Abstract
Despite its role in homogenizing populations, hybridization has also been proposed as a means to generate new species. The conceptual basis for this idea is that hybridization can result in novel phenotypes through recombination between the parental genomes, allowing a hybrid population to occupy ecological niches unavailable to parental species. Here we present an alternative model of the evolution of reproductive isolation in hybrid populations that occurs as a simple consequence of selection against genetic incompatibilities. Unlike previous models of hybrid speciation, our model does not incorporate inbreeding, or assume that hybrids have an ecological or reproductive fitness advantage relative to parental populations. We show that reproductive isolation between hybrids and parental species can evolve frequently and rapidly under this model, even in the presence of substantial ongoing immigration from parental species and strong selection against hybrids. An interesting prediction of our model is that replicate hybrid populations formed from the same pair of parental species can evolve reproductive isolation from each other. This non-adaptive process can therefore generate patterns of species diversity and relatedness that resemble an adaptive radiation. Intriguingly, several known hybrid species exhibit patterns of reproductive isolation consistent with the predictions of our model.
Author Summary
Understanding the origin of species is one of the central challenges in evolutionary biology. It has been suggested that hybridization could generate new species because hybrids can display novel combinations of traits that induce reproductive isolation from their parental species (called “hybrid speciation”). Existing models predict that this should only occur in special cases, and indeed there have been only few well-supported examples. We describe a new model of hybrid reproductive isolation that results from selection against genetic incompatibilities in hybrids, which are predicted to be common. Simulations reveal that hybrid populations rapidly and frequently become isolated from parental species by fixing combinations of genes that hinder successful reproduction with parental species. We propose that this process could be an important mechanism for the formation of new species.
Introduction
The evolutionary significance of hybridization has been a hotly debated topic for decades [1]. Homoploid hybrid speciation, speciation that occurs as a result of hybridization without a ploidy change [2, 3], is generally thought to be an exceptionally rare outcome of hybridization, and there are indeed only a handful of well-supported cases of this phenomenon [4]. Though it is not uncommon for species’ genomes to exhibit evidence of past hybridization, hybrids are often thought to be weakly isolated from parental species, though few studies have explicitly investigated this.
Empirical research on homoploid hybrid speciation over the last decade has primarily focused on the role of hybrid phenotypes in establishing reproductive isolation between hybrids and parental species [5–9]. Hybrids can have recombinant or transgressive traits that differentiate them from parental species. In some cases, these traits can allow hybrids to occupy new niches. For example, in Rhagoletis fruit flies, hybrid lineages have novel host preferences, potentially contributing to reproductive isolation between hybrids from parental species [10, 11]. Similarly, if hybrid lineages have novel mate preferences, this can isolate hybrids from parental species via assortative mating, a mechanism which has been implicated in hybrid speciation in Heliconius butterflies ([8], and see [12] for a model of this process). This work has lead to the idea that novel hybrid phenotypes are key to hybrid speciation [13].
Despite several well-documented examples [6, 8], it has been difficult to determine the evolutionary importance of hybrid speciation, in part because few theoretical models have been developed. The existing models of hybrid speciation simulate either positive selection on certain hybrid genotypes or inbreeding [9, 12, 14]. In one model [14, 15], novel combinations of underdominant parental inversions can fix in hybrid populations, particularly if the novel inversion combination is under positive selection or if rates of inbreeding (selfing) are high (see Discussion). Though there is evidence that this process combined with ecological factors was involved in the formation of hybrid Helianthus sunflower species [5, 6, 16], the basis for invoking positive selection on recombinant inversion genotypes is unclear. Later versions of this model incorporated ecological differentiation between hybrid and parental species and showed that hybrid speciation occurred frequently if hybrids had higher fitness than parental species in an unoccupied niche [9, 17]. Though hybridization often generates novel traits [18–20] it is difficult to evaluate the likelihood that these traits will be more fit than parental types (ecologically or intrinsically), making it difficult to predict the importance of hybridization in generating new species by positive selection on hybrid genotypes.
The genetic incompatibility of hybrids constitutes a key component of reproductive isolation between many species, and is the basis for the biological species concept. While previous models of hybrid speciation incorporated inversions [21], here we investigate the potential role of negative epistatic interactions, another important genetic mechanism of speciation. The first genetic model of speciation, described by Bateson, Dobzhansky and Muller (the BDM incompatibility model, S1 Fig., [22–24]), predicts that mutations at two genetic loci differentially accumulating along two lineages can negatively interact in their hybrids. Empirical research has shown that these types of negative epistatic interactions are remarkably common [25–29]; reviewed in [24, 30, 31].
Though the theory of genetic incompatibilities was originally formulated in the context of allopatrically diverging species, more recent research has investigated dynamics of these incompatibilities in the context of hybrid zones. Under the simplest BDM scenario, derived genotypes are presumed to be neutral, meaning that they have the same fitness as ancestral genotypes. When there is gene flow between species, neutral BDMs are predicted to fix for genotype combinations that are compatible with either parental species [32], rendering them ineffective barriers to gene flow [33, 34]. However, incompatibilities may also frequently arise due to adaptive evolution or coevolution of pairs of loci along lineages (S1 and S2 Figs [24, 32, 35–37]). Such incompatibilities are more effective barriers to gene flow than neutral BDM incompatibilities ([38], see also [39]).
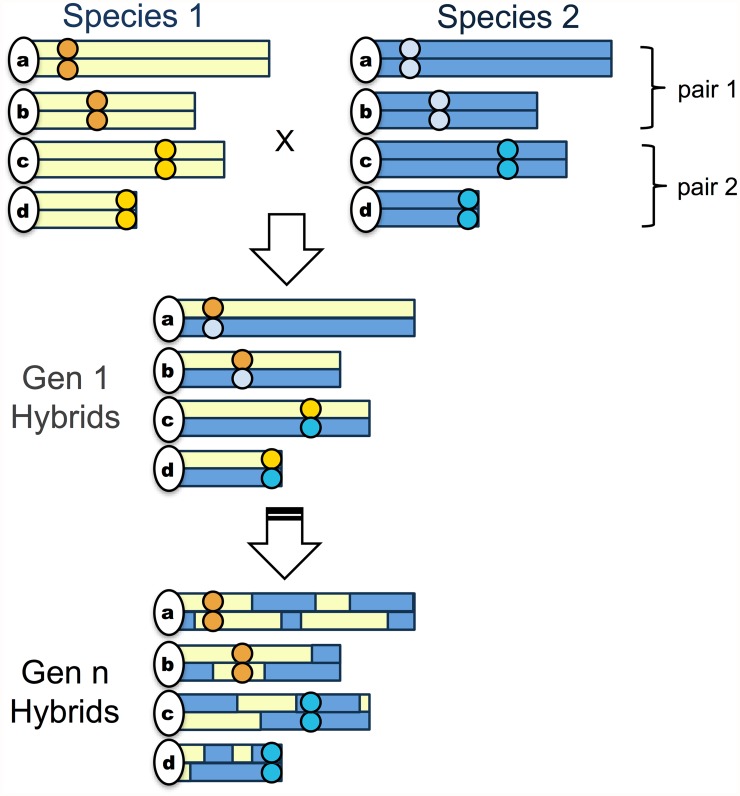
In its initial description, the BDM model envisioned incompatibilities that cause complete hybrid inviability or sterility, but many negative epistatic interactions in interspecific crosses have more moderate effects on fitness (e.g. [40–42]), allowing hybrid populations to persist. With few exceptions, previous work on genetic incompatibilities has focused on their role in maintaining reproductive isolation between parental species. As a result, hybrid populations have primarily been modeled as tension zones, but incompatibilities may also have interesting dynamics within isolated hybrid populations (i.e. hybrid swarms). Here we present a new model in which reproductive isolation between hybrid and parental populations emerges as a consequence of selection against incompatibilities in a hybrid swarm. Selection on a single adaptive or coevolving incompatibility pair can result in the fixation of genotype combinations that contribute to isolation between the hybrid population and one or the other parental species. Here, we show that in the presence of multiple pairs of such incompatibilities, this process can result in the rapid evolution of reproductive isolation of hybrid populations from both parental species (Fig. 1).
Fig 1. Schematic of the simplest “hybrid speciation by genetic incompatibility” scenario.
The simplest model hybrid reproductive isolation evolves in a hybrid swarm (S3 Fig.) via fixation of parental genetic incompatibility pairs in opposite directions. Circles indicate the location of incompatibility pairs on chromosomes; yellow shading indicates regions derived from species 1 while blue shading indicates regions derived from species 2. In the first generation, assuming random mating, 50% of individuals will be F1 hybrids if both species contribute equally to the hybrid population. In subsequent generations, recombination will break up ancestry blocks and selection will drive the fixation of parental genotypes at incompatibility loci. In some proportion of cases, incompatibility pairs will fix for opposite parental species genotypes.
Two features of this model make it particularly plausible biologically. First, as noted above, negative epistatic interactions are common, providing ample raw material for our model. Second, hybrid populations in which hybrids are abundant are common in nature (e.g. [43–45]). Though ecological and sexual selection are important factors in the few well-documented cases of hybrid speciation [6, 8], our results suggest that hybrids can evolve reproductive isolation as a result of selection against genetic incompatibilities alone.
