Abstract
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an inherited form of cerebral small vessel disease caused by mutations in conserved residues of NOTCH3. Affected arteries of CADASIL feature fibrosis and accumulation of NOTCH3. A variety of collagen subtypes (types I, III, IV, and VI) have been identified in fibrotic CADASIL vessels. Biglycan (BGN) and decorin (DCN), are Class I members of the small leucine-rich proteoglycan (SLRP) family that regulate collagen fibril size. Because DCN has been shown to deposit in arteries in cerebral small vessel disease, we tested whether BGN accumulates in arteries of CADASIL brains. BGN was strongly expressed in both small penetrating and leptomeningeal arteries of CADASIL brain. BGN protein was localized to all three layers of arteries (intima, media, and adventitia). Substantially more immunoreactivity was observed in CADASIL brains compared to controls. Immunoblotting of brain lysates showed a 4-fold increase in CADASIL brains (compared to controls). Messenger RNA encoding BGN was also increased in CADASIL and was localized by in situ hybridization to all three vascular layers in CADASIL. Human cerebrovascular smooth muscle cells exposed to purified NOTCH3 ectodomain upregulated BGN, DCN, and COL4A1 through mechanisms that are sensitive to rapamycin, a potent mTOR inhibitor. In addition, BGN protein interacted directly with NOTCH3 protein in cell culture and in direct protein interaction assays. In conclusion, BGN is a CADASIL-enriched protein that potentially accumulates in vessels by mTOR-mediated transcriptional activation and/or post-translational accumulation via protein interactions with NOTCH3 and collagen.
Keywords: CADASIL, small leucine rich proteoglycans, biglycan, collagen, Notch, arteries, protein interactions
Introduction
Cerebral small vessel disease is a major cause of stroke and an important factor in the pathogenesis of dementia [1]. Genetic causes of cerebral small vessel disease provide opportunities to discover molecules which may participate in cerebral arteriopathy. The best-recognized inherited cause of small vessel disease is cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), a condition caused by mutations in conserved residues of NOTCH3 [2].
CADASIL arteries are markedly thickened [3, 4], and there is significant loss of smooth muscle cells that are replaced by amorphous material that includes excessive NOTCH3 ectodomain [5]. These abnormally thickened vessels are dominated by hypocellular extracellular matrix, which includes type I, III, and VI collagens [6], major final components of tissue fibrosis, as well as type IV collagen [6]. At the ultrastructural level, small deposits of material known as granular osmiophilic material (GOM) accumulate around smooth muscle cells [7, 8]; these are likely consequences of accumulation and aggregation of extracellular proteins [9]. All of these changes, including accumulation of abnormal proteins in multiple layers of arteries, are present in both leptomeningeal and penetrating small arteries of the gray and white matter [9]. Molecules that contribute to arterial thickening in CADASIL are still emerging and, based on positive periodic acid-Schiff staining, may include glycoproteins [10].
The small leucine-rich proteoglycans (SLRPs) compose a class of multifunction glycoproteins secreted into the extracellular matrix in many tissues. There are 17 members in the SLRP familiy that are characterized by extensive post-translational glycosylation of a relatively small protein core backbone that is composed of repeats rich in leucine. Biglycan (BGN) and decorin (DCN) are the best characterized of the SLRP family members. Their functions include modification of extracellular matrix structure and modulation of a number of signaling systems, which have been well-documented in a wide range of tissues [11]. BGN and DCN bind collagen proteins [12–14] and are components of extracellular fibers which may in turn further modify fiber-forming complexes of the extracellular matrix. The function of BGN has been extensively examined in musculo-skeletal tissue including bone [15], cartilage [16], tendon [17], muscle [18] [19] and teeth [20, 19]. The molecular foundation for this regulation includes both structural regulation and fine-tuning of critical growth factors such as BMP-2, TGF-beta and Wnt3a [21–23]. BGN binds to Toll 2 and Toll 4 receptors to modulate the immune system [24]. Some of BGN’s functions have only been revealed by disease challenge and include the induction of myocardial infarction [25], oviarectomy [26] and bone fracture [27].
Accumulation of DCN protein and mRNA was recently demonstrated in small penetrating arteries of CADASIL [28]. Strong expression of both collagen and DCN in brain arteries in CADASIL prompted us to hypothesize that BGN may also accumulate within thickened blood vessels in this disease. Here, we characterize the expression of BGN in CADASIL and identify candidate mechanisms that may drive the accumulation of this cerebrovascular protein.
Methods
Brain histology
Formalin fixed frontal lobes sections were acquired from the Brain Bank of the National Institute for Developmental and Childhood Disorders at the University of Maryland and the Alzheimers Disease Research Core at the University of Michigan and have been previously described [6, 29]. In addition, two CADASIL brains from patients with ischemic stroke and dementia with the NOTCH3 mutations R141C and R153C were studied [28]; both of these patients were Caucasian males in their 60s who died of complications of the disease. All brains in the CADASIL group showed severe small vessel disease, with significant white matter hyperintensities on premortem MRI imaging. The ages of CADASIL and controls were on average 66 years (n=8, range 46–83) and 63 years (n=6, range 47–82). Five micron sections from frontal cortex were analyzed using chromagenic immunohistochemical staining, counterstained with hematoxylin. We verified brain tissue antigen integrity using mouse monoclonal antibody BRIC231 (anti-H; Santa Cruz). The mouse monoclonal antibody 4E1-1G7 (Abnova) against human BGN was used for immunohistochemistry at 1:1000 dilution.
