Abstract
Hypothesis
Introduction of microperforations in round window membrane (RWM) will allow reliable and predictable intracochlear delivery of pharmaceutical, molecular or cellular therapeutic agents.
Background
Reliable delivery of medications into the inner ear remains a formidable challenge. The RWM is an attractive target for intracochlear delivery. However, simple diffusion across intact RWM is limited by what material can be delivered, size of material to be delivered, difficulty with precise dosing, timing, and precision of delivery over time. Further, absence of reliable methods for measuring diffusion across RWM in vitro is a significant experimental impediment.
Methods
A novel model for measuring diffusion across guinea pig RWM, with and without microperforation, was developed and tested: cochleae, sparing the RWM, were embedded in 3D-printed acrylic holders using hybrid dental composite and light cured to adapt the round window niche to 3ml Franz diffusion cells. Perforations were created with 12.5μm diameter needles and examined with light microscopy. Diffusion of 1mM Rhodamine B across RWM in static diffusion cells was measured via fluorescence microscopy.
Results
The diffusion cell apparatus provided reliable and replicable measurements of diffusion across RWM. The permeability of Rhodamine B across intact RWM was 5.1 × 10-9 m/s. Manual application of microperforation with a 12.5μm diameter tip produced an elliptical tear removing 0.22±0.07% of the membrane and was associated with a 35x enhancement in diffusion (p<0.05).
Conclusion
Diffusion cells can be applied to the study of RWM permeability in vitro. Microperforation in RWM is an effective means of increasing diffusion across the RWM.
Keywords: Franz™ diffusion cells, inner ear delivery, methodology, Round Window Membrane, Pharmacokinetics
INTRODUCTION
Auditory and vestibular dysfunction is the most common sensory disturbance in humans. The prevalence of hearing loss is estimated to impact 538 million people in the world today and debilitating tinnitus interfering with daily activities affects 12 million in the United States alone1,2. Current treatment methods for these diseases include systemic administration, intratympanic injection (IT) and direct introduction to the cochlea of steroids or aminoglycosides. These methods are limited by systemic toxicity, unreliable dosing, and complications of traumatic disruption to the cochlea; to date, IT administration has shown the most promise and use in clinical practice. Inner ear delivery via IT injection requires filling the middle ear with the therapeutic agent to allow for subsequent diffusion across the round window membrane (RWM); inevitably, there is loss of the injectate via the Eustachian tube once the patient stands.
Office based IT administration of gentamicin has largely replaced surgical intervention for the treatment of debilitating vertigo in Ménière’s Disease3,4. Similarly, IT steroids are being used in patients with sudden sensorineural hearing loss (SSNHL), tinnitus and otologic manifestations of autoimmune disease5,6. While widely accepted and practiced, IT therapy is associated with inconsistent clinical response and toxicity with as many as 30% of patients treated for vertigo experiencing the side effect of hearing loss7,8. This is due to the great variability of diffusion across the RWM leading to unpredictable intracochlear bioavailability of the administered drug. This variability is further exacerbated by scar tissue in the RWM niche or inflammatory thickening of the RWM that directly impacts the membrane’s permeability9-11. These observations have led to significant research in the dose dependent sensitivity of different hair cell types, pharmaceutical and surgical approaches of altering the permeability of the RWM, and drug release formulations to provide precise delivery of therapeutic agents into the inner ear12,13. The development of delayed release gels placed on the RWM show promise of providing continuous drug release but are still affected by the variable permeability of the RWM14.
A means of providing safe and reliable drug delivery through the RWM will have significant clinical impact for the treatment of auditory and vestibular disorders. However, investigation of the RWM permeability and manipulation of RWM experimentally has been hindered by the high level of technical difficulty associated with working with a small membrane. The RWM is only 1mm2 in guinea pigs and 2.5mm2 in humans15. While a standardized method for studying pharmacokinetics of transdermal drug delivery is well established, it can only be used for the study of biological tissue samples greater than 6mm in diameter16,17. In this communication, we report on the adaptation of this reliable diffusion cell model for the study of RWM permeability and demonstrate the ability of microperforations to significantly enhance diffusion across the RWM.
MATERIALS AND METHODS
In this study, we investigate the use of microperforations as a means of enhancing permeability of the RWM using an in vitro guinea pig animal model and Rhodamine B (RhoB) as the therapeutic proxy. The applied method is adapted from the American Association of Pharmaceutical Scientists (AAPS) and the Federal Drug Administration (FDA) recommendations18.
