SUMMARY
Recent studies have demonstrated that natural killer (NK) cells are able to undergo clonal expansion, contraction, and generate self-renewing memory cells after infection with mouse cytomegalovirus (MCMV). It is unclear whether all or only certain subsets preferentially contribute to the generation of memory NK cells. Here we show that memory NK cells predominantly arise from killer cell lectin-like receptor G1 (KLRG1)-negative NK cell progenitors, whereas KLRG1-positive NK cells have limited capacity for expansion during infection with MCMV. Unexpectedly, the frequency of KLRG1-positive NK cells is significantly affected by the presence T cells in the host and potentially by the host microbiota. Our findings demonstrate that excessive availability of IL-15 may erode the pool of memory progenitors, resulting in the decreased efficiency of memory generation in the NK cell lineage.
INTRODUCTION
NK cells are a subset of innate lymphocytes that protect both humans and mice from certain microbial infections and tumors. Until recently, NK cells were considered exclusively as part of the innate immune defenses; however, it becomes increasingly evident that NK cells can exhibit adaptive immune-like features, including the ability to generate long-lived “memory” NK cells in response to various types of antigens (Gillard et al., 2011; O’Leary et al., 2006; Paust et al., 2010; Peng et al., 2013; Sun et al., 2009a). Mouse cytomegalovirus (MCMV) infection is a well-characterized model for studying the mechanisms of host responses against viruses. NK cell-mediated resistance to MCMV is achieved through Ly49H, an activating NK cell receptor present in MCMV-resistant C57BL/6 (B6) mice, but absent in susceptible strains such as BALB/c (Smith et al., 2000). Ly49H recognizes the MCMV-encoded glycoprotein m157 on the surface of infected cells (Arase et al., 2002; Smith et al., 2002) and delivers activating signals through the adapter proteins DAP10 and DAP12 (Orr et al., 2009). DAP12 is indispensable for stable expression of Ly49H on the cell surface (Arase et al., 2002; Orr et al., 2009). Ly49H-expressing NK cells (approximately 50% of total NK cells) preferentially expand in response to MCMV infection (Dokun et al., 2001). In the setting of adoptive transfer of NK cells into DAP12- or Ly49H-deficient hosts, Ly49H+ NK cells undergo a robust clonal expansion followed by contraction and surviving NK cells persist for several months (Sun et al., 2009a). These self-renewing mature NK cells undergo secondary expansion in response to re-challenge with MCMV and can protect neonates from MCMV infection about 10-times better than naïve NK cells (Sun et al., 2009a). Recent studies demonstrated that several factors are critical for the generation of memory NK cells in MCMV infection, including IL-12 (Sun et al., 2012), microRNA-155 (Zawislak et al., 2013), and DNAM-1 (Nabekura et al., 2014). However, whether all Ly49H+ NK cells or only certain progenitor cell population gives rise to memory NK cells remains to be elucidated.
NK cells share many traits in common with CD8+ T cells (Sun and Lanier, 2011). Naïve CD8+ T cells proliferate after antigen-specific activation and develop into short-lived effector and long-lived memory cells. In the CD8+ T cell lineage, KLRG1 has been used as a marker to distinguish short-lived effector (KLRG1+) and long-lived memory (KLRG1−) T cells. Naïve CD8+ T cells do not express KLRG1, but it is induced after antigen-specific activation. KLRG1− T cells expand more robustly and generate more memory T cells than KLRG1+ T cells (Sarkar et al., 2008).
KLRG1 has also been used as a marker for mature NK cells (Hayakawa and Smyth, 2006; Huntington et al., 2007; Robbins et al., 2004). Approximately 30–50% of NK cells in resting, uninfected mice express KLRG1 (Huntington et al., 2007) at intermediate levels (KLRG1int+) and KLRG1− NK cells give rise to KLRG1+ NK cells after adoptive transfer into Rag2−/−Il2rg−/− mice (Huntington et al., 2007). After infection with MCMV, 90–100% of NK cells express KLRG1 in very high amounts (KLRG1high) (Fogel et al., 2013; Robbins et al., 2002, 2004). Fogel et al. have noted that Ly49H+ NK cell that have specifically responded to MCMV express high levels of KLRG1, with down-regulation of SCA-1 and CD27 (Fogel et al., 2013). Furthermore, we have observed that this high level of KLRG1 is stably maintained on MCMV-specific memory NK cells for months after infection (Sun et al., 2009a; Nabekura et al., 2014). KLRG1 is not required for NK cell maturation or effector functions because Klrg1−/− C57BL/6 mice demonstrate normal NK cell development, cytolytic activity, and production of cytokines and mount a normal protective response to MCMV infection (Gründemann et al., 2010). Similarly, we have observed equivalent generation of memory NK cells in wildtype (WT) and Klrg1−/− C57BL/6 mice after infection with MCMV (Y.K., unpublished observation). Nonetheless, KLRG1 provides an informative marker to distinguish subsets of NK cells based on maturational status. Here we demonstrate that memory NK cells are predominantly derived from the KLRG1-negative progenitors, whereas the KLRG1int+ NK cells in healthy, uninfected mice have limited capacity for expansion or memory formation in response to MCMV infection. Further, our studies revealed that the presence of T cells and possibly commensal bacteria in the host might influence the generation of memory NK cells by influencing the abundance of these KLRG1− memory NK cell progenitors.
RESULTS
KLRG1− NK cells preferentially generate memory NK cells
KLRG1 is expressed at intermediate levels on 30–50% of splenic NK cells in healthy, uninfected C57BL/6 mice (Fogel et al., 2013; Robbins et al., 2002, 2004). As in the total NK cell population, a similar proportion of the Ly49H+ NK cells express KLRG1 at intermediate levels. When compared for maturation status as indicated by co-expression of CD27 and CD11b, the Ly49H+ KLRG1− NK cell subset has a higher frequency of less mature CD27+ CD11b− and CD27+ CD11b+ NK cells, whereas the Ly49H+ KLRG1int+ NK cell subset contains a higher frequency of more mature CD27− CD11b+ NK cells (Figure S1A). Both the Ly49H+ KLRG1− and Ly49H+ KLRG1int+ NK cells are capable of activation and degranulation when co-cultured in vitro with target cells expressing m157 (Figure S1B). As previously reported, both subsets comparably produce IFN-γ at day 1.5 after MCMV infection (Fogel et al., 2013) (Figure S1C). Thus, both subsets can mediate m157-specific effector functions.
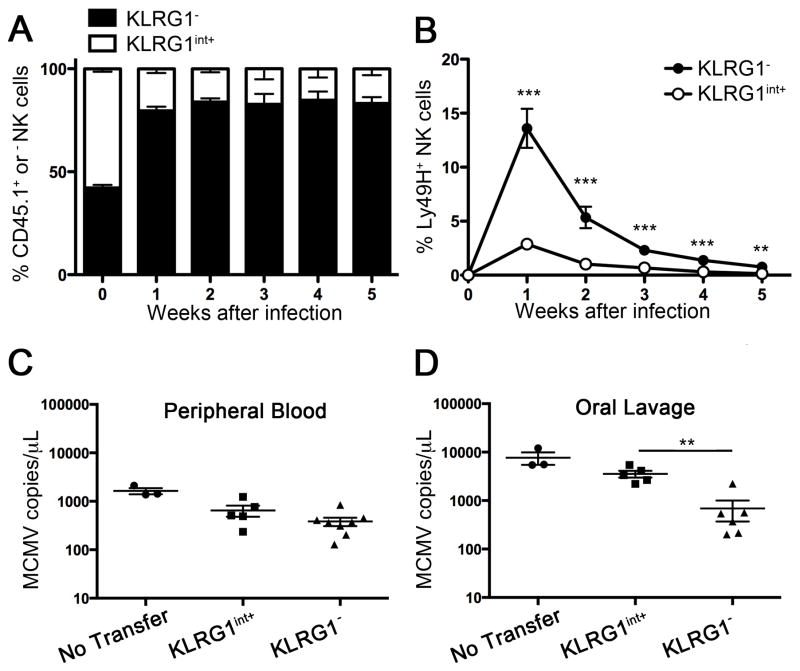
KLRG1 has been used in the CD8+ T cell lineage to discriminate naive, effector, and memory cell populations (Sarkar et al., 2008). While memory CD8+ T cell heterogeneously express KLRG1, memory NK cells homogeneously express KLRG1. To distinguish the descendant cells of KLRG1− and KLRG1int+ naïve NK cells after MCMV infection, we purified by flow cytometric cell sorting KLRG1− and KLRG1int+ NK cells from congenic CD45.2 and CD45.1 C57BL/6 mice, respectively. An equal number of CD45 congenic-marked KLRG1int+ and KLRG1− Ly49H+ NK cells was mixed and adoptively transferred into Ly49H- deficient hosts where only donor Ly49H+ NK cells can respond specifically to MCMV (Sun et al., 2009a). After infection, KLRG1− descendant Ly49H+ NK cells, but not KLRG1int+ descendant NK cells, underwent robust expansion at day 7 and preferentially generated memory NK cells (operationally defined as CD11b+, CD27−, Ly6Chigh KLRG1high Ly49H+ NK cells that persist for more than 25 days after infection with MCMV (Bezman et al., 2012; Nabekura et al., 2014; Sun et al., 2009a), In this study, we considered Ly49H+ NK cells that persisted more than 25 days as memory cells and confirmed they were KLRG1high) at day 35 (Figure 1A and B). These results indicate that KLRG1− NK cells are potent memory progenitors, whereas the KLRG1int+ NK cells that develop during steady-state in naive mice are effector cells with limited potential for expansion, resembling KLRG1+ CD8+ T cells. To compare the capacity of the KLRG1− and KLRG1int+ NK cells to control virus load, we transferred an equal number of KLRG1int+ and KLRG1− Ly49H+ NK cells into Ly49H- deficient hosts and infected with MCMV. There was no difference in viral load in peripheral blood on day 3 (Figure 1C), consistent with their equivalent ability to degranulate when stimulated with m157-bearing target cells ex vivo (Figure S1B). However, Ly49H-deficient mice receiving KLRG1− Ly49H+ NK cells showed decreased viral loads in oral lavage on day 14 compared to those receiving KLRG1int+ Ly49H+ NK cells. This result suggests that the marked proliferation by KLRG1− memory NK cell progenitors may be important for control of persistent, but not acute, virus infection.