In situ hybridization
Localization of BGN mRNA in tissue sections was performed using a system developed by Advanced Cell Diagnostics. The protocol incorporated hybridization of nucleic acid probes, multiple non-enzymatic amplification steps, and probe detection using an alkaline phosphatase-conjugated terminal probed. Only three CADASIL brain blocks that resulted in signal with ubiquitous positive control probes were used in this study.
Protein quantification
Brain protein homogenates were prepared from frozen frontal cortex and analyzed by immunoblotting using 4E1-1G7 at 1:1000 dilution. Labeled secondary antibodies were detected using a Licor Odyssey infrared scanner. Expression levels were normalized to tubulin content.
RNA quantification
RNA from frozen brain tissue or cell cultures was purified using an RNeasy kit, reverse transcribed, and the cDNA was quantified by real time PCR, using HPRT, as a control; the primer sequences were: Human BGN sense: 5'-TCCGACCTGGGTCTGAAGT- 3' and antisense: 5'-GCCTTCTCATGGATCTTGGA-3'. Human HPRT: Sense: 5'-TGGCGTCGTGATTAGTGATG-3' and antisense: 5'-AATCCAGCAGGTCAGCAAAG-3'. RNA quality was determined by agarose gel electrophoresis, and samples included here demonstrated expected ribosomal RNA banding patterns. Samples which did not show clear banding were excluded from analysis.
Cell culture and immunoprecipitation
HEK 293 cells were grown in Dulbecco’s modified Eagle’s medium (Invitrogen) with 10% fetal bovine serum. Transfections and immunoprecipitations were performed using Lipofectamine 2000 (Invitrogen) as described before [30]. In brief, cell lysates were prepared after 48 hours after transfection, antibodies were added to lysates, and protein G agarose was used to pull down immune complexes which were then analyzed by immunoblotting.
Human cerebral smooth muscle cells were purchased from ScienCell Research Laboratories and grown in smooth muscle cell medium as recommended by the manufacturer. Cells were serum starved before challenge with purified recombinant NOTCH3-Fc fusion protein (400ng/mL). This protein was purified by protein A chromatography to homogeneity from stable 293 cell lines that secreted the protein into conditioned media as described [31]. Challenge with NOTCH3-Fc or Fc control protein (R&D Systems) was performed for 24 hours prior to RNA analysis. Cells challenged with NOTCH-Fc were simultaneously treated with rapamycin dissolved in DMSO.
Solid phase binding assays
