Abstract
Sympathoexcitation, increased circulating norepinephrine, and elevated levels of reactive oxygen species are driving forces underlying numerous cardiovascular diseases including hypertension. However, the effects of elevated norepinephrine and subsequent reactive oxygen species production in splenic T-lymphocytes during hypertension are not currently understood. We hypothesized that increased systemic levels of norepinephrine inhibits the activation of splenic T-lymphocytes via redox signaling. To address this hypothesis, we examined the status of T-lymphocyte activation in spleens of a mouse model of sympathoexcitation-driven hypertension (i.e. norepinephrine infusion). Splenic T-lymphocytes from norepinephrine-infused mice demonstrated decreased proliferation accompanied by a reduction in interferon gamma and tumor necrosis factor alpha production as compared to T-lymphocytes from saline-infused mice. Additionally, norepinephrine directly inhibited splenic T-lymphocyte proliferation and cytokine production ex vivo in a dose dependent manner. Furthermore, norepinephrine caused an increase in G1 arrest in norepinephrine-treated T-lymphocytes, and this was accompanied by a decrease in pro-growth cyclin D3, E1, and E2 mRNA expression. Interestingly, norepinephrine caused an increase in cellular superoxide, which was shown to be partially-causal to the inhibitory effects of norepinephrine as antioxidant supplementation (i.e. Tempol) to norepinephrine-infused mice moderately restored T-lymphocyte growth and pro-inflammatory cytokine production. Our findings indicate that suppression of splenic T-lymphocyte activation occurs in a norepinephrine-driven model of hypertension due to, at least in part, an increase in superoxide. We speculate that further understanding of how norepinephrine mediates its inhibitory effects on splenic T-lymphocytes may elucidate novel pathways for therapeutic mimicry to suppress T-lymphocyte-mediated inflammation in an array of diseases.
Keywords: Hypertension, Reactive Oxygen Species, Superoxide, Norepinephrine, Immunosuppression, Cardiovascular Disease, Inflammation
Introduction
Over-activation of the sympathetic nervous system, or sympathoexcitation, is a hallmark of cardiovascular and cerebrovascular diseases such as heart failure, stroke, and hypertension1–4. Norepinephrine (NE) is the primary neurotransmitter of the sympathetic nervous system, and during times of chronic sympathoexcitation circulating levels of NE may increase 2–6 fold over respective controls4–6. Surges in both systemic circulating as well as localized NE can potentiate damage and stimulate reactive oxygen species (ROS) production in peripheral organs such as heart, vasculature, and kidneys7. However, while the immune system has been implicated as a potential contributor to cardiovascular diseases such as hypertension8, 9, it remains unclear how increased sympathetic outflow impacts the cell types that constitute this functional organ system.
The immune system, specifically T-lymphocytes, have been demonstrated to be a strong contributor to the complete hypertensive response8, 9. Intriguingly, T-lymphocytes express both α and β adrenergic receptors, which suggest these cells may be subject to sympathetic control by NE10. In the current study, we address the effect of chronically elevated NE on T-lymphocytes in a model of sympathoexcitation-driven hypertension (i.e. NE infusion). This model was selected to examine the effects of solely increased NE on splenic T-lymphocyte function, and to eliminate the potential for confounding factors (e.g. baroreflex suppression, salt disturbances, neurogenic feedback) that may be observed in other models of hypertension. Additionally, we focused specifically on splenic T-lymphocytes due to the unique property of the spleen being innervated by only catecholaminergic efferent nerve fibers11. This specific and restricted innervation of the spleen has shown to be critical in limiting splenic derived inflammation during a systemic immune response, and further supports the potential for significant sympathetic regulation of splenic derived T-lymphocytes12. To date, the majority of studies examining T-lymphocyte activation in hypertension specifically focus their attention in cardiovascular organs (e.g. vasculature, kidney), which leaves the status of splenic T-lymphocyte activation unknown. Furthermore, the spleen is home to a substantial proportion of resting naïve T-lymphocytes that may not be actively contributing to the hypertensive phenotype, but would be essential in the immune response towards a secondary infection. Recent evidence suggests that chronic sympathoexcitation in the context of cardiovascular disease may be a predisposition to immunodeficiency4, 13–15, which warrants further examination into the effects of increased NE on this specific population of T-lymphocytes.
Herein, we tested the hypothesis that increased systemic levels of NE and consequent ROS production inhibits splenic T-lymphocytes from normal activation. Indeed, we show NE suppresses growth and cytokine production of splenic T-lymphocytes treated with NE both in vivo and ex vivo. Furthermore, we demonstrate the novel observation that these inhibitory effects are partially facilitated through the specific ROS, superoxide (O2•−), after NE stimulation. Overall, this work suggests splenic T-lymphocytes are inhibited by NE during hypertension, and this suppression may have significant consequences on normal immune responses to secondary infections or insults.
Methods
A detailed description of the materials and methods can be found in the Online Data Supplement.
Mice
All experiments were performed using male wild-type C57BL/6 inbred mice. Hypertension was induced by the subcutaneous infusion of NE (3.8 µg/kg/min)16 using osmotic mini-pumps for 14 days. Mean arterial pressure and heart rate were recorded using intra-arterial telemetry devices in conscious unrestrained animals. All procedures were reviewed and approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee.