Figure 1. KLRG1− NK cells preferentially expand and generate memory NK cells.
(A, B) KLRG1int+ and KLRG1− NK cells from C57BL/6 mice (CD45.2+) and congenic C57BL/6.SJL mice (CD45.1+), respectively, were sorted using flow cytometry to >99% purity. An equal number (1 × 105 cells) of CD45.2+Ly49H+ KLRG1int+ and CD45.1+Ly49H+ KLRG1− NK cells were mixed and adoptively transferred into Ly49H-deficient hosts (day -1) followed by MCMV infection (day 0). (A) Percentages of CD45.1+ (KLRG1− cells-derived) and CD45.1− (KLRG1int+ cells-derived) cells in the Ly49H+ NK cell population. (B) Percentages of adoptively transferred CD45.1+ (KLRG1− cells-derived) and CD45.2+ (KLRG1int+ cells-derived) Ly49H+ NK cells within the total NK cell population. (C, D) Sorted KLRG1int+ Ly49H+and KLRG1− Ly49H+ NK cells (1 × 104 of each subset) were adoptively transferred into Ly49H-deficient hosts (day -1) and infected with MCMV (day 0). Virus copy number in peripheral blood (C) and oral lavage (D) was quantitated by real-time qPCR. Data are representative of three (A, B) and two (C, D) independent experiments. Error bars show SEM (n=3 for A, B; n=3–7 for C, D). See also Figure S1.
T cells preserve the progenitors for memory NK cells
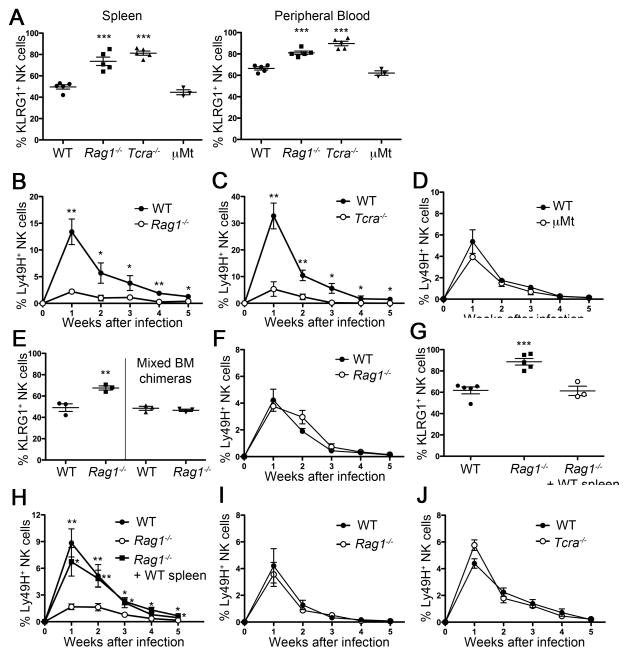
We observed that the percentage of KLRG1int+ NK cells in spleen and peripheral blood isolated from T cell-deficient mice, e.g. Rag1−/− mice and Tcra−/− mice, but not B cell-deficient mice (μMt) is higher than from syngeneic WT mice (Figure 2A). To directly compare their proliferation and memory generation, we co-transferred equal numbers of CD45-congenic WT and Rag1−/− Ly49H+ NK cells into Ly49H-deficient mice. After MCMV infection, Rag1−/− Ly49H+ NK cells expanded less and generated fewer memory NK cells than WT NK cells (Figure 2B). Similarly, Ly49H+ NK cells isolated from Tcra−/− mice were impaired in their expansion and generation of memory NK cells (Figure 2C). By contrast, normal expansion and memory generation was observed in adoptively transferred Ly49H+ NK cells derived from WT and μMt mice (Figure 2D). To determine whether intrinsic or extrinsic factors regulate KLRG1 expression in Rag1−/− mice, we generated mixed bone marrow chimeric (BMC) mice by reconstituting lethally irradiated hosts (CD45.1+ WT mice) with a mixture of bone marrow cells from WT (CD45.1+) and Rag1−/− mice (CD45.2+). The difference of KLRG1 expression between WT and Rag1−/− NK cells was abolished in mixed BMC mice (Figure 2E). When WT- and Rag1−/−-derived NK cells were isolated from these chimeric mice and adoptively transferred into Ly49H-deficient hosts followed by MCMV infection, both demonstrated equivalent expansion and generation of memory cells (Figure 2F). Similarly, the response by Tcra−/−- derived NK cells was rescued in mixed (WT and Tcra−/− cells) BMC mice (data not shown). These results demonstrate that the higher frequency of KLRG1int+ NK cells in mice lacking T lymphocytes is not NK cell intrinsic, but is caused by the absence of T cells in the host.
Figure 2. KLRG1int+ NK cells are increased in T cell-deficient mice, but not B cell-deficient mice.
(A) Percentages of KLRG1int+ cells within the total NK cell population in spleen and peripheral blood from the indicated mice. (B, C, D) NK cells were isolated from Rag1−/− mice (B), Tcra−/− mice (C), or μMt mice (D) (all CD45.2+) and co-transferred with an equal number of WT (CD45.1+) Ly49H+ NK cells. Graph shows percentages of Ly49H+ cells within the total NK cell population at the indicated time points after MCMV infection. (E) Percentages of KLRG1int+ cells within the total NK cell population in WT and Rag1−/− mice and in mixed bone marrow chimeric mice (1:1 mixture of WT (CD45.1+) and Rag1−/− mice (CD45.2+) cells). (F) WT and Rag1−/− NK cells were isolated from the mixed BMC mice (E), adoptively transferred into Ly49H- deficient hosts, and then infected with MCMV. Percentages of WT and Rag1−/− NK Ly49H+ NK cells after MCMV infection are shown. (G) Percentages of KLRG1int+ cells within the total NK cell population in WT and Rag1−/− mice and in Rag1−/− mice given WT spleen cells (5 × 107 cells). (H) WT mice, Rag1−/− mice, and the Rag1−/− mice that received WT splenocytes were treated with anti-NK1.1 mAb and rested for 8 weeks. NK cells were isolated from these three groups of mice and an equal number of Ly49H+ NK cells was adoptively transferred into Ly49H-deficient hosts (day -1) and then infected with MCMV (day 0). Percentages of transferred Ly49H+ NK cells isolated from WT mice, Rag1−/− mice, and in Rag1−/− mice given WT spleen cells after MCMV infection are shown. (I, J) KLRG1− NK cells were sorted from WT (CD45.1+), Rag1−/− (CD45.2+) and Tcra−/− (CD45.2+) mice and an equal number of KLRG1−Ly49H+ NK cells from Rag1−/− (CD45.2+) or Tcra−/− mice were mixed (1:1) with WT NK cells, transferred into Ly49H-deficient hosts (day -1), and then infected with MCMV (day 0). Percentages of transferred Ly49H+ NK cells isolated from WT and Rag1−/− mice (I) and WT and Tcra−/− mice (J) are shown. Data are representative of two (C, I, J), three (E, F) and four (A, B) independent experiments. Error bars indicate SEM (n=3–5). See also Figure S2.
Additionally, we transferred WT splenocytes into Rag1−/− mice to restore mature B and T cells. The Rag1−/− mice receiving WT splenocytes were treated with a depleting anti-NK1.1 antibody on the day of WT splenocytes transfer in order to deplete all mature endogenous NK cells. This allowed newly emerging endogenous Rag1−/− NK cells to be exposed to mature WT T and B cells. At the same time, control WT and Rag1−/− mice (not receiving adoptively transferred splenocytes) were also treated with anti-NK1.1 antibody. After eight weeks, the percentage of KLRG1int+ NK cells derived from these endogenous Rag1−/− NK cell progenitors was comparable to NK cells in WT mice (Figure 2G) and the response to MCMV was rescued (Figure 2H). These findings indicate that T cells suppress the induction of KLRG1int+ NK cells during steady-state in WT mice and maintain memory NK cell progenitors.
