Abstract
Renin cleavage of angiotensinogen (AGT) releases angiotensin I (AngI) in the initial step of producing all angiotensin peptides. It has been suggested recently that redox regulation of a disulfide bond in AGT involving Cys18-Cys137 may be important to its renin cleavage efficiency in vivo. The purpose of this study was to test this prediction in a mouse model by comparing AngII production and AngII-dependent functions in mice expressing wild-type AGT versus a mutated form of AGT lacking the disulfide bond. Wild-type (hepAGT+/+) and hepatocyte-specific AGT deficient (hepAGT−/−) littermates were developed in an LDL receptor −/− background. hepAGT+/+ mice were injected intraperitoneally with adeno-associated viral (AAV) vector containing a null insert. hepAGT−/− mice were injected with AAV containing a null insert, wild-type AGT, or Cys18Ser and Cys137Ser mutated AGT. Two weeks after AAV injection, mice were fed a Western diet for 12 weeks. Administration of AAV containing either form of AGT led to similar plasma AGT concentrations in hepAGT−/− mice. High plasma renin concentrations in hepAGT−/− mice were suppressed equally by both forms of AGT, which were accompanied by comparable increases of plasma AngII concentrations similar to hepAGT+/+ mice. AAV-driven expression of both forms of AGT led to equivalent increases of systolic blood pressure and augmentation of atherosclerotic lesion size in hepAGT−/− mice. These measurements were comparable to systolic blood pressure and atherosclerotic lesions in hepAGT+/+ mice. These data indicate that the Cys18-Cys137 disulfide bond in AGT is dispensable for AngII production and AngII-dependent functions in mice.
Keywords: Angiotensinogen, Cys18-Cys137 disulfide bond, angiotensin, blood pressure, atherosclerosis
Introduction
Angiotensinogen (AGT) is the unique precursor of all angiotensin (Ang) peptide products. This precursor is secreted predominantly by hepatocytes as a 452 amino acid protein in humans (453 amino acids in rodents).1,2 While there are several enzymes that can cleave AGT, renin is considered to be the major enzyme that cleaves the amino terminus of AGT to release AngI. AngI is predominantly cleaved by angiotensin-converting enzyme (ACE) to form the major bioactive peptide of the renin angiotensin system, AngII. Cleavage of AGT by renin is the rate-limiting step in producing AngII, implicating that the efficiency of AGT cleavage determines the rate of AngII production, and consequently influences AngII-mediated physiological and pathophysiological effects.
Recently, a renin-bound AGT structure was determined for recombinant non-glycosylated forms of the mouse, rat, and human protein.3 Intriguingly, a conserved disulfide bond between Cys18 and Cys138 in human AGT (Cys18 and Cys137 in mouse AGT) was identified,2,4 and hypothesized to be a major regulator of AGT stability that facilitates the cleavage by renin, which was consistent with previous enzymatic studies.3,5 This disulfide bond formed in the secreted protein was found to be labile, and it was proposed that its reduction by a plasma thiol-reductase system leads to decreased access to the renin cleavage site. Thus, reduced AGT (lack of the disulfide bond) was suggested to be a less efficient substrate for the generation of AngI in vivo. In support of this hypothesis, the ratio of disulfide bridged versus unbridged forms of AGT was increased in pregnant women with pre-eclampsia compared to normotensive pregnant women.3 Overall, this structural model predicted that AngII-dependent responses are regulated by the presence of a disulfide bond between Cys18 and Cys138 in humans (Cys18 and Cys137 in mice), which represents a novel mechanism of vasoconstriction. However, there is no direct evidence supporting this structural modeling-predicted difference in the in vivo conversion of these two forms of AGT to angiotensin peptides that are coupled to well established measures of AngII-induced pathophysiological effects.
AngII regulates blood pressure and promotes atherosclerosis.6–11 In the present study, we determined effects of the presence versus absence of the AGT disulfide bond on these two AngII-dependent effects. We used AGT floxed mice that we have described previously,12 in which plasma AGT concentrations were severely depleted by hepatocyte-specific expression of Albumin Cre recombinase. In these mice that had diminutive plasma AGT concentrations, adeno-associated viral (AAV) vectors were injected to populate, in a hepatocyte-specific manner, either wild-type AGT or AGT containing Cys18Ser and Cys137Ser (C18S,C137S) mutants that were unable to form the disulfide bond. In wild-type mice, plasma AGT was present in the completely oxidized form to provide a marked contrast to the mutant AGT that totally lacked a disulfide bridge. This study feature enabled a comparison of AGT proteins that were at either end of the oxidation and reduction spectrum. Comparison of these forms of AGT was unable to discern any differential effects on plasma AngII concentrations or AngII-dependent effects in mice.