Rhodamine B
RhoB (479.01g/mol, log POW 1.95, >99% purity; ACROS 29657, 50g/L in water) was selected as the therapeutic proxy as it can be accurately quantified with fluorescent microscopy (Acros Organics, Pittsburgh, PA). RhoB is considered a suitable proxy in this study due to similarities in molecular weight and diffusion coefficient to the therapeutic agents of interest (Table 1)19,20.
Table 1.
Permeability of Gentamicin, Dexamethasone and Rhodamine B
| Molecular Weight (g/mol) | Diffusion Coefficient in water at 25°C (298K) (10-6cm2s-1) | Permeability KP RWM (10-8 ms-1) | Estimated Permeability KP PORE (10-5 ms-1) | |
|---|---|---|---|---|
|
| ||||
| Gentamicin | 477.60 | 6.82 | 5~35 | 6.82 |
|
| ||||
| Dexamethasone | 392.46 | 7.20 | 3.5 ± 4.6 | 7.20 |
|
| ||||
| Rhodamine B | 479.01 | 4.5 ± 0.4 | 0.51 ± 0.41 | 4.5 |
| 4.27 ± 0.04 | 4.27 | |||
Animal Model
All experimental procedures were performed in accordance with the ethical standards of Protocol AC-AAAF9604 of theInstitutional Animal Care and Use Committee to prevent pain or discomfort to animal subjects. Hartley Guinea Pigs, sexually mature, of both sexes supplied by Charles River Laboratories International were studied (Wilmington, MA).
RWM Harvesting
Animals were euthanized with 120mg/kg Pentobarbital (1ml Euthasol Solution: 390mg Pentobarbital/50mg Phenytoin) and secondary decapitation. Total preparation of the cochlea took less than 30 minutes. The bullae were immediately excised (4 minutes) and drilled (15 minutes / cochlea) to expose the cochlea and remove the semicircular canals while leaving the round window membrane and niche intact and unobstructed (Figure 1). All drilling and handling of the excised bulla from time of excision until carrying out the diffusion was performed in a bath of normal saline solution or under humidified air to prevent desiccation of the membrane that can result in an artificial increase in the calculated permeability of fresh membranes21.
Figure 1.
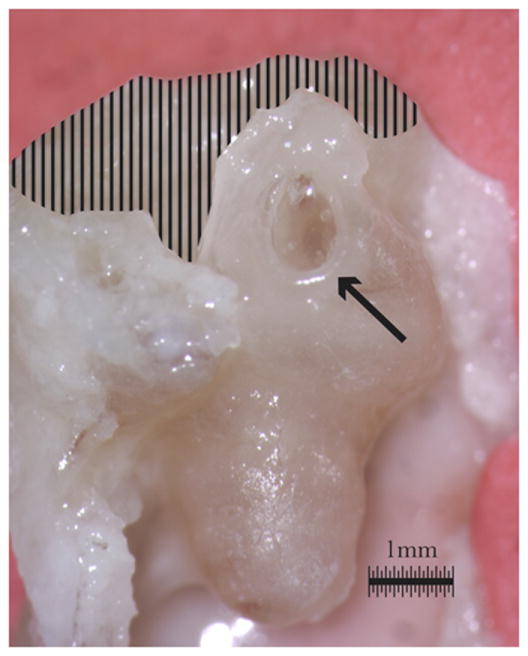
Guinea pig cochlea is shown after exposure of the RWM (arrow) via removal of the semicircular canals (shaded).
Franz™ Cell Diffusion
Diffusion of RhoB through the RWM was performed on 12 cochleas from Hartley Strain Guinea Pigs in a modified diffusion cell at room temperature. Franz™ Cell-type diffusion cells (PermeGear, Inc., Hellertown, PA) are made of borosilicate glass and have a 5mm orifice and a 3ml receptor volume with flat ground joints (Figure 2). The cell consists of three parts (Figure 3): donor chamber, membrane, and receptor chamber.
Figure 2.

Franz™Cell diffusion cell (PermeGear, Inc) shown with a pink donor chamber, blue receptor chamber and acrylic holder in place of the membrane. The diffusion cell is held together with a spring bound clamp.
Figure 3.