Recombinant proteins human BGN and Notch-3-Fc (first 11 EGF repeats) were purchased from R&D Systems. Purified unlabeled binding target proteins were adsorbed to plates in PBS at 5 ug/ml. Solid phase assays were performed as described [30, 32]. We labeled purified proteins with Alexa 700 succinimide and then removed free label by gel filtration chromatography. Protein-coated plates were blocked using 0.5 % BSA in Tris-buffered saline (50 mM Tris, 150 mM NaCl) with 2 mM CaCl2 for 1 h at room temperature. Labeled proteins were added to protein-coated ELISA plates in Tris-buffered saline with 2 mM CaCl2 at 4 °C for 16 hours. Plates were washed three times with TBS with 2 mM calcium and quantified using a LiCor flatbed infrared scanner.
Statistical analysis
Results are displayed with standard deviations. All quantitative PCR studies were done with groups of three. T-tests were applied with statistically significant differences considered for p<0.05.
Results
BGN protein in CADASIL
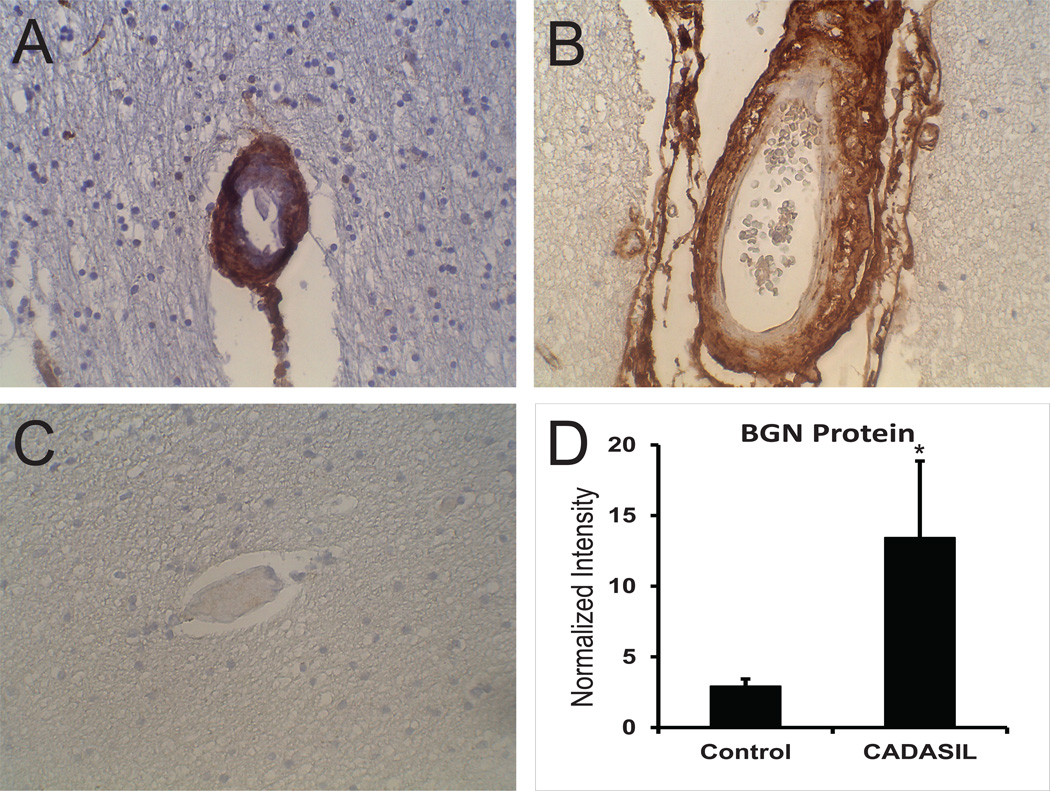
We examined the distribution of BGN in genetically-confirmed CADASIL brains (Figure 1). Immunohistochemical analysis of BGN showed abundant vascular staining predominantly in arteries affected by diseaseIn small penetrating arteries of the white matter, which typically show marked hyaline thickening, BGN was deposited densely through the entire thickness of the vessel. Capillaries in CADASIL were also stained, albeit more lightly than arteries (not shown). BGN was found predominantly in all layers of leptomeningeal arteries, with increasing density moving away from the lumen.
Figure 1.
BGN protein in CADASIL and control brain. Immunohistochemical analysis of genetically-defined CADASIL brains (A and B) and matched controls (C) was performed on frontal cortex sections with antibody 4E1-1G7. Penetrating arteries (A, C) and leptomeningeal (B) vessels are shown. Magnification is at 400x. (D) Brain lysates were analyzed by immunoblotting with 4E1-1G7 and the core BGN protein band was quantified for CADASIL and control frontal cortex, normalized to tubulin levels. Protein was not treated with glycosidases. Difference were significant (p<0.05) for CADASIL samples versus controls.
Normal appearing penetrating (Figure 1C) and leptomeninigeal arteries of controls showed light adventitial staining without smooth muscle or endothelial expression, and capillary staining was variable. Immunoblot analysis of proteins from brains demonstrated significant increases in BGN in CADASIL compared to control brain (Figure 1D).
BGN transcripts in CADASIL
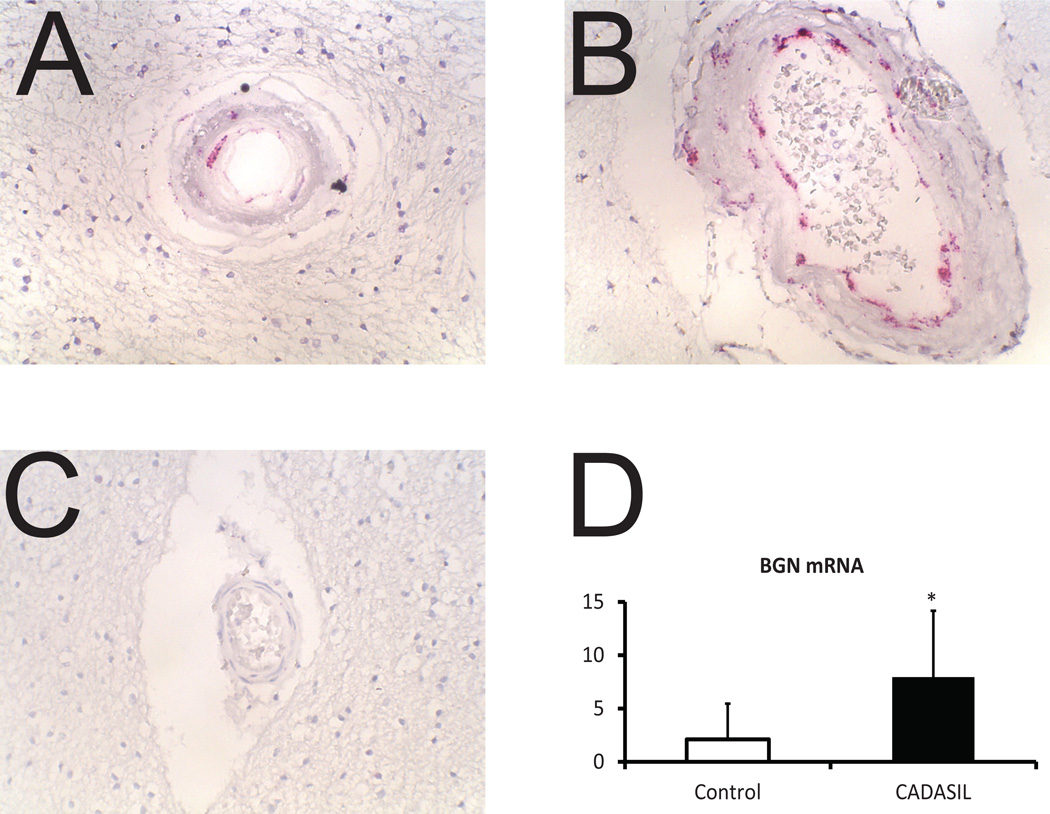
In situ hybridization revealed predominantly vascular expression of BGN in CADASIL (Figure 2A). RNA was expressed in cells located in the same vascular layers in which we identified BGN protein: the adventitia, media, and intima of leptomeningeal arteries. BGN mRNA was also detected in cells of penetrating arteries of the white matter (Figure 2B). Light capillary expression of BGN matched protein staining. In controls, BGN mRNA was reduced (Figure 2C). Quantitative RT-PCR revealed a substantial increase in BGN mRNA in CADASIL brain compared to controls (Figure 2D).