Materials and Methods
Animals
All mouse experiments reported in this manuscript were performed with the approval of the University of Kentucky Institutional Animal Care and Use Committee (University of Kentucky IACUC protocol number: 2006–0009). A detailed Methods section is available in the online-only Data Supplement.
Results
Predominance of Disulfide Bridged AGT in Mouse Plasma
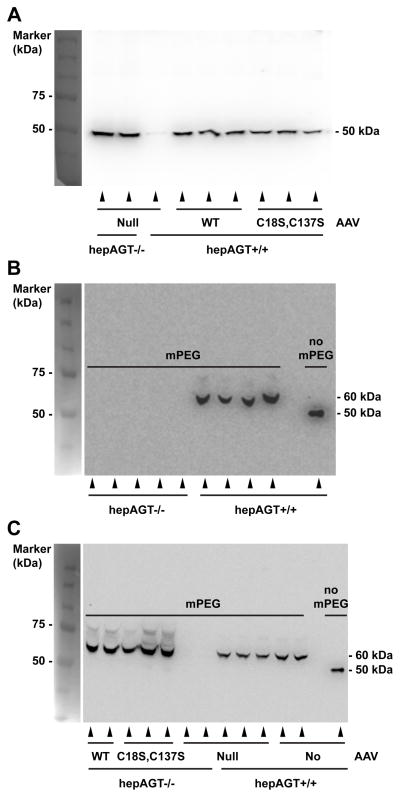
Western blotting of AGT from wild-type mouse plasma revealed a single band with molecular weight of ~50 kDa (Figure 1A). Antibody specificity against AGT was demonstrated by the lack of a discernable band in plasma harvested from hepAGT−/− mice (Figure 1A). Incubation of plasma from wild-type mice with mPEG alone increased molecular weight of AGT by 10 kDa, indicating that two of the four AGT cysteine residues are reactive to mPEG (Figure 1B). Next, mice were injected with AAV encoding either wild-type or mutated (C18S, C137S) AGT. Two weeks post AAV injection, plasmas were collected from all groups and analyzed using Western blotting after pre-incubation with mPEG. Western blotting of plasma AGT failed to detect any immunoreactive band in hepAGT−/− mice injected with AAV containing a null insert (Figure 1C). In contrast, a 60 kDa immunoreactive band was detected clearly in each hepAGT−/− mouse repopulated with wild-type AGT, demonstrating that AAV derived wild-type AGT was completely oxidized (Figure 1C). Plasma in mice repopulated with mutated (C18S, C137S) AGT showed a 60 kDa band due to interaction of mPEG with the remaining 2 cysteine residues.
Figure 1. Disulfide bridged AGT is the only form in mouse plasma.
Western blotting was performed in plasma samples from mice prior to (A and B) or 2 weeks post (C) AAV injections. Plasma samples were incubated without mPEG or with mPEG (5 kDa) for cysteine attachment. Disulfide bridged AGT and mutated AGT (Cys18Ser, Cys137Ser) gained molecular weight by 10 kDa due to addition of 2 mPEG to free Cys300 and Cys316. WT represents wild-type AGT AAV.
These data are consistent with mPEG labeling the two cysteines at positions 300 and 316 on the non renin interacting face, but being unable to interact with vicinal cysteines at position 18 and 137 due to the disulfide linkage. These results are in contrast to human AGT, which shows labeling of either two or four cysteines,3 indicating a difference between species with a stable C18S, C137S disulfide bond predominant in mouse plasma.