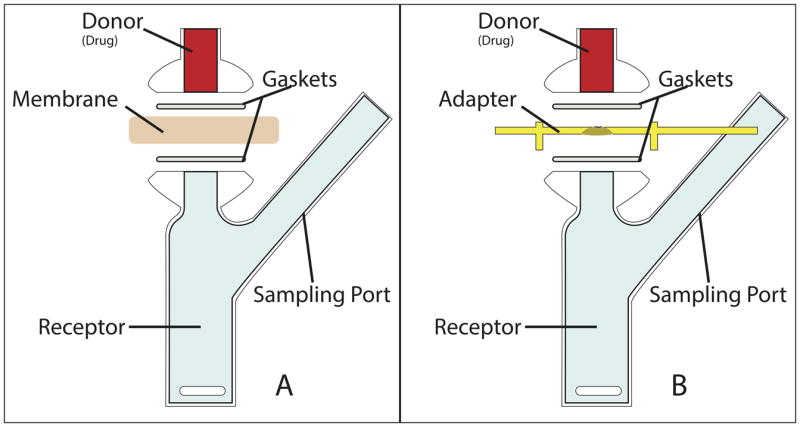
Diffusion cell schematic showing placement of PTFE gaskets and receptor/donor chambers. Also shown is the position of the membrane (A), which can be replaced by the acrylic adapter (B).The round window membrane is oriented such that its middle ear surface is facing the donor chamber, while the scala tympani surface of the round window membrane is in contact with the contents of the receptor chamber.
Donor Chamber
The donor chamber was filled with 0.3ml of 1mM RhoB in PBS meeting the conditions for continuous infinite dosing22. Donor chamber was covered with Parafilm® for the duration of the experiment. Drug application surface was determined by size of RWM.
Membrane
Guinea pig RWM was embedded (7 minutes) in an acrylic holder with dental composites (Figure 4). The acrylic holder was designed and 3D printed (Objet 24, Stratasys, MN) to allow for adaptation of the round window niche containing RWM to the diffusion cell. Dental composites (Dentsply, Milford, DE) included primer (Prime & Bond® NT™) and a urethane modified Bis-GMA resin (TPH Spectra™) which were light cured at 20s intervals with a 470nm light emitting diode greater than 500mW/cm2. The diffusion cell was assembled with the RWM oriented so that its middle ear surface was exposed to the agent in the donor chamber with two custom expanded Polytetraflurane (PTFE) gaskets, 1.5mm thick, to prevent leakage from the donor or receptor chambers at the diffusion cell/membrane interface. The acrylic holder was tested for reactivity with RhoB and was inert for >48 hours. The RWM was equilibrated in PBS solution for 30 minutes prior to application of RhoB. Membranes were embedded and equilibrated within 2 hours of euthanasia.
Figure 4.
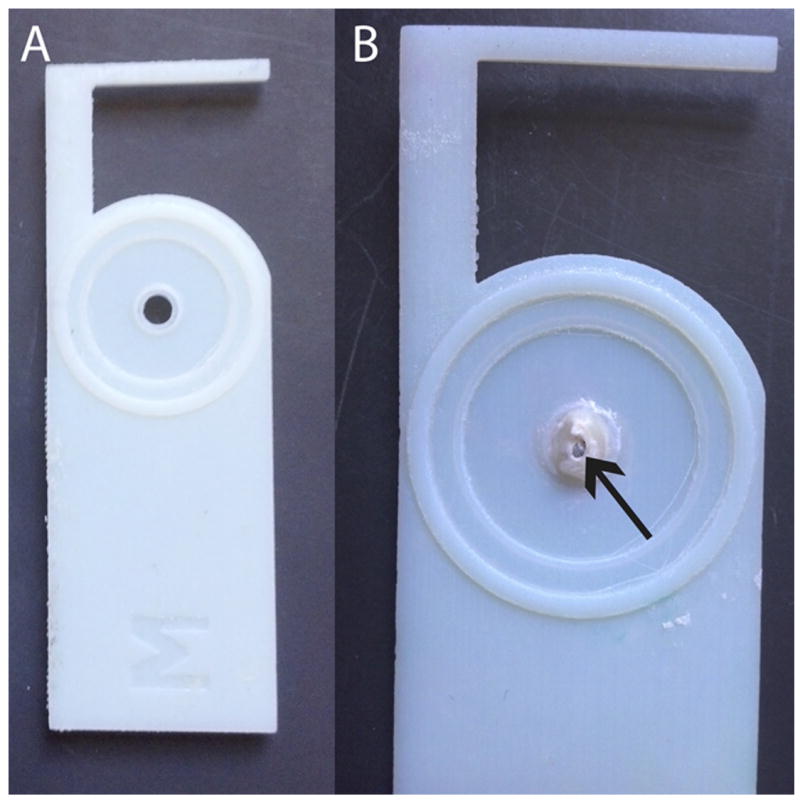
3D printed acrylic adapter allows the diffusion cell to accommodate the round window niche (RWN). The adapter is shown alone (A) and with an embedded RWM (B).
