Abstract
Skeletal muscle regrowth after atrophy is impaired in the aged and in this study we hypothesized that this can be explained by a blunted response of signaling pathways and cellular processes during reloading after hind limb suspension in muscles from old rats. Male Brown Norway Fisher 344 rats at 6 (young) and 32 (old) months of age were subjected to normal ambulatory conditions (amb), hind limb suspension for 14 days (HS), and HS followed by reloading through normal ambulation for 14 days (RE); soleus muscles were used for analysis of intracellular signaling pathways and cellular processes. Soleus muscle regrowth was blunted in old compared to young rats which coincided with a recovery of serum IGF-1 and IGFBP-3 levels in young but not old. However, the response to reloading for p-Akt, p-p70s6k and p-GSK3β protein abundance was similar between muscles from young and old rats, even though main effects for age indicate an increase in activation of this protein synthesis pathway in the aged. Similarly, MAFbx mRNA levels in soleus muscle from old rats recovered to the same extent as in the young, while Murf-1 was unchanged. mRNA abundance of autophagy markers Atg5 and Atg7 showed an identical response in muscle from old compared to young rats, but beclin did not. Autophagic flux was not changed at either age at the measured time point. Apoptosis was elevated in soleus muscle from old rats particularly with HS, but recovered in HSRE and these changes were not associated with differences in caspase-3, -8 or-9 activity in any group. Protein abundance of apoptosis repressor with caspase-recruitment domain (ARC), cytosolic EndoG, as well as cytosolic and nuclear apoptosis inducing factor (AIF) were lower in muscle from old rats, and there was no age-related difference in the response to atrophy or regrowth. Soleus muscles from old rats had a higher number of ED2 positive macrophages in all groups and these decreased with HS, but recovered in HSRE in the old, while no changes were observed in the young. Pro-inflammatory cytokines in serum did not show a differential response with age to different loading conditions. Results indicate that at the measured time point the impaired skeletal muscle regrowth after atrophy in aged animals is not associated with a general lack of responsiveness to changes in loading conditions.
Keywords: protein synthesis, hind limb suspension, protein degradation, autophagy, apoptosis, inflammation
INTRODUCTION
Sarcopenia, or the loss of skeletal muscle mass with aging (Rosenberg, 1989), has severe negative consequences for health and quality of life in the elderly; muscle mass and strength are highly correlated and predictive of functional performance, morbidity and mortality in older men and women (Bassey et al., 1992; Laukkanen et al., 1995; Metter et al., 2002; Rantanen et al., 1999; Rantanen et al., 2000; Szulc, 2010). Moreover, the potential to recover muscle mass after an atrophy-inducing event, such as bed rest or malnutrition, is reduced in aged subjects, putting them at a greater risk for falls and subsequent illnesses (Hebuterne et al., 1997; Hvid et al., 2010; Suetta et al., 2009). English and Paddon-Jones have proposed the catabolic crisis model in which the age-related inability to recover muscle mass contributes to the decrease in functional capacity (English and Paddon-Jones, 2010). They further suggested that interventions should be targeted at preventing the loss of muscle mass during disuse as well as aiding in regrowth of muscle in response to a period of inactivity. A thorough understanding of the mechanisms involved in the failed regrowth response of aged individuals is therefore warranted.