AAV Vector Infection Generated Equivalent Plasma Concentrations of Disulfide and Non-disulfide Bridged Forms of AGT
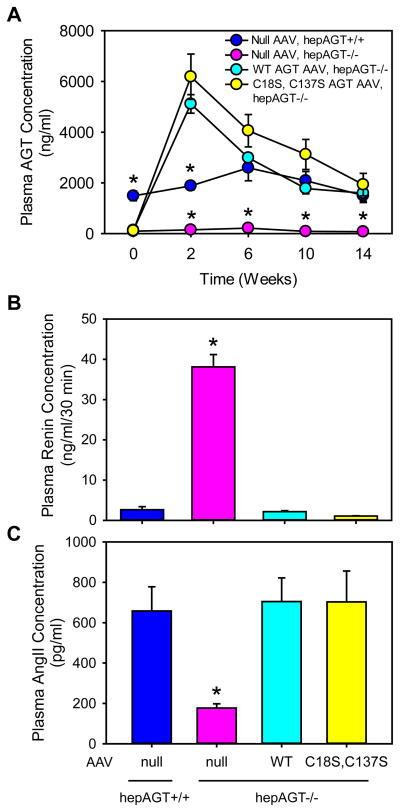
To evaluate expression efficiency and stability of AAV vectors, we measured plasma AGT concentrations prior to AAV injection, and at multiple intervals after AAV injection. At baseline (week 0), hepAGT−/− mice lacking AGT expression in hepatocytes had barely detectable plasma AGT concentrations. The low plasma AGT concentrations remained unchanged in hepAGT−/− mice receiving AAV vector containing the null insert (Figure 2A). Plasma AGT concentrations in hepAGT−/− mice receiving AAV vector expressing either wild-type or mutated AGT peaked 2 weeks post-injection and remained at concentrations comparable to hepAGT+/+ littermates from 6 to 14 weeks after AAV injections. At study endpoint, there was no difference in plasma AGT concentrations except for hepAGT−/− mice administered AAV vector containing the null insert (Figure 2A).
Figure 2. Deletion of Cys18-Cys137 disulfide bond of AGT did not affect plasma concentrations of AGT, renin, or AngII.
(A) Plasma total AGT concentrations were measured using an ELISA kit at baseline, and 2, 6, 10, and 14 weeks after AAV injection (N = 4 – 5/group). Statistical analyses were two way repeated measures ANOVA. * denotes P < 0.001 versus the other 3 groups at each time point. (B) Plasma renin concentrations were measured at termination by radioimmunoassay (N = 7 – 10/group). * denotes P < 0.001 versus the other 3 groups by one way ANOVA and post-hoc comparison of Tukey-Kramer adjustment. (C) Plasma AngII concentrations were measured at termination by radioimmunoassay (N = 3 – 5/group). * denotes P = 0.002 versus the other 3 groups by one way ANOVA with Holm-Sidak method. WT represents wild-type AGT AAV, and C18S,C137S represents AGT in AAV vector with Cys to Ser mutation at 18 and 137 residues.
Disulfide and Non-disulfide Bridged Forms of AGT Had Equivalent Effects on Plasma Renin and Plasma and Renal AngII Concentrations
Plasma renin concentrations were increased significantly in hepAGT−/− mice infected with the null AAV vector as determined 2 weeks after AAV injections (Figure S2) or at termination (Figure 2B), which were due to diminished negative feedback attributable to low concentrations of plasma AngII (Figure 2C).13 This contrasted with the renin and AngII concentrations in hepAGT+/+ mice receiving the null AAV vector (Figure 2B and 2C). HepAGT−/− mice replenished with wild-type AGT showed no significant differences in plasma renin and AngII concentrations compared to hepAGT+/+ littermates. Plasma renin and AngII concentrations in hepAGT−/− mice infected with mutated AGT AAV were not significantly different from those in hepAGT−/− mice administered AAV expressing wild-type AGT (Figure 2B, 2C).
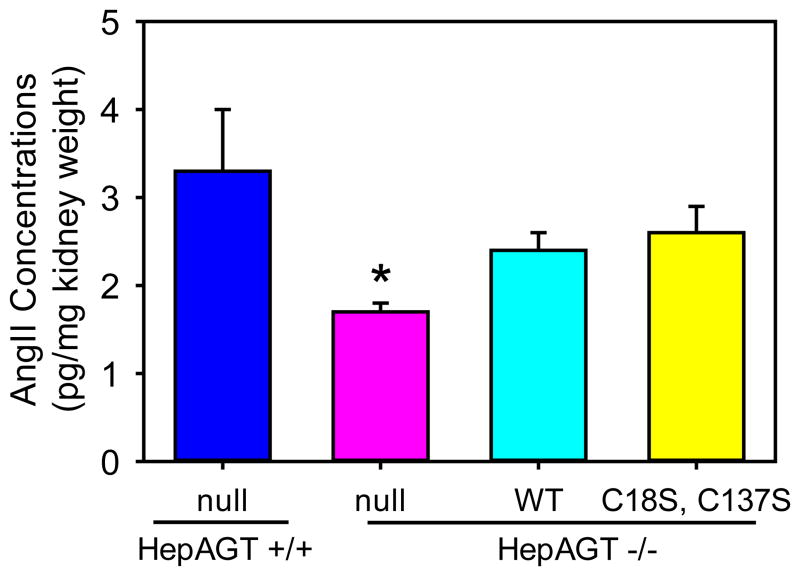
Kidney is the major source of renin and has abundant pro-renin receptors. To determine the effects of disulfide bond of AGT in local production of AngII, we measured AngII concentrations in kidney tissues of the study mice. As expected, renal AngII concentrations were lower in hepAGT−/− mice than in hepAGT+/+ mice injected with null AAVs (Figure 3). There were significant, but equivalent, increases in kidney AngII concentrations in hepAGT−/− mice injected with AAVs containing wild-type AGT versus Cys18-137Ser AGT, compared to hepAGT−/− mice injected with the null AAV (Figure 3).