To further determine whether KLRG1− NK cells from Rag1−/− mice possess the same capacity for expansion and memory generation as KLRG1− NK cells from WT mice, we sorted KLRG1− NK cells from WT (CD45.1+) and Rag1−/− mice and co-transferred them (mixed 1:1) into Ly49H-deficient hosts followed by MCMV infection. Both NK cells showed equivalent expansion and memory generation (Figure 2I). We observed equivalent results using a mixture of NK cells from WT (CD45.1+) and Tcra−/− mice (Figure 2J). These results indicate that it is the frequency of KLRG1int+ NK cells in these strains that are the important factor for differential NK cells expansion and memory generation and not an intrinsic property of the NK cells in these strains.
NOD sensors in the host affect the progenitors for memory NK cells
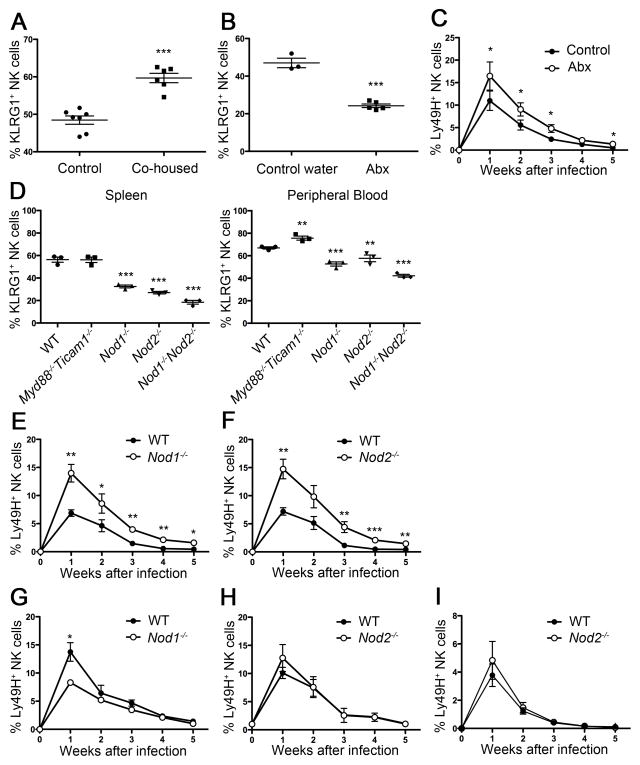
Because the frequency of KLRG1int+ NK cells in naive, uninfected mice can be influenced by extrinsic influences, we investigated whether commensal bacteria affect the generation of KLRG1int+ NK cells at steady-state. The initial clue that commensal bacteria might be involved was our observation that C57BL/6 mice obtained from different vendors or animal facilities displayed distinct percentages of KLRG1int+ NK cells in naive, uninfected mice (Figure S2). Hence, we hypothesized that commensal bacteria might be involved in the induction of KLRG1int+ NK cells in Rag1−/− mice. To test this, we co-housed WT mice with Rag1−/− mice, where the transfer of microbiota can be expected. After co-housing for three weeks, splenic NK cells from WT mice co-housed with Rag1−/− mice showed an increased frequency of KLRG1int+ NK cells compared to the frequency of KLRG1int+ NK cells in WT mice co-housed with WT mice (Figure 3A). To further address the involvement of commensal bacteria in the generation of KLRG1int+ NK cells at steady-state, we depleted commensal bacteria by treatment with a cocktail of broad-spectrum antibiotics (designated as Abx) in the drinking water. Treatment with Abx-water significantly reduced the frequency of KLRG1int+ NK cells compared to treatment with control water (Figure 3B). Of note, any single Abx treatment was not effective (data not shown). To compare their responses to MCMV, we co-transferred an equal number of Ly49H+ NK cells from control water-treated mice (CD45.2) or Abx-treated mice (CD45.1) into Ly49H-deficient recipients (receiving control water). After MCMV infection, NK cells from Abx-treated mice proliferated more robustly and generated memory NK cells more efficiently than NK cells from control water-treated mice (Figure 3C).
Figure 3. Nod proteins expressed by non-hematopoietic cells are required to induce KLRG1 expression on NK cells.
(A) Percentages of KLRG1int+ cells within total NK cell population in the WT mice housed with WT mice (control) or Rag1−/− mice (co-housed) for three weeks. (B) Percentages of KLRG1int+ cells within total NK cell population in the WT mice given control or Abx-treated water for three weeks are shown. (C) Mice were given control (CD45.2+) or Abx-treated water (CD45.1+) for three weeks. An equal number of Ly49H+ NK cells isolated from each group was mixed and transferred into Ly49H-deficient mice followed by MCMV infection. Graph shows percentages of each Ly49H+ subset within the total NK cell population. (D) Percentages of KLRG1int+ cells within the total NK cell population in spleen and peripheral blood from the indicated mice at steady-state. (E, F, G, H) Percentage of Ly49H+ NK cells derived from WT (CD45.1+) and Nod1−/− or Nod2−/− (CD45.2+) mice after MCMV infection. (I) KLRG1− NK cells were sorted from WT (CD45.1+) and Nod2−/− (CD45.2+) mice. An equal number of KLRG1−Ly49H+ NK cells from WT and Nod2−/− mice was mixed (1:1). transferred into Ly49H-deficient hosts (day -1), and then infected with MCMV (day 0). Percentages of transferred Ly49H+ NK cells derived from WT and Nod2−/− mice are shown. Data are combination (A) or representative (B–I) of two independent experiments. Error bars show SEM (n=3–5). See also Figure S3.
To investigate how commensal bacteria might induce KLRG1int+ NK cells we screened informative mutant mice lacking innate pattern-recognition receptors and their signaling molecules, which included mice lacking MyD88 and Ticam1 (also known as Trif), Nod1, Nod2, or Nod1 and Nod2. Only Nod-deficient mice showed a decreased percentage of KLRG1int+ NK cells at steady-state (data not shown and Figure 3D). NK cells isolated from Nod1−/− (Figure 3E) and Nod2−/− B6 mice (Figure 3F) proliferated more vigorously and generated more memory NK cells than NK cells isolated from WT B6 mice. To determine whether intrinsic or extrinsic factors in Nod-deficient mice regulate KLRG1 expression, we generated mixed BMC mice with bone marrow cells from WT (CD45.1+) and Nod1−/− or Nod2−/− mice (CD45.2+) introduced into lethally irradiated WT recipients. The difference in KLRG1 expression between WT and Nod-deficient NK cells was abolished in mixed BMC mice (data not shown). This indicates that Nod sensing is not NK cell intrinsic, but is due to Nod-dependent sensors on non-hematopoietic cells in the host. To compare the expansion and memory generation, we isolated WT and Nod-deficient NK cells from these chimeric mice and adoptively transferred the NK cells into Ly49H-deficient hosts. After MCMV infection, although Nod1−/− NK cells showed slightly decreased expansion, both Nod1−/− and Nod2−/− NK cells demonstrated equivalent generation of memory cells compared to WT NK cells (Figure 3G and H). To determine whether KLRG1− NK cells from WT and Nod2−/− mice possess the same capacity for expansion and memory generation, we sorted KLRG1− NK cells from WT (CD45.1+) and Nod2−/− (CD45.2+) mice and co-transferred an equal number of Ly49H+ NK cells into Ly49H-deficient hosts. Both WT and Nod2−/− KLRG1− Ly49H+ NK cells expanded and generated memory in a same manner (Figure 3I). These results suggest that commensal bacteria might promote the induction of KLRG1int+ NK cells and erode the reservoir for memory NK cell progenitors.