Regrowth of skeletal muscle after an atrophy-inducing event is impaired with aging in animals as well as in humans. Muscle size lost due to starvation, glucocorticoid treatment, hind limb suspension, and limb immobilization was not recovered to the same extent in old as in young rats (Dardevet et al., 1995; Hao et al., 2011; Magne et al., 2011; Mosoni et al., 1999; Zarzhevsky et al., 2001a; Zarzhevsky et al., 2001b) and the growth response to intermittent mobility during atrophy is also inhibited (Gallegly et al., 2004). Similarly, muscle hypertrophy in response to overload is impaired in aged animals (Chale-Rush et al., 2009; Degens and Alway, 2003; Thomson and Gordon, 2006) indicating that aged muscle responds differently to a similar growth stimulus than young muscle. However, previous studies have shown contradictory results when investigating cellular mechanisms potentially responsible for the impaired growth response. Some reports indicate that translational signaling, particularly through the Akt-mTOR related pathway, is impaired or delayed in aged animals undergoing hypertrophy (Funai et al., 2006; Haddad and Adams, 2006; Hwee and Bodine, 2009; Thomson and Gordon, 2006), while others show no differences with young (Chale-Rush et al., 2009; Hornberger et al., 2005); also, the potential to sense mechanical activity was not changed with age (Hornberger et al., 2005). Very few studies have directly compared mechanisms in muscles from young and old to identify potential differences during regrowth after atrophy. In a human study, it was concluded that a decrease in the proliferative response of satellite cells in combination with a change in the regulation of myostatin was responsible for the impaired regrowth in the aged, but intracellular pathways involved in protein synthesis and degradation were not studied (Suetta et al., 2013). In rats, data are sparse and contradictory; while the impaired regrowth response after immobilization was associated with a lower level of IGFBP5 and attenuated p70s6k levels in the aged, changes in Akt phosphorylation were not different from young (Morris et al., 2004; Spangenburg et al., 2003). During muscle recovery from starvation, protein synthesis levels in muscles from aged rats increased to the same extent as young rats, while proteolysis was not returned to baseline in the aged, despite similar responses in protein degradation markers after refeeding (Mosoni et al., 1999). Similarly, the lack of muscle recovery after immobilization in aged rats was not associated with a lack of normalization of proteasome-mediated degradation markers and the caspase-dependent apoptosis pathway, but these findings were not compared to young animals (Magne et al., 2011). Thus, it is currently unclear how intracellular mechanisms differ in muscles from aged and young rats during regrowth after hind limb suspension-induced atrophy. In addition, inflammation-related events and autophagy-mediated changes have not been investigated under impaired regrowth conditions. Therefore, the purpose of our current study was to determine if there are differences between young and old muscle in the regrowth response after atrophy. We hypothesized that muscles from aged rats exhibited a blunted recovery in intracellular pathways changed in response to disuse-induced atrophy.
METHODS
Animals and experimental procedures
All procedures were performed in accordance with institutional guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the University of Kentucky. Male Fischer 344 X Brown Norway rats (6 months and 32 months) were purchased from the National Institute on Aging. This strain of rat was chosen because it has increased longevity and decreased cumulative lesion incidence compared with other strains; therefore, aging aspects can be studied in the relative absence of disease (Lipman et al., 1996). The different ages were chosen to reflect a mature rat, post long bone growth (young: 6 months) and an old rat at about 50% mortality (old: 32 months). Rats of both ages were divided into 3 groups (n=8–10 per group): non-suspended ambulatory controls (amb), hind limb suspended for 14 days (HS), and rats that were hind limb suspended for 14 days and subsequently reloaded and allowed to move around the cage freely for 14 days (HSRE). Rats were allowed free access to food and water at all times and were housed in a 12:12hr light:dark cycle. Hind limb suspension was performed as previously described with minor modifications (Dupont-Versteegden et al., 2006; Hofer et al., 2008). Briefly, a tail device containing a hook was attached with gauze and cynoacrylate glue while the animals were anesthetized with isoflurane (2% by inhalation). After the animal regained consciousness, the tail device was connected via a thin cable to a pulley sliding on a vertically adjustable stainless steel bar running longitudinally above a high-sided cage. The system was designed in such a way that the rats could not rest their hind limbs against any side of the cage. Rats in the HSRE group were released from the tail suspension device after 14 days of unloading and they were allowed to maintain normal ambulation for 14 days (reloading). At the end of the experimental period, rats were anesthetized with sodium pentobarbital and serum was collected through a cardiac puncture; soleus muscles were then dissected, weighed and frozen. Muscles were either frozen in liquid nitrogen and stored at -80°C for biochemical analyses or were embedded in a freezing medium, frozen in liquid nitrogen-cooled isopentane, and stored at -80°C for immunohistochemical and histological analyses.