Figure 3. Deletion of Cys18-Cys137 disulfide bond of AGT did not affect renal AngII concentrations.
Renal AngII concentrations (n = 5 – 6/group) were measured using radioimmuno-assay method. * denotes P < 0.05 versus the other 3 groups by one way ANOVA with Benjamini-Hochberg method. WT represents wild-type AGT AAV, and C18S,C137S represents AGT in AAV vector with Cys to Ser mutation at 18 and 137 residues.
Disulfide and Non-disulfide Bridged Forms of AGT Had Equivalent Effects on AngII Mediated Effects
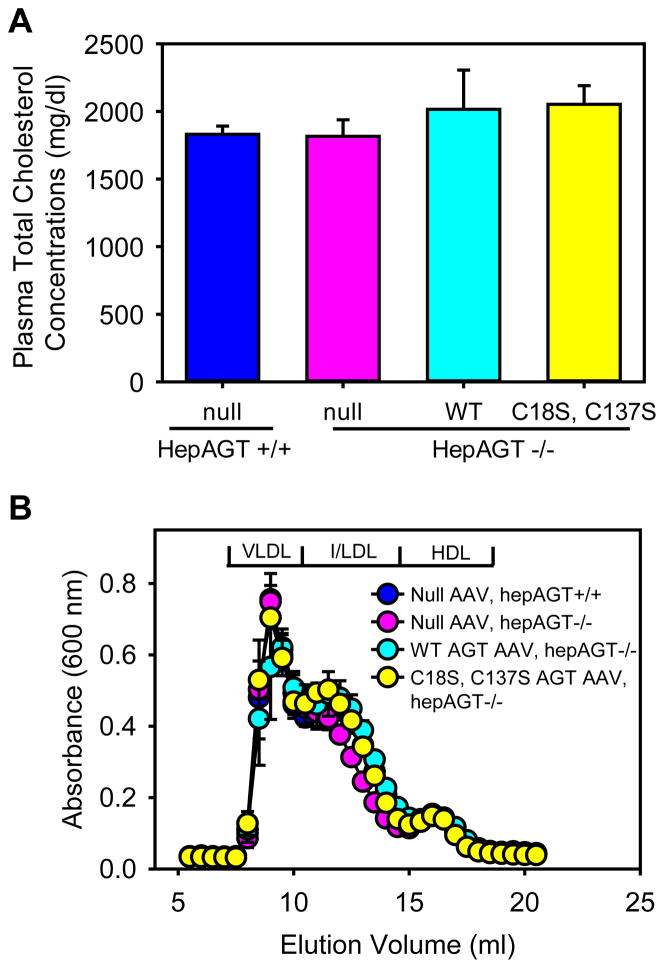
Two weeks after AAV injections prior to Western diet feeding, plasma cholesterol concentrations were equivalently low in all study groups (Figure S3). Consistent with our previous findings,6,14,15 LDL receptor−/− mice fed Western diet for 12 weeks exhibited severe hypercholesterolemia that were attributed to increases of very low-density lipoproteins (VLDL) and low-density lipoproteins (LDL) as demonstrated by plasma total cholesterol measurements and lipoprotein distribution analyses (Figure 4A and 4B). Mouse genotype or AAV infection had no effect on plasma cholesterol concentrations and lipoprotein distribution.
Figure 4. Deletion of Cys18-Cys137 disulfide bond of AGT did not affect plasma cholesterol concentrations.