Figure 2.
Localization of BGN mRNA in CADASIL and control brain vessels. In situ hybridization was performed on frontal cortex of genetically-defined CADASIL brains (A and B) and controls (C). Small penetrating arteries (A and C) and leptomeningeal vessels (B) are shown. Magnification is at 400x. (D) Quantitative RT-PCR analysis of BGN mRNA from controls and CADASIL brains are shown; differences were significant with p<0.05.
Regulation of BGN mRNA by excessive NOTCH3 ectodomain
NOTCH3 is dramatically deposited in CADASIL vessels [5], suggesting a pathogenic role of NOTCH3 excess in pathogenesis of small vessel disease. We investigated the effects of excessive NOTCH3 on BGN and related factors in human cerebrovascular smooth muscle cells (HC-SMC) grown in culture (Figure 3). Incubation of HC-SMC with purified NOTCH3-Fc fusion protein significantly increased BGN mRNA relative to levels in cells treated with Fc protein.
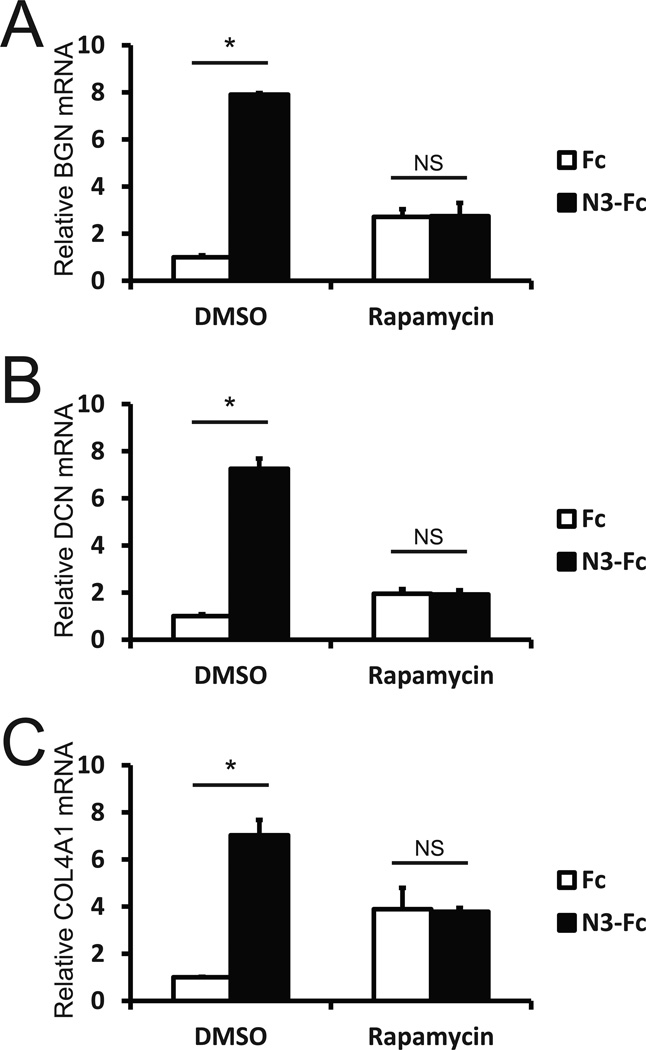
Figure 3.
Regulation of BGN mRNA levels in human cerebrovascular smooth muscle cells. Primary vascular smooth muscle cells from human brain were serum starved and then challenged with purified NOTCH3-Fc fusion protein or Fc control protein. One day after challenge with proteins, BGN (A), DCN (B), and COL4A1 (C) mRNA levels were assessed using quantitative RT-PCR. During the protein challenge, cells were exposed to DMSO vehicle or rapamycin to assess the role of PI3K or mTOR signaling on BGN transcript induction. Differences between NOTCH3 and control treated cells were all significant with p<0.05. There were no significant differences between these groups after rapamycin treatment.
Rapamycin, a potent and specific inhibitor of mTOR signaling, has been used clinically to block stent stenosis by intimal hyperplasia, which is associated with BGN elevation [33, 34]. We therefore investigated whether this drug would affect NOTCH3 ectodomain-driven BGN gene activation (Figure 3, bars on the right). The elevation of BGN transcripts in response to NOTCH3-Fc was blocked by rapamycin. Messenger RNA encoding additional components of pathological vessels, DCN [28] and COL4A1 [6], were also upregulated by incubation with NOTCH3 ectodomain. Stimulation of both DCN and COL4A1 message was also blocked by rapamycin.