Results
Modeling selection against hybrid incompatibilities
In the simplest model of a hybrid population, an equal mixture of individuals from both parental species form a new isolated population and mate randomly with respect to genotype (Figs. 1 and S3), such that the first mating event generates 50% F1 hybrids and 25% each parental species. Using theory of two-locus selection [46, 47], hereafter the “deterministic two-locus model”, one can model the effect of selection at two polymorphic loci on gamete frequencies of a diploid sexual population (see Methods and S1 Text). This model describes the dynamics of two loci subject to any arbitrary fitness matrix. Here, we focus on fitness matrices for three types of incompatibilities that may commonly arise between species (S1 and S2 Figs; [30, 35]): 1. BDM incompatibilities arising from neutral substitutions, 2. BDM incompatibilities arising from adaptive substitutions, and 3. BDM incompatibilities arising from coevolution between loci. Applying the two-locus selection model to these incompatibility types, one can see that the direction of fixation depends on the initial frequency of the parental alleles (f, see S3 and S4 Figs) and dominance at each locus (h, S4 Fig.; see also [48]).
This purely deterministic model of selection on hybrid incompatibilities is unrealistic because even large populations experience some degree of genetic drift. We thus extended the model to include genetic drift, which can affect the speed and direction of fixation of incompatibility pairs (S5 Fig.). For neutral BDM incompatibilities (S1 Fig.), this model does not predict fixation of genotypes incompatible with either parental species (S4 Fig.). In contrast, for coevolving or adaptive BDM incompatibilities (S1 Text, S1 and S2 Figs), the two-locus finite population model predicts that at equal admixture proportions (f = 0.5), a single incompatibility pair has a 50% chance of fixing for either parental allele combination (Fig. 2, S2 Text, S1 Table). Interestingly, while genetic drift in small populations could accomplish the same thing (9), the process described here occurs rapidly in large populations and is driven by deterministic selection (Fig. 2). Given these dynamics, it is clear that large hybrid populations with two or more of these types of hybrid incompatibilities could, in principle, fix for one parental genotype at one incompatibility pair and the other parental genotype at the other incompatibility pair (Fig. 1). This outcome would result in reproductive isolation of the hybrid population from both parental species. With two codominant incompatibility pairs and equal admixture proportions, the probability that a hybrid population will become isolated can be predicted by a simple binomial. However the binomial prediction breaks down when there is variation in dominance, admixture proportions, or linkage between incompatibilities, and thus we explore these further by simulation.
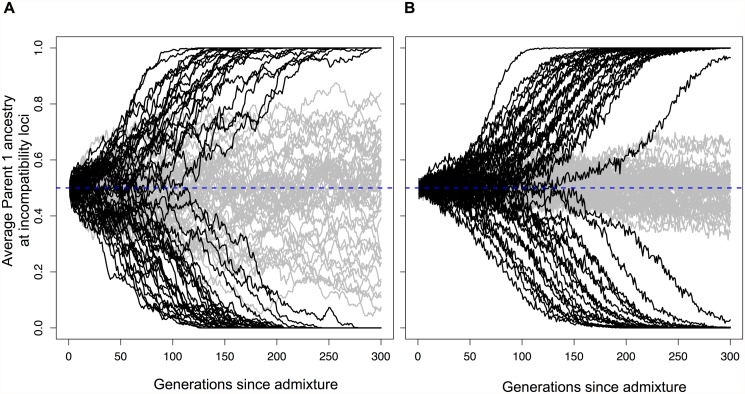
Fig 2. Hybrid populations rapidly fix for hybrid incompatibility locus pairs.
Selection drives hybrid incompatibility loci to fixation, even when a hybrid population forms at equal admixture proportions (f = 0.5). Black lines show average parent 1 ancestry at a hybrid incompatibility pair (h = 0.5, s = 0.1) in 50 replicate populations of (A) N = 1,000 or (B) N = 10,000 diploid individuals. Gray lines show results for this same population size with no selection. Because of this behavior, two incompatibility pairs may fix for opposite parents, resulting in reproductive isolation of hybrids from both parents (see Fig. 1).
Simulations of an isolated hybrid population
To investigate the dynamics of multiple incompatibility pairs, we simulated a large, randomly-mating and spatially isolated hybrid population with two pairs of unlinked hybrid incompatibility loci (see Methods; S3 Fig., setting m 1 = m 2 = 0). The fitness scheme used is that of a coevolutionary incompatibility model (S2 Fig.), assuming that incompatibilities are codominant (i.e. h = 0.5), that fitness is symmetric with respect to the parental source of alleles (i.e. w ij = w ji) and that the cumulative fitness effects of multiple incompatibility pairs is multiplicative. If hybrid populations fixed for the parent species 1 genotype at one incompatibility pair and the parent species 2 genotype at the other, we considered the hybrid population as having evolved reproductive isolation from both parental species (albeit weaker than between the two parental species).
While selection against hybrids will sometimes be so extreme that few hybrids will survive (or reproduce) in the population (see simulations below), selection against hybrids can also be more moderate, allowing hybrids to persist [41, 45, 49–53]. In simulations of this moderate selection scenario, reproductive isolation between hybrid and parental populations can evolve frequently and rapidly (Fig. 3). For example, for two incompatibility pairs with selection coefficients (s) of 0.1, 47±2% of simulated hybrid populations became isolated from both parental species within an average of ~200 generations. Exploring a range of s (0.1–0.5, S6 Fig., S2 Table), initial admixture proportions (f = 0.3–0.7, S7 Fig.), and population sizes (100–10,000 diploids, S3 Table), we conclude that, unless fitness of hybrids is low (i.e. F1 fitness <0.5) or ancestry of the founding population is substantially skewed (>60% one parental species), reproductive isolation evolves rapidly and with surprisingly high probability (27±2% to 43±2% of the time; on average within 75 ± 16 to 258 ± 38 generations, see S3 Text).
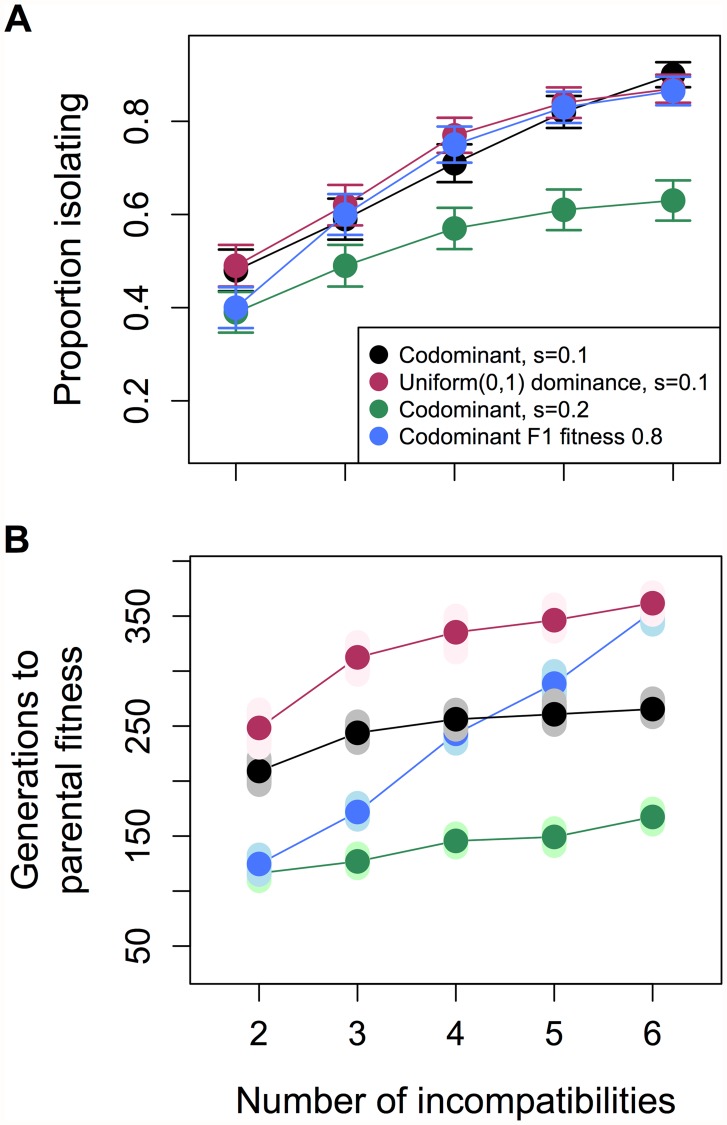
Fig 3. Relationship between the number of hybrid incompatibility pairs and probability of evolving isolation from both parents.
With an increasing number of hybrid incompatibility pairs, reproductive isolation from both parents increases in likelihood (A) but populations require longer periods of time to reach parental fitness levels (B). In these simulations two to six hybrid incompatibility pairs distinguish the hybridizing species and hybrid populations formed at equal admixture proportions (f = 0.5, 1,000 diploid individuals). Simulations labeled F1 indicate that the selection coefficients were set such that the fitness of F1 hybrids between the two parental species equaled 0.8 regardless of the number of incompatibilities. Results are based on 500 replicate simulations. In (A) whiskers represent two standard errors; in (B) smears represent the means of 1,000 bootstrap samples.
The effect of dominance and asymmetry in selection intensity
In the above simulations, we assume that selection on different hybrid incompatibility interactions is symmetrical (s 1 = s 2, S2 Fig.), but it is unlikely that selection is truly equal on different hybrid genotype combinations. When fitness is completely asymmetrical (i.e. s 1 = 0 in S1 Fig., as for neutral BDM incompatibilities), only strong genetic drift can cause the fixation of genotype pairs that are incompatible with either parental species, as selection cannot do so (see S4, S8, S9 Figs, S4 Text). This reliance on genetic drift implies that this process will be slow unless an extreme bottleneck is invoked.
