Abstract
HIV+ persons with co-occurring bipolar disorder (HIV+/BD+) have elevated rates of medication nonadherence. We conducted a 30-day randomized controlled trial of a two-way, text messaging system, iTAB (n = 25), compared to an active comparison (CTRL) (n = 25) to improve antiretroviral (ARV) and psychotropic (PSY) adherence and dose timing. Both groups received medication adherence psychoeducation and daily texts assessing mood. The iTAB group additionally received personalized medication reminder texts. Participants responded to over 90 % of the mood and adherence text messages. Mean adherence, as assessed via electronic monitoring caps, was high and comparable between groups for both ARV (iTAB 86.2 % vs. CTRL 84.8 %; p = 0.95, Cliff’s d = 0.01) and PSY (iTAB 78.9 % vs. CTRL 77.3 %; p = 0.43, Cliff’s d = −0.13) medications. However, iTAB participants took ARVs significantly closer to their intended dosing time than CTRL participants (iTAB: 27.8 vs. CTRL: 77.0 min from target time; p = 0.02, Cliff’s d = 0.37). There was no group difference on PSY dose timing. Text messaging interventions may represent a low-burden approach to improving timeliness of medication-taking behaviors among difficult-to-treat populations. The benefits of improved dose timing for long-term medication adherence require additional investigation.
Keywords: Medication adherence, HIV/AIDS, Bipolar disorder, mHealth, Behavior modification, Randomized controlled trial, Intervention research
Introduction
Adherence to combination antiretroviral therapy (ART) is critical for successful HIV viral suppression [1]. Nonadherence to ART poses several potentially serious health consequences, including higher HIV viral loads, faster progression to AIDS, and a heightened risk for HIV treatment resistance [2]. Although less than perfect ART adherence can still help to treat HIV [3], the best health outcomes are associated with optimal adherence levels [4]. In various ART adherence-enhancing interventions, adherence is frequently defined as the percentage of prescribed doses taken during a given period (i.e., percent adherence) [5]; however, another aspect of adherence is whether a prescribed dose is taken on time (i.e., dose-timing adherence) [6]. Dose-timing adherence is important to consider given varying half-lives of ART agents [7] and data suggesting that sustained treatment interruptions better predict viral rebound than interspersed missed doses [8]. Additionally, smaller delays in dose timing were associated with long-term suppression of viral load in a 48-month longitudinal study of HIV+ persons initiating ART [6].
Multiple factors are associated with ART nonadherence, including demographics (e.g., younger age), psychiatric comorbidities (e.g., bipolar disorder; BD), psychosocial factors (e.g., social support), and active substance use disorders [9-13]. The prevalence of serious mental illness (SMI), including BD, is elevated among HIV-infected populations [12, 14, 15] and is associated with poor ART adherence [16]. A recent systematic review underscored the need to evaluate the impact of SMI conditions and their treatment on HIV outcomes (e.g., adherence and virologic control) [16]. To that end, HIV-infected individuals with co-occurring BD (HIV+/BD+), when compared to demographically similar HIV+/BD− controls, demonstrated poorer ART and psychotropic (PSY) medication adherence and were twice as likely to be non-adherent to their ART regimen [11]. Moreover, patterns of non-adherence to PSY medications are found to correlate with patterns of ART non-adherence in individuals with psychiatric and substance use disorders [17].
Poor PSY medication adherence is also common among people with SMI; it has been estimated that 40 % of those with BD do not take their mood stabilizer as prescribed, and one third take less than 30 % of their medication [18]. Among persons with BD, nonadherence to PSY medications can lead to greater risk for manic and depressive episodes, decreased quality of life, suicide attempts, and hospitalization [19, 20]. This suggests HIV+/BD+ individuals are particularly at-risk for PSY non-adherence, and there is a critical need to develop interventions to improve adherence in this population.
Several interventional approaches have been taken to improve medication adherence among HIV-infected individuals including peer support, directly administered ART, and interventions with cognitive-behavioral components (e.g., adherence education and problem-solving) [21, 22]. These interventions, although promising, suffer from limited reach in the community. Less clinician-intensive is the utilization of mobile health (i.e., mHealth) technologies. mHealth interventions capitalize on technology already incorporated into most people’s daily lives (e.g., cell phones) to assist people with behavior modification and disease self-management [23]. Text messaging, in particular, may support daily ART adherence by delivering reminders at precise times to match an individuals’ dosing schedule [24, 25].
The initial evidence for using text messaging to improve ART medication adherence has been compelling [26]. Specifically, randomized trials (RCT) in Kenya have shown that simple, once-weekly text messages improves ART adherence and was associated with viral load suppression within a HIV-infected population [27, 28]. A recent U.S.-based study also showed that daily text messages significantly increased self-reported adherence to ART in a young HIV-infected population (aged 14–29 years) over a 24-week period [29]. Though preliminary, these studies support the feasibility and efficacy of text message interventions to promote adherence in HIV and warrant further exploration of the effectiveness of various intervention components (e.g., frequency of messages, interactive communication, tailored message content, and delivery of messages to match dosage time) [25].
Researchers and clinicians have also started employing technology-based approaches to improve treatment for individuals with BD. High compliance rates among BD persons using a home computer software system to report mood, medication, sleep, life events, and menstrual data demonstrated the potential use of technology-based methods to provide on-going feedback [30]. More recently, text messages have been used to track weekly changes in mood symptoms (i.e., ratings of mania and depression) among persons with BD [31]. The feasibility of mHealth interventions to improve adherence among BD individuals are just now emerging [32], and studies show that BD individuals are receptive to use of technological devices for adherence intervention [33].
Taken together, a distinct need for RCTs utilizing text messaging to improve medication adherence within a difficult-to-treat HIV population is warranted. Individualized Texting for Adherence Building (iTAB), based in the theory of planned behavior (TPB), was therefore developed as an automated, personalized, two-way, text messaging system designed to send text messages directly to participant’s mobile phones. We hypothesized that HIV+/BD+ individuals assigned to the iTAB condition, which included psychoeducation, text medication reminders and texts inquiring about current mood, would show better adherence rates and better medication dose timing for both antiretroviral (ARV) and PSY medications as compared to matched HIV+/BD+ individuals assigned to the active control condition in which participants received psychoeducation and mood inquiries, but did not receive medication reminders. We also aimed to determine whether mood was related to medication adherence among HIV+/BD+ persons, and postulated that greater affective distress, either depressive or manic, would correlate with poorer adherence.
Methods
Participants
HIV+/BD+ participants were recruited from ongoing studies at the UCSD HIV Neurobehavioral Research Program (HNRP). Inclusion criteria were the capacity to provide informed consent, age 18 years or older at enrollment, documented HIV infection, diagnosis of BD I or II via the Composite International Diagnostic Interview (CIDI) (described below), and currently taking at least one ARV and one PSY medication to treat HIV and BD, respectively. Medication nonadherence was not a study entry requirement. Participants had to be willing to respond to text messages and utilize electronic medication tracking devices [i.e., medication event system monitoring, (MEMS)] for the identified medications over the 30-day study period. Participants were excluded from the study if they met diagnostic criteria for psychotic disorders (e.g., schizophrenia), had a neurological condition known to impact cognitive functioning (e.g., stroke, seizure disorder, closed head injury with loss of consciousness greater than 30 min), or had a mood disorder due to a General Medical Condition with manic features (i.e., HIV-induced mood disorder). Participants were recruited from a parent study that excluded for a current substance abuse or dependence diagnosis.