Interactions between BGN and NOTCH3 proteins
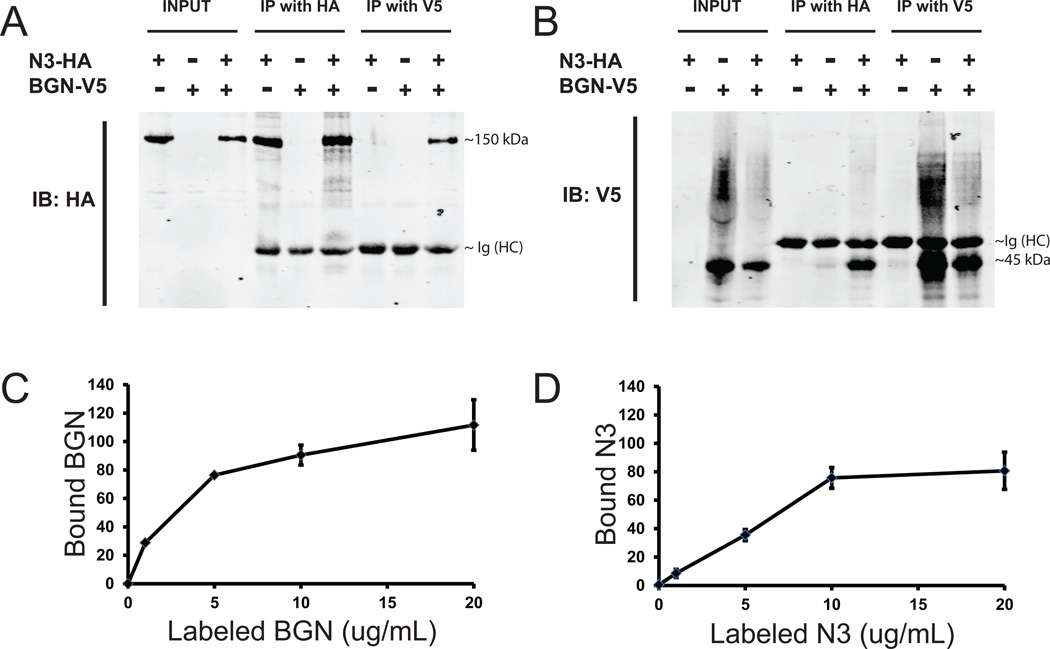
In addition to regulation at the transcriptional level, CADASIL-enriched proteins may accumulate in vessels by virtue of protein-protein interactions that trap molecules with the degenerating arterial matrix. A large increase in NOTCH3 protein has been reported in CADASIL arteries [5]; thus, binding to NOTCH3 may result in anchoring of proteins in diseased arteries [35]. To test whether BGN binds to NOTCH3, we performed co-immunoprecipitation assays in cells expressing both proteins. Protein immunopurified with BGN antibodies contained NOTCH3 protein. Conversely, NOTCH3 immunoprecipitates also contained BGN protein (Figure 4A–B).
Figure 4.
Molecular interaction between BGN and NOTCH3 proteins. (A-B) 293 cells were transfected with genes encoding the indicated epitope-tagged proteins, as indicated in the legend above. Expression levels in cell lysates are shown on the left of each panel (INPUT). After treatment of lysates with indicated antibodies (IP with HA or IP with V5), proteins were pulled down using protein G-agarose were analyzed by immunoblotting (IB) with either HA (A) or V5 (B) monoclonal antibodies. NOTCH3 ectodomain tagged with HA (N3-HA) migrates at approximately 150kDa. The 50kDa band in lanes with IP proteins corresponds to the heavy chain of immunoglobulin used for the IP (Ig (HC)). The tagged BGN (BGN-V5) band migrates at approximately 45 kDa, with an upward smear that corresponds to glycosylated product. (C-D) Direct protein binding assays were performed by first adsorbing pure recombinant NOTCH3 (C) or BGN (D) to plastic dishes. Labeled BGN (in C) or NOTCH3 (in D), at increasing concentrations shown on the X-axis, was incubated with coated plates, which were washed and then quantitatively imaged to assess protein binding (on the Y-axis). Labeled Fc protein was used as a negative control and resulted in negligible binding to BGN- and NOTCH3-coated plates.
To confirm molecular interactions between BGN and NOTCH3, we tested the ability of labeled BGN to bind to purified NOTCH3 in vitro. We observed dose dependent binding of BGN to NOTCH3 but not to Fc (control protein) (Figure 4C). Similar results were obtained when labeled NOTCH3 was applied to BGN coated wells (Figure 4D).
Discussion
Thickening of degenerating arteries of the brain is a striking feature of CADASIL. In extracerebral vessels, proteoglycans have been implicated in atherosclerosis [36, 37]. Studies identifying a number of types of collagen [38, 6, 35] in CADASIL vessels led us to hypothesize that proteoglycans known to bind collagen may localize to diseased arteries. Here, we demonstrate abundant accumulation of the collagen-binding proteoglycan BGN in CADASIL arteries.