(A) Plasma total cholesterol concentrations at termination were measured using an enzymatic kit. Histobars are mean and error bars are SEM. N = 7 – 10/group. P = 0.59 by Kruskal-Wallis One way ANOVA on Ranks. (B) Plasma lipoprotein distributions were resolved by size exclusion chromatography. Circles and error bars are means ± SEM. WT represents wild-type AGT AAV, and C18S,C137S represents AGT in AAV vector with Cys to Ser mutation at 18 and 137 residues.
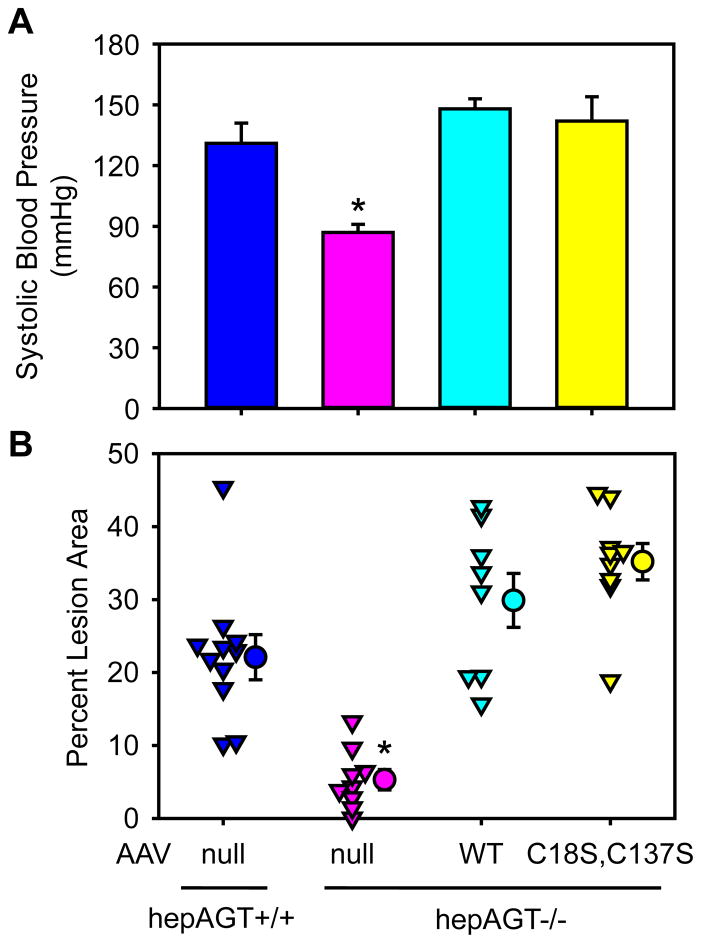
In agreement with the low plasma AngII concentrations, administration of AAV containing the null vector to hepAGT−/− mice had lower systolic blood pressure both at 2 weeks post AAV injection (Figure S4) and the endpoint (Figure 5A), compared to their hepAGT+/+ littermates. Administration of AAV vectors containing either wild-type or the mutated form of AGT increased systolic blood pressure in hepAGT−/− mice to levels equivalent to hepAGT+/+ littermates. However, systolic blood pressures were not significantly different between mice expressing wild-type or mutated AGT (Figure S4 and 5A).
Figure 5. Deletion of Cys18-Cys137 disulfide bond of AGT did not affect systolic blood pressure or atherosclerosis.
(A) Systolic blood pressures were measured using a non-invasive tail-cuff system 8 weeks after AAV injection. N = 4 – 6/group. * denotes P ≤ 0.01 versus the other 3 groups by one way ANOVA with Holm-Sidak method. (B) Atherosclerotic lesion areas were measured on the intimal surface of the aortic arch region using an en face method. Percent lesion area = lesion area/the entire intimal area × 100%. Triangles represent values of individual mice (N = 8 – 11/group), circles represent means, and error bars are SEM. * denotes P < 0.001 versus the other 3 groups by one way ANOVA with Holm-Sidak method. WT represents wild-type AGT AAV, and C18S,C137S represents AGT in AAV vector with Cys to Ser mutations at 18 and 137 residues.
LDL receptor−/− mice develop profound atherosclerotic lesions in the aortic arch region after 12 weeks of Western diet feeding.7,14,16 hepAGT−/− mice administered AAV containing the null vector had significantly smaller lesions, compared to hepAGT+/+ littermates, as measured by percent lesion area in the aortic arch region. In contrast, hepAGT−/− mice receiving either wild-type or mutated AGT developed pronounced atherosclerotic lesions, and percent lesion areas were not different between these two groups, as well as compared to hepAGT+/+ mice (Figure 5B).