Serum analysis
Serum IGF-1 was determined using a radioimmunoassay (RIA; ALPCO Diagnostics, Salem, NH) as described previously (Delahunty et al., 2006). The analytical sensitivity of the assay is 0.02 ng/ml and 10 μl serum was used to determine IGF-I concentration (ng/ml). Serum IGFBP-3 was determined using the IGFBP-3 (Mouse/Rat) ELISA kit (ALPCO Diagnostics, Salem, NH) according to the manufacturer’s instructions. The analytical sensitivity of the assay is 0.018 ng/ml and 5 μl serum was used to determine IGFBP-3 concentration (ng/ml). Serum insulin levels were determined using the Rat/Mouse Insulin ELISA (Millipore, Saint Charles, MO) according to the manufacturer’s instructions. The analytical sensitivity of the assay is 0.2 ng/ml and 10 μl serum was used to determine the insulin concentration (ng/ml). Serum levels of tumor necrosis factor (TNF)-α, interleukin-6 (IL-6) and regulated-on-activation normal T cell-expressed and secreted (RANTES) were measured using a multiplex kit (EMD Millipore, Billerica, MA) according to the manufacturer’s recommendation. The analytical linear range of the assay is 4.88 – 20,000 pg/ml.
Immunohistochemistry
Cross sectional area determination
Mean fiber cross sectional area (CSA) was determined as described in (Jackson et al., 2012) and adapted for rats. Briefly, cross sections from the mid belly area of soleus muscles were cut on a cryostat (7μm), air dried, and stored at -20°C until further analysis. Sections were rehydrated in phosphate buffered saline (PBS) and incubated in dystrophin antibody (1:50, Vector Laboratories, Burlingame, CA) for 1 hour at room temperature and overnight at 4°C. Secondary antibody (1:200, directly conjugated Texas Red goat anti-mouse, Rockland Immunochemicals, Gilbertsville, PA) was applied and sections were coverslipped. Images were captured using a Zeiss AxioImager MI upright fluorescent microscope (Zeiss, Göttingen, Germany) and analysis was performed using AxioVision software (Zeiss). CSA was determined by manually tracing the dystrophin stained sarcolemma of about 200 fibers in 4 different areas of the muscle.
Immune cell detection
Soleus cross sections (7μm) were fixed in ice-cold acetone and blocked in 3% H2O2 in PBS and horse serum, followed by incubation in primary antibody overnight at 4°C. For detection of immune cells the following antibodies were used: neutrophils (CD43, 1:200, Serotec, Raleigh, NC), ED1 macrophages (CD68, 1:200, Serotec) and ED2 macrophages (CD163, 1:200, Serotec). The Tyramide Signal Amplification (TSA, Invitrogen, Carlsbad, CA) system was used for horse radish peroxidase signal amplification and detection using Cyanine-3 (Cy-3) according to the manufacturer’s instructions. Muscle sections were then reacted with DAPI (10nM: 4′, 6-diamidino-2- phenylindole, Invitrogen) to identify nuclei.
Apoptotic index and caspase activities
Cell death ELISA
Cytosolic fractions of muscles were obtained as previously described (McMullen et al., 2009). Soleus muscles were homogenized using a Polytron in isolation buffer: 220 mM D-mannitol, 75 mM sucrose, 0.1% fatty acid-free bovine serum albumin, 0.5 mM EGTA, and 2 mM HEPES, pH 7.4 (1:10 wt/vol). The homogenate was centrifuged at 700 g at 4°C for 10 min, and the supernatant was centrifuged again at 8,000 g at 4°C for 10 min. PMSF (1mM) and leupeptin (1μg/ml) were added to the supernatant and protein concentration was determined according to the Bradford method (Bradford, 1976). Cell death detection ELISA kit (Roche Applied Science, Indianapolis, IN) was used to quantitatively determine DNA fragmentation by measuring the cytosolic histone-associated mono- and oligonucleosomes as described previously (Leeuwenburgh et al., 2005; McMullen et al., 2009). Apoptotic index was expressed as absorbance at OD405, measured using Spectra Max M2 Fluorescent Microplate reader (Molecular Devices, Sunnyvale, CA), normalized to micrograms of protein.