Our studies suggest two mechanisms by which BGN could accumulate in CADASIL vessels. First, transcripts encoding BGN are increased in CADASIL; furthermore, in situ hybridization demonstrates an increase in vascular BGN mRNA specifically in intimal, medial and adventitial cells within arteries. Thus, arterial BGN transcriptional upregulation likely contributes to protein accumulation in vivo. Our studies suggest that in CADASIL, accumulation of NOTCH3 ectodomain in arterial smooth muscle cells may be sufficient to trigger increased BGN expression. Furthermore, experiments in cell culture implicate involvement of the mTOR pathway in activation of BGN mRNA. Stimulation of mTOR initiated by NOTCH3 ectodomain also resulted in upregulation of additional components of thickened vessels in CADASIL, DCN and COL4A1. Thus, BGN, DCN, and COL4A1 appear to be coordinately regulated in cerebral smooth muscle cells via a common signaling pathway. To our knowledge, this is the first signaling pathway identified that regulates transcripts enriched in CADASIL vessels. The mTOR pathway has been previously implicated in the development of intimal hyperplasia [39], which has resulted in deployment of rapamycin-eluting stents [40]; this is the first example of interactions between mTOR, Notch proteins, and BGN, and these studies suggest that pharmacological blockade of mTOR could modify intimal hyperplasia through BGN regulation. Of note, intimal hyperplasia is a core feature of CADASIL pathology [3].
Secondly, BGN accumulation in CADASIL may result from post-translational mechanisms. Specifically, BGN could become anchored to the vessel wall via protein binding partners that are up-regulated in CADASIL. These partners include collagen [6] and NOTCH3, which we show here for the first time is a BGN molecular partner. Attachment of BGN to extracellular components of CADASIL vessels may impair clearance of the protein. Of note, several additional proteins that build up in CADASIL vessels have been shown to physically interact with NOTCH3 protein (eg. vWF [41], collagen [42], TIMP3 [35], and NOTCH3 itself [31]). The binding of BGN to both NOTCH3 and collagen suggests the possibility that complex multimolecular complexes may form in CADASIL. The complexes, which likely feature multiple domains of interactions, could amplify protein accumulation and vascular thickening.
As a hydrophilic proteoglycan, BGN alter properties of the extracellular matrix by tissue-and gender-specific modulation of collagen structure [16, 43, 44] which may, in the brain, contribute to arterial thickening. On the other hand, the possibility of a homeostatic role of BGN is supported by Heegaard et al [44] who described a potential role of BGN in stabilizing vascular smooth muscle in mice and preventing aneurysm formation. In addition, in vitro, BGN binds TGFbeta [45] and blocks signaling [46], which is implicated in human small vessel disease [47]. The role of BGN in inhibiting TGFbeta mediated fibrosis in vivo is not as clear as for other SLRPs [46]. Assignment of a functional role of BGN awaits overexpression and knockout studies of BGN in the context of accelerated CADASIL animal models.
In conclusion, we have identified for the first time the accumulation the proteoglycan BGN in arteries of CADASIL patients. Accumulation of BGN may result from a combination of mTOR-dependent transcriptional upregulation and/or binding to proteins that contribute to molecular networks that drive vascular wall thickening.
Acknowledgements
NIH (NS052681, NS054724, and NS062816) and the Department of Veterans Affairs (5I01BX000375) provided funding for these studies. This research was supported, in part, by the DIR, NIDCR of the IRP, NIH, DHHS.
Footnotes
Compliance with Ethics Requirements
Xiaojie Zhang, Soo Jung Lee, Marian F.Young, and Michael M. Wang declare that they have no conflict of interest.
This article does not contain any studies with living human or animal subjects.
References
- 1.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi: 10.1016/S1474-4422(10)70104-6. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 2.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707–710. doi: 10.1038/383707a0. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 3.Dong H, Ding H, Young K, Blaivas M, Christensen PJ, Wang MM. Advanced intimal hyperplasia without luminal narrowing of leptomeningeal arteries in CADASIL. Stroke. 2013;44(5):1456–1458. doi: 10.1161/STROKEAHA.111.000721. doi: 10.1161/STROKEAHA.111.0007211 STROKEAHA.111.000721 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao Q, Paloneva T, Tuominen S, Poyhonen M, Tuisku S, Viitanen M, et al. Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain pathology. 2004;14(4):358–364. doi: 10.1111/j.1750-3639.2004.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105(5):597–605. doi: 10.1172/JCI8047. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong H, Blaivas M, Wang MM. Bidirectional encroachment of collagen into the tunica media in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Res. 2012;1456:64–71. doi: 10.1016/j.brainres.2012.03.037. doi: S0006-8993(12)00546-X [pii]10.1016/j.brainres.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baudrimont M, Dubas F, Joutel A, Tournier-Lasserve E, Bousser MG. Autosomal dominant leukoencephalopathy and subcortical ischemic stroke. A clinicopathological study. Stroke. 1993;24(1):122–125. doi: 10.1161/01.str.24.1.122. [DOI] [PubMed] [Google Scholar]