Antibiotic treatment suppresses homeostatic proliferation of NK cells
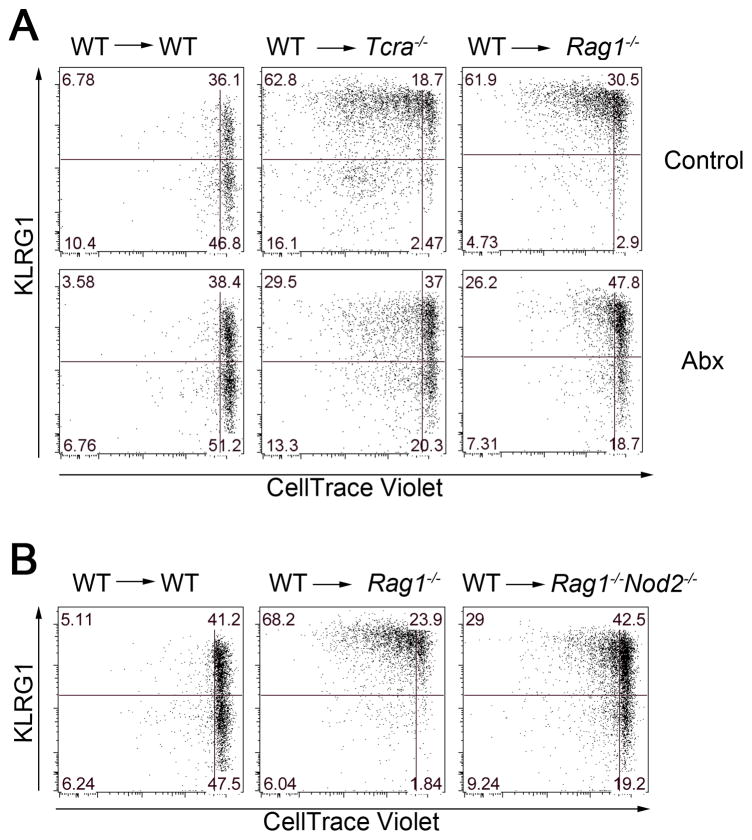
Our results indicate that T cells suppress the induction of KLRG1int+ NK cells, whereas in WT mice commensal bacteria might promote the generation of KLRG1int+ NK cells. To test whether a T cell-deficient environment is sufficient to explain the increased frequency of KLRG1int+ NK cells, we transferred WT CD45.1-congenic NK cells into WT, Rag1−/−, or Tcra−/− mice. Donor NK cells transferred into Rag1−/− or Tcra−/− mice, but not WT mice, demonstrated a dramatic increase in the percentage of KLRG1int+ NK cells (Figure 4A upper panels). Unexpectedly, donor WT NK cells underwent homeostatic proliferation in Rag1−/− and Tcra−/− mice, which contain normal numbers of endogenous NK cells (Figure 4A upper panels). This increased baseline proliferation in Rag1−/− and Tcra−/− mice was further confirmed by the increased expression of Ki67 in NK cells isolated from these mice compared to those from WT mice (Figure S3). Furthermore, donor WT NK cells transferred into Abx water-treated Rag1−/− or Tcra−/− mice showed a decreased frequency of KLRG1int+ NK cells and less homeostatic proliferation compared to NK cells from control Rag1−/− or Tcra−/− mice (Figure 4A lower panels). These results suggest that commensal bacteria might be responsible for generating KLRG1int+ NK cells in mice lacking T cells and that T cells suppress this process.
Figure 4. Commensal bacteria are partially responsible for homeostatic proliferation of NK cells and induction of KLRG1 expression.
(A) Enriched WT (CD45.1+) NK cells were labeled with CellTrace Violet and 5 × 105 cells were transferred via intravenous injection into WT mice (CD45.2+), Tcra−/− mice (CD45.2+), or Rag1−/− mice (CD45.2+) and given either control or Abx-treated water for 14 days prior to transfer. (B) CellTrace-Violet-labeled WT NK cells were transferred into WT mice (CD45.2+), Rag1−/− mice (CD45.2+), or Rag1−/−Nod2−/− mice (CD45.2+). KLRG1 expression and dilution of CellTrace-Violet in splenic CD45.1+ NK cells were analyzed 7 days after transfer. Data are representative of three independent experiments with 3 mice in each experiment. See also Figure S4.
To determine the involvement of Nod2 in this system, we transferred CD45.1-congenic WT NK cells into Rag1−/−Nod2−/− CD45.2+ mice. Donor WT NK cells showed a decreased frequency of KLRG1int+ NK cells and less homeostatic proliferation when transferred into Rag1−/−Nod2−/− mice compared with WT NK cells transferred into Rag1−/− mice (Figure 4B). Therefore, Nod2 in the recipient Rag-deficient mice enhances the homeostatic proliferation and generation of KLRG1int+ NK cells. Collectively, these findings suggest that both T cells and possibly commensal bacteria regulate KLRG1 induction and homeostatic proliferation.
Availability of IL-15 regulates generation of KLRG1int+ NK cells
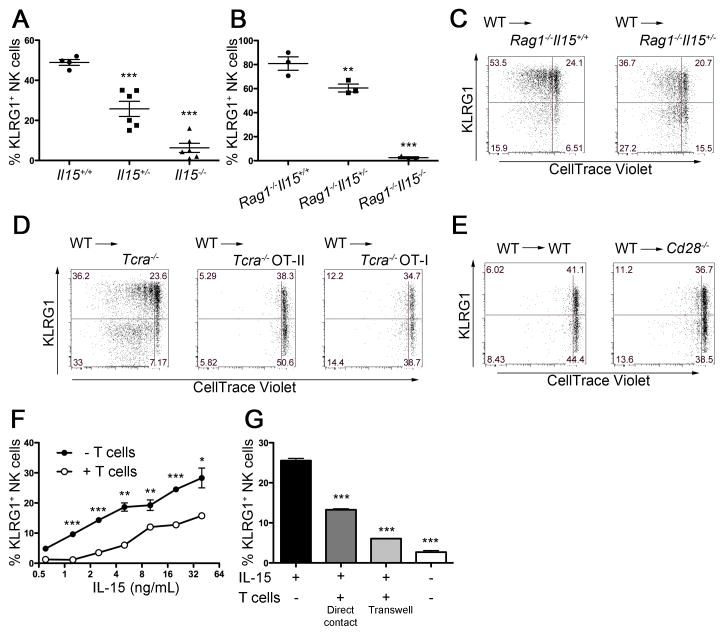
Because IL-15 is known to be important in the development and homeostasis of NK cells and IL-15 is also used by T cells to efficiently develop memory T cells we investigated whether T cells might suppress the induction of KLRG1 on NK cells by competition for IL-15 in WT mice. Disruption of the Il15 gene results in a severe reduction, but not the complete elimination of NK cells (Sun et al., 2009b). We found that the few NK cells present in Il15−/− mice lacked KLRG1 expression (Figure S4). Interestingly, Il15 heterozygous mice demonstrated a decrease in the percentage of KLRG1int+ NK cells compared to WT mice (Figure 5A), suggesting that IL-15 might be involved in the generation of NK cells KLRG1int+ NK cells at steady-state. To address whether IL-15 is also responsible for elevated KLRG1 expression in Rag−/− mice, we crossed Il15−/− mice with Rag1−/− mice. We found that KLRG1int+ NK cells were undetectable in Rag1−/−Il15−/− mice. Furthermore Rag1−/−Il15+/− mice exhibited a decreased frequency of KLRG1int+ NK cells compared with Rag1−/−Il15+/+ mice (Figure 5B). To test whether IL-15 is required for the homeostatic proliferation and KLRG1 induction as observed in Figure 4, we transferred CD45.1-congenic WT NK cells into Rag1−/−Il15+/− CD45.2+ mice. Donor NK cells demonstrated a decreased frequency of KLRG1int+ NK cells and less homeostatic proliferation in Rag1−/−Il15+/− mice than in Rag1−/−Il15+/+ mice (Figure 5C). Note that donor NK cells were undetectable if transferred into Rag1−/−Il15−/− mice (data not shown), consistent with prior studies (Burkett et al., 2004; Sandau et al., 2004). These findings indicate that the availability of IL-15 might regulate the abundance of KLRG1int+ NK cells at steady-state.
Figure 5. IL-15 induces KLRG1 expression.
(A) Percentages of KLRG1int+ cells within the total NK cell population from mice with the indicated Il15 genotype. (B) Percentages of KLRG1int+ cells within the total NK cell population from Rag1−/− mice with the indicated Il15 genotype. (C, D, E) Enriched NK cells were labeled with CellTrace Violet and 5 × 105 cells cell were transferred via intravenous injection into Rag1−/−Il15+/+ mice (CD45.2+) or Rag1−/−Il15+/− mice (CD45.2+) (C); Tcra−/− mice (CD45.2+), Tcra−/− OT-II mice (CD45.2+), or Tcra−/− OT-I mice (CD45.2+) (D); WT mice (CD45.2+) or Cd28−/− mice (CD45.2+) (E). KLRG1 expression and dilution of CellTrace-Violet in splenic CD45.1+ NK cells were analyzed 7 days after transfer. (F) Sorted KLRG1− NK cells (4 × 104/well) were cultured in the indicated doses of IL-15 in the presence or absence of T cells (8 × 105/well) (a ratio of 20:1 T cells to NK cells to represent relative proportions in the spleen) for 3 days. (G) KLRG1− NK cells and T cells were co-cultured in the same well or in trans-well (0.4 μm pore size) cultures in the presence of IL-15 (5 ng/mL) for 3 days. Data are representative of three independent experiments with three mice (C, D), two independent experiments with three mice (E) and triplicates (F, G) per experiment. Error bars show SEM.
Although it is not essential for the development of naïve T cells, IL-15 can promote survival of both naïve and memory T cells (Berard et al., 2003). We hypothesized that T cells might suppress the differentiation or activation of NK cells by competitively consuming IL-15 rather than a unique subset of T cells actively suppressing the induction of KLRG1int+ NK cells. Consistent with this hypothesis, we found that CD45.1-congenic WT NK cells transferred into Tcra−/− OT-I or Tcra−/− OT-II TcR-transgenic CD45.2+ mice failed to undergo homeostatic proliferation and induce KLRG1 (Figure 5D). To determine the involvement of regulatory T cells (Treg) cells in the suppression, we transferred CD45.1-congenic WT NK cells into Cd28−/− CD45.2+ mice, which have severely impaired Treg development (Tai et al., 2005). Compared with the robust homeostatic proliferation observed when donor NK cells were introduced into T cell-deficient hosts, the expansion of donor NK cells and acquisition of KLRG1 were equally impaired when NK cells were introduced into either WT or Cd28−/− recipients (Figure 5E). These results indicate that either mono-specific conventional CD4+ T cells or CD8+ T cells are sufficient for the suppression of homeostatic proliferation and acquisition of KLRG1 by NK cells, and that Treg cells are not required.