Caspase activity measurement
Caspase activities for caspase-3, -8, and -9 were measured in the cytosolic fraction of the muscle homogenate using fluorometric substrates as described previously (McMullen et al., 2009). The following substrates were used for caspase-3, -8, and -9 respectively, Ac-DEVD-AMC, Ac-IETD-AMC, Ac-LEHD-AMC (Peptides International, Louisville, KY), and the fluorescence of free AMC was measured and compared to a standard curve for free AMC (Sigma, St Louis, MO). For determination of caspases 100 μg protein was incubated for 2 hours in caspase buffer (100mM HEPES, 10% sucrose, 10 mM DTT, 0.1% CHAPS, 1 μg/ml leupeptin, 1 mM PMSF). Fluorescence was determined with an excitation wavelength of 380 nm and an emission wavelength of 460 nm for AMC using a Spectra Max M2 Fluorescent Microplate reader (Molecular Devices). Values were expressed as nmoles AMC per μg of protein.
Western analysis
Subcellular fractionations
Fractionations from soleus muscles were obtained according to (Siu et al., 2005a; Xiao et al., 2011). Briefly, muscles were pulverized and homogenized in lysis buffer (10 mM NaCl, 1.5 mM MgCl2, 20 mM HEPES, pH 7.4, 20% glycerol, 0.1% Triton X-100 and 1 mM dithiothreitol) and centrifuged for 5 minutes at 4°C. Supernatants were collected as the cytosolic fractions. The nuclear pellet was resuspended in lysis buffer and 5M NaCl was added to lyse the nuclei. The mixture was rotated for 1 hour at 4°C and centrifuged at 14,000rpm for 15 minutes at 4°C. The supernatant containing the nuclear protein was collected. Purity of the fractions was confirmed with histone and CuZnSOD antibodies for nuclear and cytosolic fractions, respectively. Cytosolic and nuclear fractions were supplemented with 1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin and 1 μg/ml pepstatin. Protein concentration of the subcellular fractions was determined using the Bradford assay (Bradford, 1976). Cytosolic and nuclear fractions were used for Western analysis of apoptosis repressor with CARD domain (ARC), apoptosis inducing factor (AIF) and endonuclease G (EndoG), while the nuclear fraction was used to assess AIF and EndoG localization.
Western analysis
Western analysis was performed as described previously (Xiao et al., 2011) and adapted as follows. For ARC, AIF and EndoG the subcellular fractions as described above were used. For phospho-p70s6k, total p70s6k, phospho-Akt, total Akt, phospho-GSK3β, and total GSK3β, soleus muscles were homogenized in RIPA buffer (Boston Bioproducts, Ashland, MA) with 10μl/ml protease inhibitor cocktail (Roche, Indianapolis, IN), 5mM benzamidine, 5mM, N-Ethylmaleimide, 50mM NaF, 25mM B-glycerophosphate, 1mM EDTA, and 1mM PMSF added and centrifuged 10,000g. For quantification of protein abundance, 10–100 μg total protein was loaded and separated on 4–15% acrylamide gradient gels (Bio-Rad, Hercules, CA), followed by transfer proteins to PVDF membranes with 0.22 μm pore size. Membranes were incubated in Odyssey Blocking Buffer (Li-Cor, Lincoln, NE) followed by incubation with apoptosis repressor with CARD domain (ARC; 1:500; ProSci Incorporated, Poway, CA), endonuclease G (EndoG; 1:500, Millipore, Billerica, MA), apoptosis inducing factor (AIF; 1:500; Santa Cruz Biotechnologies, Santa Cruz, CA) p70s6K (1:500; Santa Cruz Biotechnologies), pp70s6K (Thr389) (1:3,000; Cell Signaling, Danvers, MA), glycogen synthase kinase (GSK)-3β (1:1,000; Cell Signaling), pGSK3β (Ser9) (1:1,000; Cell Signaling), AKT (1:1,000; Cell Signaling), or pAKT (Ser473) (1:1,000; Cell Signaling), washed, and further incubated with highly cross-absorbed infra red-labeled secondary antibodies (1:20,000; Li-Cor). Membranes were scanned using Odyssey infrared imaging system (Li-Cor) to detect specific antibody binding and quantification. Two different methods were used to ensure equal protein loading of lanes. For whole muscle extracts derived from RIPA buffer, actin antibody (C-11) (1:2,000; Santa Cruz Biotechnologies) was used and these results are depicted in the figures; however, actin staining was not possible for the subcellular fractionations and therefore Ponceau S staining of the membranes was utilized to ensure equal loading (not shown). Each gel contained samples from every group. Gel-to-gel corrections were performed by determining the density of all bands per gel and using it as a standard for normalization. To indicate when representative samples did not come from continuous sets, we left space between the bands in the Figures.