The UCSD Human Research Protection Program approved the current study. After meeting study inclusion/exclusion requirements, interested participants provided written informed consent to participate. Participants received monetary compensation for both the initial and follow-up assessments. Participants were encouraged to use their own cell phones and were reimbursed for any additional costs incurred by participating in the study over their regular cell phone use. A mobile phone with a comprehensive texting plan was loaned to those participants that did not own a cell phone or were unable to receive text messages on their current phone (n = 6 control and n = 7 iTAB participants received a loan phone).
Medication Adherence Intervention
Randomized Controlled Trial
After enrollment, participants were randomized into two groups; 30 were assigned to psychoeducation plus daily text message medication reminders and mood assessments for 30 days (iTAB) while 28 were assigned to an active control with a standard of care adherence psychoeducation and daily text mood inquiries (CTRL). Figure 1 provides a study CONSORT diagram detailing study enrollment and retention [34]. As planned before study initiation, we continued enrolling participants until 25 participants completed both baseline and follow-up data in both study arms. The demographic and clinical characteristics of the 50 study participants who completed follow-up are provided in Table 1.
Fig. 1.
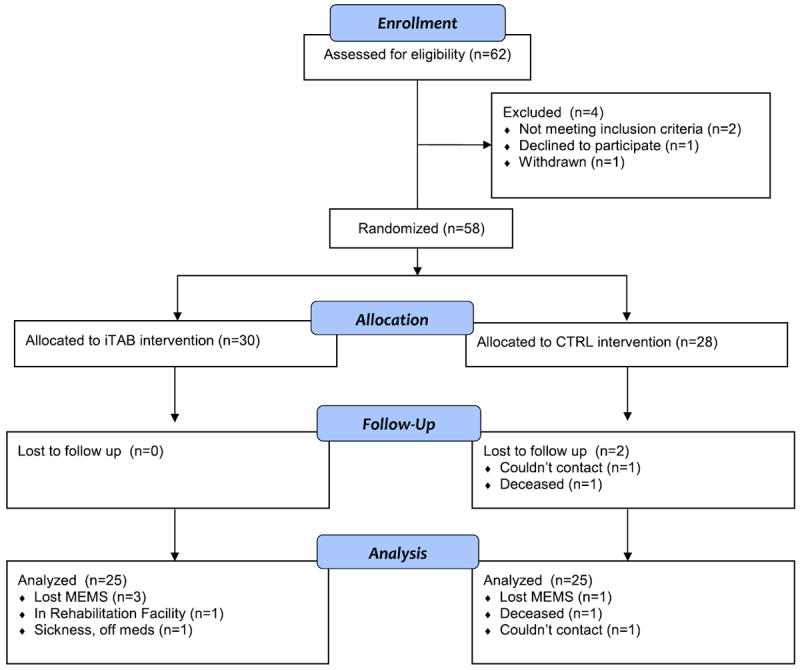
Consort diagram showing study enrollment.
Table 1.
Baseline participant characteristics by intervention arm
| iTAB (n = 25) | CTRL (n = 25) | p value | |
|---|---|---|---|
| Descriptive | |||
| Age; mean (SD) | 48.4 (9.2) | 45.9 (10.2) | 0.36 |
| Education; mean (SD) | 13.3 (2.2) | 13.0 (3.1) | 0.67 |
| Male; % (#) | 92.0 % [23] | 84.0 % [21] | 0.38 |
| Caucasian; % (#) | 64.0 % [16] | 44.0 % [11] | 0.16 |
| HCV infected; % (#) | 24.0 % [6] | 40.0 % [10] | 0.22 |
| Lifetime substance abuse/dependence; % (#) | |||
| Alcohol | 48.0 % [12] | 83.3 % [20] | <.01 |
| Marijuana | 32.0 % [8] | 37.5 % [9] | 0.69 |
| Cocaine | 40.0 % [10] | 45.8 % [11] | 0.68 |
| Opioid | 12.0 % [3] | 8.3 % [2] | 0.67 |
| Methamphetamine | 48.0 % [12] | 58.3 % [14] | 0.47 |
| HIV disease characteristics | |||
| CD4 count; median (IQR) (n = 40) | 463.5 (273.3, 912.0) | 613.5 (346.3, 959.8) | 0.47 |
| Nadir CD4 count; median (IQR) (n = 38) | 212 (94, 450) | 188 (98, 330) | 0.79 |
| HIV RNA plasma; median (IQR)a | 1.6 (1.6, 1.7) | 1.6 (1.6, 1.6) | 0.51 |
| Proportion undetectable % (#) b | 75.0 % [18] | 95.8 % [23] | 0.10 |
| AIDS % (#) | 66.7 % [14] | 60.9 % [14] | 0.69 |
| Psychiatric | |||
| Number non-ARV Medications; mean (SD) | 7.2 (4.7) | 6.4 (4.5) | 0.54 |
| YMRS; mean (SD) | 6.4 (7.6) | 5.2 (4.5) | 0.49 |
| BDI-II Total; mean (SD) | 17.0 (12.6) | 18.0 (10.6) | 0.77 |
| GAF; mean (SD) | 68.0 (10.0) | 67.3 (10.9) | 0.82 |
ART antiretroviral Therapy, BDI-II beck depression inventory-II, HCV hepatitis C virus, YMRS young mania rating scale, BDI-II beck depression inventory II, GAF global assessment of functioning, Nadir CD4 count is calculated as the lowest of self reported or laboratory generated value. IQR interquartile range
In log copies/ml
<50 cp/ml
Both the iTAB and CTRL groups received psychoeducation on the importance of medication adherence, which was delivered face-to-face by the study examiner (AP) using a standard script and a powerpoint presentation. The adherence education information was drawn from multiple components of previously successful medication interventions [35, 36]. The adherence education included information on: the importance of attention to medication maintenance; health benefits of adherence to PSY and ART medications; strategies for coping with BD, HIV symptoms, and adverse medication effects; and practical medication adherence strategies. The psychoeducation lasted approximately 30 min and participants were provided opportunities to ask questions and speak about their own experiences. To prevent bias, the examiner was blinded to the participant’s group assignment (i.e., iTAB or CTRL) at the time the psychoeducation intervention was administered.
In order to provide equivalent interaction time with the examiner and to provide the opportunity to use information gathered from the adherence psychoeducation, both groups were provided with a list of potential adherence reminders (see Table 2 for examples of reminders). Among participants assigned to the iTAB condition, the selected personalized reminders were delivered via text message (described below). Reminders were written down for the participants assigned to the CTRL condition and participants were informed that they could use these messages as they desired.
Table 2.