In contrast, the dynamics of BDM incompatibilities resulting from adaptation within parental lineages can be quite different (S1 Fig.). Notably, while selection may also be highly asymmetric in such cases [38, 54], derived alleles have higher fitness than ancestral alleles, allowing for the fixation of genotype combinations that are incompatible with both parental species. We find that isolation evolves with similar frequency under asymmetric selection as long as selection is strong relative to drift (S3 Text, S4 Table), because even weak selection will prevent the fixation of the ancestral genotype.
Above we simulated codominant hybrid incompatibilities (h = 0.5), but the two-locus model (S4 Fig.) shows that patterns of fixation are different depending on the value of h. In particular, when h is zero or unity, fixation is not strongly dependent on admixture proportions (S4 Fig.). When we simulate variation in dominance among incompatibility interactions (see S3 Text, S5 Table), we find that reproductive isolation between hybrid populations and parental species evolves with comparable frequency (42–48±2% vs 47±2% under the codominant scenario).
Increasing the number of hybrid incompatibilities
Recent empirical studies have suggested that most species are distinguished by multiple hybrid incompatibilities [30, 41, 55–59]. Theoretically, barring extinction of the hybrid population (see simulations below), increasing the number of pairs of incompatibilities should increase the probability that a hybrid population will evolve isolation from both parental species. In order to illustrate this, we simulated 3–6 unlinked hybrid incompatibility pairs (S5 Text). As expected, increasing the number of hybrid incompatibilities increases the probability that the hybrid population will be isolated from each parental species by at least one incompatibility pair (>90% with 6 incompatibility pairs, Figs. 3, S6, S5 Text).
We assume in most of our simulations that loci involved in hybrid incompatibilities are completely unlinked. As the number of incompatibilities increases, this becomes unlikely. Genetic linkage between loci involved in different epistatic interactions can reduce the frequency at which hybrid populations evolve isolation because alleles are more likely to fix for the same parental genotype (S10 Fig., S5 Text, S6 Table). Interestingly, when coevolving incompatibility loci are linked to a neutral BDM incompatibility, this does not significantly lower the frequency at which hybrid populations evolve reproductive isolation (S5 Text). Furthermore, linkage between coevolving incompatibilities and neutral BDM incompatibilities can more frequently result in fixation of neutral BDM incompatibilities for a parental genotype (16±2%), resulting in stronger isolation between hybrid and parental populations (S5 Text).
The above simulations focus on simple models that show this process can occur in principle. To capture more biological realism in the number and types of incompatibilities, we simulated 20 incompatibility pairs with randomly determined genomic position and dominance, exponentially distributed selection coefficients (mean s = 0.05) and variation in asymmetry of selection (see above and S5 Text). In these simulations, 95% of populations developed isolation from both parental species. On average, the hybrid population first evolved isolation from both parental species after ~250 generations and was isolated from each by 7 incompatibility pairs within 1000 generations. Since incompatibility pairs with the largest fitness effects tend to fix first, hybrid populations developed considerable reproductive isolation from parental species even before all incompatibilities were fixed in the population (Figs. 4 and S11). Overall, our simulations suggest that rapid evolution of reproductive isolation of hybrid populations is likely when parental species are separated by several hybrid incompatibility pairs.
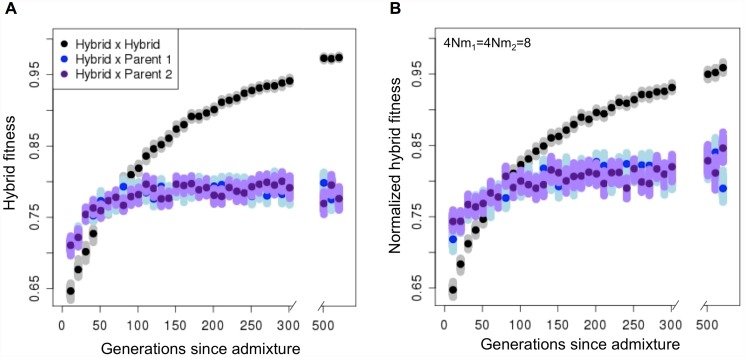
Fig 4. Hybrid populations rapidly develop reproductive isolation from both parental species, even in the presence of migration.
Once hybrid populations diverge in ancestry at hybrid incompatibility loci from parental populations, individual hybrids have higher fitness on average when they mate with other hybrids in their population compared to either parent. (A) No migration and (B) ongoing migration (4Nm 1 = 4Nm 2 = 8) from parental populations. Dark points represent the mean fitness, and smears represent the means of 1,000 bootstrap samples. In B, fitness is normalized to the mean fitness of individuals in the parental populations. Simulation parameters: 100 replicates per time point, N = 1000, 20 hybrid incompatibility pairs, s 1, s 2 and h drawn from distributions (exponential, exponential and uniform, respectively, see details S5 Text).
Reproductive isolation between hybrids and parental species is less likely to evolve as the fitness of hybrids decreases. For example, if we repeat the simulations above (i.e. the 20 incompatibility pairs with exponentially distributed selection coefficients), if = 0.1, the average fitness of an F1 hybrid between the two parental species is 0.38 and isolation evolves in only 56±2% of simulations. When = 0.2, the average fitness of hybrids is 0.1, and only 1.4±0.5% of simulated populations develop isolation and parental genotypes dominated in these populations. Thus, this process is unlikely to occur between species in which post-zygotic isolation is nearly complete.
Similarly, if parental individuals in the simulated hybrid population mate assortatively with conspecifics, reproductive isolation between hybrids and parental species is significantly less likely to evolve (S6 Text). The reasons for this are two-fold: assortative mating prevents the formation of a large hybrid population, and parentals outcompete early generation hybrids that are still segregating for parental incompatibilities.
Simulations of hybrid populations with ongoing migration
We model a completely isolated hybrid swarm, but many hybrid populations experience gene flow with parental species. Ongoing migration may impede the evolution of reproductive isolation by preventing the fixation of genetic incompatibilities. To evaluate this, we simulated hybridization scenarios with ongoing migration (S3 and S13 Figs, 4Nm = 8–20). Even with substantial gene flow from parental populations, hybrid populations evolved reproductive isolation from them at high probability (i.e. 38±2% of simulations with two incompatibility pairs, s = 0.1 and 4Nm = 8; S6 Text; S7 Table).
In the above simulations, we assume that migration is symmetrical from both parental species, but asymmetrical migration may be common in hybrid zones (e.g. [60–62]). To explore how asymmetrical migration could influence our results, we varied asymmetry in migration rates (S6 Text). As expected, when migration rates were high and strongly asymmetrical (S12 Fig.), hybrid reproductive isolation from both parental species evolved infrequently. However, in less extreme cases, hybrid reproductive isolation was still observed frequently (e.g. >20% of simulations with 4Nm<20, S12 Fig.).
It is interesting to consider the fact that chance plays an important role in the direction that incompatibility pairs fix. As a result, one would expect that two or more independently formed hybrid populations from the same pair of parental species could evolve isolation from each other. To demonstrate this effect, we simulated two hybrid populations formed from the same pair of parental species (S14 Fig.). In the absence of migration, the two hybrid populations evolved isolation from each other frequently (50±5%, as expected given two hybrid incompatibility pairs, see S6 Text; S8 Table). Remarkably, this outcome is still observed with relatively high gene flow between the two hybrid populations (24±4% with 4Nm = 8 and two hybrid incompatibility pairs, S6 Text; S8 Table).
Discussion
We describe a new model of the evolution of reproductive isolation of hybrid populations, a first step towards hybrid speciation. Unlike previous models of hybrid speciation, our model does not assume positive selection on hybrid genotypes or inbreeding, but rather deterministic selection against hybrid incompatibilities in randomly mating hybrid populations. With moderate selection (i.e. s≤0.2) on two or more incompatibility pairs in an allopatric hybrid population, reproductive isolation from both parental species emerges with ~50% (or higher) probability. Hybrid reproductive isolation also evolves frequently with substantial levels of ongoing migration between hybrids and parental species (4Nm < 20 each parent).
Another striking result of our simulations is the speed with which reproductive isolation evolves between hybrids and parental species. Depending on parameters, reproductive isolation can emerge in fewer than 100 generations with moderate selection (S3 Text). The idea that hybrid speciation can occur rapidly has been supported by experimental results [14, 63, 64] and to some extent by previous models of hybrid speciation [9, 14]. Our model suggests that simple selection on incompatibilities in hybrid populations could also lead to rapid reproductive isolation on timescales much faster than expected for allopatric speciation due to the accumulation of neutral BDM incompatibilities. Given that epistatic incompatibilities are common, our results on the probability and speed of isolation suggest that this process may frequently occur in hybrid populations.