Microperforations were introduced in the RWM with manual application of a minutien insect pin (Size 000, 12.5μm diameter tip, 250μm diameter shaft; Austerlitz, Czech Republic) under a binocular microscope. All membranes were imaged before and after diffusion study using bright field, phase contrast or fluorescent microscopy and Z-stacking. Imaging was used to confirm the presence or absence of membrane perforation. 60 images at 5μm intervals were taken and compiled to create one image. ImageJ (Rasband, W.S., Image J, U.S. National Institutes of Health, Bethesda, MD) was used to calculate the size of the membrane and, if present, perforation.
Receptor Chamber
The receptor chamber was filled with 3ml of PBS and continuously stirred at 650 rpm with a cylindrical Teflon® magnetic stirbar to allow rapid mixing of contents without development of a vortex. Sampling port allowed for extraction of fluid samples at various time points to monitor progression of diffusion. The set up and sampling conditions were validated for consistency in the following categories: 1) diffusion cell dimensions, 2) stir bar speed, and 3) sampling frequency, to increase the precision of results across experiments23. Sink conditions, defined as concentration in the receptor chamber <10% solubility concentration, were satisfied at all times during the experiment.
Sampling of the receiver chamber was made at 12 predetermined times over 24 hours and analyzed with fluorescent microscopy. At each time point 90μL was removed using a stretched syringe from the center of the receptor chamber and the volume was replaced with 90μL of fresh PBS. These diffusion experiments are considered static as solution from the receptor chamber is renewed by removal of a set quantity (90μL) at sampling times and replacement of this volume with fresh medium. The removed 90μL was then diluted with 360μL PBS to reduce the effects of evaporation and maintain concentrations within the acceptable detection range of the microscope. The samples were placed on a tilt table to enhance mixing after dilution. Right and Left cochleae were run in parallel.
Quantitative Analysis of Rhodamine B
Samples from each time point were sealed in 0.6mm square capillary tubes to allow detection with fluorescent microscopy. Tubes were sealed to a glass microscope slide with Norland Optical Glue 63 (Norland, Cranbury, NJ). Square capillary tubes allow direct measurement of fluorescence without distortion of light. Samples were imaged with a Bio Imaging Navigator FSX-100 fluorescent microscope (Olympus America, Center Valley, PA). Three samples taken at each time point were imaged and averaged. Calibration curves were constructed using standards of RhoB concentrations between 0.01μM and 10μM.
Statistical Analysis
Statistical analysis was performed with Microsoft Excel. All data are presented as their mean ± standard deviation (SD). Least squares regression analysis was performed to determine the slope and standard errors (SE) of sample data. F-tests were performed to assess for equal variance prior to conducting a two sample T-test, two tails, alpha = 0.05, for equal or unequal variance (as determined by F-test) between perforated and unperforated samples. Statistical differences were considered significant at the p<0.05 level. The coefficient of variance (CV) was calculated to assess variability of measurements:
| (1) |
RESULTS
Adaptation of Diffusion Cell Method
The diffusion cell was adapted to the size and shape of the RWM using an acrylic adapter. The additional height of the RWM niche necessitated the use of thicker gaskets to provide a watertight seal. Expanded PTFE gaskets (1.5mm thick) were able to seal the diffusion cell to the adapter without reacting to the test substance. Additionally, a new clamp was built to hold together the components of the diffusion cell while providing enough force to compress the PTFE gaskets.
Permeability of RWM to Rhodamine B
1mM RhoB solution was applied to the RWM and concentration of RhoB in the receptor chamber was measured over time. RhoB concentration increased with time as RhoB entered the receptor chamber through the RWM to reach steady-state concentration. The diffusion of RhoB across intact RWM in a diffusion cell setup was monitored for up to 24 hours (n=6). The diffusion progression is shown in Figure 5.
Figure 5.