Discussion
In the present study, we used a mouse model with nearly undetectable plasma AGT concentrations due to hepatocyte-specific disruption of the mouse Agt gene, to determine whether the presence of the disulfide bond in AGT is critical in determining AngII production and AngII-dependent effects in mice. Plasma AGT was repopulated in these mice by injection with AAVs expressing wild-type or a mutated form of AGT in a hepatocyte-specific manner. The mutated form of AGT lacked the disulfide bond between Cys18 and Cys137 due to their substitutions by serines. Since plasma AGT in mice exists exclusively in a disulfide bridged form, this system provides two extreme conditions of AGT, namely, completely disulfide bridged form versus unbridged form, to determine its functional consequences. Using this in vivo system, the combined findings of multiple parameters demonstrate that the absence of Cys18-Cys137 disulfide link has no discernable effects on plasma and local AngII concentrations and AngII-dependent functions in mice. Therefore, although all AGT in mouse plasma has the disulfide bridge, this fact did not negate the equivalent capability of mouse renin to cleave non disulfide bonded AGT, implicating that disulfide bond is not necessary for renin to get access to the cleavage site of AGT as proposed by Zhou et al.3
Cys18 and Cys138 disulfide linkage (Cys18-Cys137 in mouse) of AGT is conserved across species.3 In vitro studies of disulfide-bridged versus unbridged form of AGT by Zhou et al.3 revealed minor changes of the Km and Kcat when incubated with renin alone. Although no statistical significance was stated, differences in reaction kinetics of these two forms of AGT with renin appeared to be enhanced in the presence of pro-renin receptors, and accompanied by increases in AngI release.3 Currently, a role of pro-renin receptors in AngI release in vivo has not been determined, 17 although the high abundance of pro-renin receptors in the kidney is consistent with a possible role in blood pressure regulation. It has been demonstrated recently that hepatocyte-derived AGT is required for blood pressure maintenance via a kidney based production of AngII.18 Therefore, the system used in the present study provided an opportunity for both forms of AGT to engage with renal pro-renin receptors to regulate blood pressure through an AngII-dependent mechanism. Since kidney is the major source of renin and has abundant presence of pro-renin receptors, we determined whether the disulfide bond of AGT would affect local AngII productions as proposed by Zhou et al.3 Renal AngII concentrations were not diminished by removal of the disulfide bond in AGT, providing compelling evidence that the presence of this disulfide bond does not enhance the effectiveness of AGT cleavage by renin and pro-renin receptor interaction.
Development of atherosclerosis in LDL receptor −/− mice fed Western diet is profoundly influenced by AngII interacting with AT1a receptors.6,7 Therefore, the effects of AGT manipulations on atherosclerosis are attributable to generation of AngII. Unlike blood pressure responses, there has been no inference of a role for pro-renin receptors on the local response of atherogenic AngII. However, the reduced lesion size in LDL receptor −/− mice with renin deficient macrophages implies a local production of this AngII.14 Therefore, the effect of AGT on atherosclerosis appears to be due to its cleavage at a cellular level.6 Altogether, the system designed in this study provides an in vivo approach to test effects of the two forms of AGT on two major AngII-dependent end points, blood pressure and atherosclerosis. Neither was affected by the presence versus absence of the disulfide bridge in AGT.
The extent of the disulfide bond formation between Cys18 and Cys137 of AGT has been proposed as a novel mechanism of blood pressure regulation in humans.3,19 Our data indicate that this is not the case in mice. There are several differences of AGT between mouse and human. These differences provided an optimal environment to determine whether the disulfide bond of AGT plays a critical role in the generation of AngII. First, as demonstrated in the present study, only the disulfide bridged form of AGT is present in mouse plasma. In contrast, humans have both disulfide bridged and non bridged forms in a ratio that is approximately 60:40.3 The exclusively disulfide bonded form of AGT in mouse plasma did not negate the ability of mouse renin to cleave non disulfide bonded AGT applied exogenously, implicating that the disulfide bond does not facilitate renin access to the cleavage site of AGT as proposed by Zhou et al.3 Second, plasma AGT concentrations in humans are approximately 1 μM,20,21 which is close to the Michaelis-Menton constant of human renin in plasma (1.25 μM).22 Plasma AGT concentrations in mice are approximately 40 nM,23 which is about 25 times lower than its concentrations in humans. Therefore, AGT is the rate-limiting factor for the generation of AngI in mouse plasma,24 while renin is the rate-limiting enzyme to cleave AGT in humans. A shortcoming of existing methodologies is that these data represent measurements of total AGT concentrations and do not discriminate between intact and renin-cleaved des(AngI)AGT forms. Hence, concentrations of intact AGT that serves as the renin substrate are unknown in either species. More importantly, in contrast to what was hypothesized by the study of Zhou et al., 3 local production of AngII in kidney tissues was equivalent between mice injected with wild-type and Cys18Ser and Cys137Ser mutated AGT AAVs. We conclude that the disulfide bond of mouse AGT does not alter AGT-renin interactions and cleavage, either in circulation or local tissues such as kidney.