Results
Increased circulating NE in vivo inhibits activation of splenic T-lymphocytes
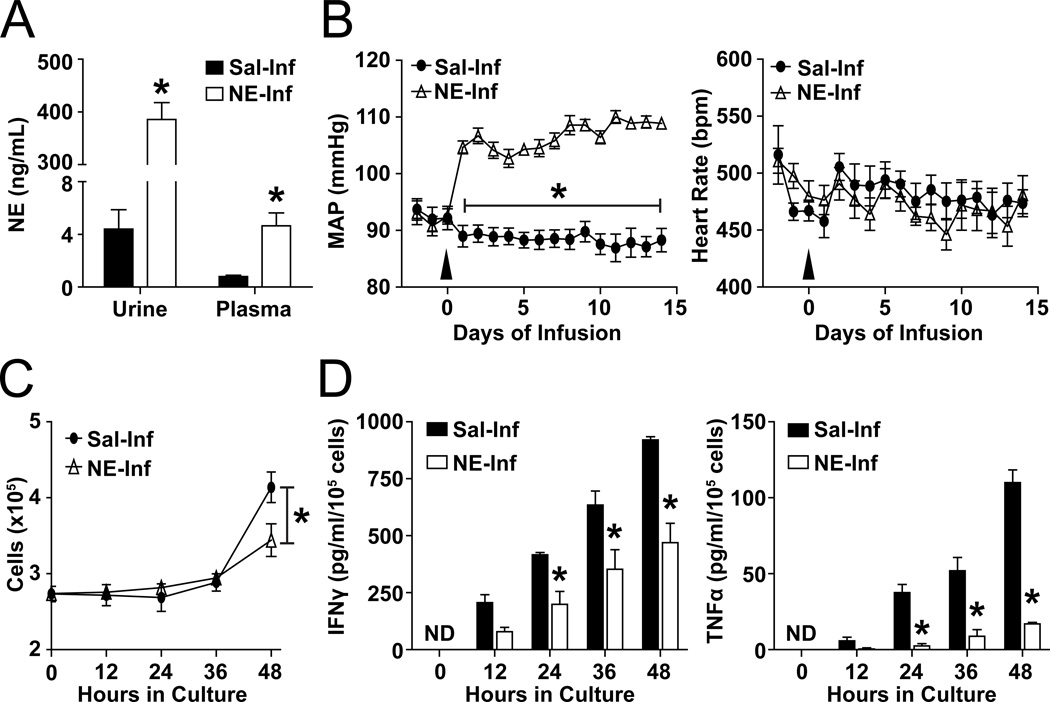
To model the effects of increased sympathetic drive, we used a mouse model subcutaneously infused with a dose of NE previously demonstrated to lead to the reported elevated levels of circulating NE during sympathoexcitation16. After two weeks of NE infusion, urine NE levels increased approximately two orders of magnitude from 4.4 ± 1.5 ng/mL in saline-infused mice to 385.4 ± 32.4 ng/mL in NE-infused mice, while steady-state plasma levels increased roughly 6-fold (saline: 0.8 ± 0.1 ng/mL; NE: 4.6 ± 1.0 ng/mL) (Figure 1A). As previously reported in this animal model, NE infusion produced a significant, rapid, and consistent rise in mean arterial pressure (MAP) that averaged 20.6 ± 0.6 mmHg on day 14 with no change in heart rate (Figure 1B). Because of the significant rise in systemic levels of NE and evident hypertension (hypertension defined by the Eighth Joint National Committee as a rise in MAP ≥15 mmHg17) in our model, we examined splenic T-lymphocytes on day 14 of NE infusion. T-lymphocytes were cultured ex vivo and activated by CD3 stimulation (10 µg/mL; optimal activation dose identified in Figure S1A–D) to understand the effects of NE infusion on early stages of T-lymphocyte activation. Total splenic T-lymphocytes isolated from mice on day 14 of NE infusion and cultured ex vivo under optimized CD3 stimulation demonstrated a 20% ± 5% decrease in cell numbers after 48 hours (Figure 1C). Moreover, we observed significant decreases in the pro-inflammatory cytokines interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) at every time point beyond 24-hour post-plating (Figure 1D). Of note, T-lymphocyte CD28 co-stimulation has demonstrated importance in the perpetuation of hypertension18. Due to this, we replicated these ex vivo experiments with the addition of 2 µg/mL soluble anti-CD28 antibody (optimal dose identified in Figure S1A–D) in addition to plate-bound anti-CD3. We observed similar decreases in cell numbers and pro-inflammatory cytokine levels from T-lymphocytes isolated from NE-infused animals independent of CD28 stimulation (Figure S2A–B). Last, we observed no change in the expression level of α or β adrenergic receptors with saline or NE-infusion (Figure S3). Overall, these data suggest that increased circulating NE in vivo reprograms splenic T-lymphocytes to an inhibitory state, which leads to a blunted response during canonical (i.e. CD3 ± CD28) activation.
Figure 1. NE-induced hypertension suppresses splenic T-lymphocyte activation.
Mice were infused with saline or NE (3.8 µg/kg/min) for 14 days. A. Urine and plasma NE levels 14 days after saline or NE infusion. N=5. B. Mean arterial pressure (MAP) and heart rate (HR) during 14 days of saline or NE infusion. Arrow indicates start of NE infusion. N=8. C. T-lymphocyte numbers at 0–48 hours of ex vivo culture with CD3 stimulation. T-lymphocytes were isolated on day 14 after the start of saline or NE infusion, and plated for 48 hours with CD3 stimulation. N=4. D. IFNγ and TNFα levels in media at 0–48 hours of T-lymphocyte ex vivo culture. ND indicates non-detectable. N=4. *p<0.05 vs. saline-infused.