Sample text message reminder stems
| Theme | Stem |
|---|---|
| Celebrate health | Stay healthy! It’s time 2 take ur meds, pls take ur [med] now. |
| Time and focus | It’s pill time! Take ur [med] now. |
| Control disease | Taking ur meds helps control ur disease. Rmber 2 take ur [med] now. |
| Empowering | It’s med time, only u can control this. Rmber 2 take ur [med] now. |
| Importance of adherence | Adherence is impt. Pls take ur… |
Personalization of iTAB Reminders
The iTAB system broadly draws from the TPB, which posits that behavior is driven by behavioral intentions and that individual motivational factors interact with cognitive impairment, mood disruption, and substance use to create both intentional and unintentional nonadherence [37]. Sample TPB reminder messages were provided to pilot HIV + persons who provided feedback during the initial development and refinement phase of the iTAB intervention; although these messages provided a foundation for the general theme of the iTAB texts, they were ultimately streamlined to increase participant comprehension. Individualized text message reminders and reinforcement messages were identified for the iTAB group that were built around this theoretical model. The participant and examiner identified an appropriate time for the ARV and PSY reminders (once daily or twice daily, depending on the regimen instructions), and an appropriate description of the medications for use in the message (e.g., “the round, white pill”), to protect against potentially stigmatizing medication names appearing in the content of the text message. Participants chose their preferred name for use in the text message, and selected five modifiable reminder stems from a list of 17 predetermined reminders, which were originally derived from focus groups conducted with the target population (see examples in Table 2). Participants were also encouraged to write their own adherence reminders with the assistance of the study coordinator. Similarly, participants chose five reinforcement stems from a list of 15 options (e.g., “Nice work, [Name],” “Good job, every dose helps!” and “U R awesome! Keep up the good work!”). As with the reminder messages, participants could modify existing reinforcement stems or create their own within the character limits. For example, a reminder message might read, “John, your meds r important. It is time to take ur meds. Take ur big blue pill now. Pls reply (A) took (D) didn’t (G) snooze.” A reinforcement message might read, “Great job! Ur current adherence: 75 %. Adhr when u take ur next dose: 80 % (4/5 doses)” (see Fig 2 for text-messaging decision tree). Separate messages were sent for each medication (ARV and PSY) at each dosing time and were automated by a central server.
Fig. 2.
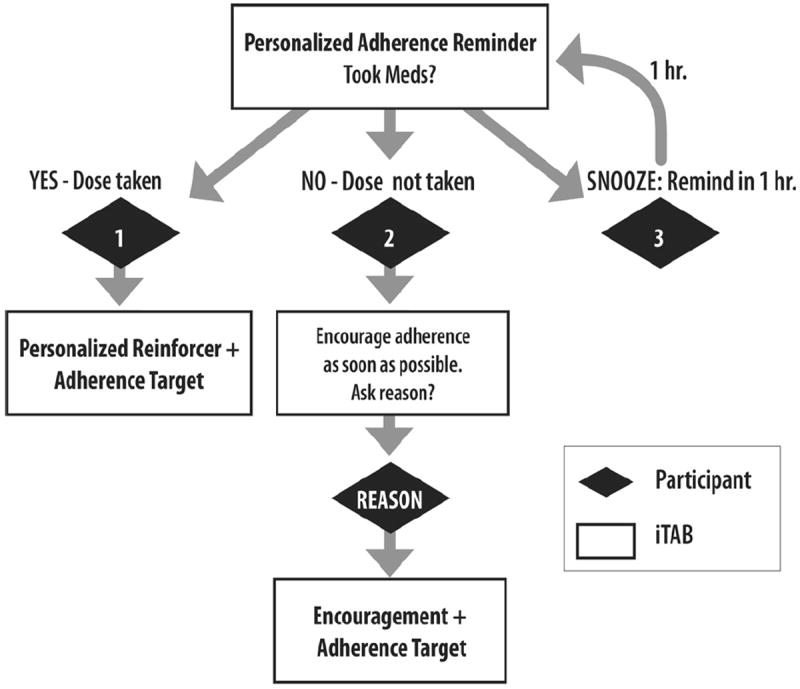
iTAB reminder system decision tree
The text messaging system had additional automated features designed to optimize adherence. These features included the option to “snooze” the adherence reminder (i.e., the reminder was re-sent 1 hour later). The iTAB system also had protections against multiple or inaccurate responses, wherein the participant would receive a message saying that a given text was not understood. If participants indicated they did not take their medication, they were sent an additional message to identify the reason for the missed dose. After three consecutive days of missed messages, the automated system sent out an “adherence alert” message to the participant, and an alert was sent to the study coordinator. The study coordinator called the participant after 5 days of failure to respond to text messages and each subsequent 5 days until the participant began responding to the system or the study period closed. The study coordinator had real-time access to participant response logs to identify problems with responding and to be able to contact participants who were having difficulties using the system.
As a rigorous control for the novelty of receiving text messages, both groups were sent a text message at noon every day, and asked to evaluate their mood (i.e., manic, depressed, mixed state, or neither). A participant’s overall mood endorsement during the study period was calculated for each of the mood states (i.e., depressed, manic, or “both”) as a percentage and used in analyses (e.g., depressed mood during study period calculation: [(# days endorsed “depressed”)/(# days monitored)]*100 %).
Additional Assessments
Psychiatric and Substance Use Assessment
Psychiatric diagnoses were assigned using the lay administered Composite International Diagnostic Interview (CIDI version 2.1, WHO, 1998); ambiguous diagnoses were resolved via case discussion with a diagnostic expert (JHA). Current mood was assessed via the CIDI and classified as euthymic, hypomanic, manic, or depressive episode, or one with mixed (i.e., concurrent manic and depressive symptoms) features. Severity of current mood symptomatology was assessed via the Young Mania Rating Scale (YMRS) [38], and the Beck Depression Inventory-II (BDI-II) [39]. Scores of less than 14 on each of these measures are considered to be of “minimal severity” [38, 39].
Medication Adherence Assessments
Objective Medication Adherence
Medication Event Monitoring System (MEMS, AARDEX, Sion, Switzerland) TrackCaps, which provide an electronic record of the date and time the cap is removed, were used to track medication adherence to both ARV and PSY medications over the study period. Separate MEMS caps were used for each medication. Medications were selected using “sentinel” strategy criteria that has been previously detailed [11].
Overall MEMS Adherence Calculation
One of the primary outcome variables for analysis was a MEMS-derived ARV and PSY percent adherence over the study period (i.e., ([# of bottle openings)/(# of prescribed doses)]*100 %). Adherence reports were adjusted by a verbal review with participants at the follow-up visit to correct for any openings that were done when medication was not taken (e.g., if the participant opened the bottle to refill his/her prescription).
MEMS Dose Timing Windows
The second primary outcome variable was participants’ “dose timing window.” This was calculated by subtracting the time at which the MEMS cap was opened (i.e., “dose taken”) from the previously indicated targeted time for dosing (i.e., the time at which participants received adherence text messages or indicated that they would take his/her medication for the CTRL group). ARV and PSY dose timing windows were used in analyses to indicate the discrepancy between intended dosing time and actual dosing time (in minutes) such that higher values indicate more variable dosing (i.e., decreased therapeutic coverage). At the group level, the median and interquartile range as well as the mean and standard deviation, of a participant’s dose timing are reported.
Self-Reported Adherence
In addition to MEMS data for the 30-day study period, participants used a visual analogue scale (VAS) scale [40] to report adherence to both ARV and PSY medications independently at the baseline visit (representing 30 days prior to the study start) and the follow-up visit (covering the 30-days while on intervention). Participants were asked mark a line anchored from 0 to 100 representing their adherence to the tracked medications. Change in VAS scores between the two study visits and the proportion of individuals reporting less than 100 % adherence at baseline are reported here.
Neuromedical Evaluation
Each participant completed a standardized medical history interview, structured neurological and medical examination, as well as collection of blood and urine samples consistent with previous studies at the HNRP [41]. Trained research staff conducted all medical history interviews, and a clinician (i.e., RN, NP or MD) performed the neuromedical examination.