In summary, we sought to directly determine the role of Cys18-Cys137 disulfide bridge of AGT in vivo on AngII-dependent functions. Using a system in which AGT was present at either end of the oxidation-reduction spectrum, we did not distinguish any differences between the two forms on plasma AngII production and AngII-dependent functions in mice. This does not negate a role for the AGT disulfide bridge in humans where further research is needed to determine the contribution of this disulfide bridge to blood pressure regulation. The provocative hypothesis that liability of the disulfide bridge of AGT is a modulator of blood pressure was primarily based on a cross sectional study that determined the ratio of oxidized to reduced AGT in 12 women with pre-eclampsia compared to 12 normotensive pregnant women.3 Confirmation in a larger cohort study would be enhanced by measurements of absolute concentrations of oxidized forms of AGT, coupled with plasma concentrations of AngII.
Supplementary Material
Perspectives.
Using an in vivo mouse system, we demonstrated that structural modulation by formation or removal of the disulfide bridge between Cys18 and Cys137 did not influence AngII production and AngII-dependent blood pressure regulation and atherosclerosis. Since multiple divergences exist between humans and mice, future studies are necessary to determine the importance/relevance of this structural modulation of AGT in regulation of AngII production and functions in human subjects.
Novelty and Significance.
What Is New?
This manuscript reports a new mouse model of hepatocyte-specific deficiency of AGT in a hypercholesterolemic background that provides an excellent experimental approach to studying AGT-related physiological and pathophysiological functions.
This is the first study revealing that AGT in mouse plasma is mainly disulfide bridged.
This is the first study that directly determined effects of the disulfide bond of AGT in local production of AngII.
This is the first study providing in vivo evidence that the disulfide bridging of AGT does not influence AngII production and AngII-dependent regulation of blood pressure and atherosclerosis in mice.
What is Relevant?
AGT is the only known precursor of the renin angiotensin system, a critical hormonal system in blood pressure regulation and the development of atherosclerosis.
The pathophysiological role of the disulfide bond of AGT has been highlighted as the “linchpin” of a recent study published in the Nature by Zhou et al. This published prediction has become a debate in multiple reviews whether this is applicable to hypertension or other angiotensin-mediated conditions in humans.
Summary
In mice with hepatocyte-specific deficiency of AGT, repopulation of AGT that contains (Cys18-Cys137) or does not contain (Ser18-Ser137) the disulfide bond has equivalent effects on renin regulation, AngII production, blood pressure regulation, and the development of atherosclerosis.
Acknowledgments
We thank Dr. Richard Charnigo in the Department of Statistics for advising on statistical analysis, Ms. Victoria English for measuring plasma angiotensin II and renin concentrations, and Ms. Debra L. Rateri for manuscript editing.
Sources of Funding
This research work was supported by an American Heart Association predoctoral fellowship (12PRE12030364) to Congqing Wu, grants (HL062846 and HL073085) to Alan Daugherty and Lisa A. Cassis, respectively, from the National Institutes of Health of the United States of America, and a grant (81320108003) to Jian’an Wang from the Major International (Regional) Joint Research Project of the People’s Republic of China.
Footnotes
The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest/Disclosures
None.