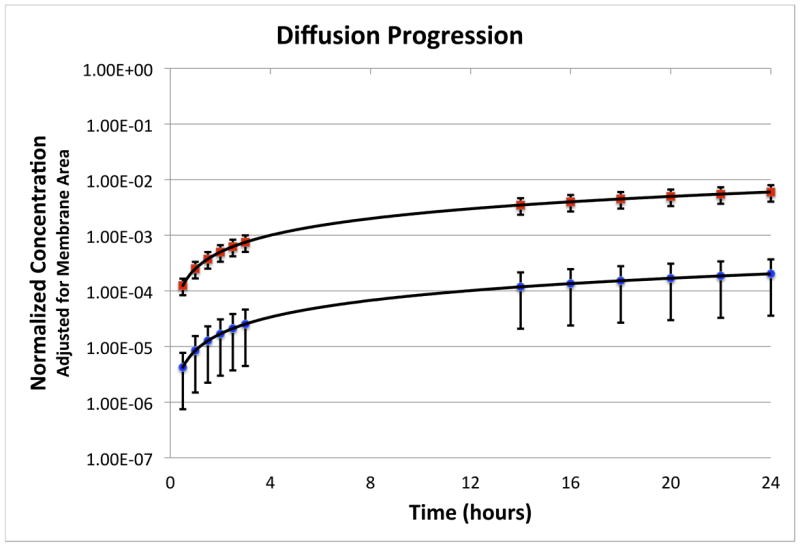
Progression of the diffusion experiment is shown with the normalized concentration as a function of time for the perforated (squares) and unperforated (circles) membranes. Error bars indicate standard deviation.
A linear regression was used to model the change in quantity over time of RhoB. The permeability coefficient (KP) was derived from this regression by
| (2) |
where Q is the mass (pg)of compound transported through the membrane in time (t) across area of exposed membrane (A). Co-Ciare the concentrations on the outer (donor) and inner (receptor) side of the membranes and can be simplified to Co in infinite dosing conditions. The permeability of the RWM to RhoB was determined to be 0.51±0.41 × 10-8 m/s. Animals in this group had a coefficient of variance of 80%. Results are summarized in Table 2.
Table 2.
Summary of Membrane and Perforation Size Characteristics and Permeability Coefficient (KP)
| Size (mm2) | Perforation (10-3 mm2) | Perforation (%) | KP RWM (10-8 ms-1) | KP PORE (ms-1) | |
|---|---|---|---|---|---|
| Unperforated (n=6) | 1.24±0.24 | N/A | N/A | 0.51±0.41 * | N/A |
| Perforated (n=6) | 1.17±0.05 | 2.53±0.73 | 0.22±0.07 | 18±6.1 * | 8.3±2.2 × 10-5 |
Statistically significant p<0.05
Application of Microperforations
The RWM was imaged and sized with bright field microscopy to determine surface area of diffusion and relative size of perforations. RWM size overall was 1.19±0.17 mm2 (n=12; CV 14%). No statistically significant difference in size of membrane was seen between the unperforated and perforated groups (Table 2). Perforations were elliptical, 2.53±0.73 × 10-3 mm2 (n=6; CV 29%) (Table 3, Figure 6).
Table 3.
Membrane and Perforation Characteristics of Perforated RWM
| # | Size (mm2) | Perforation (mm2) | Perforation (%) | KP PORE (10-5 ms-1) |
|---|---|---|---|---|
| 1 | 1.19 | 0.00142 | 0.119 | 6.82 |
| 2 | 1.18 | 0.00241 | 0.204 | 9.65 |
| 3 | 1.23 | 0.00294 | 0.238 | 11.1 |
| 4 | 1.12 | 0.00348 | 0.311 | 5.10 |
| 5 | 1.05 | 0.00288 | 0.273 | 7.75 |
| 6 | 1.10 | 0.00204 | 0.186 | 9.45 |
Figure 6.
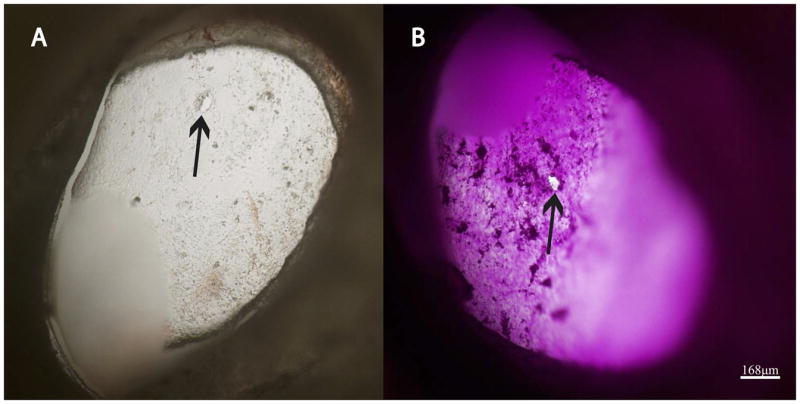
RWM is shown with microperforation(arrows) before (A) and after (B) diffusion experiment. RWM was imaged with Z-stacking (Olympus FSX-100, Center Valley, PA); 60 images at 5μm intervals were taken and compiled to one image.