NE directly inactivates naïve T-lymphocytes
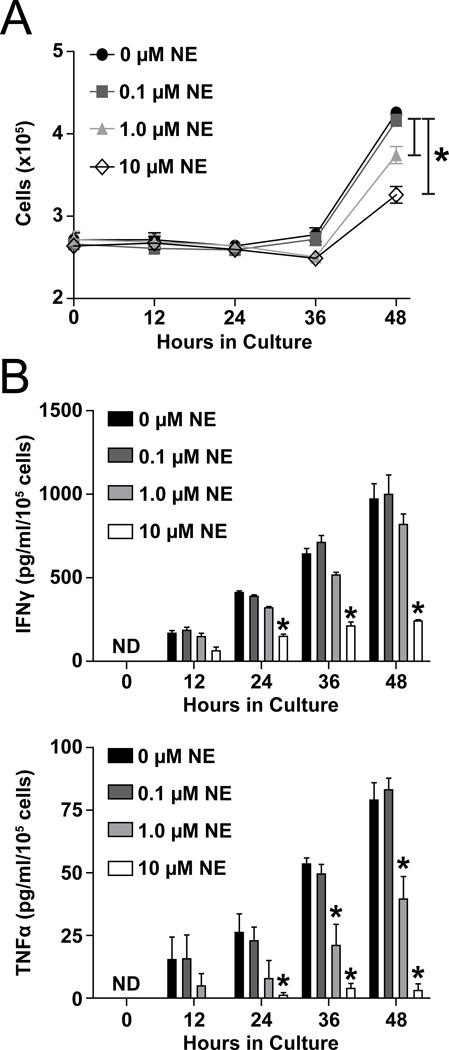
To address the direct effects of NE on T-lymphocytes, we isolated splenic T-lymphocytes from unchallenged mice and cultured the cells as previously described with increasing doses of NE. Similar to what we observed with T-lymphocytes from NE-infused animals, we detected a NE dose-dependent decrease in numbers of T-lymphocytes after 48 hours of culture with CD3 ± CD28 stimulation (Figure 2A, S4B). IFNγ and TNFα production per cell were also decreased by increasing doses of NE, with 10 µM NE producing similar inhibitory effects to what was observed with in vivo NE infusion (Figure 2B, S4A). As such, our subsequent ex vivo experiments were performed using 10 µM NE. It should be noted that although the exact concentration of NE that splenic T-lymphocytes are exposed to is unknown, we posit that due to the synaptic terminals of catecholaminergic nerves terminating directly upon the white pulp centers of the spleen that these T-lymphocytes are exposed to a significant amount of NE.
Figure 2. NE has direct inhibitory effects on T-lymphocytes.
T-lymphocytes were isolated from unchallenged mice and plated for 48 hours with CD3 stimulation with increasing doses of NE. A. T-lymphocyte cell counts 0–48 hours of ex vivo culture. N=4. B. IFNγ and TNFα levels in media at 0–48 hours of T-lymphocyte ex vivo culture. ND indicates non-detectable. N=4. *p<0.05 vs. 0 µM NE.
TH2 lymphocytes are significantly increased with NE stimulation
The decrease in excreted cytokines (i.e. TNFα and IFNγ) from NE-treated T-lymphocytes could be due to an alteration in cellular function or polarization. To examine this, splenic T-lymphocytes from NE-infused animals were immunophenotyped. Total number of splenocytes was unchanged when comparing NE-infused and saline-infused spleens (Figure S5A), which demonstrates that increased circulating NE does not cause significant atrophy of the spleen. Furthermore, total spleen immunophenotyping showed no significant change in percentage of CD3+, CD4+, or CD8+ lymphocytes (Figure S5B, Figure S6A–B). Moreover, screening CD4+ T-lymphocytes for intracellular markers of polarization displayed no significant changes between NE and saline-infused spleens (Figure S5C, Figure S6C). These findings strongly suggest that NE alters the internal function of T-lymphocytes prior to activation and polarization. Due to this, we examined the same immunophenotyping parameters on purified T-lymphocytes activated ex vivo via CD3 stimulation in the presence of NE. It was first observed that ex vivo culture of T-lymphocytes increased the percentage of CD8+ relative to CD4+ cells, but NE did not significantly affect this distribution (Figure S5D, Figure S7A–B). Additionally, while NE had no impact on the early polarization of TReg, TH1, or TH17 cells, it did significantly increase the proportion of TH2 cells (Figure S5E, Figure S7C), which are known to limit pro-inflammatory TH1 differentiation. Taken together, these data indicate that NE does not appear to affect T-lymphocyte polarization in vivo prior to activation and that ex vivo exposure to NE during initial (i.e. 48 hours) CD3 activation may drive TH2 differentiation. In addition, these results suggest that NE reprograms naïve splenic T-lymphocytes predisposing them to inhibited canonical activation, and as such may influence their ability to function properly in the event of a secondary infection.