Data Analyses
Due to the lack of conformity to assumptions of parametric methods (Shapiro–Wilk p < 0.05 and Levene’s test p < 0.05), we conducted Wilcoxon rank sum (Mann–Whitney) tests in order to examine group (i.e., iTAB vs. CTRL) differences on overall adherence and dose timing windows for both ARV and PSY medications. Next, we conducted Spearman’s rho correlations in order to explore the relationships between dose timing windows on overall adherence for ARVs and PSYs, respectively. Additionally, we examined the impact of mood symptomology (i.e., self-reported symptoms on questionnaires and mood state endorsed via text messaging) on our ARV and PSY adherence outcomes in each group via Spearman’s rho correlations. Last, the impact of HIV disease variables on our adherence outcomes was examined in each group using Mann–Whitney tests and Spearman’s rho correlations, as appropriate. Spearman’s rho values quantify the strength of relationships (i.e., effect sizes) for all continuous variable analyses (range: −1 to +1), while Cliff’s d was calculated to estimate the effect size for between-group comparisons (range: −1 to +1; [42]). With regard to the latter, Cliff’s d represents the size of an effect between two groups with non-parametric data. “Small,” “medium,” and “large” Cliff’s d effect sizes are defined as 0.147, 0.330, and 0.474, respectively [43, 44]. It estimates the probability of scores in a given group being larger than scores in another group (i.e., dominance), and is particularly robust to violations of normality and heterogeneity of variance, and thus was appropriate for these adherence outcomes.
Results
Within the iTAB group, participants were sent a median of 30 ARV and 31 PSY adherence text messages across the 30-day study period; participants responded to the messages 92.3 % (ART) and 96.7 % (PSY; medians) of the time, respectively. Among the mood messages sent out to participants in both the iTAB and CTRL groups (median 30 messages per participant in both group), participants responded 90.0 and 87.0 % (medians) of the time, respectively.
Within the iTAB group, the most common response to ARV and PSY medication reminders was ‘Took’ at 82.0 and 83.9 %, respectively. The second most common response to ARV and PSY medication reminders was non-response at 15.2 and 12.2 %, respectively. ‘Snooze’ and ‘Didn’t Take’ responses were uncommon for both medications, occurring for only 1.6 and 1.0 % of ARV reminders and 0.6 and 3.3 % of PSY reminders, respectively. Among those who responded ‘Didn’t Take’, reasons provided for non-adherence to ARVs were evenly split among ‘Ran out’, ‘Medication not with me’, and no response. Among those who responded they ‘Didn’t Take’ their PSY medication, reasons provided for non-adherence were ‘Ran out’ (46.7 %), ‘Medication not with me’ (26.7 %), no response (20.0 %), and ‘Other’ (6.7 %).
With regard to adherence, the iTAB and CTRL groups both showed high levels of overall 30-day MEMS adherence, but did not significantly differ on for ARV (Means: iTAB 86.2 % (SD = 12.7) vs. CTRL 84.8 % (SD = 18.1); Medians: iTAB 90.3 % [IQR: 75.8, 96.5] vs. CTRL 90.0 % [IQR: 77.6, 93.3]; p = 0.95, Cliff’s d = 0.01) or PSY (Means: iTAB 78.9 % (SD = 22.2) vs. CTRL 77.3 % (SD = 30.2); Medians: iTAB 83.9 % [IQR: 70.2, 95.1] vs. CTRL 90.0 % [IQR: 74.5, 96.7]; p = 0.43, Cliff’s d = -0.13) medications. Importantly though, iTAB individuals were more likely to take their ARV closer to their designated dosing time (i.e., time they intended to take dose) than the CTRL group (Means: iTAB 65.7 (SD = 76.5) vs. CTRL 120.8 (SD = 145.0); Medians: iTAB 27.8 [IQR: 15.0, 105.5] vs. CTRL 77.0 [IQR: 36.3, 137.8] minutes from dosing target time; p = 0.02, Cliff’s d = 0.37; Fig 3). There was no difference between the iTAB and CTRL groups on PSY dose timing windows [Means: iTAB 130.7 (SD = 213.8) vs. CTRL 122.9 (160.0); Medians: iTAB: 46.8 (IQR: 18.7, 121.3) vs. CTRL: 66.5 (IQR: 24.0, 135.0) minutes from dosing target time; p = 0.42, Cliff’s d = 0.14; Fig 3]. In the whole sample, greater overall ARV adherence was associated with smaller dose timing windows (i.e., ARV taken closer to the designated dosing time; ρ = −0.36, p = 0.01; however, overall PSY adherence was not significantly associated with dose timing windows (ρ = −0.20, p = 0.19). Given that prevalence of lifetime alcohol use disorders significantly differed between the two groups, we explored the relationship between alcohol use disorder and adherence outcomes within each of the groups. We found that lifetime alcohol use disorders were not associated with ARV (p = 0.33) or PSY (p = 0.59) dose-timing, or MEMS ARV (p = 0.94) or PSY (p = 0.57) overall adherence. Therefore, it appears that alcohol use was not driving the group differences reported above.
Fig. 3.
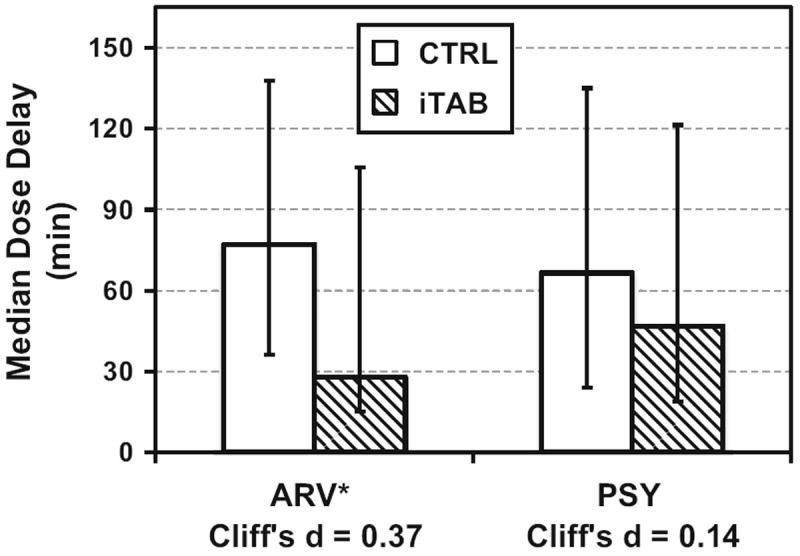
Dose timing windows are significantly smaller for antiretroviral medications among iTAB participants as compared to control *p < 0.05. ARV antiretroviral medications, CTRL participants assigned to the control arm, iTAB participants assigned to the individualized Texting for Adherence Building arm, PSY psychotropic medications
Among the participants assigned to the iTAB group, 60 % (15/25;) received three prompt messages per day (1 mood message, 1 medication prompt for ARV, and 1 medication prompt for PSY), 32 % (8/25) received four prompt messages per day (1 mood message, 1–2 ARV and 1–2 PSY messages depending on dosing), and 8 % (2/25) received five messages per day (1 mood, 1–2 ARV, 1–3 PSY). We examined mean MEMS adherence across these three groups and observed that receiving more reminders (i.e., more complicated medication regimens) was significantly associated with worse PSY adherence [χ2 = 6.52, p = 0.04; 3 reminders Mean MEMS = 87.3 % (S.D. = 11.1), 4 reminders = 73.9 % (S.D. = 19.1), 5 reminders = 35.9 % (S.D. = 50.7)], but not ARV adherence [χ2 = 4.99, p = 0.08; 3 reminders = 90.8 % (S.D. = 9.3), 4 reminders = 80.9 % (S.D. = 15.9), 5 reminders = 73.1 % (S.D. = 1.6)].