Previous empirical work has emphasized the importance of ecological differentiation between hybrid and parental populations or positive selection on hybrid genotypes as a route to hybrid reproductive isolation [6, 8–10, 12, 63, 65]. The novel finding of our simulations is that reproductive isolation evolves readily in hybrid populations without positive selection on hybrids. However, the two are not mutually exclusive and ecological factors, which have been shown to underlie several cases of hybrid speciation [6, 8, 63], may complement selection on genetic incompatibilities to further strengthen reproductive isolation. For example, in Helianthus, a combination of chromosomal rearrangements and novel hybrid phenotypes are important in distinguishing hybrid and parental species [6, 66]. Like other models ([9, 14]), our model predicts that isolation between hybrids and parental species is inherently weaker than isolation between the two parental species. We propose that fixation of incompatibilities could be a crucial step in initially limiting gene flow between hybrids and parental species, allowing for the development of other isolating mechanisms. For example, theoretical work predicts that reinforcement can develop even when selection against gene flow is moderate [67–70].
Previous models of hybrid speciation have incorporated species-specific inversions that are assumed to be underdominant. Under this “underdominant inversion” model, hybrid populations can fix for novel inversion combinations, resulting in isolation between hybrid and parental species [15]. Simulation results under this model have suggested that inbreeding [14] or positive selection on hybrid genotypes [9, 14] is important for the evolution of hybrid reproductive isolation. However, past simulation efforts focused on hybrids in a tension zone, either with no spatial isolation from parental species [14] or with high migration rates from parental species [17]. To investigate the dynamics of the underdominant inversion model in situations where migration is more restricted, we simulate the underdominant inversion model in an isolated hybrid swarm scenario that is similar to our epistatic incompatibility model (S7 Text). Interestingly, we find that isolation evolves frequently under this model even without positive selection (~40% of simulations, see S7 Text). These results show that, in hybrid-dominated populations, the inversion model has similar behavior to our model of selection against negative epistatic interactions (S7 Text). Which mechanism of isolation is more prevalent in hybrid populations will depend on the frequency of hybrid incompatibilities of each type. Empirical evidence suggests that while underdominance can be a common isolating mechanism in plants (reviewed in [21]), negative epistatic interactions may be a more common mechanism of reduced hybrid fitness in animals [24].
It is important to note several factors that may influence how common our epistatic interactions model of hybrid speciation will be in natural populations. First, our model assumes that hybrids are abundant in a population and, while this appears to be reasonably common (see S6 Text; S9 Table), this is clearly not a feature of all hybrid zones. We also note that our model only represents fitness in terms of genetic incompatibilities and that hybrid populations can have lower fitness as a result of ecological or sexual selection. For example, in our simulations, we assumed random mating between hybrids and parentals. But when parental species exert negative sexual selection against hybrids, hybrid populations are significantly more likely to be outcompeted by parentals (S10 Table). There is substantial variation in the mating preferences of parentals for hybrids [71]. In two species of cyprinidontiform fishes, male and female parentals mate readily with hybrids [45, 72, 73], while mice discriminate against them [74]. This suggests that the likelihood of this process will depend in part on the biology of the hybridizing species.
An additional consideration is that hybrid reproductive isolation is most likely to evolve during a particular window of divergence between parental species. When the fitness of hybrid populations is low (i.e. corresponding to high levels of divergence between parental species), they are more prone to extinction or displacement by parentals (S6 Fig., S5 Text). This suggests that the evolution of hybrid reproductive isolation through this mechanism is most likely to occur in a period of evolutionary divergence during which species have accumulated some hybrid incompatibilities but have not diverged to the point at which hybrids are largely inviable. The most detailed work characterizing genetic incompatibilities has been between Drosophila species, where hybrids generally have substantially reduced fitness compared to parents [56, 57, 75]. Hybrids between several other species studied to date, however, are affected by fewer incompatibilities or incompatibilities of weaker effects [26, 55, 59, 76–79]. Such groups may be more likely to form hybrid populations, and should be the focus of future empirical research. In addition, even species that currently have strong isolation may have historically produced hybrid populations, though investigating ancient hybrid speciation by the mechanism we describe would be challenging. This is because if parental and hybrid lineages have diverged substantially since the time of initial hybridization it may not be possible to determine whether or not incompatibilities were initially derived from parental genomes.
It is interesting to note that reduced frequency of reproductive isolation with increasing selection on hybrids can be mitigated to some extent by an increase in the total number of hybrid incompatibility pairs. In our simulations, we see a positive relationship between the number of interactions and the probability of developing reproductive isolation, and a negative relationship between the total strength of selection on hybrids and the probability of developing reproductive isolation (Figs. 3 and S6). This tradeoff suggests that reproductive isolation can evolve between hybrid and parental populations even when the fitness of hybrids is low (as in Figs. 3, 4, and S6, keeping in mind that extinction occurs frequently when hybrid fitness is nearly zero).
Similarly, our model is sensitive to skewed initial admixture proportions, but increasing the number of hybrid incompatibility pairs increases the probability that skewed hybrid populations will be isolated from both parental species by at least one incompatibility (S7 Fig.). For example, with two incompatibility pairs, the probability of isolation from both parental species in an ancestry-skewed population (65% parent 1) was 7% while with four incompatibility pairs the probability rose to 15%. In addition, because discrete populations in a cline often span a range of admixture proportions (e.g. [80–82]), it is likely that some hybrid populations will fall in the range where we predict that isolation can evolve. On the other hand, our results show that high levels of migration (as might be observed in continuous clines) can prevent isolation; future research should investigate the dynamics of this process in a range of hybrid zone structures.
Finally, our model assumes that coevolving incompatibilities or BDM incompatibilities arising from adaptive evolution frequently occur between species. Accumulating evidence suggests that incompatibilities arising from coevolution may be common [30, 36, 83–86]. For example, in marine copepods, coevolution between cytochrome c and cytochrome c oxidase results in a reciprocal breakdown of protein function in hybrids [86]. In addition, the fact that many known incompatibility genes involve sexual conflict, selfish genetic elements, or pathogen defense suggests an important role for coevolution in the origin of incompatibilities [36, 83, 87, 88]. Our model also applies to BDM incompatibilities that arise due to within-lineage adaptation, assuming that the fitness advantage of the derived alleles is not dependent on the parental environment. It is currently unknown whether incompatibilities are more likely to be neutral or adaptive. Though there is evidence for asymmetric selection on many hybrid incompatibilities [28, 29, 89], neutrality has not been established in these cases. Anecdotal evidence supports the idea that adaptive incompatibilities are common, since many of the genes underlying hybrid incompatibilities identified so far show evidence of positive selection within lineages [90], but the relative frequency of adaptive and neutral BDM incompatibilities awaits answers from further empirical research. Intriguingly, theoretical work also suggests that neutral BDM incompatibilities are unlikely to persist if there is gene flow between species [32].
The patterns predicted by our model are testable with empirical approaches. A large number of studies have successfully mapped genetic incompatibilities distinguishing species [25, 26, 41, 56, 57, 79, 91]. Ancestry at these sites can be determined in putative hybrid species, and the relative contribution of parental-derived incompatibilities to reproductive isolation can be determined experimentally. For some species, it may be possible to evaluate the dynamics of incompatibilities relative to the genetic background in experimentally generated hybrid swarms [92]. We predict that many hybrid populations exhibiting postzygotic isolation from parental species will have fixed incompatibility pairs for each parental species. Several cases of hybrid speciation report reduced fitness of offspring between parental and hybrid species consistent with the mechanism described here [6, 16, 53, 93] and are promising cases for further empirical research. Strikingly, a recent study on Italian sparrows concludes that reproductive isolation between parental and hybrid species is partly due to the fixation of parental-derived incompatibilities [94].
An intriguing implication of our model is that independently formed hybrid populations between the same parental species can develop reproductive isolation from each other. The likelihood of this outcome increases with the number of incompatibility pairs. In sunflowers, empirical studies of ecologically-mediated hybrid speciation have identified multiple hybrid species derived from the same parental species [95]. It is interesting to note that selection against hybrid incompatibilities could generate the same pattern in replicate hybrid populations. In fact, this mechanism could generate a species phylogeny pattern similar to that expected from an adaptive radiation, with multiple closely related species arising in a relatively short evolutionary window. This finding is striking because our model does not invoke adaptation and suggests that non-adaptive processes (i.e. selection against incompatibilities) could also explain clusters of rapidly arising, closely-related species.
Methods
Mathematical model of selection on hybrid incompatibilities
To characterize evolution at hybrid incompatibility loci in hybrid populations without drift, we used the equations described by Karlin and others [46, 47] to calculate changes in allele frequency as a result of two-locus selection. The frequency of gamete i at generation t is given by
| (1) |
where ε 1 = ε 4 = -ε 2 = -ε 3 = -1, the marginal fitness of allele i,
| (2) |
the mean fitness of the population,
| (3) |
w 14is the fitness of a double heterozygote, r is the recombination rate and D is linkage disequilibrium between the two loci. These equations assume random mating, non-overlapping generations and that fitness depends only on two-locus genotype and not on whether the chromosome was maternally or paternally inherited (i.e. w ij = w ji). To model changes in allele frequencies over time, we developed a custom R script (available from github: https://github.com/melop/twolocusmodel). Iterating through the change in allele frequencies each generation as a result of selection gives the expected patterns of fixation at incompatibility loci without genetic drift (S4 Fig.; see also [48]).
The deterministic two-locus model of fixation of hybrid incompatibilities does not realistically predict expected patterns in natural populations because even large populations will have some level of genetic drift. To model drift, we added multinomial sampling of N diploid individuals and recalculated allele frequencies each generation (available from github: https://github.com/melop/twolocusmodel). Patterns of fixation incorporating genetic drift through multinomial sampling show similar dynamics to the model lacking genetic drift, with the exception of several equilibrium states specific to the latter (see S5 Fig., S2 Text).