Decreased T-lymphocyte numbers are due to cell cycle arrest
The decrease in T-lymphocyte numbers observed with NE stimulation could be due to either increased cell death or decreased cell proliferation. We first examined apoptosis using annexin V and propidium iodide (PI) staining. After 48 hours of NE treatment ex vivo with CD3 ± CD28 co-stimulation, no significant changes in annexin V or PI positive T-lymphocytes were identified (Figure S8). These data suggest cell death is not a major contributor to the decrease in T-lymphocytes following NE stimulation. Next, we assessed the status of cell cycle progression in T-lymphocytes using the Krishan PI method19. NE treatment led to an approximate 20% increase in T-lymphocytes in G1 phase, while proportionally decreasing the number of cells in both S and G2 phase (Figure S9A–B). We also investigated cellular proliferation by carboxyfluorescein succinimidyl ester (CFSE) staining. NE treatment ex vivo (±CD28 co-stimulation) significantly decreased the proportion of dividing cells and the proliferative index in both CD4+ and CD8+ lymphocytes (Figure S10A–B). To elucidate a possible mechanism behind the G1 arrested T-lymphocytes, we examined the mRNA expression level of the cyclin D and E families. These transcriptionally-regulated proteins have been shown to be critical in the progression of the cell cycle from G1 to S phase20. In T-lymphocytes from NE-infused animals, cyclin D3, E1, and E2 mRNA was significantly lower than saline-infused T-lymphocytes with cyclin D1 and D2 remaining unchanged (Figure S11A). Moreover, steady-state mRNA levels of cyclins D3, E1, and E2 were also significantly reduced with direct stimulation of NE on splenic T-lymphocytes during ex vivo culture (Figure S11B). In summary, NE does not increase T-lymphocyte death, but limits cellular proliferation and arrests cell cycle progression at the G1-S checkpoint through a possible down-regulation of specific cyclins.
NE increases steady-state superoxide (O2•−) levels in T-lymphocytes
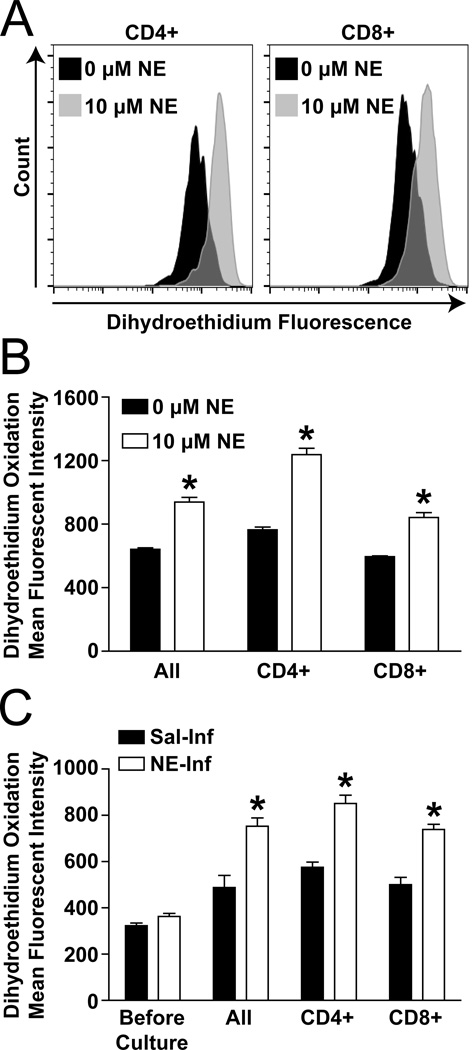
O2•− has been implicated as a primary signaling intermediate during NE-stimulation in an array of cell types21–23, but its presence in NE-treated T-lymphocytes has not been explored. Using the O2•− sensitive dye dihydroethidium (DHE) and flow cytometry, we observed an approximate 70% increase in cellular O2•− levels in both CD4+ and CD8+ NE-treated T-lymphocytes ex vivo compared to control (Figure 3A–B). When examining T-lymphocytes from the in vivo sympathoexcitation model before culture, we observed a slight but non-significant increase (p=0.07) in cellular O2•− in T-lymphocytes from NE-infused mice compared with saline-infused (Figure 3C). However, once cultured ex vivo with CD3 ± CD28 stimulation the T-lymphocytes from the NE-infused animals demonstrated a significant increase in steady-state O2•− levels compared to saline-infused, and this response was independent of T-lymphocyte subtype (Figure 3C, S12A–B). Interestingly, while long-term infusion of NE did not significantly increase steady-state levels of O2•− in freshly isolated T-lymphocytes, the acute treatment (30 minutes) of splenic T-lymphocytes with NE did produce a significant increase in O2•− (Figure S12C). This observation of variable O2•− levels at different time points of NE-treatment suggests the potential for temporal control of steady-state O2•− flux or even ROS-induced-ROS production in T-lymphocytes with NE-stimulation24. Overall, NE causes an elevation in T-lymphocyte cellular O2•− levels, and these increases are correlated with a suppression of T-lymphocyte growth and pro-inflammatory cytokine production.
Figure 3. O2•− is increased in NE-stimulated T-lymphocytes.