Self-Reported Adherence
Randomization procedure resulted in comparable baseline ARV and PSY adherence between the two groups as indicated by self-reported adherence data on the VAS [Mean VAS ARV: CTRL = 92.4 % (S.D. = 13.0) vs. iTAB = 95.8 % (S.D. = 6.6), p = 0.44; Mean VAS PSY CTRL = 85.3 % (S.D. = 23.2) vs. iTAB = 92.2 % (S.D. = 16.8), p = 0.29. Using any VAS score <100 % to indicate some adherence difficulty showed that 47.8 % of the cohort reported less than perfect ARV adherence; nonadherence rates by VAS were comparable across the two groups (CTRL = 54.5 % nonadherence vs. iTAB = 41.7 % nonadherence; p = 0.38). Findings were similar with the VAS PSY adherence data with 54.3 % of the overall cohort reporting less than perfect adherence (CTRL = 59.1 % vs. iTAB = 50.0 %; p = 0.54).
Consistent with the MEMS findings, there was little change in self-reported overall medication adherence rates by intervention group. At 30-day follow-up, the CTRL group reported 5.7 points less (i.e., worse adherence) on the VAS ARV scale as compared to baseline whereas the iTAB group reported 1.1 points less. For PSY VAS scores, the CTRL group reported 2.2 points higher, whereas the iTAB group reported a mean of 1.4 points higher. None of these findings were statistically different from one another.
Mood and Adherence Outcomes
The study groups demonstrated comparable mood symptoms (i.e., depressive and manic) at both baseline (BDI-II p = 0.77; YMRS p = 0.49 and follow-up (BDI-II p = 0.57; YMRS p = 0.07), and, in general, our sample was dysthymic rather than manic as a cohort (i.e., BDI-II scores higher than YMRS scores). For analytic purposes, we utilized mood symptoms reported at the follow-up visit given that the BDI-II asks participants about the previous 2 weeks and portions of this evaluation would overlap with the MEMS days tracked. Among the iTAB individuals, current depressive (i.e., BDI-II) and manic (i.e., YMRS) symptoms were not associated with ARV or PSY adherence (ARV BDI-II p = 0.27; ARV YMRS p = 0.38; PSY BDI-II p = 0.06; PSY YMRS p = 0.92) or dose timing (ARV BDI-II p = 0.42; ARV YMRS p = 0.33; PSY BDI-II p = 0.65; PSY YMRS p = 0.50). However, within the CTRL group, more severe manic symptoms were associated with larger PSY dose timing windows (ρ = 0.42, p = 0.048), and greater depressive symptoms were associated with poorer overall PSY adherence (ρ = −0.44, p = 0.03). On the other hand, mood symptoms were not associated with ARV adherence indicators in the CTRL cohort (overall adherence BDI-II p = 0.14; dose timing BDI-II p = 0.07; overall adherence YMRS p = 0.31; dose timing YMRS p = 0.13).
We also examined the relationship between medication adherence and overall mood across the study period as reported via text messages [e.g., (# messages indicated depressed)/(total # mood messages)] within each of the study groups. Among the iTAB participants, a greater proportion of reported manic days was associated with larger ARV dose timing windows (ρ = 0.49, p = 0.02), while in the CTRL group, a larger proportion of days reported as depressed was associated with larger PSY dose timing periods (ρ = 0.45, p = 0.03).
HIV Disease and Adherence Outcomes
Finally, HIV disease indicators were not associated with ARV or PSY dose timing windows or overall adherence in the iTAB or CTRL groups (i.e., AIDS status, baseline CD4, nadir CD4, plasma viral load; ps > 0.05).
Discussion
Our study supports the feasibility of implementing a personalized adherence text messaging intervention among HIV+/BD+ individuals. Adherence to study visits and intervention responsiveness were extremely high; over 80 % of our participants were retained on study and participants demonstrated >90 % response rate to study text messages. Additionally, our findings show that ARV dose timing is significantly improved with our intervention as compared to control.
Smaller dose timing windows indicate that participants were taking their ARVs closer to their assigned medication time each day. Previous work among HIV+ persons has shown that smaller dose timing windows may lead to superior therapeutic coverage of ARVs, which is in turn linked to better HIV disease outcomes [45]. Importantly, we found that greater dose timing windows correlated with poorer overall adherence (i.e., increased likelihood of missing a dose). Increasing dose timing accuracy via text messaging may lead to overall improved ART adherence behaviors and better HIV disease indicators when examined over longer periods of time.
Interestingly, dose-timing windows were not significantly improved for PSY medication in the iTAB group as compared to controls suggesting that persons have differential adherence behaviors for different medications. This is consistent with our previous work showing that HIV+/BD+ persons demonstrate higher adherence rates for ARV than PSY medications [11]. It is possible that HIV+ persons view ARV medications as a “life or death” health behavior and discount (or are unaware of) the importance of PSY medications, consequently ignoring the potential cyclical relationship between PSY nonadherence, mood dysregulation and subsequent ARV nonadherence. Research among HIV-uninfected individuals with BD reveals several predictors of medication nonadherence including younger age, substance use and personality disorders, illness severity, PSY side effects, patient insight and poor therapeutic alliance [46]. Pertinent to our findings, persons with more complex medication regimens appeared to have the worst medication adherence. Worse adherence among those with the most complex medication regimens may be a result of medication burden, an indication of historical difficulty adhering to medications, or negative attitudes toward medications and adherence. It is possible that our system, which used more general medication reminders, may need to be revised to more directly target these and other predictors of PSY non-adherence.
Although our findings did not indicate a benefit of the personalized intervention for overall medication adherence, this lack of significance may be in part due to our rigorous control condition. More specifically, all participants in the study received text messages as well as medication adherence psychoeducation specific to their conditions (i.e., HIV infection and BD); we tested the added benefit of tailored medication reminder messages as a supplement to these other approaches. The high rates of self-reported adherence may have also contributed to our inability to find differences between the two groups, although we recognize the limitations of self-report measures of adherence. We believe that more studies need to effectively test components of interventions and use comparison groups that (1) receive the highest possible level of standard of care (e.g., adherence counseling), and (2) control for novelty and time equivalence (e.g., receiving text messages as part of an adherence study). This is an approach that moves away from designing studies simply to show statistical significance.
Perhaps as a result of the rigor of our control group, we found that participation in this daily text messaging adherence intervention in combination with psychoeducation was associated with high adherence rates among HIV+/BD+ individuals regardless of group assignment. That is, the combination of baseline psychoeducation and a text message asking about mood were also associated with high ARV adherence rates. Specifically, adherence rates from the present study for both ARV (~85 % regardless of intervention arm) and PSY (~78 % regardless of intervention arm) medications were superior to those found in an observational study among HIV+/BD+ using the same methodology to track adherence (i.e., MEMS caps) over the same time period of 30 days (i.e., mean ART = 75 % adherence; PSY = 67 %) [11]. The approximately 10 % higher mean adherence rates in both the intervention and control groups among individuals enrolled in this study as compared to the previous observational work may be related to the fact that both groups received: (1) psychoeducation about the importance of medication adherence at the beginning of the 30-day monitoring period, (2) the parallel process of generating reminders that were focused on the factors that would best motivate adherence, and/or (3) the daily text messages asking the participant to evaluate his or her mood that may have served as an indirect medication reminder. It is important to note, that while these rates reflect improved adherence while on study, they still represent suboptimal overall adherence in terms of optimal therapeutic coverage.