RNA isolation and analysis
RNA isolation
Total RNA was isolated as described (Dupont-Versteegden et al., 2006) with modifications. RNA isolation was performed using Totally RNA isolation kit (Ambion, Austin, TX) according to the manufacturer’s instructions. Total RNA was treated with DNase (Ambion) and RNA integrity and concentration was determined using the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA).
Real-time RT-PCR (reverse transcriptase polymerase chain reaction)
Quantitative real-time RT-PCR was performed using the protocols, chemistries, and the amplification and detection systems of Applied Biosystems (Applied Biosystems, Foster City, CA). For each sample, cDNA was synthesized from 1 μg of DNase-treated total RNA using iScript Reverse Transcriptase (Biorad) according to the manufacturer’s instructions. cDNA samples were aliquoted and stored at −80°C. Primer sequences were selected from the accession numbers in NCBI database using the Taqman Probe and Primer Design function of the Primer Express v1.5 software (Applied Biosystems) and were as follows: β-2 microglobulin (NM_012512) forward, cgtgcttgccattcagaaaa, reverse, gaagttgggcttcccattctc; muscle atrophy F-box (MAFbx), forward, gacctgcatgtgctcagtgaag, reverse, ggatctgccgctctgagaagt; 18S (M11188) forward, ttcggacgtctgccctatcaa, reverse, atggtaggcacggcgacta; NADH subunit 1 (X14848) forward, agaacggaaaatcctaggctacatac, reverse ccatatgggccttcgttgtt; tubulin (NM_022298) forward, ggcatggaggagggagagtt, reverse, ccaacctcctcataatccttctctag; Atg5 (AF43433) forward, agaaggttatgagac, reverse tgatgttccaaggcagagc; Atg7 (NM001012097) forward, ccagtctgttgaagttctc, reverse, atacatccgctgaggttc; beclin (NM_001034117) forward agcacgccatgtatagcaaaga, reverse ggaagagggaaaggacagcat; Murf-1 (Ay05627) forward tgccctgccagcacaac, reverse ggattggcagcctggaagat. PCR reactions were assembled using the SYBR Green PCR Master Mix that required only the addition of cDNA template and primers. Data points were generated using four-fold serial dilutions of cDNA. The reactions were performed using the ABI PrismTM 7700 Sequence Detection System (Applied Biosystems) and the instrument’s universal cycling conditions. RNA abundance for each gene of interest is expressed as a ratio normalized to the Geometric mean of NADH subunit 1, tubulin, 18S and β-2 microglobulin, as described previously (Dupont-Versteegden et al., 2008; Xiao et al., 2011).
Statistical Analyses
For the comparison of all groups a two-way ANOVA was performed with age (6 and 32 months) as factor A and experimental group (amb, HS, or HSRE) as factor B using SigmaStat statistical analysis software. Main effects as well as interaction effects are reported as appropriate and in case of statistically significant differences the Holm-Sidak pairwise multiple comparison procedure was employed to determine group differences. If normality was not achieved for the samples, log transformation was performed before applying two-way ANOVA. All values reported are mean ± standard error (SE) and statistical significance was assumed at p<0.05.