T-lymphocytes were isolated from unchallenged (A–B) or saline/NE-infused (C; 3.8 µg/kg/min – day 14) mice and plated for 48 hours with CD3 stimulation. A. Representative dihydroethidium (DHE) flow cytometry analysis of CD4+ and CD8+ T-lymphocytes after 48 hours in ex vivo culture with 0 or 10 µM NE. B. Quantification of DHE oxidation in T-lymphocytes 48 hours after ex vivo culture with 0 or 10 µM NE. N=5. C. Quantification of DHE oxidation in T-lymphocytes from saline or NE-infused animals before and 48 hours after ex vivo culture. N=5. *p<0.05 vs. 0 µM NE.
Superoxide-specific antioxidant supplementation rescues T-lymphocytes from NE-mediated inhibition
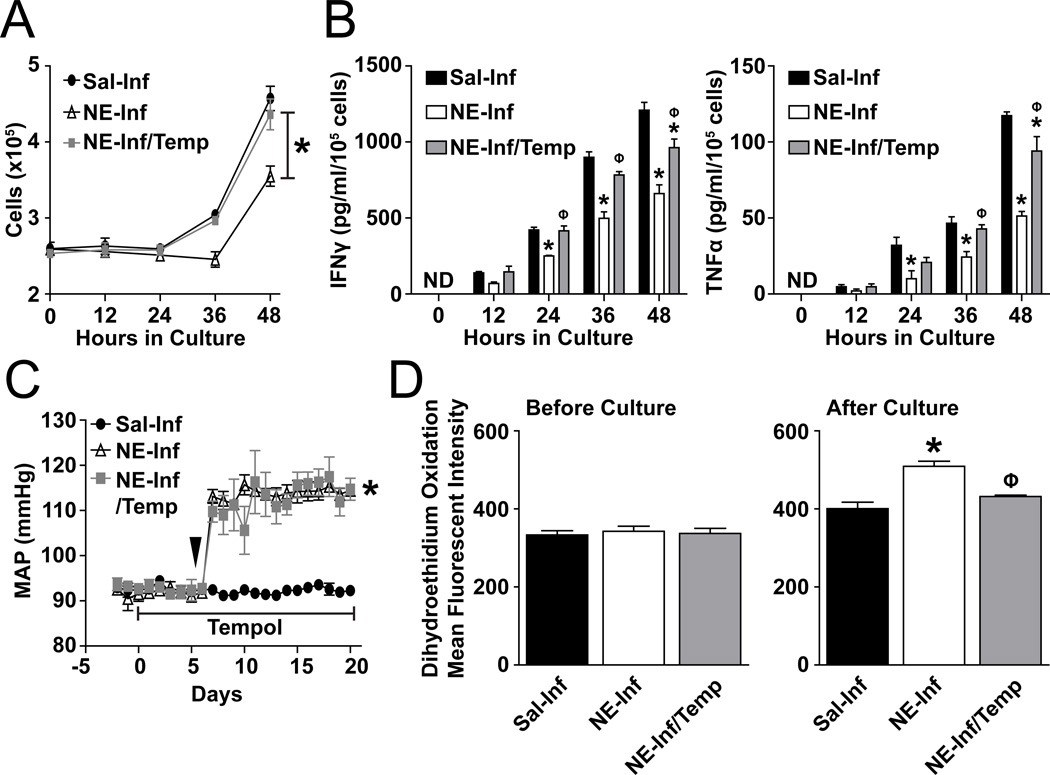
To identify a potential mechanistic role for O2•− in the NE-driven inhibition of T-lymphocytes, we treated mice with Tempol, a O2•−-scavenging antioxidant, concurrently with NE infusion. T-lymphocytes isolated from mice administered Tempol demonstrated a complete rescue in growth when cultured ex vivo with CD3 ± C28 stimulation (Figure 4A, S13B). In addition, levels of the pro-inflammatory cytokines IFNγ and TNFα were significantly increased with Tempol compared to NE infusion alone (Figure 4B, S13A). In contrast, Tempol did not affect MAP suggesting the observed partial rescue in the splenic T-lymphocytes was due to a potential redox mechanism as opposed to alleviation of the induced hypertension (Figure 4C). Indeed, while NE infusion increased DHE oxidation in T-lymphocytes after ex vivo culture, T-lymphocytes from Tempol treated mice had attenuated DHE oxidation, suggesting decreased levels of cellular O2•− (Figure 4D, Figure S13C). Taken together, these data infer a partially causal role for increased O2•− in inducing the inhibitory effect of NE on splenic T-lymphocyte proliferation and cytokine production.
Figure 4. Scavenging of O2•− rescues the inhibitory phenotype of T-lymphocytes from NE-infused animals.
Mice were infused with saline or NE (3.8 µg/kg/min) for 14 days. Drinking water was supplemented with 1 mM Tempol (Temp) 5 days prior and throughout the entire infusion. T-lymphocytes were isolated on day 14 after the start of saline or NE infusion and cultured for 48 hours with CD3 stimulation. A. T-lymphocyte numbers at 0–48 hours of ex vivo culture. N=4. B. IFNγ and TNFα levels in media at 0–48 hours of T-lymphocyte ex vivo culture. ND indicates non-detectable. N=4. C. Mean arterial pressure (MAP) during 14 days of saline or NE infusion with Tempol supplementation. Arrow indicates start of NE infusion. N=4. D. Dihydroethidium (DHE) oxidation in T-lymphocytes before and after 48 hours ex vivo culture. N=4. *p<0.05 vs. saline-infused. Φp<0.05 vs. NE-infused.