Even though adherence rates did not differ between the two groups on our self-report measure of ARV and PSY adherence (i.e., VAS), there is a slight indication that the iTAB group may have had worse baseline adherence despite the study randomization. Specifically, a detectable baseline plasma viral load was found among the 25 % of iTAB group as compared to 4 % of the CTRL group. Ideally, only participants with detectable viral loads would be included in the study. However, viral load detectability is dynamic and thus a baseline measurement may not sufficiently capture and classify those at significant risk for medication non-adherence, such as persons with BD.
Numerous studies have discussed the negative impact that mood (particularly depressed mood) can have on medication adherence [16]. In our iTAB intervention arm, we did not see specific associations between mood and medication adherence. On the other hand, all of our participants were engaged in mental health care, had been prescribed PSY medications for BD, and had mood scores that were generally below the clinically relevant range. This engagement in care alone, and subclinical psychiatric symptoms, decreases the likelihood for associations between mood and adherence. It is interesting, though, that the associations that we observed between mood variables and adherence were only apparent in the Control arm. In particular, manic symptoms were associated with larger dose timing windows. This could suggest that manic symptoms may be disruptive to specific aspects of planful daily functioning. Overall, electronically-monitored PSY adherence levels among those assigned to the Control arm appeared to be associated with depressive symptoms perhaps suggesting that the lower adherence levels relate to worse depressive symptoms and vice versa.
Our results are also consistent with other text messaging intervention studies among HIV-infected persons that have suggested that components of adherence can be improved using this method [26-28]. One possible next step is to determine which theoretical models best enhance the utility of this technology for adherence. In this regard, the TPB model may be a useful guide for development of mobile interventions with respect to health behavioral changes; however, the specific components of TPB need to be evaluated directly and many of these components were diluted in the present study based on initial pilot work with participant message preferences. As in our intervention, this approach supports the use of individualized messages, supporting pre-stated behavioral intentions. It allows for assessment of reasons for missed doses in real time, while the behavioral context is fresh. It is possible that this process allows individuals to examine the exact barriers to their own medication adherence while on study and to change their maladaptive medication-taking behaviors to avoid encountering these in the future. For example, a person that acknowledges that his or her substance use may have led to a missed dose may consider this prior to subsequent substance use behaviors. With this said, our experience suggests individuals rarely indicated that they did not take their medications via the texting system. It appears that participants were more likely to simply not reply to the question of adherence rather than acknowledge nonadherence. Therefore, novel approaches to capture information regarding both intentional and nonintentional missed doses are clearly warranted in order to inform points of adherence intervention, and could be easily implemented. This also allows for balanced interventions that require an equivalent number of text responses regardless of the initial response, to see if this improves the likelihood of all responses.
We believe that there may be specific advantages of implementing personalized text messaging for medication adherence support into the HIV clinical care. Although there was no clear benefit of the personalized messages for overall ARV or PSY adherence, the improvement in dose timing may have a beneficial effect for HIV disease outcomes. Moreover, there is little downside to implementing a text messaging system; patients are making increasing demands for personalized and technology-driven care; and decreased costs associated with better patient outcomes are likely to outweigh the costs of implementing an automated system as has been shown with other mHealth adherence interventions [47]. In agreement with recommendations from recent SMS adherence interventions [25], future SMS systems should be grounded in specific behavior change techniques (e.g., goals and planning) [48] and allow for personalized tailoring of message content and frequency [46]. Moreover, a recent study of text message preferences underscored the importance of message structure, linguistic content, and overall tone in the development of messages for goal-directed behaviors, e.g., avoiding “textese” [49].
There are several limitations of the current study that must be considered. Our study design does not allow us to tease apart the impact of the text messaging versus psychoeducation components for improving adherence. As has been previously suggested [21], the influence of the psychoeducational component likely deteriorates over time and requires additional resources and time to be re-administered in order to support its potential effect. On the other hand, we hypothesize that the text-messaging component can provide improved support over longer time periods with little to no additional burden for the provider or patient. Future studies that examine text messaging to improve adherence for longer periods of time may therefore be able to address the likely impact of texting over psychoeducation. Additionally, due to the nature of the MEMS technology, our adherence data is based on the number of openings and does not directly indicate whether the medication was ingested. Studies have shown that MEMS might underestimate adherence [50, 51] and may interfere with already established adherence routines such as pill organizers or blister packs. Both of these factors may have minimized the true impact of our intervention for adherence; however, MEMS technology is widely used in HIV research [52] as it has shown strong ecological validity with pill counts, self- and other-reports, and pharmacy refills [53]. Completer analysis was planned, so participants that did not complete the study were replaced to ensure that at least 25 participants were included in each study arm. The differences between groups in terms of dropout were minimal with the only difference being that two more persons in the iTAB group lost their MEMS caps as compared to the control condition. This slightly increased likelihood of losing MEMS caps may reflect greater engagement in the study (e.g., carrying MEMS with them to maintain adherence). Finally, the current study included a relatively small sample size of HIV+/BD+ individuals who were relatively healthy in terms of both HIV disease and BD symptoms, thereby potentially limiting the generalizability of our findings. Larger studies including individuals with a range of HIV disease and mental health symptomology severity are needed to determine the applicability of text messaging interventions to improve adherence in lower functioning individuals.
Conclusions
mHealth interventions are gaining traction and represent a promising new healthcare intervention direction; however, as was recently noted [9], the question of whether mHealth interventions will truly transform healthcare is still very much an open question. The present study suggests that daily contact via text messaging is feasible even in difficult populations, and that text messaging in conjuction with psychoeducation improves ARV does timing in a group of individuals who are at high-risk for nonadherence to important medications. Subsequent, larger-scale interventions with similarly rigorous control groups are necessary to determine the long-term efficacy of such interventions. A tiered, integrative approach, that uses mHealth as an initial intervention and transitions to a more intensive face-to-face, and costly, intervention may be effective in improving health behaviors such as medication adherence among HIV+/BD+ persons.
Acknowledgments
The present work was supported by California HIV/AIDS Research Program IDEA Award ID09-SD-047 (D.J. Moore, PI) as well as the HIV Neurobehavioral Research Center (HNRC) NIMH/CSPAR Award Number P30MH062512 (R.K. Heaton, PI).
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Appendix: HIV Neurobehavioral Research Program (HNRP) Group
The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S.
Contributor Information
David J. Moore, Email: djmoore@ucsd.edu, Department of Psychiatry, School of Medicine, University of California, San Diego, La Jolla, CA 92093, USA; HIV Neurobehavioral Research Program, 220 Dickinson St., Suite B, Mail Code 8231, San Diego, CA 92103-8231, USA.
Amelia Poquette, Department of Psychiatry, School of Medicine, University of California, San Diego, La Jolla, CA 92093, USA; HIV Neurobehavioral Research Program, 220 Dickinson St., Suite B, Mail Code 8231, San Diego, CA 92103-8231, USA.