- 8.Estes ML, Chimowitz MI, Awad IA, McMahon JT, Furlan AJ, Ratliff NB. Sclerosing vasculopathy of the central nervous system in nonelderly demented patients. Arch Neurol. 1991;48(6):631–636. doi: 10.1001/archneur.1991.00530180087022. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto Y, Craggs LJ, Watanabe A, Booth T, Attems J, Low RW, et al. Brain microvascular accumulation and distribution of the NOTCH3 ectodomain and granular osmiophilic material in CADASIL. J Neuropathol Exp Neurol. 2013;72(5):416–431. doi: 10.1097/NEN.0b013e31829020b5. doi: 10.1097/NEN.0b013e31829020b5. [DOI] [PubMed] [Google Scholar]
- 10.Brulin-Fardoux P, Godfrain C, Maurage CA, De Reuck J, Hauw JJ, Kaltner H, et al. Glycohistochemical characterization of vascular muscle cell destruction in CADASIL subjects by lectins, neoglycoconjugates and galectin-specific antibodies. Neuropathology and applied neurobiology. 2003;29(4):400–410. doi: 10.1046/j.1365-2990.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 11.Nastase MV, Young MF, Schaefer L. Biglycan: a multivalent proteoglycan providing structure and signals. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2012;60(12):963–975. doi: 10.1369/0022155412456380. doi: 10.1369/0022155412456380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schonherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. Interaction of biglycan with type I collagen. J Biol Chem. 1995;270(6):2776–2783. doi: 10.1074/jbc.270.6.2776. [DOI] [PubMed] [Google Scholar]
- 13.Wiberg C, Heinegard D, Wenglen C, Timpl R, Morgelin M. Biglycan organizes collagen VI into hexagonal-like networks resembling tissue structures. J Biol Chem. 2002;277(51):49120–49126. doi: 10.1074/jbc.M206891200. doi: 10.1074/jbc.M206891200. [DOI] [PubMed] [Google Scholar]
- 14.Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegard D, et al. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem. 2003;278(39):37698–37704. doi: 10.1074/jbc.M304638200. doi: 10.1074/jbc.M304638200. [DOI] [PubMed] [Google Scholar]
- 15.Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nature genetics. 1998;20(1):78–82. doi: 10.1038/1746. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 16.Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16(7):673–680. doi: 10.1096/fj.01-0848com. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 17.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature medicine. 2007;13(10):1219–1227. doi: 10.1038/nm1630. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 18.Casar JC, McKechnie BA, Fallon JR, Young MF, Brandan E. Transient up-regulation of biglycan during skeletal muscle regeneration: delayed fiber growth along with decorin increase in biglycan-deficient mice. Dev Biol. 2004;268(2):358–371. doi: 10.1016/j.ydbio.2003.12.025. doi: 10.1016/j.ydbio.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 19.Young MF, Fallon JR. Biglycan: a promising new therapeutic for neuromuscular and musculoskeletal diseases. Current opinion in genetics & development. 2012;22(4):398–400. doi: 10.1016/j.gde.2012.07.008. doi: 10.1016/j.gde.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg M, Septier D, Rapoport O, Iozzo RV, Young MF, Ameye LG. Targeted disruption of two small leucine-rich proteoglycans, biglycan and decorin, excerpts divergent effects on enamel and dentin formation. Calcified tissue international. 2005;77(5):297–310. doi: 10.1007/s00223-005-0026-7. doi: 10.1007/s00223-005-0026-7. [DOI] [PubMed] [Google Scholar]
- 21.Berendsen AD, Fisher LW, Kilts TM, Owens RT, Robey PG, Gutkind JS, et al. Modulation of canonical Wnt signaling by the extracellular matrix component biglycan. Proc Natl Acad Sci U S A. 2011;108(41):17022–17027. doi: 10.1073/pnas.1110629108. doi: 10.1073/pnas.1110629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi Y, Stuelten CH, Kilts T, Wadhwa S, Iozzo RV, Robey PG, et al. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280(34):30481–30489. doi: 10.1074/jbc.M500573200. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- 23.Chen XD, Fisher LW, Robey PG, Young MF. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18(9):948–958. doi: 10.1096/fj.03-0899com. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115(8):2223–2233. doi: 10.1172/JCI23755. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westermann D, Mersmann J, Melchior A, Freudenberger T, Petrik C, Schaefer L, et al. Biglycan is required for adaptive remodeling after myocardial infarction. Circulation. 2008;117(10):1269–1276. doi: 10.1161/CIRCULATIONAHA.107.714147. doi: 10.1161/CIRCULATIONAHA.107.714147. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen KL, Allen MR, Bloomfield SA, Andersen TL, Chen XD, Poulsen HS, et al. Biglycan deficiency interferes with ovariectomy-induced bone loss. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2003;18(12):2152–2158. doi: 10.1359/jbmr.2003.18.12.2152. doi: 10.1359/jbmr.2003.18.12.2152. [DOI] [PubMed] [Google Scholar]