To directly evaluate the effect of IL-15 and T cells on the induction of KLRG1 by NK cells, we sorted KLRG1− NK cells and cultured them in the presence of various concentration of IL-15 with or without T cells. After 72 hours in culture with IL-15, KLRG1 was induced in a dose-dependent manner on NK cells (Figure 5F, filled circle) and the addition of T cells suppressed the generation of KLRG1int+ NK cells (Figure 5G, open circle). To determine whether T cells require cell-contact with NK cells to inhibit the induction of KLRG1int+ NK cells, we performed trans-well experiments. T cells suppressed the IL-15-dependent induction of KLRG1int+ NK cells in the absence of cell-cell contact (Figure 5G). Thus, T cells might competitively consume IL-15 and therefore block NK cell acquisition of KLRG1.
Antibiotics improve control of MCMV infection
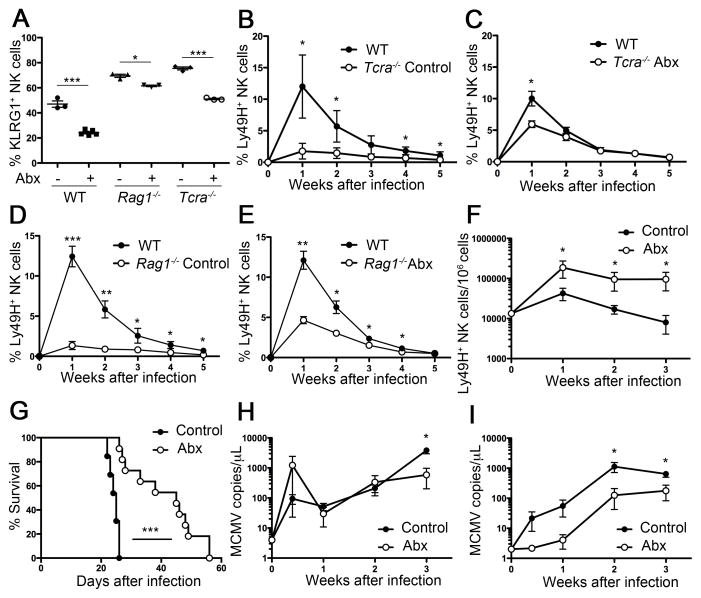
Our findings suggest that commensal bacteria might drive the generation of KLRG1int+ NK cells, which we have shown are less responsible to MCMV infection than KLRG1− NK cells. Although Ly49H+ NK cells are responsible for early phase control of MCMV infection, SCID or Rag-deficient mice are unable to completely control MCMV and die within a month after infection. Although mutation in m157, the viral ligand of Ly49H, can be observed in MCMV isolated from SCID or Rag-deficient mice (French et al., 2004), we considered the possibility that the poor proliferation of Rag1−/− NK cells due to the increased frequency of KLRG1int+ NK cells might be another reason for the failure to control MCMV in Rag1−/− mice. Prior studies have reported that NK cell control of MCMV infection is dependent on robust NK cell proliferation (Orr et al., 2009), therefore, suggesting that mice bearing a higher frequency of more responsive KLRG1− NK cells might control infection more efficiently. We found that Abx treatment increased the frequency of KLRG1− NK cells in Rag1−/− mice and Tcra−/− mice (Figure 6A). Moreover, Ly49H+ NK cells isolated from Abx water-treated Tcra−/− and Rag1−/− mice showed improved MCMV-driven proliferation and memory generation compared to those from control water-treated Tcra−/− and Rag1−/− mice (Figure 6B, C, D, E).
Figure 6. Antibiotic treatment improves NK cell responses to MCMV in T cell-deficient mice.
(A, B, C) WT, Tcra−/−, and Rag1−/− mice were given control or Abx-treated water for three weeks. (a) Percentages of KLRG1int+ cells within the total NK cell population in the indicated mice are indicated. (B) An equal number of Ly49H+ NK cells from Tcra−/− mice (CD45.2+) given control (B) or Abx-treated water (C) were mixed (1:1) with NK cells from WT mice (CD45.1+) and transferred into Ly49H-deficient mice, followed by MCMV infection. (D) An equal number of Ly49H+ NK cells from Rag1−/− mice (CD45.2+) given control (D) or Abx-treated water (E) were mixed with NK cells from WT mice (CD45.1+) and transferred into Ly49H-deficient mice, followed by MCMV infection. Graph shows percentages of each Ly49H+ population within the total NK cell population. (F, G, H, I) Rag1−/− mice were treated with control or Abx-treated water three weeks before MCMV infection and treatment was continued throughout the infection. (F) Number of Ly49H+ NK cells per million peripheral blood leukocytes (red blood cells-depleted fraction). (G) Survival of Rag1−/− mice treated either control water (n=13) or Abx-treated water (n=11). (H, I) Copy numbers of MCMV genomic DNA in peripheral blood (H) or oral lavage (I) were quantified by qPCR. Data are compiled from two independent experiments (G) or representative of two independent experiments with three to five (B, C, D, E), five (Abx-treated group in F, H, I) or seven (control group in F, H, I) mice per experiment. Error bars show SEM. See also Figure S5.
We tested whether Abx treatment of Rag1−/− mice would affect their ability to control MCMV infection. Rag1−/− mice were given either control or Abx-treated water before infection (starting at day -21) and continued after MCMV infection (day 0) throughout the experiment. We monitored survival, the percentage of Ly49H+ NK cells, and viral DNA copy numbers in peripheral blood and oral lavage. Ly49H+ NK cells in control Rag1−/− mice expanded but diminished at day 21, whereas those in Abx water-treated Rag1−/− mice increased and persisted at day 21 (Figure 6F). Furthermore, Abx water-treated Rag1−/− mice clearly survived longer than control Rag1−/− mice (median survival days: 45 vs. 25, p<0.0001) (Figure 6G). The viral load in blood was readily detectable at day 3, temporally decreased at day 7, and increased after day 14, but Abx treatment did not reduce the viremia (Figure 6H). Although the viral load in oral lavage was increased after MCMV infection (reflecting the accumulation of virus in salivary glands), a lower viral load was detected in Abx water-treated Rag1−/− mice than in control water-treated Rag1−/− mice (Figure 6I). Additionally, to test whether prolonged survival by Abx-water treatment is NK cell-dependent, we injected anti-NK1.1 antibody to control or Abx water-treated Rag1−/− mice one day before MCMV infection. In the absence of NK cells, both control and Abx water-treated Rag1−/− mice died with the same kinetics (Figure S5). These results indicate that Abx treatment allows for a more robust NK cell response, possibly due to increasing the abundance of the more responsive KLRG1− NK cell subset, and is beneficial for NK cell control of persistent virus infection.
DISCUSSION
Recently, several types of NK cells have been reported to possess immunological memory. Peng et al. reported that a subset of DX5− NK cells in liver are responsible for hapten-specific responses (Peng et al., 2013). These DX5− hepatic NK cells (or ILC1 cells) do not express Ly49H (Sojka et al., 2014) and lack Eomes (Daussy et al., 2014), in contrast with conventional NK cells that are Eomes-positive and express DX5 and activating Ly49 receptors. In this report, we demonstrate that memory progenitors identified as KLRG1− Ly49H+ NK cells with the capacity for MCMV-specific expansion and memory generation. The KLRG1int+ Ly49H+ NK cells express a more mature phenotype (CD27− CD11b+) than the KLRG1−Ly49H+ NK cells, suggesting that once Ly49H+ NK cells have reached a mature stage of differentiation they lose their potential for memory generation. It is unlikely the KLRG1 receptor itself is responsible for this lack of responsiveness because memory NK cells express high levels of KLRG1 and we have shown that they are capable of secondary expansion equivalent to naive NK cells when they are re-challenged with MCMV in vivo (Sun et al., 2009a, 2010). Unexpectedly, we found that T cells and possibly commensal bacteria reciprocally regulate the pool of memory NK cell progenitors.