Kaitlin B. Casaletto, HIV Neurobehavioral Research Program, 220 Dickinson St., Suite B, Mail Code 8231, San Diego, CA 92103-8231, USA SDSU/UCSD Joint Doctoral Program in Clinical Psychology, San Diego, CA 92120, USA.
Ben Gouaux, Department of Psychiatry, School of Medicine, University of California, San Diego, La Jolla, CA 92093, USA; HIV Neurobehavioral Research Program, 220 Dickinson St., Suite B, Mail Code 8231, San Diego, CA 92103-8231, USA.
Jessica L. Montoya, HIV Neurobehavioral Research Program, 220 Dickinson St., Suite B, Mail Code 8231, San Diego, CA 92103-8231, USA SDSU/UCSD Joint Doctoral Program in Clinical Psychology, San Diego, CA 92120, USA.
Carolina Posada, HIV Neurobehavioral Research Program, 220 Dickinson St., Suite B, Mail Code 8231, San Diego, CA 92103-8231, USA; SDSU/UCSD Joint Doctoral Program in Clinical Psychology, San Diego, CA 92120, USA.
Alexandra S. Rooney, Department of Psychiatry, School of Medicine, University of California, San Diego, La Jolla, CA 92093, USA HIV Neurobehavioral Research Program, 220 Dickinson St., Suite B, Mail Code 8231, San Diego, CA 92103-8231, USA.
Jayraan Badiee, Department of Psychiatry, School of Medicine, University of California, San Diego, La Jolla, CA 92093, USA; HIV Neurobehavioral Research Program, 220 Dickinson St., Suite B, Mail Code 8231, San Diego, CA 92103-8231, USA.
Reena Deutsch, Department of Psychiatry, School of Medicine, University of California, San Diego, La Jolla, CA 92093, USA; HIV Neurobehavioral Research Program, 220 Dickinson St., Suite B, Mail Code 8231, San Diego, CA 92103-8231, USA.
Scott L. Letendre, HIV Neurobehavioral Research Program, 220 Dickinson St., Suite B, Mail Code 8231, San Diego, CA 92103-8231, USA Department of Medicine, School of Medicine, University of California, San Diego, La Jolla, CA 92093, USA.
Colin A. Depp, Department of Psychiatry, School of Medicine, University of California, San Diego, La Jolla, CA 92093, USA
Igor Grant, Department of Psychiatry, School of Medicine, University of California, San Diego, La Jolla, CA 92093, USA; HIV Neurobehavioral Research Program, 220 Dickinson St., Suite B, Mail Code 8231, San Diego, CA 92103-8231, USA.
J. Hampton Atkinson, Department of Psychiatry, School of Medicine, University of California, San Diego, La Jolla, CA 92093, USA; HIV Neurobehavioral Research Program, 220 Dickinson St., Suite B, Mail Code 8231, San Diego, CA 92103-8231, USA; Psychiatry Service, VA San Diego Healthcare System, San Diego, CA 92161, USA.
References
- 1.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 2.Bangsberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. J Infect Dis. 2008;197(Suppl 3):S272–8. doi: 10.1086/533415. [DOI] [PubMed] [Google Scholar]
- 3.Bangsberg DR, Deeks SG. Is average adherence to HIV antiretroviral therapy enough? J Gen Intern Med. 2002;17(10):812–3. doi: 10.1046/j.1525-1497.2002.20812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002;34(8):1115–21. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 5.Mathes T, Pieper D, Antoine SL, Eikermann M. Adherence-enhancing interventions for highly active antiretroviral therapy in HIV-infected patients—a systematic review. HIV Med. 2013;14(10):583–95. doi: 10.1111/hiv.12051. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Miller LG, Hays RD, Golin CE, Wu T, Wenger NS, et al. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. J Acquir Immune Defic Syndr. 2006;41(3):315–22. doi: 10.1097/01.qai.0000197071.77482.6e. [DOI] [PubMed] [Google Scholar]
- 7.Bangsberg DR, Kroetz DL, Deeks SG. Adherence-resistance relationships to combination HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4(2):65–72. doi: 10.1007/s11904-007-0010-0. [DOI] [PubMed] [Google Scholar]
- 8.Parienti JJ, Das-Douglas M, Massari V, Guzman D, Deeks SG, Verdon R, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS ONE. 2008;3(7):e2783. doi: 10.1371/journal.pone.0002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghidei L, Simone MJ, Salow MJ, Zimmerman KM, Paquin AM, Skarf LM, et al. Aging, antiretrovirals, and adherence: a meta analysis of adherence among older HIV-infected individuals. Drugs Aging. 2013;30(10):809–19. doi: 10.1007/s40266-013-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, Heaton RK, et al. Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS Care. 2012;24(12):1504–13. doi: 10.1080/09540121.2012.672718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore DJ, Posada C, Parikh M, Arce M, Vaida F, Riggs PK, et al. HIV-infected individuals with co-occurring bipolar disorder evidence poor antiretroviral and psychiatric medication adherence. AIDS Behav. 2012;16(8):2257–66. doi: 10.1007/s10461-011-0072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiser SD, Wolfe WR, Bangsberg DR. The HIV epidemic among individuals with mental illness in the United States. Curr HIV/AIDS Rep. 2004;1(4):186–92. doi: 10.1007/s11904-004-0029-4. [DOI] [PubMed] [Google Scholar]
- 13.Woodward EN, Pantalone DW. The role of social support and negative affect in medication adherence for HIV-infected men who have sex with men. AIDS Care. 2012;23(5):388–96. doi: 10.1016/j.jana.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beyer JL, Taylor L, Gersing KR, Krishnan KR. Prevalence of HIV infection in a general psychiatric outpatient population. Psychosomatics. 2007;48(1):31–7. doi: 10.1176/appi.psy.48.1.31. [DOI] [PubMed] [Google Scholar]
- 15.Walkup J, Crystal S, Sambamoorthi U. Schizophrenia and major affective disorder among Medicaid recipients with HIV/AIDS in New Jersey. Am J Public Health. 1999;89(7):1101–3. doi: 10.2105/ajph.89.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Springer SA, Dushaj A, Azar MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS Behav. 2012;16(8):2119–43. doi: 10.1007/s10461-012-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellins CA, Havens JF, McDonnell C, Lichtenstein C, Uldall K, Chesney M, et al. Adherence to antiretroviral medications and medical care in HIV-infected adults diagnosed with mental and substance abuse disorders. AIDS Care. 2009;21(2):168–77. doi: 10.1080/09540120802001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott J, Pope M. Nonadherence with mood stabilizers: prevalence and predictors. J Clin Psychiatry. 2002;63(5):384–90. doi: 10.4088/jcp.v63n0502. [DOI] [PubMed] [Google Scholar]
- 19.Colom F, Vieta E, Tacchi MJ, Sanchez-Moreno J, Scott J. Identifying and improving non-adherence in bipolar disorders. Bipolar Disord. 2005;7(Suppl 5):24–31. doi: 10.1111/j.1399-5618.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- 20.Li J, McCombs JS, Stimmel GL. Cost of treating bipolar disorder in the California Medicaid (Medi-Cal) program. J Affect Disord. 2002;71(1-3):131–9. doi: 10.1016/s0165-0327(01)00394-9. [DOI] [PubMed] [Google Scholar]
- 21.Binford MC, Kahana SY, Altice FL. A systematic review of antiretroviral adherence interventions for HIV-infected people who use drugs. Curr HIV/AIDS Rep. 2012;9(4):287–312. doi: 10.1007/s11904-012-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC) Compendium of evidence-based HIV behavioral interventions. 2013 http://www.cdc.gov/hiv/prevention/research/compendium/ma/complete.html.