Permeability with Microperforations
Diffusion experiments were carried out on RWM samples after manual creation of microperforations. The procedure of the diffusion experiment in perforated group was identical to unperforated group. The permeability coefficient of the RWM with microperforations was measured at 18.1±6.1 × 10-8 m/s with a coefficient of variance of 34%. This represents a statistically significant increase in permeability of 35 times with 0.22% microperforation in the round window membrane. F-test showed equal variance between groups; Two Tailed T-test for equal variances performed with alpha 0.05, p<0.05 (Table 2).
DISCUSSION
Our study focuses on the methodology and enhancement of drug delivery to the inner ear. After adapting the standardized static diffusion cell method to the study of inner ear drug delivery, we applied this method to the study of microperforations as a means of permeability enhancement. RWM is permeable to a large range of materials, including various antimicrobials, steroids, anesthetics, tracers, albumin, horseradish peroxidase, latex spheres, germicidal solutions, water, ions and macromolecules24. The extent of permeability to these various materials is dependent on the size, charge, liposolubility and morphology of the compound as well as RWM thickness25. The adapted Franz cell method provides a standardized, controlled means to study permeability enhancers, both pharmaceutical and surgical, on the RWM. This method can also be applied to the study of drug release formulations with new models that extrapolate the standard diffusion cell to the unique physical dimensions of the cochlea17.
The observed RWM permeability coefficient to RhoB is smaller than those seen in previous studies for gentamicin and dexamethasone (Table 1). This is consistent with the lower diffusion coefficient of RhoB and may also be attributed to significant variability in RWM permeability21,26. In addition to its similarity to medicines routinely used in the ear, RhoB is also relatively inexpensive and easy to detect and measure in small concentrations. While an increase in permeability with macroperforation (>1%) has been shown in the past, a potential concern of pores within the RWM is leakage of perilymph from the scala tympani into the middle ear due to perilymph pressure27. A micropore, by nature, is suited to prevent perilymph leakage without slowing the diffusive transport of therapeutic reagents. In smaller pores, the viscous resistance to the fluid flow due to the close presence of the walls of the pore causes a decrease in the flow rate. In other words, the Reynolds number, which is the ratio of the inertial forces to the viscous forces in the flowing fluid, quantifies this behavior. A small Reynolds number due to large viscous forces leads to laminar rather than turbulent flow of liquid through a pore. Under such circumstances, the fluidic resistance of a circular pore is inversely proportional to the 4th power of the pore diameter. Thus, decreasing a pore diameter by a factor of 10 while increasing the number of holes by 100 times to keep the total area constant, increases the fluid resistance 100 times28; this is one of the reasons why we favor a design with multiple smaller perforations instead of one large hole.
Permeability with a single microperforation was 35 times that of an intact RWM, with permeability across the area of the perforation itself at 8.3±2.2 × 10-5 m/s (CV 26%), 16,000 times the permeability across an intact membrane (Table 2). This change is double the predicted value of 4 × 10-5 m/s based on the diffusion coefficient (Table 1). This may represent a very small quantity of fluid flow across the perforation despite no noticeable change in donor or receptor volumes during the experiment. If we assume ototoxicity from aminoglycosides applied directly to hair cells occurs with 10μM of compound (a documented ototoxic dose in explanted hair cells) then with the dosing regimen seen in this experiment applied to the RWM, an intact membrane would require 7 days to diffuse 10μM across the RWM while a membrane with a single microperforation of approximately 2.5 × 10-3 mm2 would require just under 5 hours29. Assuming minimal interaction between perforations, an increase in the number of perforations will inversely decrease the time necessary to achieve a target dose. Thus, three perforations are expected to reach 10μM in 1.5 hours, and a 3×3 array of perforations in only 30 minutes, a time span more consistent with the capabilities of surgical administration of medication to the RWM and new delayed and continuous release drug formulations14.
The coefficient of variance among intact RWM samples (80%) was higher than the recommended value of <30% capable with diffusion cell studies. We recommend the use of barrier integrity measurements in future studies. Variability in measured permeability results of intact membranes may be from desiccation of the membrane and consequent damage to the epithelial layer during preparation or imaging despite precautions taken to protect the membrane during these steps15. The RWM is a three-layered membrane composed of an outer epithelial layer, thick middle fibrous collagen layer and inner epithelium30. Both the outer and inner epithelial layers play a role in the diffusion of molecules across the RWM, ranging from passive diffusion to pinocytosis. Of note, the coefficient of variance was significantly lower (34%) in perforated samples, which may represent either an alternate method of diffusion compared to the intact membrane or simply the minuteness of the variation at a significantly higher permeability.