Description of simulation program
Exact results for more than two loci have proven difficult to obtain [96–99]. As a result, we developed a custom c++ program, called admix’em (github: https://github.com/melop/admixem), to simulate more complex scenarios. The code allows one to specify the number and length of chromosomes and the genomic locations of hybrid incompatibilities and neutral markers. The current implementation assumes non-overlapping generations and diploid sexual individuals. When modeling linkage, we assume a uniform recombination rate and one recombination event per chromosome per meiosis. Unless otherwise specified, we model all pairs of hybrid incompatibility loci as unlinked. As we are interested in short-term dynamics, we do not implement mutation.
Selection coefficients are assigned to particular allelic combinations according to a hybrid fitness matrix (see S1 and S2 Figs). Based on each individual’s genotype at the hybrid incompatibility loci, we calculate total individual fitness w, defined as the probability of survival of that individual. Total fitness across multiple incompatibility pairs is assumed to be multiplicative. Each female mates with one randomly selected male (but we also accommodate assortative mating, see S6 Text), and produces a Poisson distributed number of offspring with a mean = 2. After selection, if the carrying capacity (N) is not reached, additional offspring from the same mating events are drawn from a Poisson distribution with a new mean = (carrying capacity—current population size)/number of females. This process is repeated until carrying capacity is reached or females have no available gametes (set to a maximum of 10). A potential concern with this approach for maintaining a constant population size is that it could artificially preserve a hybrid population that would otherwise be ephemeral by continuing to sample offspring (up to 10 per female in our simulations). However, because parentals are present in each population (see below, at 50% frequency each parental species in the initial population), this allows for out-competition of hybrids by parentals when hybrid fitness is low.
All reported results are based on 500 replicate simulations, which were conducted for 2000 generations. In the majority of simulations (except S3 and S4 Texts) the hybrid population is initially colonized by 500 individuals of each parental species. Hybrid and parental populations were modeled as spatially distinct with migration parameters between them; most simulations specified one hybrid population formed between two parental populations (S3 Fig.) but we also simulated a stepping-stone model and a model with multiple independently formed hybrid populations (S6 Text, S13–S14 Figs). In simulations with migration, the number of migrating individuals each generation was determined by drawing from a binomial distribution with a mean equal to the number of migrating individuals. Details on individual simulations and results can be found in the supporting text. Hybrid populations are considered to have evolved reproductive barriers from both parental species if they fix at least one incompatibility from each parental type; the strength of reproductive isolation between hybrids and parental species will depend on the selection coefficient and number of incompatibilities.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(A) One of two possible mutational paths to the development of a two-locus BDM incompatibility (Not shown is the case where both mutations occur on one lineage). These incompatibilities can arise as the result of neutral fixation (wparental = wancestral) or as the result of adaptive evolution (wparental>wancestral). (B) Potential selection patterns on hybrid genotypes between the two parentals (assuming wparental>wancestral); genotypes corresponding to selection coefficients s 1 and s 2 are indicated in blue and red respectively. For BDM incompatibilities, s 1 and s 2 can be asymmetric, and in neutral BDM incompatibilities either s 1 or s 2 will equal zero. (C) Fitness of hybrid individuals with each genotype will depend on the intensity of selection (s 1, s 2) and dominance (h A, h B) at the two loci. We assume for simplicity that the fitness advantage of all derived genotypes (here, xB and Ax) is equal.
(JPG)
(A) One of two possible mutational paths to the development of a two-locus coevolved incompatibility. Not shown is the case where B2 precedes A (see S4 Text). (B) Potential epistatic interactions among hybrid genotypes. Incompatibilities corresponding to s 1 and s 2 are indicated in blue and red, respectively. (C) Fitness of hybrid individuals with each genotype will depend the intensity of selection (s 1, s 2) and dominance (h A, h B) at the two loci.
(JPG)
The simplest model of hybrid speciation evolves in a hybrid swarm via fixation of parental genetic incompatibility pairs in opposite directions (see Fig. 1). f is the proportion of the hybrid (H) population colonized by parent 1 (P1), m 1,2 denotes migration rates between the parental and hybrid populations over n generations.
(JPG)
The expected parent 1-derived allele trajectories for two unlinked hybrid incompatibilities under the deterministic two-locus model depend on starting admixture proportions (f = 0.3–0.7 shown here), dominance parameters (h), and the intensity of selection (i.e. s 1, s 2, see S1 and S2 Figs). The solid line tracks ancestry at locus 1 and the dashed line shows ancestry at locus 2. (A) Neutral BDM incompatibility pairs do not fix if f ≠ 0.5; at f = 0.5 they fix for a hybrid genotype pair that is not incompatible with either parental species (see S4 Text). When incompatibilities are codominant (B, C), the two incompatibility loci fix deterministically for the major parent. At certain values of h (D, E, F), fixation is less dependent on initial admixture proportions.
(JPG)
The expected patterns of fixation for a coevolving hybrid incompatibility (S2 Fig.) under the two-locus model depend on starting admixture proportions (f = 0.3–0.7 shown here), dominance parameters (h), and asymmetry in selection (s 1 ≠ s 2). The solid line shows ancestry at locus 1 of an incompatibility and the dashed line shows ancestry at locus 2 of an incompatibility. (A) Parent 1 allele trajectories predicted by the two-locus model for a given set of parameters. (B) Results for the same parameters incorporating multinomial sampling of 10,000 individuals at each generation. (C) Results for the same parameters incorporating multinomial sampling of 1,000 individuals at each generation. Patterns of fixation depend less on initial admixture proportions as drift increases. The equilibrium at f = 0.5 in the selection-only model is unstable in the presence of drift.
(JPG)
With increasing selection on F1 hybrids between the parental species, the probability that hybrid populations will develop reproductive isolation from both parents decreases. However, reproductive isolation is more likely to evolve with a greater number of hybrid incompatibilities pairs (HI) when controlling for the total strength of selection against F1 hybrids. Error bars show two standard errors. Simulation parameters were h = 0.5, s 1 = s 2, f = 0.5, and N = 1,000.
(JPG)
Proportion of hybrid populations developing isolation from both parents as a function of admixture proportions, dominance (h) and population size (two incompatibility pairs, s 1 = s 2) with two (A) and four (B) incompatibility pairs. Isolation occurs most frequently at equal admixture proportions, but can occur in ancestry-skewed populations, especially if the populations are small, there is variation in dominance, or larger numbers of incompatibility pairs. Error bars show two standard errors.
(JPG)
(A) When hybrid populations form at equal admixture proportions, the deterministic model predicts that neutral BDM incompatibilities will fix for the ancestral genotype in a two-lineage model (left) and a genotype that is compatible with both species in a one-lineage model (right). (B) In a coevolution scenario, certain mutation orders result in an identical fitness matrix to A and thus do not result in reproductive isolation in hybrid populations. In all cases depicted, mutations in lineage 1 could occur in lineage 2 and vice versa but the expected effects on isolation from parental species do not change.
(JPG)
As drift increases, the proportion of hybrid populations isolated from parentals by fixation of neutral BDM incompatibilities increases. However, this process does not occur as rapidly as deterministic selection on other types of hybrid incompatibilities. Simulation parameters: two neutral BDMI pairs (S8 Fig.), s = 0.1, f = 0.5, h = 0.5 for 500 replicate simulations.
(JPG)
Linkage between incompatibility pairs can change the probability of hybrid populations evolving reproductive isolation (S6 Table). (A) In scenario 1, linkage between loci in the same incompatibility pair does not influence the frequency of hybrid populations evolving reproductive isolation. (B) In linkage scenario 2, linkage between loci in different incompatibility pairs significantly decreases the frequency at which hybrid populations evolve reproductive isolation. The probability of recombination between two sites is indicated as r.
(JPG)
(A) Change in average hybrid population fitness over time in a simulation of 20 incompatibility pairs with dominance and selection coefficients drawn from an exponential distribution (see S5D Text). (B) The same hybrid population with a one generation burst of migrants from parent 1 (4Nm1 = 400) at generation 300. (C) The same hybrid population with a one generation burst of migrants from parent 2 (4Nm2 = 400) at generation 300. Notably, hybrid populations have lower average fitness after gene flow with either parent, but recover rapidly.
(JPG)
Proportion of hybrid populations evolving isolation from both parents as a function of asymmetry in migration rates from parental populations. When migration is highly asymmetric hybrid populations are less likely to evolve reproductive isolation from parental species. Simulation conditions: two incompatibility pairs, h = 0.5, s 1 = s 2 = 0.1, f = 0.5, N = 1000 for 500 replicate simulations.
(JPG)
Model of hybrid zone structure used in simulations of complex hybrid zone structures (see S6B Text). This structure of a gradient of hybrid populations with ongoing gene flow from parental and other hybrid populations is similar to many naturally occurring hybrid populations.
(JPG)
Hybrid zone structure used in simulations of reciprocal hybrid isolation (S6C Text).
(JPG)
Proportion of hybrid populations developing isolation from both parents as a function of admixture proportion with two underdominant inversions. Simulation conditions: s 1 = s 2 = 0.05, N = 1000 for 500 replicate simulations. Error bars show two standard errors.