- 23.Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemed J E Health. 2009;15(3):231–40. doi: 10.1089/tmj.2008.0099. [DOI] [PubMed] [Google Scholar]
- 24.Coomes CM, Lewis MA, Uhrig JD, Furberg RD, Harris JL, Bann CM. Beyond reminders: a conceptual framework for using short message service to promote prevention and improve healthcare quality and clinical outcomes for people living with HIV. AIDS Care. 2012;24(3):348–57. doi: 10.1080/09540121.2011.608421. [DOI] [PubMed] [Google Scholar]
- 25.Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS ONE. 2014;9(2):e88166. doi: 10.1371/journal.pone.0088166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev. 2014;14 doi: 10.1002/14651858.CD009756. CD009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1):a randomised trial. Lancet. 2010;376(9755):1838–45. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 28.Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, de Walque D, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25(6):825–34. doi: 10.1097/QAD.0b013e32834380c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dowshen N, Kuhns LM, Johnson A, Holoyda BJ, Garofalo R. Improving adherence to antiretroviral therapy for youth living with HIV/AIDS: a pilot study using personalized, interactive, daily text message reminders. J Med Internet Res. 2012;14(2):e51. doi: 10.2196/jmir.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer M, Grof P, Gyulai L, Rasgon N, Glenn T, Whybrow PC. Using technology to improve longitudinal studies: self-reporting with ChronoRecord in bipolar disorder. Bipolar Disord. 2004;6(1):67–74. doi: 10.1046/j.1399-5618.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 31.Bopp JM, Miklowitz DJ, Goodwin GM, Stevens W, Rendell JM, Geddes JR. The longitudinal course of bipolar disorder as revealed through weekly text messaging: a feasibility study. Bipolar Disord. 2010;12(3):327–34. doi: 10.1111/j.1399-5618.2010.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenze SJ, Armey MF, Miller IW. Feasibility and acceptability of a mobile intervention to improve treatment adherence in bipolar disorder: a pilot study. Behavior Modif. 2014 doi: 10.1177/0145445513518421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depp CA, Mausbach B, Granholm E, Cardenas V, Ben-Zeev D, Patterson TL, et al. Mobile interventions for severe mental illness: design and preliminary data from three approaches. J Nerv Mental Dis. 2010;198(10):715–21. doi: 10.1097/NMD.0b013e3181f49ea3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz KF, Altman DG, Moher D Group C. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balfour L, Kowal J, Silverman A, Tasca GA, Angel JB, Macpherson PA, et al. A randomized controlled psycho-education intervention trial: improving psychological readiness for successful HIV medication adherence and reducing depression before initiating HAART. AIDS Care. 2006;18(7):830–8. doi: 10.1080/09540120500466820. [DOI] [PubMed] [Google Scholar]
- 36.Colom F, Vieta E, Reinares M, Martinez-Aran A, Torrent C, Goikolea JM, et al. Psychoeducation efficacy in bipolar disorders: beyond compliance enhancement. J Clin Psychiatry. 2003;64(9):1101–5. doi: 10.4088/jcp.v64n0917. [DOI] [PubMed] [Google Scholar]
- 37.Glanz K, Rimer BK. Theory at a glance: a guide for health promotion practice. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 1997. [Google Scholar]
- 38.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 39.Beck AT, Steer RA, Brown GK. BDI: Beck Depression Inventory: Manual. Psychological Corporation; San Antonio: 1996. [Google Scholar]
- 40.Badiee J, Riggs PK, Rooney AS, Vaida F, Grant I, Atkinson JH, et al. Approaches to identifying appropriate medication adherence assessments for HIV infected individuals with comorbid bipolar disorder. AIDS Patient Care STDs. 2012;26(7):388–94. doi: 10.1089/apc.2011.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cliff N. Dominance statistics: ordinal analyses to answer ordinal questions. Psychol Bull. 1993;114(3):494. [Google Scholar]
- 43.Hoogenhout EM, de Groot RH, van der Elst W, Jolles J. Effects of a comprehensive educational group intervention in older women with cognitive complaints: a randomized controlled trial. Aging Mental Health. 2012;16(2):135–44. doi: 10.1080/13607863.2011.598846. [DOI] [PubMed] [Google Scholar]
- 44.Romano J, Kromrey JD, Coraggio J, Skowronek J, Devine L. Exploring methods for evaluating group differences on the NSSE and other surveys: Are the t-test and Cohen’s d indices the most appropriate choices?. Paper presented at the annual meeting of the Southern Association for Institutional Research; October 14–17, 2006; Arlington, Virginia. [Google Scholar]
- 45.Gill CJ, Sabin LL, Hamer DH, Keyi X, Jianbo Z, Li T, et al. Importance of dose timing to achieving undetectable viral loads. AIDS Behav. 2010;14(4):785–93. doi: 10.1007/s10461-009-9555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pompili M, Venturini P, Palermo M, Stefani H, Seretti ME, Lamis DA, et al. Mood disorders medications: predictors of nonadherence—review of the current literature. Expert Rev Neurother. 2013;13(7):809–25. doi: 10.1586/14737175.2013.811976. [DOI] [PubMed] [Google Scholar]
- 47.Garfein R, Collins K, Muñoz F, Moser K, Cerecer-Callu P, Sullivan M, et al., editors. Wireless Health. Baltimore, MD: 2013. Nov 1–3, Use of mobile phones for video directly observed therapy among tuberculosis patients in high and low income countries. [Google Scholar]
- 48.Bobrow K, Brennan T, Springer D, Levitt NS, Rayner B, Namane M, et al. Efficacy of a text messaging (SMS) based intervention for adults with hypertension: protocol for the StAR (SMS Text-message Adherence suppoRt trial) randomised controlled trial. BMC Public Health. 2014;14:28. doi: 10.1186/1471-2458-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muench F, van Stolk-Cooke K, Morgenstern J, Kuerbis AN, Markle K. Understanding messaging preferences to inform development of mobile goal-directed behavioral interventions. J Med Internet Res. 2014;16(2):e14. doi: 10.2196/jmir.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wendel CS, Mohler MJ, Kroesen K, Ampel NM, Gifford AL, Coons SJ. Barriers to use of electronic adherence monitoring in an HIV clinic. Ann Pharmacother. 2001;35(9):1010–5. doi: 10.1345/aph.10349. [DOI] [PubMed] [Google Scholar]
- 51.Bova CA, Fennie KP, Knafl GJ, Dieckhaus KD, Watrous E, Williams AB. Use of electronic monitoring devices to measure antiretroviral adherence: practical considerations. AIDS Behav. 2005;9(1):103–10. doi: 10.1007/s10461-005-1685-0. [DOI] [PubMed] [Google Scholar]
- 52.Levine AJ, Hinkin CH, Castellon SA, Mason KI, Lam MN, Perkins A, et al. Variations in patterns of highly active antiretroviral therapy (HAART) adherence. AIDS Behav. 2005;9(3):355–62. doi: 10.1007/s10461-005-9009-y. [DOI] [PubMed] [Google Scholar]
- 53.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16(2):269–77. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]