Rag-deficient mice have been used in NK cell studies to exclude the involvement of T and B cells. However, NK cell development and responsiveness in the absence of T and B cells has not been extensively examined. It was reported that Rag plays a cell-intrinsic role in the development of NK cells because accumulation of NK cells in spleen, liver, and bone marrow of Rag-deficient mice is slower than in WT mice during ontogeny (Andrews and Smyth, 2009). Although Rag is not expressed in mature NK cells, a subset of NK cell precursors in bone marrow experience incomplete TcR V(D)J recombination (Pilbeam et al., 2008). Furthermore, Karo et al. reported that Ly49H+ NK cells derived from precursor cells that had transiently expressed RAG during their early development preferentially expand and generate memory NK cells during MCMV infection, whereas NK cells that arose from precursor cells that failed to express Rag are more prone to apoptosis, possibly due to less efficient repair of DNA damage (Karo et al., 2014). Our current findings, however, suggest that the absence of T cells, in Rag-deficient or TcRα-deficient mice, impacts the frequency of memory NK cell progenitors in a cell-extrinsic manner, potentially by competing for IL-15. Although IL-15 is necessary for the development and homeostasis of NK cells at steady-state, we observed that IL-15 might also drive the generation of KLRG1int+ NK cells at the expense of memory progenitors. Consistent with our observations, it has also been reported that continuous injection of IL-15 causes up-regulation of KLRG1 expression and hypo-responsiveness of NK cells (Elpek et al., 2010). Gasteiger and colleagues recently reported that Treg and NK cells compete for IL-2 in the host and that this affects the cytotoxicity of NK cells (Gasteiger et al., 2013). In our study, conventional T cells, rather than Treg cells, appear to play an important role in regulating the development of KLRG1int+ NK cells from KLRG1− memory progenitors. Prior studies have demonstrated that there is competition between NK cells and T cells for the limited amounts of IL-15 available in the host (French et al., 2005). These findings, together with our observations that IL-15 can directly induce expression of KLRG1 on NK cells and that NK cells in Il15−/− and Il15+/− mice possess more memory progenitors support the hypothesis that Rag-deficient mice might possess fewer memory progenitors due to the increased availability of this cytokine.
NK cells adoptively transferred into Rag2−/−Il2rg−/− mice undergo homeostatic proliferation and acquire the expression of KLRG1 (Sun et al., 2011). Unexpectedly, we observed that WT NK cells also underwent homeostatic proliferation when adoptively transferred into Rag1−/− or Tcra−/− hosts, despite the presence of endogenous host NK cells, which was dependent on IL-15 and was diminished by Abx treatment. Recently, commensal bacteria have been implicated as a novel regulator of the immune system (Atarashi et al., 2013; Ivanov et al., 2009; Lathrop et al., 2011; Round and Mazmanian, 2010); however, the impact of commensal bacteria on NK cell development, homeostasis, and effector function are poorly understood. Ganal and colleagues reported that NK cells in germfree mice are poorly activated by various PAMPS due to impaired cytokine production by non-mucosal myeloid cells, resulting in decreased activation of NK cells and poor clearance of MCMV in spleen at day 3 post-infection compared to specific pathogen-free mice (Ganal et al., 2012). Similar impairment of myeloid cells was also induced by Abx treatment and resulted in a decreased number and activation of influenza and LCMV-specific CD8+ T cells (Abt et al., 2012). We observed a similar trend at day 3 post-infection of Rag-deficient mice with MCMV; however, Abx treatment improved control of MCMV infection at later time points and prolonged survival of the mice. French and colleagues have reported that m157-mutated MCMV outgrow in C57BL/6-SCID mice by day 28 post-infection (French et al., 2004). Although both control and Abx-treated Rag-deficient mice succumbed to MCMV infection, we have demonstrated that Abx treatment increased the frequency of memory progenitors and improved expansion of Ly49H+ NK cells that might allow for better control of MCMV and result in prolonged survival.
Although Ganal and colleagues did not address how commensal bacteria influence myeloid cells in distal sites, it is possible that systemically circulating Nod ligands (Clarke et al., 2010) deliver instructive signals to peripheral myeloid cells. Nod1 and Nod2 are intracellular pattern-recognition receptors sensing D-glutamyl- meso-diaminopimelic acid (iE-DAP) and muramyl dipeptide (MDP), respectively, which are unique motifs within bacterial peptidoglycan. Nod1 is expressed ubiquitously, whereas Nod2 is limited to myeloid subsets and intestinal epithelial cells. Once ligand binds, Nod proteins phosphorylate Rip2 and activate NF-κB, resulting in the expression of a variety of pro-inflammatory cytokines. The Il15 promoter contains a NF-κB-binding element that is necessary for the efficient transcription of IL-15 (Azimi et al., 2000; Washizu et al., 1998). Recently, Jiang and colleagues demonstrated that Nod2 is important for sensing gut microbiota and essential for the induction of IL-15 in intestinal macrophages (Jiang et al., 2013); however, it remains to be elucidated why Nod, but not Toll-like receptors, apparently play a role in the induction of IL-15. It is also unclear how IL-15 induced by commensal bacteria in the intestine might affect the frequency of memory progenitors in spleen and blood. However, Yokoyama et al. reported that enterocyte-specific overexpression of IL-15 results in significant increase of NK cell- number in peripheral blood and enlargement of spleen (Yokoyama et al., 2009). Further studies are needed to understand whether NK cells circulate through the intestine to encounter IL-15 or if blood-borne signals, as suggested by Ganal and colleagues, induce IL-15 in myeloid cells located distally. Collectively, these prior observations and our findings in this study suggest a model in which NK cells are regulated both by T cells in the host and the microbiota. We speculate that Nod-dependent sensing of commensal bacteria might regulate the production of IL-15 by myeloid cells. In steady-state T cells and NK cells compete for the limiting amounts of IL-15, thereby restraining the maturation of NK cells and preserving memory NK cell progenitors (Figure 7). However, in the absence of T cell competition, the availability of excess IL-15 drives terminal maturation of NK cells in the host. Controlling the balance of effector NK cells versus memory progenitor NK cells might be beneficial for optimal responsiveness of NK cells to microbial infections.
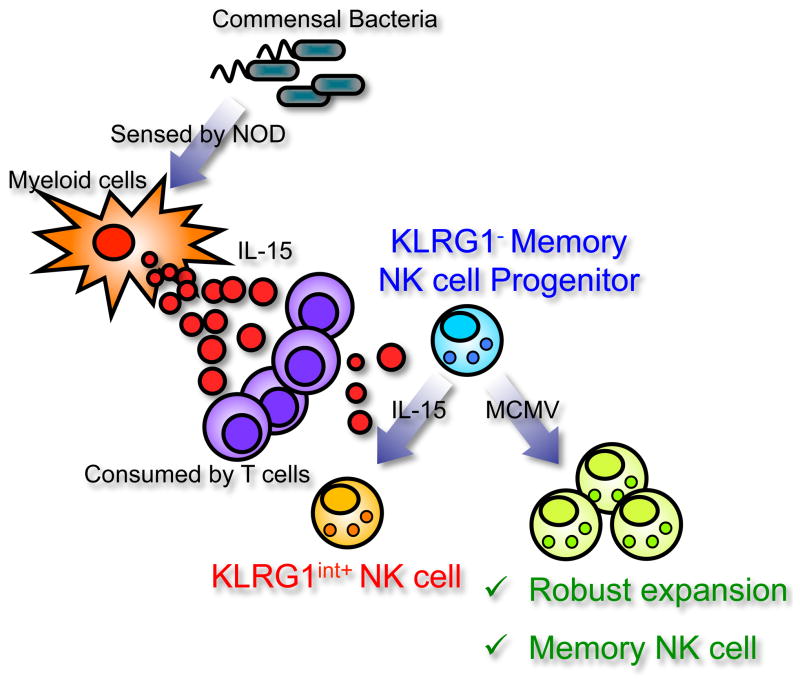
Figure 7. Reciprocal regulation of memory NK cell progenitors by commensal bacteria and T cells – a hypothetical model.
Nod receptors on host non-hematopoietic cells sense commensal bacteria and enhance IL-15 production in myeloid cells. Competitive consumption of IL-15 by T cells inhibits the induction of KLRG1int+ NK cells and preserves progenitor KLRG1− NK cells that preferentially develop into KLRG1high memory NK cells.
EXPERIMENTAL PROCEDURES
Mice and Infections
CD45.1-congenic C57BL/6 mice were purchased from the National Cancer Institute and bred at UCSF. Rag1−/−, Tcra−/−, μMt, Rag2−/−Il2rg−/−, Il15−/−, Rag1−/−Nod2−/−, Rag1−/− Il15+/−, Rag1−/−Il15−/−, Cd28−/−, Tcra−/− OT-I TcR transgenic, Tcra−/− OT-II TcR transgenic, and Klra8−/− (Ly49H-deficient) (Fodil-Cornu et al., 2008) mice on a C57BL/6 background were bred at UCSF. Nod1−/−, Nod2−/−, Nod1−/−Nod2−/−, Myd88−/−Ticam1−/− mice (Adachi et al., 1998; Chamaillard et al., 2003; Kobayashi et al., 2005; Yamamoto et al., 2003) on a C57BL/6 background were generously provided by Dr. Gregory M. Barton (University of California Berkley, California). All mice were maintained in accordance with guidelines of the Institutional Animal Care and Use Committee. Mixed bone marrow chimeric mice were generated as described previously (Sun et al., 2009a). Briefly, recipient (CD45.1+) mice were irradiated at 550 rad twice with a 4 hour interval. WT (CD45.1+) and mutant (CD45.2+) bone marrow cells were mixed (1:1) and adoptively transferred (total 2 × 106 cells/mouse) into recipient mice after irradiation. One tablet of SCIDS MD’s™ (6 mg Sulfamethoxazole and 1.2 mg Trimethoprim, Bio-Serve) was added to each cage one day before irradiation. Mice were rested for eight weeks and then used for experiments. For MCMV infection, mice were infected by i.p. injection with 1 × 104 pfu Smith strain MCMV.