RESULTS
Muscle size
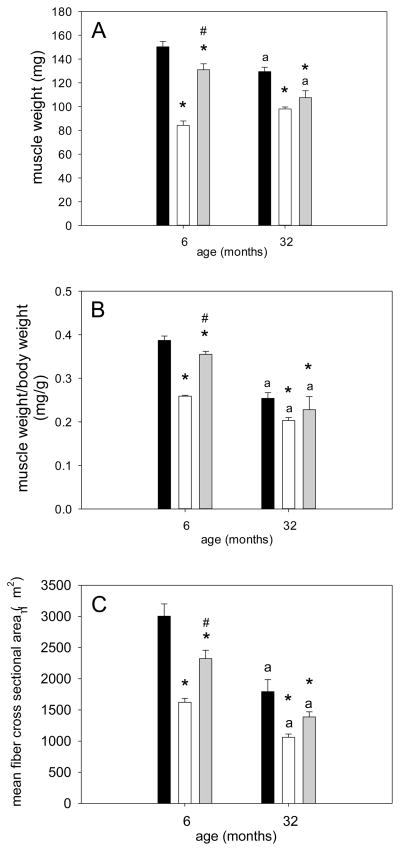
Soleus muscle weight, muscle-to-body weight (mw/bw) ratio, as well as muscle fiber cross sectional area (CSA) were lower in aged rats compared to their young control ambulatory animals (Figures 1A, B & C). Muscle weight of soleus muscle showed a significant decrease of 44% in young rats and 24% in aged rats with hind limb suspension (Figure 1A). Recovery of soleus muscle weight with reloading was severely blunted in the aged rats: muscle weight significantly increased by 56% in HSRE compared to HS at a young age, while the 9% recovery in the old HSRE rats was not statistically significant (Figure 1A). This finding was mimicked by mw/bw ratio which decreased by 33% and showed a statistically significant 37% recovery with reloading in young rats, while the 12% recovery in the aged (after a 20% decrease with suspension) was not statistically significant (Figure 1B). Moreover, a similar lack of muscle size recovery with age was observed for muscle fiber cross sectional area (CSA). A decrease of 46% with HS was observed in young animals and a statistically significant 43% increase with reloading; in aged rats, however, the 31% increase with reloading after 41% decrease with suspension was not statistically significant (Figure 1C). Taken together these data indicate that the recovery of muscle size after a period of disuse is impaired with age.
Figure 1. Restoration of muscle size after atrophy is impaired in soleus muscle of aged rats.
Soleus muscle weight (A), muscle weight-to-body weight ratio (B), and mean muscle fiber cross sectional area (C) of ambulatory (amb, black bars), hind limb suspended (HS, white bars), and reloaded (HSRE, grey bars) rats at 6 and 32 months are depicted (n=8–10). Values are means ± SE; * indicates significant difference from ambulatory, # indicates significant difference from HS, and ‘a’ indicates a difference from young within the same group; p<0.05.
Serum insulin, IGF- and IGFBP-3
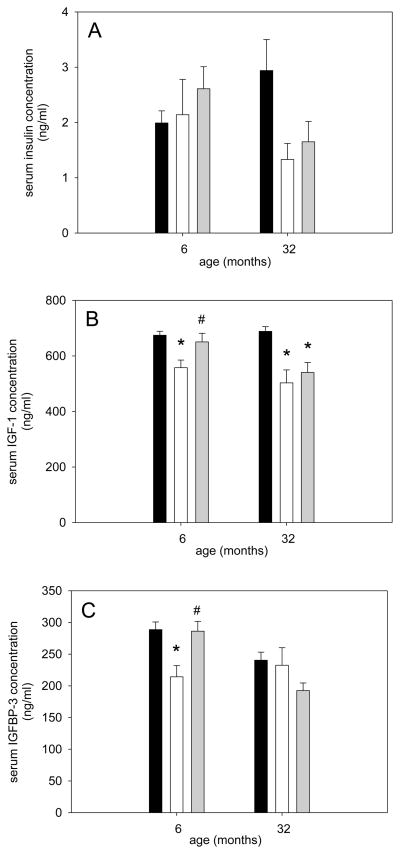
Since it is known that IGF-1 is anabolic and IGFBP-3 aids in this response (Clemmons, 2009; Yamada and Lee, 2009; Zdanowicz and Teichberg, 2003) we investigated whether these two molecules and insulin were changed in response to different loading conditions in young and old rats. Serum insulin was not different at different ages or in response to disuse and reloading (Figure 2A). However, serum IGF-1 decreased with disuse in both young and old and recovered to ambulatory levels in the young but not the old (Figure 2B). IGFBP-3 showed a very similar response to IGF-1 in young rats, but did not change at all in the aged (Figure 2C). Thus, the response to IGF-1 and IGFBP-3 is different between young and old and could potentially explain some of the inhibited recovery of muscle size in the aged. Therefore, we investigated the intracellular signaling pathways downstream of IGF-1.