(JPG)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank Molly Przeworski and members of the Rosenthal and Andolfatto labs for helpful discussion; as well as Yaniv Brandvain, Guy Sella, Stephen Wright, Clair Han, Ying Zhen, and three anonymous reviewers for helpful comments on earlier versions of this manuscript.
Data Availability
Simulator program is available at github: https://github.com/melop/admixem. Other scripts used to generate and analyze data for this project are available at github: https://github.com/melop/twolocusmodel
Funding Statement
This work was supported by NSF GRFP (DGE0646086) and NSF DDIG (DEB-1405232) to MS and an NSF IOS-0923825 to GGR. website url: nsf.gov. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Arnold ML (2006) Evolution through genetic exchange. Oxford, U.K.: Oxford University Press. [Google Scholar]
- 2. Mallet J (2007) Hybrid speciation. Nature 446: 279–283. [DOI] [PubMed] [Google Scholar]
- 3. Nolte AW, Tautz D (2010) Understanding the onset of hybrid speciation. Trends Genet 26: 54–58. 10.1016/j.tig.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 4. Schumer M, Rosenthal GG, Andolfatto P (2014) How common is homoploid hybrid speciation? Evolution 68: 1553–1560. 10.1111/evo.12399 [DOI] [PubMed] [Google Scholar]
- 5. Rieseberg LH, Vanfossen C, Desrochers AM (1995) Hybrid speciation accompanied by genomic reorganization in wild sunflowers. Nature 375: 313–316. [Google Scholar]
- 6. Rieseberg LH, Raymond O, Rosenthal DM, Lai Z, Livingstone K, et al. (2003) Major ecological transitions in wild sunflowers facilitated by hybridization. Science 301: 1211–1216. [DOI] [PubMed] [Google Scholar]
- 7. Lexer C, Welch ME, Raymond O, Rieseberg LH (2003) The origin of ecological divergence in Helianthus paradoxus (Asteraceae): Selection on transgressive characters in a novel hybrid habitat. Evolution 57: 1989–2000. [DOI] [PubMed] [Google Scholar]
- 8. Mavarez J, Salazar CA, Bermingham E, Salcedo C, Jiggins CD, et al. (2006) Speciation by hybridization in Heliconius butterflies. Nature 441: 868–871. [DOI] [PubMed] [Google Scholar]
- 9. Buerkle CA, Morris RJ, Asmussen MA, Rieseberg LH (2000) The likelihood of homoploid hybrid speciation. Heredity 84: 441–451. [DOI] [PubMed] [Google Scholar]
- 10. Schwarz D, Matta BM, Shakir-Botteri NL, McPheron BA (2005) Host shift to an invasive plant triggers rapid animal hybrid speciation. Nature 436: 546–549. [DOI] [PubMed] [Google Scholar]
- 11. Schwarz D, Shoemaker KD, Botteri NL, McPheron BA (2007) A novel preference for an invasive plant as a mechanism for animal hybrid speciation. Evolution 61: 245–256. [DOI] [PubMed] [Google Scholar]
- 12. Duenez-Guzman EA, Mavarez J, Vose MD, Gavrilets S. (2009) Case studies and mathematical models of ecological speciation. 4. Hybrid speciation in butterflies in a jungle. Evolution. 63:2611–26. 10.1111/j.1558-5646.2009.00756.x [DOI] [PubMed] [Google Scholar]
- 13. Jiggins CD, Salazar C, Linares M, Mavarez J (2008) Hybrid trait speciation and Heliconius butterflies. Philos T Roy Soc B 363: 3047–3054. 10.1098/rstb.2008.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCarthy EM, Asmussen MA, Anderson WW (1995) A theoretical assessment of recombinational speciation. Heredity 74: 502–509. [Google Scholar]
- 15. Grant V (1971) Plant Speciation. New York: Columbia University Press. [Google Scholar]
- 16. Lai Z, Nakazato T, Salmaso M, Burke JM, Tang SX, et al. (2005) Extensive chromosomal repatterning and the evolution of sterility barriers in hybrid sunflower species. Genetics 171: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buerkle CA, Wolf DE, Rieseberg LH (2003) The origin and extinction of species through hybridization In: Brigham CA, Schwarz MW, editors. Population viability in plants: Conservation, management, and modeling of rare plants pp. 117–141. [Google Scholar]
- 18. Carr DE, Rieseberg LH, Abbott RJ (2003) The genetic architecture necessary for transgressive segregation is common in both natural and domesticated populations—Discussion. Philos T Roy Soc B 358: 1147–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rieseberg LH, Widmer A, Arntz AM, Burke JM (2003) The genetic architecture necessary for transgressive segregation is common in both natural and domesticated populations. Philos T Roy Soc B 358: 1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stelkens R, Seehausen O (2009) Genetic distance between species predicts novel trait expression in their hybrids. Evolution 63: 884–897. 10.1111/j.1558-5646.2008.00599.x [DOI] [PubMed] [Google Scholar]
- 21. Hoffmann AA, Rieseberg LH (2008) Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Ann Rev Ecol Evol Syst 39:21–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dobzhansky T (1937) Genetics and the origin of species. New York: Columbia University Press. [Google Scholar]
- 23. Muller HJ (1942) Isolating mechanisms, evolution and temperature. Biol Symp 6: 71–125. [Google Scholar]
- 24. Coyne JA, Orr HA (2004) Speciation. Sunderland, MA: Sinaeur Associates. [Google Scholar]
- 25. Fishman L, Willis JH (2001) Evidence for Dobzhansky-Muller incompatibilites contributing to the sterility of hybrids between Mimulus guttatus and M. nasutus . Evolution 55: 1932–1942. [DOI] [PubMed] [Google Scholar]
- 26. Sweigart AL, Fishman L, Willis JH (2006) A simple genetic incompatibility causes hybrid male sterility in Mimulus . Genetics 172: 2465–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brideau NJ, Flores HA, Wang J, Maheshwari S, Wang X, et al. (2006) Two Dobzhansky-Muller genes interact to cause hybrid lethality in Drosophila . Science 314: 1292–1295. [DOI] [PubMed] [Google Scholar]
- 28. Meiklejohn CD, Holmbeck MA, Siddiq MA, Abt DN, Rand DM, et al. (2013) An incompatibility between a mitochondrial tRNA and its nuclear-encoded tRNA synthetase compromises development and fitness in Drosophila . PLoS Genet 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sawamura K, Yamamoto MT (1997) Characterization of a reproductive isolation gene, zygotic hybrid rescue, of Drosophila melanogaster by using minichromosomes. Heredity 79: 97–103. [Google Scholar]
- 30. Presgraves DC (2010) The molecular evolutionary basis of species formation. Nat Rev Genet 11: 175–180. 10.1038/nrg2718 [DOI] [PubMed] [Google Scholar]
- 31. Orr HA, Presgraves DC (2000) Speciation by postzygotic isolation: forces, genes and molecules. Bioessays 22: 1085–1094. [DOI] [PubMed] [Google Scholar]
- 32. Bank C, Buerger R, Hermisson J (2012) The limits to parapatric speciation: Dobzhansky-Muller incompatibilities in a continent-island model. Genetics 191: 845–U345. 10.1534/genetics.111.137513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gavrilets S (1997) Single locus clines. Evolution 51: 979–983. [DOI] [PubMed] [Google Scholar]
- 34. Gompert Z, Parchman TL, Buerkle CA (2012) Genomics of isolation in hybrids. Philos T Roy Soc B 367: 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seehausen O, Butlin RK, Keller I, Wagner CE, Boughman JW, et al. (2014) Genomics and the origin of species. Nat Rev Genet 15: 176–192. 10.1038/nrg3644 [DOI] [PubMed] [Google Scholar]
- 36. Rieseberg LH, Blackman BK (2010) Speciation genes in plants. Ann Bot 106: 439–455. 10.1093/aob/mcq126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnson NA, Wolf JB, Brodie ED III, Wade MJ (2000) Gene interactions and the origin of species In: Wolf J, Brodie EI, Wade M, editors. Epistasis and the evolutionary process. New York: Oxford University Press; pp. 197–212. [Google Scholar]
- 38. Gavrilets S (1997) Hybrid zones with Dobzhansky-type epistatic selection. Evolution 51: 1027–1035. [DOI] [PubMed] [Google Scholar]
- 39.Paixão T, Bassler KE, Azevedo RBR (2014) Emergent speciation by multiple Dobzhansky-Muller incompatibilities. bioRxiv doi: 10.1101/008268. [DOI]
- 40. Schartl M (2008) Evolution of Xmrk: an oncogene, but also a speciation gene? Bioessays 30: 822–832. 10.1002/bies.20807 [DOI] [PubMed] [Google Scholar]
- 41. Schumer M, Cui R, Powell D, Dresner R, Rosenthal G, et al. (2014) High-resolution mapping reveals hundreds of genetic incompatibilities in hybridizing fish species. eLife 3: e02535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fang S, Yukilevich R, Chen Y, Turissini DA, Zeng K, et al. (2012) Incompatibility and competitive exclusion of genomic segments between sibling Drosophila species. PLoS Genet 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fisher HS, Wong BBM, Rosenthal GG (2006) Alteration of the chemical environment disrupts communication in a freshwater fish. Proc R Soc London Ser B 273: 1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hasselman DJ, Argo EE, McBride MC, Bentzen P, Schultz TF, et al. (2014) Human disturbance causes the formation of a hybrid swarm between two naturally sympatric fish species. Mol Ecol 23: 1137–1152. 10.1111/mec.12674 [DOI] [PubMed] [Google Scholar]
- 45. Rosenfield JA, Kodric-Brown A (2003) Sexual selection promotes hybridization between Pecos pupfish, Cyprinodon pecosensis and sheepshead minnow, C. variegatus . J Evolution Biol 16: 595–606. [DOI] [PubMed] [Google Scholar]
- 46. Karlin S (1975) General 2-locus selection models—Some objectives, results and interpretations. Theor Popul Biol 7: 364–398. [DOI] [PubMed] [Google Scholar]