Discussion
Herein, we describe the potent inhibitory effect of NE on splenic T-lymphocytes in a model of sympathoexcitation-driven hypertension. Additionally, we show NE has a direct consequence in modulating the canonical activation of splenic T-lymphocytes. This direct effect is important in the context of hypertension because it has been demonstrated that angiotensin II, a pro-hypertensive peptide, does not have a significant direct effect on the modulation of T-lymphocyte activity16. NE effects on T-lymphocytes have been examined previously in other models of disease, but the consensus is conflicted as to the exact function of NE on T-lymphocyte development and activation25. For example, our work confirms and extends that of others who show NE acts to suppress activation of naïve T-lymphocytes in unfractionated populations26, 27. Additionally, work using specific T-lymphocytes subsets such as naïve CD4+ or CD8+ cells have shown NE-mediated suppression of immune activity primarily mediated through β2-adrenergic signaling28, 29. However, under different conditions such as specific systemic infections, stress, or targeted T-lymphocyte differentiation ex vivo NE has been shown to enhance the pro-inflammatory activation of T-lymphocytes30–32. While this evidence is inconsistent, numerous variables are at play in these studies that may explain the differences in conclusions.
First, while T-lymphocytes express both α and β adrenergic receptors, specific isoforms of these receptors have been found in different quantities on T-lymphocyte subsets. For example, naïve T-lymphocytes highly express the β2-adrenergic receptor25, and our data suggest stimulation with NE during T-lymphocyte activation predisposes differentiation to the TH2 lineage. Notably, it has been observed that TH2-differentiated, but not TH1-differentiated, CD4+ T-lymphocytes lack the β2-adrenergic receptor, and thus may be a mechanism to become resistant to this catecholamine once polarized33. Understanding that NE demonstrates differential immunomodulatory effects on different subclasses, mixtures, and stages of differentiated T-lymphocytes increases the likelihood of conflicting results between different experimental setups. Second, a temporal and developmental component of NE-stimulation may be critical in the phenotype rendered in T-lymphocytes. In one clinical study, short-term administration of NE augmented the number of CD8+ circulating T-lymphocytes, but long-term administration resulted in decreased T-lymphocyte numbers34. Additionally, the majority of evidence, including our data presented herein, suggests that naïve unchallenged T-lymphocytes are suppressed by NE-stimulation, but the functionality of pre-existing activated T-lymphocytes (e.g. during infection) is exacerbated30. Last, a spatial component of NE-mediated effects on T-lymphocytes may also be at play. We demonstrate that T-lymphocytes in the spleen, a catecholaminergic-innervated lymphoid organ, are suppressed by NE infusion in vivo. In contrast, Marvar et al. demonstrated increased T-lymphocyte numbers and activation in the aorta of NE-infused mice16. While appearing conflicting, both situations of T-lymphocyte activation and inhibition are most likely occurring concurrently, but in different locations. This hypothesis would be supported if specific T-lymphocytes (i.e. vasculature or renal positioned) were activated prior to the increased sympathoexcitation and NE outflow associated with hypertension35. Under these circumstances, NE would potentiate the effects of the activated T-lymphocytes in these cardiovascular-related organs, but suppress the inactivated naïve T-lymphocytes located in the spleen. As such, it is tempting to speculate that the NE-mediated inhibition of splenic T-lymphocytes, as we observed, is a compensatory mechanism attempting to inhibit resting T-lymphocytes so that they cannot further add to the inflammation contributing to the hypertension. Overall, further research examining more detailed parameters such as specific T-lymphocyte subsets, timing of NE-administration, and organ-specific effects of NE on T-lymphocytes are highly warranted in the context of various models of hypertension.
Current dogma suggests that hypertension is a systemic inflammatory disease; whereas, our data implies that not all peripheral organs demonstrate increased inflammation. That is, we demonstrate that T-lymphocytes in the spleens of NE-driven hypertensive mice show no signs of increased inflammatory parameters, and in fact are significantly suppressed. These findings are consistent with the observation that hypertensive mice and humans do not exhibit constitutional symptoms (e.g. fever, malaise, myalgia) associated with increased systemic immune activation and circulating pro-inflammatory cytokines. These observations further support the notion that hypertension leads to a site-specific (e.g. vascular or renal) and localized activation of T-lymphocyte inflammation as opposed to systemic. In this manner, while administration of systemic immunosuppressants may be indicated as a possible therapy for hypertension, it may further the sympathoexcitation-mediated immunocompromised state in lymphoid organs such as the spleen and predispose hypertensive patients to secondary infections. In summary, we present evidence that T-lymphocytes are not uniformly activated during hypertension, and this finding may preclude systemic targeting of the immune system for hypertension therapy.
Finally, we observe the novel finding that O2•− is increased with NE stimulation of T-lymphocytes, and that increased O2•−-scavenging via Tempol significantly restores the original pro-inflammatory potential of the cells, but does not decrease the elevated blood pressure in NE-infused mice. We interpret these findings to mean that O2•− is a partial mediator of the inhibitory phenotype in T-lymphocytes exposed to NE and this inhibition is not due solely to changes in blood pressure. ROS have become well accepted as intracellular signaling molecules, and play a primary role in various cell types during hypertension. In fact, alterations in the redox environment have been directly linked to changes in cell cycle regulation similar to what we have observed in our NE-stimulated T-lymphocytes36. Furthermore, NE has been shown to increase levels of ROS in an array of cell types, but until now had not been examined in T-lymphocytes. Studies are currently underway in our laboratory, which are designed to aid in the further understanding of specific redox-sensitive intracellular signaling pathways affected by NE-mediated ROS production. Additionally, we observed that a NE-induced increase in O2•− leads to a suppression of immune function, while others have observed angiotensin II-induced escalations in O2•− causing enhanced lymphocyte inflammation9. These findings fully support the notion that not all ROS-inducing events are created equal, and in fact, may have very specific intracellular signaling pathways and subcellular molecules in which they target. Together, these observations may explain why antioxidant therapy has demonstrated minimal clinical success for diseases such as hypertension37, as global targeting of ROS may inhibit both pro- and anti-inflammatory pathways throughout the body.