Flow cytometry
Fc receptors were blocked with anti-CD16+CD32 mAb (2.4G2) before staining. To monitor NK cells in peripheral blood weekly after MCMV infection, 50 μL of heparinized blood was lysed by ACK buffer and stained with anti-TCRβ (H57-597; BioLegend), -NK1.1 (PK136; BioLegend), -Ly49H (3D10; eBioscience), -CD45.1 (A20; BioLegend), -CD45.2 (104; Tonbo Biosciences) and -KLRG1 (2F1; BioLegend). Cells were analyzed on a BD LSR II flow cytometer using FlowJo software (Tree Star).
Cell preparation
NK cells were enriched with an NK Cell Isolation kit (Miltenyi Biotec) or a method where spleen cells were incubated with purified rat antibodies against mouse CD4, CD5, CD8, CD19, Gr-1, and Ter119, followed by anti–rat IgG conjugated to magnetic beads (QIAGEN). Purity of enriched NK cells was analyzed by staining with anti-TCRβ-NK1.1, and -Ly49H. After enrichment, TCRβ− NK1.1+ cells were typically 50–70% and half expressed Ly49H. One hundred thousand Ly49H+ NK cells were injected intravenously into Ly49H-deficient recipients 1 day before viral infection. In some experiments, NK cells were labeled with 1 μM CellTrace Violet (Invitrogen), according to the manufacturer’s instructions and 5 × 105 cells were injected into recipients via intravenous injection. In some experiments, enriched NK cells were further sorted as TCRβ− KLRG1+ or TCRβ− KLRG1− cells using a BD Aria III flow cytometer.
Commensal bacteria depletion
Mice were given broad-spectrum Abx (ampicillin 1 g/mL, Sigma; neomycin sulfate 1 g/mL, Sigma; metronidazole 1 g/mL, Sigma; and vancomycin 0.5 g/mL, Goldbio) in drinking water for 3 weeks. Depletion of bacteria was confirmed by culturing of feces and determining CFU on BHI culture plates (NEOGEN).
Co-housing experiment
Female WT C57BL/6 mice purchased from the NCI (which served as ‘recipients’) were co-housed with female Rag1−/− (which served as ‘donors’) for 3 weeks. Three WT ‘recipients’ and two Rag1−/− ‘donors’ were housed per cage. The frequency of KLRG1int+ NK cells in splenocytes isolated from the recipients was analyzed.
Quantitation of MCMV
MCMV viral loads were monitored sequentially by real-time qPCR as previously described (Kamimura and Lanier, 2014). For oral lavage collection, the mouse sublingual cavity was extensively washed with 20 μL of sterile saline under anesthesia. Five to ten μL of lavage fluid was collected and viral load was determined by qPCR analysis. To measure viral load in peripheral blood, 10 μL of peripheral blood was processed with a GenElute™ Blood Genomic DNA kit (Sigma), eluted with 20 μL of sterile nuclease-free water, and analyzed by quantitative PCR (qPCR). Primers use; MCMV-IE1 Forward: 5′-AGCCACCAACATTGACCACGCAC-3′, MCMV-IE1 Reverse: 5′-GCCCCAACCAGGACACACAACTC-3′. To establish a standard curve for quantitation of viral load full-length MCMV-IE1 DNA was amplified by primers; IE1-full Forward: 5′-TGTCGCCAACAAGATCCTCG-3′, IE1-full Reverse: 5′-CCCTGCCTGCTGTTCTT-3′ and inserted into the TOPO-PCR2.1 vector (Invitrogen).
Statistical method
The unpaired t test was used to compare 2 groups. The Newman-Keuls test was used to compare 3 or more groups. The Log-Rank test was used to compare survival between groups of mice. P values provided in figures are reported as *P<0.05, **P<0.001 and ***P<0.0001, respectively.
Supplementary Material
Figure S1. Equivalent effector function between KLRG1int+ and KLRG1− NK cells, related to Figure 1.
(A) CD27 and CD11b expression by splenic naïve KLRG1int+ and KLRG1− Ly49H+ NK cells. (B) Degranulation by splenic naïve KLRG1int+ (upper panel) and KLRG1− (lower panel) Ly49H+ NK cells stimulated ex vivo with RMA-m157 target cells. (C) IFN-γ production by splenic KLRG1int+ (upper panel) and KLRG1− (lower panel) Ly49H+ NK cells at day 1.5 after MCMV infection. Data are representative of two independent experiments.
Figure S2. Differential frequency of KLRG1int+ NK cells in WT B6 mice from different animal vendors, related to Figure 2.
Expression of KLRG1 on TCRβ− NK1.1+ gated NK cells in peripheral blood (left panel) and spleen (right panel) of C57BL/6 mice purchased from the Jackson Laboratory (Jax), the NCI Mouse Repository, and Taconic (Tac) and C57BL/6 mice bred in our animal facility at UCSF. All mice were 8 week-old females. Data are representative of two independent experiments with 3 mice from each strain. Error bars show S.E.M.
Figure S3. Increased baseline proliferation of NK cells in T cell-deficient mice, related to Figure 3.
Expression of Ki67 in splenic NK cells was detected by intracellular staining. A representative histogram plot (left panel; shaded: WT, bold: Rag1−/−, dotted: Tcra−/−) and graph summarizing results from individual mice (right panel) are shown. Data are representative of two independent experiments with 4 mice from each strain. Error bars show S.E.M.
Figure S4. Severely decreased frequency of KLRG1int+ NK cells in Il15−/− mice, related ot Figure 4.
Expression of KLRG1 and Ly49H by splenic NK cells isolated from WT and Il15−/− mice. Percentage of TCRβ−NK1.1+, KLRG1+, or Ly49H+ cells is shown in each panel. Data are representative of two independent experiments (n=4–6).
Figure S5. Effect of antibiotics on the survival of NK cell-depleted Rag1−/− mice, related to Figure 6.
Rag1−/− mice were treated with control or Abx-treated water for three weeks before MCMV infection (day 0) and treatment was continued throughout the experiment. Anti-NK1.1 antibody (200 μg/mouse) was injected i.p. one day before infection (day -1). Data are compiled from two independent experiments (control: n=10, Abx: n=12, control+anti-NK1.1: n=15, Abx + anti-NK1.1: n=17).
Acknowledgments
We thank Ms. J. Arakawa-Hoyt for assistance with cell sorting; Drs. D. W. Hendricks, G. Min-Oo, T. Nabekura, H. Jensen, Y. Zhang, N. Bezman, J. C. Sun, and V. Lam for critical reading of the manuscript. Drs. B. R. Russell and G. M. Barton (University of California, Berkley, California) for generously providing Nod-deficient mice and Myd88−/−Ticam1−/− mice; Dr. A. Lee and A. Ma (University of California, San Francisco) for Il15−/− mice; and Dr. S. Vidal (McGill University) for Ly49H-deficient mice. Supported by the National Institutes of Health AI068129. L.L.L. is an American Cancer Society Professor.
Footnotes
AUTHOR CONTRIBUTIONS
Y. K. planned and performed experiments and wrote the manuscript and L. L. L. contributed to experimental design, data evaluation, and writing of the manuscript.
COMPETING FINANCIAL INTEREST
The authors declare no competing financial interests.