- 47. Bodmer WF, Felsenstein J (1967) Linkage and selection—theoretical analysis of deterministic 2 locus random mating model. Genetics 57: 237–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gavrilets S (2004) Fitness landscapes and the origin of species. Princeton, NJ, USA: Princeton University Press; 1–432 p. [Google Scholar]
- 49. James JK, Abbott RJ (2005) Recent, allopatric, homoploid hybrid speciation: The origin of Senecio squalidus (Asteraceae) in the British Isles from a hybrid zone on Mount Etna, Sicily. Evolution 59: 2533–2547. [PubMed] [Google Scholar]
- 50. Lexer C, Joseph JA, van Loo M, Barbara T, Heinze B, et al. (2010) Genomic Admixture Analysis in European Populus spp. Reveals Unexpected Patterns of Reproductive Isolation and Mating. Genetics 186: 699–U391. 10.1534/genetics.110.118828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kunte K, Shea C, Aardema ML, Scriber JM, Juenger TE, et al. (2011) Sex Chromosome Mosaicism and Hybrid Speciation among Tiger Swallowtail Butterflies. PLoS Genetics 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rubidge EM, Taylor EB (2004) Hybrid zone structure and the potential role of selection in hybridizing populations of native westslope cutthroat trout (Oncorhynchus clarki lewisi) and introduced rainbow trout (O. mykiss). Mol Ecol 13: 3735–3749. [DOI] [PubMed] [Google Scholar]
- 53. Trier CN, Hermansen JS, Sætre G-P, Bailey RI (2014) Evidence for mito-nuclear and sex-linked reproductive barriers between the hybrid Italian sparrow and its parent species. PLoS Genet 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Unckless RL, Orr HA (2009) Dobzhansky-Muller incompatibilities and adaptation to a shared environment. Heredity 102: 214–217. 10.1038/hdy.2008.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moyle LC, Graham EB (2006) Genome-wide associations between hybrid sterility QTL and marker transmission ratio distortion. Mol Biol Evol 23: 973–980. [DOI] [PubMed] [Google Scholar]
- 56. Masly JP, Presgraves DC (2007) High-resolution genome-wide dissection of the two rules of speciation in Drosophila . PLoS Biol 5: 1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Presgraves DC (2003) A fine-scale genetic analysis of hybrid incompatibilities in Drosophila . Genetics 163: 955–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gardner K, Buerkle A, Whitton J, Rieseberg L (2000) Inferring epistasis in wild sunflower hybrid zones In: Wolf JB, Brodie ED III, Wade MJ, editors. Epistasis and the evolutionary process. New York: Oxford University Press; pp. 264–279. [Google Scholar]
- 59. Payseur BA, Hoekstra HE (2005) Signatures of reproductive isolation in patterns of single nucleotide diversity across inbred strains of mice. Genetics 171: 1905–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Teeter KC, Payseur BA, Harris LW, Bakewell MA, Thibodeau LM, et al. (2008) Genome-wide patterns of gene flow across a house mouse hybrid zone. Genome Res 18: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ostberg CO, Slatton SL, Rodriguez RJ (2004) Spatial partitioning and asymmetric hybridization among sympatric coastal steelhead trout (Oncorhynchus mykiss irideus), coastal cutthroat trout (O. clarki clarki) and interspecific hybrids. Mol Ecol 13: 2773–2788. [DOI] [PubMed] [Google Scholar]
- 62. Field DL, Ayre DJ, Whelan RJ, Young AG (2011) Patterns of hybridization and asymmetrical gene flow in hybrid zones of the rare Eucalyptus aggregata and common E. rubida . Heredity 106: 841–853. 10.1038/hdy.2010.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Greig D, Louis EJ, Borts RH, Travisano M (2002) Hybrid speciation in experimental populations of yeast. Science 298: 1773–1775. [DOI] [PubMed] [Google Scholar]
- 64. Buerkle CA, Rieseberg LH (2008) The rate of genome stabilization in homoploid hybrid species. Evolution 62: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gompert Z, Fordyce JA, Forister ML, Shapiro AM, Nice CC (2006) Homoploid hybrid speciation in an extreme habitat. Science 314: 1923–1925. [DOI] [PubMed] [Google Scholar]
- 66. Rieseberg LH, Whitton J, Gardner K (1999) Hybrid zones and the genetic architecture of a barrier to gene flow between two sunflower species. Genetics 152: 713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Servedio MR (2000) Reinforcement and the genetics of nonrandom mating. Evolution 54: 21–29. [DOI] [PubMed] [Google Scholar]
- 68. Servedio MR, Noor MAF (2003) The role of reinforcement in speciation: Theory and data. Annu Rev Ecol Evol S 34: 339–364. [Google Scholar]
- 69. Kirkpatrick M, Servedio MR (1999) The reinforcement of mating preferences on an island. Genetics 151: 865–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nosil P, Yukilevich R (2008) Mechanisms of reinforcement in natural and simulated polymorphic populations. Biol J Linnean Soc 95: 305–319. [Google Scholar]
- 71. Rosenthal GG (2013) Individual mating decisions and hybridization. J Evol Biol 26: 252–255. 10.1111/jeb.12004 [DOI] [PubMed] [Google Scholar]
- 72. Fisher HS, Mascuch SJ, Rosenthal GG (2009) Multivariate male traits misalign with multivariate female preferences in the swordtail fish, Xiphophorus birchmanni . Anim Behav 78: 265–269. [Google Scholar]
- 73. Culumber ZW, Ochoa OM, Rosenthal GG (2014) Assortative mating and the maintenance of population structure in a natural hybrid zone. Am Nat 184: 225–232. 10.1086/677033 [DOI] [PubMed] [Google Scholar]
- 74. Ganem G, Litel C, Lenormand T (2008) Variation in mate preference across a house mouse hybrid zone. Heredity 100: 594–601. 10.1038/hdy.2008.20 [DOI] [PubMed] [Google Scholar]
- 75. Matute DR, Butler IA, Turissini DA, Coyne JA (2010) A test of the snowball theory for the rate of evolution of hybrid incompatibilities. Science 329: 1518–1521. 10.1126/science.1193440 [DOI] [PubMed] [Google Scholar]
- 76. Corbett-Detig RB, Zhou J, Clark AG, Hartl DL, Ayroles JF (2013) Genetic incompatibilities are widespread within species. Nature 504: 135–137. 10.1038/nature12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Harushima Y, Nakagahra M, Yano M, Sasaki T, Kurata N (2001) A genome-wide survey of reproductive barriers in an intraspecific hybrid. Genetics 159: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Janousek V, Wang L, Luzynski K, Dufkova P, Vyskocilova MM, et al. (2012) Genome-wide architecture of reproductive isolation in a naturally occurring hybrid zone between Mus musculus musculus and M. m. domesticus . Mol Ecol 21: 3032–3047. 10.1111/j.1365-294X.2012.05583.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Turner LM, White MA, Tautz D, Payseur BA (2014) Genomic networks of hybrid sterility. PLoS Genet 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gay L, Crochet P-A, Bell DA, Lenormand T (2008) Comparing clines on molecular and phenotypic traits in hybrid zones: A window on tension zone models. Evolution 62: 2789–2806. 10.1111/j.1558-5646.2008.00491.x [DOI] [PubMed] [Google Scholar]
- 81. Nolte AW, Gompert Z, Buerkle CA (2009) Variable patterns of introgression in two sculpin hybrid zones suggest that genomic isolation differs among populations. Mol Ecol 18: 2615–2627. 10.1111/j.1365-294X.2009.04208.x [DOI] [PubMed] [Google Scholar]
- 82. Vines TH, Kohler SC, Thiel A, Ghira I, Sands TR, et al. (2003) The maintenance of reproductive isolation in a mosaic hybrid zone between the fire-bellied toads Bombina bombina and B. variegata . Evolution 57: 1876–1888. [DOI] [PubMed] [Google Scholar]
- 83. Bomblies K, Lempe J, Epple P, Warthmann N, Lanz C, et al. (2007) Autoimmune response as a mechanism for a Dobzhansky-Muller-type incompatibility syndrome in plants. PLoS Biol 5: 1962–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sweigart AL (2010) Simple Y-Autosomal incompatibilities cause hybrid male sterility in reciprocal crosses between Drosophila virilis and D. americana . Genetics 184: 779–U225. 10.1534/genetics.109.112896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lee H-Y, Chou J-Y, Cheong L, Chang N-H, Yang S-Y, et al. (2008) Incompatibility of nuclear and mitochondrial genomes causes hybrid sterility between two yeast species. Cell 135: 1065–1073. 10.1016/j.cell.2008.10.047 [DOI] [PubMed] [Google Scholar]
- 86. Rawson PD, Burton RS (2002) Functional coadaptation between cytochrome c and cytochrome c oxidase within allopatric populations of a marine copepod. Proc Natl Acad Sci USA 99: 12955–12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Johnson NA (2010) Hybrid incompatibility genes: remnants of a genomic battlefield? Trends Genet 26: 317–325. 10.1016/j.tig.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 88. Crespi B, Nosil P (2013) Conflictual speciation: species formation via genomic conflict. Trends Ecol Evol 28: 48–57. 10.1016/j.tree.2012.08.015 [DOI] [PubMed] [Google Scholar]
- 89. Barbash DA, Roote J, Ashburner M (2000) The Drosophila melanogaster hybrid male rescue gene causes inviability in male and female species hybrids. Genetics 154: 1747–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Maheshwari S, Barbash DA (2011) The genetics of hybrid incompatibilities. Ann Rev Genet 45: 331–355. 10.1146/annurev-genet-110410-132514 [DOI] [PubMed] [Google Scholar]