Microperforations were manually applied with ease and created irregular tears of similar sizes. Studies on the mechanical properties and shape of the RWM may be able to create more reliable perforations in the RWM31. The RWM is known to spontaneously heal from large perforations32. By creating microperforations, we may be able to transiently increase the permeability of the round window membrane while reducing the possibility of complications (infection, perilymph leakage) due to the natural healing properties of the membrane. In vivo application of microperforations with varying healing times followed by in vitro diffusion across RWM and histology would provide insight into the healing capabilities of RWM. Investigations into the timescale of microperforation healing, and the creation of precise holes rather than tears may allow the introduction of a transient, self-healing opening into the cochlea for controlled drug delivery. The findings of our study open the door to novel manipulation of the RWM for the treatment of inner ear diseases.
Acknowledgments
We would like to thank Dr. Keith Yeager for manufacturing the 3D acrylic holders.
Funding: This project was funded in part by Columbia-Coulter Translational Research Partnership. Catherine M. Kelso was supported by the Dean’s Research Fellowship from the Columbia University College of Physicians and Surgeons. Zhen J. Qian was supported by an NIH T35 Training Grant.
Footnotes
Relevant Financial Disclosures: Dr. Anil Lalwani serves on the Medical Advisory Board of Advanced Bionics Corporation. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1.Stevens G, Flaxman S, Brunskill E, et al. Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. European journal of public health. 2013 Feb;23(1):146–152. doi: 10.1093/eurpub/ckr176. [DOI] [PubMed] [Google Scholar]
- 2.Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. The American journal of medicine. 2010 Aug;123(8):711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein H, Lewis WB, Jackson LE, Rosenberg SI, Thompson JH, Hoffmann KK. Changing trends in the surgical treatment of Meniere’s disease: results of a 10-year survey. Ear, nose, & throat journal. 2003 Mar;82(3):185–187. 191–184. [PubMed] [Google Scholar]
- 4.Giannuzzi AL, Merkus P, Falcioni M. The use of intratympanic gentamicin in patients with vestibular schwannoma and disabling vertigo. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2013 Aug;34(6):1096–1098. doi: 10.1097/MAO.0b013e3182804c41. [DOI] [PubMed] [Google Scholar]
- 5.Rauch SD. Intratympanic steroids for sensorineural hearing loss. Otolaryngologic clinics of North America. 2004 Oct;37(5):1061–1074. doi: 10.1016/j.otc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Plontke S, Lowenheim H, Preyer S, et al. Outcomes research analysis of continuous intratympanic glucocorticoid delivery in patients with acute severe to profound hearing loss: basis for planning randomized controlled trials. Acta oto-laryngologica. 2005 Aug;125(8):830–839. doi: 10.1080/00016480510037898. [DOI] [PubMed] [Google Scholar]
- 7.Jackson LE, Silverstein H. Chemical perfusion of the inner ear. Otolaryngologic clinics of North America. 2002 Jun;35(3):639–653. doi: 10.1016/s0030-6665(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 8.Light JP, Silverstein H, Jackson LE. Gentamicin perfusion vestibular response and hearing loss. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2003 Mar;24(2):294–298. doi: 10.1097/00129492-200303000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter AM, Muchow D, Goycoolea MV. Ultrastructural studies of the human round window membrane. Archives of otolaryngology--head & neck surgery. 1989 May;115(5):585–590. doi: 10.1001/archotol.1989.01860290043012. [DOI] [PubMed] [Google Scholar]
- 10.Sahni RS, Paparella MM, Schachern PA, Goycoolea MV, Le CT. Thickness of the human round window membrane in different forms of otitis media. Archives of otolaryngology--head & neck surgery. 1987 Jun;113(6):630–634. doi: 10.1001/archotol.1987.01860060056015. [DOI] [PubMed] [Google Scholar]
- 11.Yoda S, Cureoglu S, Shimizu S, et al. Round window membrane in Meniere’s disease: a human temporal bone study. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2011 Jan;32(1):147–151. doi: 10.1097/MAO.0b013e318200a0e0. [DOI] [PubMed] [Google Scholar]