Perspectives
In recent years, research examining how the immune system contributes to hypertension has grown exponentially, and it has become mostly accepted that the immune system systemically is contributing to the hypertensive phenotype. However, our findings support a hypertension model of site-specific and localized inflammation as opposed to systemic. More specifically, we have elucidated that increased systemic levels of NE in a mouse model of sympathoexcitation-driven hypertension directly desensitizes splenic T-lymphocytes to canonical activation, while in this same model it has previously been shown that vascular and renal T-lymphocytes are activated16. Furthermore, we report that the inhibitory effects of NE on T-lymphocytes are mediated in part by increased steady-state O2•− flux, as increased O2•−-scavenging significantly restores the original pro-inflammatory potential of the cells. We speculate that further understanding of how NE mediates its effects on organ-specific localized T-lymphocytes may elucidate novel pathways for therapeutic mimicry to modulate T-lymphocyte-mediated inflammation in various pathologies including hypertension.
Supplementary Material
Novelty and Significance.
1) What is new?
The majority of recent research examining inflammation in hypertension has primarily focused on how T-lymphocytes contribute to elevated blood pressure and end organ damage. Herein, we present new data showing that increased levels of norepinephrine in a model of sympathoexcitation-driven hypertension inhibits splenic derived T-lymphocytes activity and the redox-mediated regulation of this inhibition.
2) What is relevant?
T-lymphocytes localized to the vasculature and kidneys have been demonstrated to have pro-inflammatory effects that exacerbate the hypertensive phenotype, but the status of splenic T-lymphocytes in hypertension remains unclear. We demonstrate that splenic T-lymphocytes exposed to elevated levels of circulating norepinephrine are blunted in growth and cytokine production upon canonical activation. Furthermore, norepinephrine increases levels of splenic T-lymphocyte superoxide, which we show is mechanistic in the inhibitor phenotype of these immune cells during sympathoexcitation.
3) Summary
T-lymphocytes from the spleens of hypertensive animals are subject to activation-suppression by increased circulating levels of norepinephrine in a redox-dependent manner.
Acknowledgments
We thank Dr. Jun Tian for her technical expertise in assisting with animal procedures. We are grateful to the University of Nebraska Medical Center Flow Cytometry Research Facility for their assistance in all flow cytometric analyses.
Sources of Funding
This work is supported by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) R01HL103942 (to MCZ), the American Heart Association (AHA) 13POST17060015 (to AJC), and NIH NHLBI F32HL122021 (to AJC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of Interest/Disclosures
None
References
- 1.Guyenet PG. The sympathetic control of blood pressure. Nature reviews. Neuroscience. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 2.Osborn JW, Fink GD. Region-specific changes in sympathetic nerve activity in angiotensin ii-salt hypertension in the rat. Experimental physiology. 2010;95:61–68. doi: 10.1113/expphysiol.2008.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- 4.Walter U, Kolbaske S, Patejdl R, Steinhagen V, Abu-Mugheisib M, Grossmann A, Zingler C, Benecke R. Insular stroke is associated with acute sympathetic hyperactivation and immunodepression. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2013;20:153–159. doi: 10.1111/j.1468-1331.2012.03818.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu JL, Zucker IH. Regulation of sympathetic nerve activity in heart failure: A role for nitric oxide and angiotensin ii. Circulation Research. 1999;84:417–423. doi: 10.1161/01.res.84.4.417. [DOI] [PubMed] [Google Scholar]
- 6.Mathias CJ. Role of sympathetic efferent nerves in blood pressure regulation and in hypertension. Hypertension. 1991;18:III22–III30. doi: 10.1161/01.hyp.18.5_suppl.iii22. [DOI] [PubMed] [Google Scholar]
- 7.Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens. 1998;16:1979–1987. doi: 10.1097/00004872-199816121-00019. [DOI] [PubMed] [Google Scholar]
- 8.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin ii-dependent hypertension. American journal of physiology. Regulatory, integrative and comparative physiology. 2010;298:R1089–R1097. doi: 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. The Journal of experimental medicine. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders VM. The role of adrenoceptor-mediated signals in the modulation of lymphocyte function. Advances in neuroimmunology. 1995;5:283–298. doi: 10.1016/0960-5428(95)00019-x. [DOI] [PubMed] [Google Scholar]
- 11.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain, behavior, and immunity. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martelli D, Yao ST, McKinley MJ, McAllen RM. Reflex control of inflammation by sympathetic nerves, not the vagus. J Physiol. 2014;592:1677–1686. doi: 10.1113/jphysiol.2013.268573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mracsko E, Liesz A, Karcher S, Zorn M, Bari F, Veltkamp R. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain, behavior, and immunity. 2014;41:200–209. doi: 10.1016/j.bbi.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Vahdat K, Pourbehi MR, Ostovar A, Hadavand F, Bolkheir A, Assadi M, Farrokhnia M, Nabipour I. Association of pathogen burden and hypertension: The persian gulf healthy heart study. Am J Hypertens. 2013;26:1140–1147. doi: 10.1093/ajh/hpt083. [DOI] [PubMed] [Google Scholar]