- 91. Staubach F, Lorenc A, Messer PW, Tang K, Petrov DA, et al. (2012) Genome patterns of selection and introgression of haplotypes in natural populations of the house mouse (Mus musculus). PLoS Genet 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pritchard VL, Edmands S (2013) The genomic trajectory of hybrid swarms: outcomes of repeated crosses between populations of Tigriopus californicus . Evolution 67: 774–791. 10.1111/j.1558-5646.2012.01814.x [DOI] [PubMed] [Google Scholar]
- 93. Schwander T, Suni SS, Cahan SH, Keller L (2008) Mechanisms of reproductive isolation between an ant species of hybrid origin and one of its parents. Evolution 62: 1635–1643. 10.1111/j.1558-5646.2008.00387.x [DOI] [PubMed] [Google Scholar]
- 94. Hermansen JS, Haas F, Trier CN, Bailey RI, Nederbragt AJ, et al. (2014) Hybrid speciation through sorting of parental incompatibilities in Italian sparrows. Mol Ecol 10.1111/mec.12910 [DOI] [PubMed] [Google Scholar]
- 95. Gross BL, Rieseberg LH (2005) The ecological genetics of homoploid hybrid speciation. J Hered 96: 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hastings A (1984) Linkage disequilibrium, selection and recombination at three loci. Genetics 106: 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kirkpatrick M, Johnson T, Barton N (2002) General models of multilocus evolution. Genetics 161: 1727–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gardner A, West SA, Barton NH (2007) The relation between multilocus population genetics and social Evolution Theory. Am Nat 169: 207–226. [DOI] [PubMed] [Google Scholar]
- 99. Kopp M, Gavrilets S (2006) Multilocus genetics and the coevolution of quantitative traits. Evolution 60: 1321–1336. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(A) One of two possible mutational paths to the development of a two-locus BDM incompatibility (Not shown is the case where both mutations occur on one lineage). These incompatibilities can arise as the result of neutral fixation (wparental = wancestral) or as the result of adaptive evolution (wparental>wancestral). (B) Potential selection patterns on hybrid genotypes between the two parentals (assuming wparental>wancestral); genotypes corresponding to selection coefficients s 1 and s 2 are indicated in blue and red respectively. For BDM incompatibilities, s 1 and s 2 can be asymmetric, and in neutral BDM incompatibilities either s 1 or s 2 will equal zero. (C) Fitness of hybrid individuals with each genotype will depend on the intensity of selection (s 1, s 2) and dominance (h A, h B) at the two loci. We assume for simplicity that the fitness advantage of all derived genotypes (here, xB and Ax) is equal.
(JPG)
(A) One of two possible mutational paths to the development of a two-locus coevolved incompatibility. Not shown is the case where B2 precedes A (see S4 Text). (B) Potential epistatic interactions among hybrid genotypes. Incompatibilities corresponding to s 1 and s 2 are indicated in blue and red, respectively. (C) Fitness of hybrid individuals with each genotype will depend the intensity of selection (s 1, s 2) and dominance (h A, h B) at the two loci.
(JPG)
The simplest model of hybrid speciation evolves in a hybrid swarm via fixation of parental genetic incompatibility pairs in opposite directions (see Fig. 1). f is the proportion of the hybrid (H) population colonized by parent 1 (P1), m 1,2 denotes migration rates between the parental and hybrid populations over n generations.
(JPG)
The expected parent 1-derived allele trajectories for two unlinked hybrid incompatibilities under the deterministic two-locus model depend on starting admixture proportions (f = 0.3–0.7 shown here), dominance parameters (h), and the intensity of selection (i.e. s 1, s 2, see S1 and S2 Figs). The solid line tracks ancestry at locus 1 and the dashed line shows ancestry at locus 2. (A) Neutral BDM incompatibility pairs do not fix if f ≠ 0.5; at f = 0.5 they fix for a hybrid genotype pair that is not incompatible with either parental species (see S4 Text). When incompatibilities are codominant (B, C), the two incompatibility loci fix deterministically for the major parent. At certain values of h (D, E, F), fixation is less dependent on initial admixture proportions.
(JPG)
The expected patterns of fixation for a coevolving hybrid incompatibility (S2 Fig.) under the two-locus model depend on starting admixture proportions (f = 0.3–0.7 shown here), dominance parameters (h), and asymmetry in selection (s 1 ≠ s 2). The solid line shows ancestry at locus 1 of an incompatibility and the dashed line shows ancestry at locus 2 of an incompatibility. (A) Parent 1 allele trajectories predicted by the two-locus model for a given set of parameters. (B) Results for the same parameters incorporating multinomial sampling of 10,000 individuals at each generation. (C) Results for the same parameters incorporating multinomial sampling of 1,000 individuals at each generation. Patterns of fixation depend less on initial admixture proportions as drift increases. The equilibrium at f = 0.5 in the selection-only model is unstable in the presence of drift.
(JPG)
With increasing selection on F1 hybrids between the parental species, the probability that hybrid populations will develop reproductive isolation from both parents decreases. However, reproductive isolation is more likely to evolve with a greater number of hybrid incompatibilities pairs (HI) when controlling for the total strength of selection against F1 hybrids. Error bars show two standard errors. Simulation parameters were h = 0.5, s 1 = s 2, f = 0.5, and N = 1,000.
(JPG)
Proportion of hybrid populations developing isolation from both parents as a function of admixture proportions, dominance (h) and population size (two incompatibility pairs, s 1 = s 2) with two (A) and four (B) incompatibility pairs. Isolation occurs most frequently at equal admixture proportions, but can occur in ancestry-skewed populations, especially if the populations are small, there is variation in dominance, or larger numbers of incompatibility pairs. Error bars show two standard errors.
(JPG)
(A) When hybrid populations form at equal admixture proportions, the deterministic model predicts that neutral BDM incompatibilities will fix for the ancestral genotype in a two-lineage model (left) and a genotype that is compatible with both species in a one-lineage model (right). (B) In a coevolution scenario, certain mutation orders result in an identical fitness matrix to A and thus do not result in reproductive isolation in hybrid populations. In all cases depicted, mutations in lineage 1 could occur in lineage 2 and vice versa but the expected effects on isolation from parental species do not change.
(JPG)
As drift increases, the proportion of hybrid populations isolated from parentals by fixation of neutral BDM incompatibilities increases. However, this process does not occur as rapidly as deterministic selection on other types of hybrid incompatibilities. Simulation parameters: two neutral BDMI pairs (S8 Fig.), s = 0.1, f = 0.5, h = 0.5 for 500 replicate simulations.
(JPG)
Linkage between incompatibility pairs can change the probability of hybrid populations evolving reproductive isolation (S6 Table). (A) In scenario 1, linkage between loci in the same incompatibility pair does not influence the frequency of hybrid populations evolving reproductive isolation. (B) In linkage scenario 2, linkage between loci in different incompatibility pairs significantly decreases the frequency at which hybrid populations evolve reproductive isolation. The probability of recombination between two sites is indicated as r.
(JPG)
(A) Change in average hybrid population fitness over time in a simulation of 20 incompatibility pairs with dominance and selection coefficients drawn from an exponential distribution (see S5D Text). (B) The same hybrid population with a one generation burst of migrants from parent 1 (4Nm1 = 400) at generation 300. (C) The same hybrid population with a one generation burst of migrants from parent 2 (4Nm2 = 400) at generation 300. Notably, hybrid populations have lower average fitness after gene flow with either parent, but recover rapidly.
(JPG)
Proportion of hybrid populations evolving isolation from both parents as a function of asymmetry in migration rates from parental populations. When migration is highly asymmetric hybrid populations are less likely to evolve reproductive isolation from parental species. Simulation conditions: two incompatibility pairs, h = 0.5, s 1 = s 2 = 0.1, f = 0.5, N = 1000 for 500 replicate simulations.
(JPG)
Model of hybrid zone structure used in simulations of complex hybrid zone structures (see S6B Text). This structure of a gradient of hybrid populations with ongoing gene flow from parental and other hybrid populations is similar to many naturally occurring hybrid populations.
(JPG)
Hybrid zone structure used in simulations of reciprocal hybrid isolation (S6C Text).
(JPG)
Proportion of hybrid populations developing isolation from both parents as a function of admixture proportion with two underdominant inversions. Simulation conditions: s 1 = s 2 = 0.05, N = 1000 for 500 replicate simulations. Error bars show two standard errors.
(JPG)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Simulator program is available at github: https://github.com/melop/admixem. Other scripts used to generate and analyze data for this project are available at github: https://github.com/melop/twolocusmodel