Homeostatic control of memory cell progenitors in the NK cell lineage Yosuke Kamimura and Lewis L. Lanier
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. Commensal Bacteria Calibrate the Activation Threshold of Innate Antiviral Immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted Disruption of the MyD88 Gene Results in Loss of IL-1- and IL-18-Mediated Function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Andrews DM, Smyth MJ. A potential role for RAG-1 in NK cell development revealed by analysis of NK cells during ontogeny. Immunol Cell Biol. 2009;88:107–116. doi: 10.1038/icb.2009.94. [DOI] [PubMed] [Google Scholar]
- Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- Azimi N, Tagaya Y, Mariner J, Waldmann TA. Viral Activation of Interleukin-15 (IL-15): Characterization of a Virus-Inducible Element in the IL-15 Promoter Region. J Virol. 2000;74:7338–7348. doi: 10.1128/jvi.74.16.7338-7348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard M, Brandt K, Paus SB, Tough DF. IL-15 Promotes the Survival of Naive and Memory Phenotype CD8+ T Cells. J Immunol. 2003;170:5018–5026. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- Bezman NA, Kim CC, Sun JC, Min-Oo G, Hendricks DW, Kamimura Y, Best JA, Goldrath AW, Lanier LL Immunological Genome Project Consortium. Molecular definition of the identity and activation of natural killer cells. Nat Immunol. 2012;13:1000–1009. doi: 10.1038/ni.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate Expression and Trans Presentation of Interleukin (IL)-15Rα and IL-15 Supports Natural Killer Cell and Memory CD8+ T Cell Homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, Bienvenu J, Henry T, Debien E, Hasan UA, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med. 2014;211:563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokun AO, Kim S, Smith HRC, Kang HSP, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- Elpek KG, Rubinstein MP, Bellemare-Pelletier A, Goldrath AW, Turley SJ. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Rα complexes. Proc Natl Acad Sci. 2010;107:21647–21652. doi: 10.1073/pnas.1012128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodil-Cornu N, Lee SH, Belanger S, Makrigiannis AP, Biron CA, Buller RM, Vidal SM. Ly49h-deficient C57BL/6 mice: a new mouse cytomegalovirus-susceptible model remains resistant to unrelated pathogens controlled by the NK gene complex. J Immunol. 2008;181:6394–6405. doi: 10.4049/jimmunol.181.9.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel LA, Sun MM, Geurs TL, Carayannopoulos LN, French AR. Markers of nonselective and specific NK cell activation. J Immunol. 2013;190:6269–6276. doi: 10.4049/jimmunol.1202533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AR, Pingel JT, Wagner M, Bubic I, Yang L, Kim S, Koszinowski U, Jonjic S, Yokoyama WM. Escape of Mutant Double-Stranded DNA Virus from Innate Immune Control. Immunity. 2004;20:747–756. doi: 10.1016/j.immuni.2004.05.006. [DOI] [PubMed] [Google Scholar]
- French JD, Roark CL, Born WK, O’Brien RL. γδ T cell homeostasis is established in competition with αβ T cells and NK cells. Proc Natl Acad Sci. 2005;102:14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37:171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Gasteiger G, Hemmers S, Firth MA, Floc’h AL, Huse M, Sun JC, Rudensky AY. IL-2–dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J Exp Med. 2013;210:1167–1178. doi: 10.1084/jem.20122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard GO, Bivas-Benita M, Hovav AH, Grandpre LE, Panas MW, Seaman MS, Haynes BF, Letvin NL. Thy1+ NK Cells from Vaccinia Virus-Primed Mice Confer Protection against Vaccinia Virus Challenge in the Absence of Adaptive Lymphocytes. PLoS Pathog. 2011;7:e1002141. doi: 10.1371/journal.ppat.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründemann C, Schwartzkopff S, Koschella M, Schweier O, Peters C, Voehringer D, Pircher H. The NK receptor KLRG1 is dispensable for virus-induced NK and CD8+ T-cell differentiation and function in vivo. Eur J Immunol. 2010;40:1303–1314. doi: 10.1002/eji.200939771. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y, Smyth MJ. CD27 Dissects Mature NK Cells into Two Subsets with Distinct Responsiveness and Migratory Capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli-Esposti MA, Smyth MJ, Tarlinton DM, Nutt SL. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol. 2007;178:4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Wang X, Zeng B, Liu L, Tardivel A, Wei H, Han J, MacDonald HR, Tschopp J, Tian Z, et al. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. J Exp Med. 2013;210:2465–2476. doi: 10.1084/jem.20122490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura Y, Lanier LL. Rapid and sequential quantitation of salivary gland-associated mouse cytomegalovirus in oral lavage. J Virol Methods. 2014;205C:53–56. doi: 10.1016/j.jviromet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karo JM, Schatz DG, Sun JC. The RAG Recombinase Dictates Functional Heterogeneity and Cellular Fitness in Natural Killer Cells. Cell. 2014;159:94–107. doi: 10.1016/j.cell.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-Dependent Regulation of Innate and Adaptive Immunity in the Intestinal Tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura T, Kanaya M, Shibuya A, Fu G, Gascoigne NRJ, Lanier LL. Costimulatory Molecule DNAM-1 Is Essential for Optimal Differentiation of Memory Natural Killer Cells during Mouse Cytomegalovirus Infection. Immunity. 2014;40:225–234. doi: 10.1016/j.immuni.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell–and B cell–independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- Orr MT, Sun JC, Hesslein DGT, Arase H, Phillips JH, Takai T, Lanier LL. Ly49H signaling through DAP10 is essential for optimal natural killer cell responses to mouse cytomegalovirus infection. J Exp Med. 2009;206:807–817. doi: 10.1084/jem.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, et al. Critical role for CXCR6 in NK cell-mediated antigen-specific memory to haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, Sun R, Yokoyama WM, Tian Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilbeam K, Basse P, Brossay L, Vujanovic N, Gerstein R, Vallejo AN, Borghesi L. The Ontogeny and Fate of NK Cells Marked by Permanent DNA Rearrangements. J Immunol. 2008;180:1432–1441. doi: 10.4049/jimmunol.180.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SH, Nguyen KB, Takahashi N, Mikayama T, Biron CA, Brossay L. Cutting Edge: Inhibitory Functions of the Killer Cell Lectin-Like Receptor G1 Molecule During the Activation of Mouse NK Cells. J Immunol. 2002;168:2585–2589. doi: 10.4049/jimmunol.168.6.2585. [DOI] [PubMed] [Google Scholar]
- Robbins SH, Tessmer MS, Mikayama T, Brossay L. Expansion and Contraction of the NK Cell Compartment in Response to Murine Cytomegalovirus Infection. J Immunol. 2004;173:259–266. doi: 10.4049/jimmunol.173.1.259. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting Edge: Transpresentation of IL-15 by Bone Marrow-Derived Cells Necessitates Expression of IL-15 and IL-15Rα by the Same Cells. J Immunol. 2004;173:6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HRC, Chuang HH, Wang LL, Salcedo M, Heusel JW, Yokoyama WM. Nonstochastic Coexpression of Activation Receptors on Murine Natural Killer Cells. J Exp Med. 2000;191:1341–1354. doi: 10.1084/jem.191.8.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HRC, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife. 2014;3:e01659. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009a;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Ma A, Lanier LL. Cutting Edge: IL-15-independent NK cell response to mouse cytomegalovirus infection. J Immunol. 2009b;183:2911–2914. doi: 10.4049/jimmunol.0901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Immune memory redefined: characterizing the longevity of natural killer cells. Immunol Rev. 2010;236:83–94. doi: 10.1111/j.1600-065X.2010.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med. 2011;208:357–368. doi: 10.1084/jem.20100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- Washizu J, Nishimura H, Nakamura N, Nimura Y, Yoshikai Y. The NF-κB binding site is essential for transcriptional activation of the IL-15 gene. Immunogenetics. 1998;48:1–7. doi: 10.1007/s002510050393. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. Role of Adaptor TRIF in the MyD88-Independent Toll-Like Receptor Signaling Pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Watanabe N, Sato N, Perera PY, Filkoski L, Tanaka T, Miyasaka M, Waldmann TA, Hiroi T, Perera LP. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc Natl Acad Sci. 2009;106:15849–15854. doi: 10.1073/pnas.0908834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawislak CL, Beaulieu AM, Loeb GB, Karo J, Canner D, Bezman NA, Lanier LL, Rudensky AY, Sun JC. Stage-specific regulation of natural killer cell homeostasis and response against viral infection by microRNA-155. Proc Natl Acad Sci. 2013;110:6967–6972. doi: 10.1073/pnas.1304410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Equivalent effector function between KLRG1int+ and KLRG1− NK cells, related to Figure 1.
(A) CD27 and CD11b expression by splenic naïve KLRG1int+ and KLRG1− Ly49H+ NK cells. (B) Degranulation by splenic naïve KLRG1int+ (upper panel) and KLRG1− (lower panel) Ly49H+ NK cells stimulated ex vivo with RMA-m157 target cells. (C) IFN-γ production by splenic KLRG1int+ (upper panel) and KLRG1− (lower panel) Ly49H+ NK cells at day 1.5 after MCMV infection. Data are representative of two independent experiments.
Figure S2. Differential frequency of KLRG1int+ NK cells in WT B6 mice from different animal vendors, related to Figure 2.
Expression of KLRG1 on TCRβ− NK1.1+ gated NK cells in peripheral blood (left panel) and spleen (right panel) of C57BL/6 mice purchased from the Jackson Laboratory (Jax), the NCI Mouse Repository, and Taconic (Tac) and C57BL/6 mice bred in our animal facility at UCSF. All mice were 8 week-old females. Data are representative of two independent experiments with 3 mice from each strain. Error bars show S.E.M.
Figure S3. Increased baseline proliferation of NK cells in T cell-deficient mice, related to Figure 3.
Expression of Ki67 in splenic NK cells was detected by intracellular staining. A representative histogram plot (left panel; shaded: WT, bold: Rag1−/−, dotted: Tcra−/−) and graph summarizing results from individual mice (right panel) are shown. Data are representative of two independent experiments with 4 mice from each strain. Error bars show S.E.M.
Figure S4. Severely decreased frequency of KLRG1int+ NK cells in Il15−/− mice, related ot Figure 4.
Expression of KLRG1 and Ly49H by splenic NK cells isolated from WT and Il15−/− mice. Percentage of TCRβ−NK1.1+, KLRG1+, or Ly49H+ cells is shown in each panel. Data are representative of two independent experiments (n=4–6).
Figure S5. Effect of antibiotics on the survival of NK cell-depleted Rag1−/− mice, related to Figure 6.
Rag1−/− mice were treated with control or Abx-treated water for three weeks before MCMV infection (day 0) and treatment was continued throughout the experiment. Anti-NK1.1 antibody (200 μg/mouse) was injected i.p. one day before infection (day -1). Data are compiled from two independent experiments (control: n=10, Abx: n=12, control+anti-NK1.1: n=15, Abx + anti-NK1.1: n=17).