- 15.Rosenne E, Sorski L, Shaashua L, Neeman E, Matzner P, Levi B, Ben-Eliyahu S. In vivo suppression of nk cell cytotoxicity by stress and surgery: Glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain, behavior, and immunity. 2014;37:207–219. doi: 10.1016/j.bbi.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of t-lymphocyte activation and vascular inflammation produced by angiotensin ii-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 18.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the b7/cd28 t-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. The Journal of cell biology. 1975;66:188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boonstra J. Progression through the g1-phase of the on-going cell cycle. Journal of cellular biochemistry. 2003;90:244–252. doi: 10.1002/jcb.10617. [DOI] [PubMed] [Google Scholar]
- 21.Schraml E, Quan P, Stelzer I, Fuchs R, Skalicky M, Viidik A, Schauenstein K. Norepinephrine treatment and aging lead to systemic and intracellular oxidative stress in rats. Experimental gerontology. 2007;42:1072–1078. doi: 10.1016/j.exger.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Xiong F, Xiao D, Zhang L. Norepinephrine causes epigenetic repression of pkcepsilon gene in rodent hearts by activating nox1-dependent reactive oxygen species production. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:2753–2763. doi: 10.1096/fj.11-199422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deo SH, Jenkins NT, Padilla J, Parrish AR, Fadel PJ. Norepinephrine increases nadph oxidase-derived superoxide in human peripheral blood mononuclear cells via alpha-adrenergic receptors. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;305:R1124–R1132. doi: 10.1152/ajpregu.00347.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassegue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin ii-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal. 2014;20:281–294. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padro CJ, Sanders VM. Neuroendocrine regulation of inflammation. Semin Immunol. 2014;26:357–368. doi: 10.1016/j.smim.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldman RD, Hunninghake GW, McArdle WL. Beta-adrenergic-receptor-mediated suppression of interleukin 2 receptors in human lymphocytes. Journal of immunology. 1987;139:3355–3359. [PubMed] [Google Scholar]
- 27.Takayanagi Y, Osawa S, Ikuma M, Takagaki K, Zhang J, Hamaya Y, Yamada T, Sugimoto M, Furuta T, Miyajima H, Sugimoto K. Norepinephrine suppresses ifn-gamma and tnf-alpha production by murine intestinal intraepithelial lymphocytes via the beta(1) adrenoceptor. Journal of neuroimmunology. 2012;245:66–74. doi: 10.1016/j.jneuroim.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Ramer-Quinn DS, Swanson MA, Lee WT, Sanders VM. Cytokine production by naive and primary effector cd4+ t cells exposed to norepinephrine. Brain, behavior, and immunity. 2000;14:239–255. doi: 10.1006/brbi.2000.0603. [DOI] [PubMed] [Google Scholar]
- 29.Sheridan JF, Dobbs C, Jung J, Chu X, Konstantinos A, Padgett D, Glaser R. Stress-induced neuroendocrine modulation of viral pathogenesis and immunity. Ann N Y Acad Sci. 1998;840:803–808. doi: 10.1111/j.1749-6632.1998.tb09618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alaniz RC, Thomas SA, Perez-Melgosa M, Mueller K, Farr AG, Palmiter RD, Wilson CB. Dopamine beta-hydroxylase deficiency impairs cellular immunity. Proc Natl Acad Sci U S A. 1999;96:2274–2278. doi: 10.1073/pnas.96.5.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanson MA, Lee WT, Sanders VM. Ifn-gamma production by th1 cells generated from naive cd4+ t cells exposed to norepinephrine. Journal of immunology. 2001;166:232–240. doi: 10.4049/jimmunol.166.1.232. [DOI] [PubMed] [Google Scholar]
- 32.Madden KS, Moynihan JA, Brenner GJ, Felten SY, Felten DL, Livnat S. Sympathetic nervous system modulation of the immune system. Iii. Alterations in t and b cell proliferation and differentiation in vitro following chemical sympathectomy. Journal of neuroimmunology. 1994;49:77–87. doi: 10.1016/0165-5728(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 33.Ramer-Quinn DS, Baker RA, Sanders VM. Activated t helper 1 and t helper 2 cells differentially express the beta-2-adrenergic receptor: A mechanism for selective modulation of t helper 1 cell cytokine production. Journal of immunology. 1997;159:4857–4867. [PubMed] [Google Scholar]
- 34.Maisel AS, Michel MC. Beta-adrenergic receptors in congestive heart failure: Present knowledge and future directions. Cardiology. 1989;76:338–346. doi: 10.1159/000174517. [DOI] [PubMed] [Google Scholar]
- 35.Trott DW, Harrison DG. The immune system in hypertension. Advances in physiology education. 2014;38:20–24. doi: 10.1152/advan.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiffrin EL. Antioxidants in hypertension and cardiovascular disease. Molecular interventions. 2010;10:354–362. doi: 10.1124/mi.10.6.4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.