References
- 1.Wu C, Lu H, Cassis LA, Daugherty A. Molecular and pathophysiological features of angiotensinogen: a mini review. N Am J Med Sci (Boston) 2011;4:183–190. doi: 10.7156/v4i4p183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clouston WM, Evans BA, Haralambidis J, Richards RI. Molecular cloning of the mouse angiotensinogen gene. Genomics. 1988;2:240–248. doi: 10.1016/0888-7543(88)90008-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, Stein PE, Broughton Pipkin F, Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–111. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clouston WM, Fournier RE, Richards RI. The angiotensinogen gene is located on mouse chromosome 8. FEBS Lett. 1989;255:419–422. doi: 10.1016/0014-5793(89)81136-6. [DOI] [PubMed] [Google Scholar]
- 5.Streatfeild-James RM, Williamson D, Pike RN, Tewksbury D, Carrell RW, Coughlin PB. Angiotensinogen cleavage by renin: importance of a structurally constrained N-terminus. FEBS Lett. 1998;436:267–270. doi: 10.1016/s0014-5793(98)01145-4. [DOI] [PubMed] [Google Scholar]
- 6.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- 7.Lu H, Balakrishnan A, Howatt DA, Wu C, Charnigo R, Liau G, Cassis LA, Daugherty A. Comparative effects of different modes of renin angiotensin system inhibition on hypercholesterolaemia-induced atherosclerosis. Br J Pharmacol. 2012;165:2000–2008. doi: 10.1111/j.1476-5381.2011.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103:448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- 9.Hillaert MA, Lentjes EG, Beygui F, et al. Measuring and targeting aldosterone and renin in atherosclerosis-A review of clinical data. Am Heart J. 2011;162:585–596. doi: 10.1016/j.ahj.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Wassmann S, Czech T, van Eickels M, Fleming I, Bohm M, Nickenig G. Inhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/angiotensin II type 1A receptor double-knockout mice. Circulation. 2004;110:3062–3067. doi: 10.1161/01.CIR.0000137970.47771.AF. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama F, Haraoka S, Watanabe T, Shiota N, Taniguchi K, Ueno Y, Tanimoto K, Murakami K, Fukamizu A, Yagami K. Acceleration of atherosclerotic lesions in transgenic mice with hypertension by the activated renin-angiotensin system. Lab Invest. 1997;76:835–842. [PubMed] [Google Scholar]
- 12.Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, Daugherty A, Cassis LA. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012;60:1524–1530. doi: 10.1161/HYPERTENSIONAHA.112.192690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrmann HC, Dzau VJ. The feedback regulation of angiotensinogen production by components of the renin-angiotensin system. Circ Res. 1983;52:328–334. doi: 10.1161/01.res.52.3.328. [DOI] [PubMed] [Google Scholar]
- 14.Lu H, Rateri DL, Feldman DL, Charnigo RJ, Jr, Fukamizu A, Ishida J, Oesterling EG, Cassis LA, Daugherty A. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest. 2008;118:984–993. doi: 10.1172/JCI32970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XC, Lu H, Zhao M, Tashiro K, Cassis LA, Daugherty A. Angiotensin-converting enzyme promotes atherosclerosis through an angiotensin I to angiotensin II pathway involving leukocytes. Arterioscler Thromb Vasc Biol. 2013;33:2075–2080. doi: 10.1161/ATVBAHA.113.301777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu H, Wu C, Howatt DA, Balakrishnan A, Charnigo RJ, Jr, Cassis LA, Daugherty A. Differential effects of dietary sodium intake on blood pressure and atherosclerosis in hypercholesterolemic mice. J Nutr Biochem. 2013;24:49–53. doi: 10.1016/j.jnutbio.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–1189. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sigmund CD. Structural biology: On stress and pressure. Nature. 2010;468:46–47. doi: 10.1038/468046a. [DOI] [PubMed] [Google Scholar]
- 20.Michel FS, Norton GR, Majane OH, Badenhorst M, Vengethasamy L, Paiker J, Maseko MJ, Sareli P, Woodiwiss AJ. Contributions of circulating angiotensinogen concentrations to variations in aldosterone and blood pressure in a group of African ancestry depends on salt intake. Hypertension. 2012;59:62–69. doi: 10.1161/HYPERTENSIONAHA.111.181230. [DOI] [PubMed] [Google Scholar]
- 21.Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–60. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cumin F, Le-Nguyen D, Castro B, Menard J, Corvol P. Comparative enzymatic studies of human renin acting on pure natural or synthetic substrates. Biochim Biophys Acta. 1987;913:10–19. doi: 10.1016/0167-4838(87)90226-3. [DOI] [PubMed] [Google Scholar]
- 23.Kobori H, Katsurada A, Miyata K, Ohashi N, Satou R, Saito T, Hagiwara Y, Miyashita K, Navar LG. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol. 2008;294:F1257–F1263. doi: 10.1152/ajprenal.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lum C, Shesely EG, Potter DL, Beierwaltes WH. Cardiovascular and renal phenotype in mice with one or two renin genes. Hypertension. 2004;43:79–86. doi: 10.1161/01.HYP.0000107401.72456.50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.