergoing transcatheter aortic valve implantation: An analysis of TAVI Karlsruhe (TAVIK) patients. Clin Res Cardiol. 2014 doi: 10.1007/s00392-014-0729-8. , et al.; doi: 10.1007/s00392-014-0729-8. [DOI] [PubMed] [Google Scholar]
- 5.Barbanti M, Yang TH, Rodes Cabau J. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation. 2013;128:244–253. doi: 10.1161/CIRCULATIONAHA.113.002947. [DOI] [PubMed] [Google Scholar]
- 6.Gurvitch R, Tay EL, Wijesinghe N. Transcatheter aortic valve implantation: Lessons from the learning curve of the first 270 high-risk patients. Catheter Cardiovasc Interv. 2011;78:977–984. doi: 10.1002/ccd.22961. [DOI] [PubMed] [Google Scholar]
- 7.Nombela-Franco L, Barbosa Ribeiro H. Role of balloon postdilation following trancatheter aortic valve implantation. Minerva Cardioangiol. 2013;61:499–512. [PubMed] [Google Scholar]
- 8.Lasa G, Gaviria K, Sanmartin JC. Postdilatation for treatment of perivalvular aortic regurgitation after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2014;83:E112–118. doi: 10.1002/ccd.25173. [DOI] [PubMed] [Google Scholar]
- 9.Nombela-Franco L, Rodes-Cabau J, DeLarochelliere R. Predictive factors, efficacy, and safety of balloon post-dilation after transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc Interv. 2012;5:499–512. doi: 10.1016/j.jcin.2012.02.010. [DOI] [PubMed] [Google Scholar]