- 12.Hoffer ME, Allen K, Kopke RD, Weisskopf P, Gottshall K, Wester D. Transtympanic versus sustained-release administration of gentamicin: kinetics, morphology, and function. The Laryngoscope. 2001 Aug;111(8):1343–1357. doi: 10.1097/00005537-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Mikulec AA, Hartsock JJ, Salt AN. Permeability of the round window membrane is influenced by the composition of applied drug solutions and by common surgical procedures. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2008 Oct;29(7):1020–1026. doi: 10.1097/MAO.0b013e31818658ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowe SN, Jacob A. Round window perfusion dynamics: implications for intracochlear therapy. Current opinion in otolaryngology & head and neck surgery. 2010 Oct;18(5):377–385. doi: 10.1097/MOO.0b013e32833d30f0. [DOI] [PubMed] [Google Scholar]
- 15.Salt AN, Hale SA, Plonkte SK. Perilymph sampling from the cochlear apex: a reliable method to obtain higher purity perilymph samples from scala tympani. Journal of neuroscience methods. 2006 May 15;153(1):121–129. doi: 10.1016/j.jneumeth.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franz TJ. Percutaneous absorption on the relevance of in vitro data. The Journal of investigative dermatology. 1975 Mar;64(3):190–195. doi: 10.1111/1523-1747.ep12533356. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Feng L, Tolia G, Liddell MR, Hao J, Li SK. Evaluation of intratympanic formulations for inner ear delivery: methodology and sustained release formulation testing. Drug development and industrial pharmacy. 2013 Apr 30; doi: 10.3109/03639045.2013.789054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Recommended Protocol for In vitro Percutaneous Absorption Rate Studies. Federal Register. 1996;61(65) [Google Scholar]
- 19.Gendron PO, Avaltroni F, Wilkinson KJ. Diffusion coefficients of several rhodamine derivatives as determined by pulsed field gradient-nuclear magnetic resonance and fluorescence correlation spectroscopy. Journal of fluorescence. 2008 Nov;18(6):1093–1101. doi: 10.1007/s10895-008-0357-7. [DOI] [PubMed] [Google Scholar]
- 20.Culbertson CT, Jacobson SC, Michael Ramsey J. Diffusion coefficient measurements in microfluidic devices. Talanta. 2002 Feb 11;56(2):365–373. doi: 10.1016/s0039-9140(01)00602-6. [DOI] [PubMed] [Google Scholar]
- 21.Plontke SK, Mynatt R, Gill RM, Borgmann S, Salt AN. Concentration gradient along the scala tympani after local application of gentamicin to the round window membrane. The Laryngoscope. 2007 Jul;117(7):1191–1198. doi: 10.1097/MLG.0b013e318058a06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.OECD. Test No 428: Skin Absorption: In Vitro Method. OECD Publishing; [Google Scholar]
- 23.Ng SF, Rouse JJ, Sanderson FD, Meidan V, Eccleston GM. Validation of a static Franz diffusion cell system for in vitro permeation studies. AAPS PharmSciTech. 2010 Sep;11(3):1432–1441. doi: 10.1208/s12249-010-9522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goycoolea MV. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta oto-laryngologica. 2001 Jun;121(4):437–447. doi: 10.1080/000164801300366552. [DOI] [PubMed] [Google Scholar]
- 25.Goycoolea MV, Muchow D, Schachern P. Experimental studies on round window structure: function and permeability. The Laryngoscope. 1988 Jun;98(6 Pt 2 Suppl 44):1–20. doi: 10.1288/00005537-198806001-00002. [DOI] [PubMed] [Google Scholar]
- 26.Hahn H, Kammerer B, DiMauro A, Salt AN, Plontke SK. Cochlear microdialysis for quantification of dexamethasone and fluorescein entry into scala tympani during round window administration. Hearing research. 2006 Feb;212(1-2):236–244. doi: 10.1016/j.heares.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salt AN, Sirjani DB, Hartsock JJ, Gill RM, Plontke SK. Marker retention in the cochlea following injections through the round window membrane. Hearing research. 2007 Oct;232(1-2):78–86. doi: 10.1016/j.heares.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sia SK, Whitesides GM. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis. 2003 Nov;24(21):3563–3576. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- 29.Wood JW, Bas E, Gupta C, et al. Otoprotective Properties of Mannitol Against Gentamicin Induced Hair Cell Loss. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2014 Mar 21; doi: 10.1097/MAO.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 30.Goycoolea MV, Lundman L. Round window membrane. Structure function and permeability: a review. Microscopy research and technique. 1997 Feb 1;36(3):201–211. doi: 10.1002/(SICI)1097-0029(19970201)36:3<201::AID-JEMT8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe H, Kysar JW, Lalwani AK. Microanatomic analysis of the round window membrane by white light interferometry and microcomputed tomography for mechanical amplification. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2014 Apr;35(4):672–678. doi: 10.1097/MAO.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 32.Gyo K. Healing of experimentally produced round window membrane rupture. Acta oto-laryngologica. 1989 Jan-Feb;107(1-2):85–89. doi: 10.3109/00016488909127483. [DOI] [PubMed] [Google Scholar]