- 27.Berendsen AD, Pinnow EL, Maeda A, Brown AC, McCartney-Francis N, Kram V, et al. Biglycan modulates angiogenesis and bone formation during fracture healing. Matrix biology : journal of the International Society for Matrix Biology. 2014;35:223–231. doi: 10.1016/j.matbio.2013.12.004. doi: 10.1016/j.matbio.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SJ, Zhang X, Wang MM. Vascular accumulation of the small leucine-rich proteoglycan decorin in CADASIL. Neuroreport. 2014 doi: 10.1097/WNR.0000000000000230. doi: 10.1097/WNR.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Meng H, Blaivas M, Rushing EJ, Moore BE, Schwartz J, et al. Von Willebrand Factor permeates small vessels in CADASIL and inhibits smooth muscle gene expression. Transl Stroke Res. 2012;3(1):138–145. doi: 10.1007/s12975-011-0112-2. doi: 10.1007/s12975-011-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng H, Zhang X, Hankenson KD, Wang MM. Thrombospondin 2 potentiates notch3/jagged1 signaling. J Biol Chem. 2009;284(12):7866–7874. doi: 10.1074/jbc.M803650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng H, Zhang X, Yu G, Lee SJ, Chen YE, Prudovsky I, et al. Biochemical characterization and cellular effects of CADASIL mutants of NOTCH3. PLoS One. 2012;7(9):e44964. doi: 10.1371/journal.pone.0044964. doi: 10.1371/journal.pone.0044964 PONE-D-11-06949 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng H, Zhang X, Lee SJ, Strickland DK, Lawrence DA, Wang MM. Low density lipoprotein receptor-related protein-1 (LRP1) regulates thrombospondin-2 (TSP2) enhancement of Notch3 signaling. J Biol Chem. 2010;285(30):23047–23055. doi: 10.1074/jbc.M110.144634. doi: M110.144634 [pii]10.1074/jbc.M110.144634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung IM, Gold HK, Schwartz SM, Ikari Y, Reidy MA, Wight TN. Enhanced extracellular matrix accumulation in restenosis of coronary arteries after stent deployment. Journal of the American College of Cardiology. 2002;40(12):2072–2081. doi: 10.1016/s0735-1097(02)02598-6. [DOI] [PubMed] [Google Scholar]
- 34.Yamakawa T, Bai HZ, Masuda J, Sawa Y, Shirakura R, Ogata J, et al. Differential expression of proteoglycans biglycan and decorin during neointima formation after stent implantation in normal and atherosclerotic rabbit aortas. Atherosclerosis. 2000;152(2):287–297. doi: 10.1016/s0021-9150(99)00475-x. [DOI] [PubMed] [Google Scholar]
- 35.Monet-Lepretre M, Haddad I, Baron-Menguy C, Fouillot-Panchal M, Riani M, Domenga-Denier V, et al. Abnormal recruitment of extracellular matrix proteins by excess Notch3 ECD: a new pathomechanism in CADASIL. Brain. 2013;136(Pt 6):1830–1845. doi: 10.1093/brain/awt092. doi: 10.1093/brain/awt092awt092 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Brien KD, Olin KL, Alpers CE, Chiu W, Ferguson M, Hudkins K, et al. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques: colocalization of biglycan with apolipoproteins. Circulation. 1998;98(6):519–527. doi: 10.1161/01.cir.98.6.519. [DOI] [PubMed] [Google Scholar]
- 37.Riessen R, Isner JM, Blessing E, Loushin C, Nikol S, Wight TN. Regional differences in the distribution of the proteoglycans biglycan and decorin in the extracellular matrix of atherosclerotic and restenotic human coronary arteries. Am J Pathol. 1994;144(5):962–974. [PMC free article] [PubMed] [Google Scholar]
- 38.Arboleda-Velasquez JF, Manent J, Lee JH, Tikka S, Ospina C, Vanderburg CR, et al. Hypomorphic Notch 3 alleles link Notch signaling to ischemic cerebral small-vessel disease. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(21):E128–E135. doi: 10.1073/pnas.1101964108. doi: 10.1073/pnas.1101964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gregory CR, Huie P, Billingham ME, Morris RE. Rapamycin inhibits arterial intimal thickening caused by both alloimmune and mechanical injury. Its effect on cellular, growth factor, and cytokine response in injured vessels. Transplantation. 1993;55(6):1409–1418. doi: 10.1097/00007890-199306000-00037. [DOI] [PubMed] [Google Scholar]
- 40.Sousa JE, Costa MA, Abizaid A, Abizaid AS, Feres F, Pinto IM, et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001;103(2):192–195. doi: 10.1161/01.cir.103.2.192. [DOI] [PubMed] [Google Scholar]
- 41.Meng H, Zhang X, Lee SJ, Wang MM. Von Willebrand factor inhibits mature smooth muscle gene expression through impairment of Notch signaling. PLoS One. 2013;8(9):e75808. doi: 10.1371/journal.pone.0075808. doi: 10.1371/journal.pone.0075808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Meng H, Wang MM. Collagen represses canonical Notch signaling and binds to Notch ectodomain. Int J Biochem Cell Biol. 2013;45(7):1274–1280. doi: 10.1016/j.biocel.2013.03.020. doi: 10.1016/j.biocel.2013.03.020S1357-2725(13)00095-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002;17(7):1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 44.Heegaard AM, Corsi A, Danielsen CC, Nielsen KL, Jorgensen HL, Riminucci M, et al. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation. 2007;115(21):2731–2738. doi: 10.1161/CIRCULATIONAHA.106.653980. doi: 10.1161/CIRCULATIONAHA.106.653980. [DOI] [PubMed] [Google Scholar]
- 45.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolb M, Margetts PJ, Sime PJ, Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. American journal of physiology Lung cellular and molecular physiology. 2001;280(6):L1327–L1334. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- 47.Hara K, Shiga A, Fukutake T, Nozaki H, Miyashita A, Yokoseki A, et al. Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N Engl J Med. 2009;360(17):1729–1739. doi: 10.1056/NEJMoa0801560. doi: 10.1056/NEJMoa0801560. [DOI] [PubMed] [Google Scholar]