Figure 2. Serum IGF-1 and IGFBP-3 concentrations do not recover after disuse in the aged rat.
Serum insulin (A), IGF-1 (B) and IGFBP-3 (C) of ambulatory (black bars), hind limb suspended (HS, white bars), and reloaded (HSRE, grey bars) rats at 6 and 32 months are depicted (n=8–10). Values are means ± SE; * indicates significant difference from ambulatory and # indicates significant difference from HS; p<0.05.
Protein synthesis markers
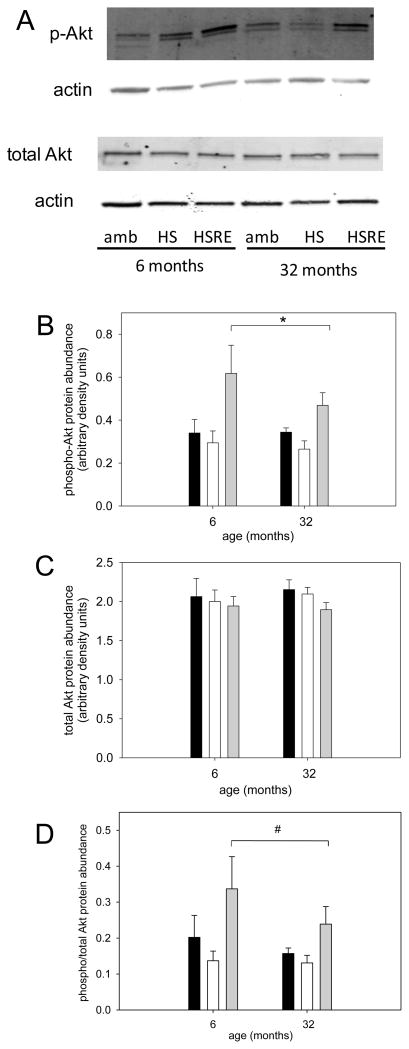
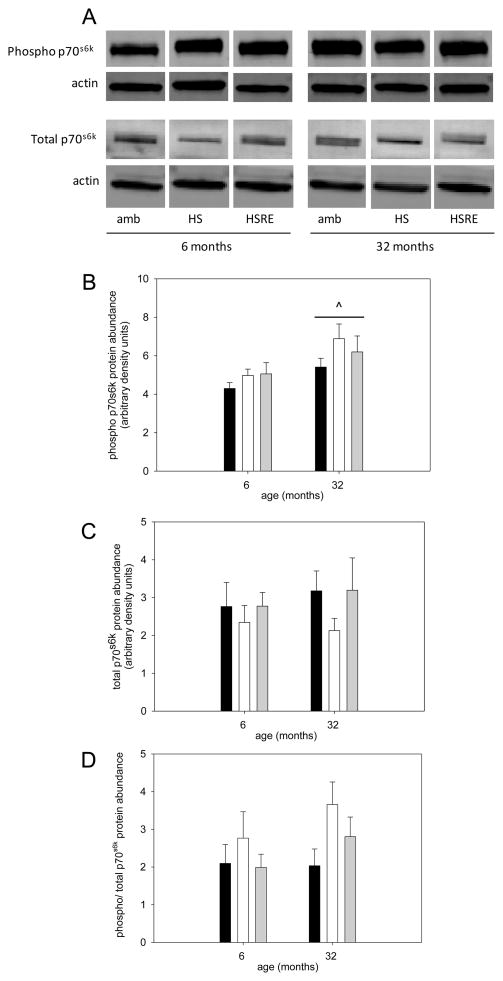
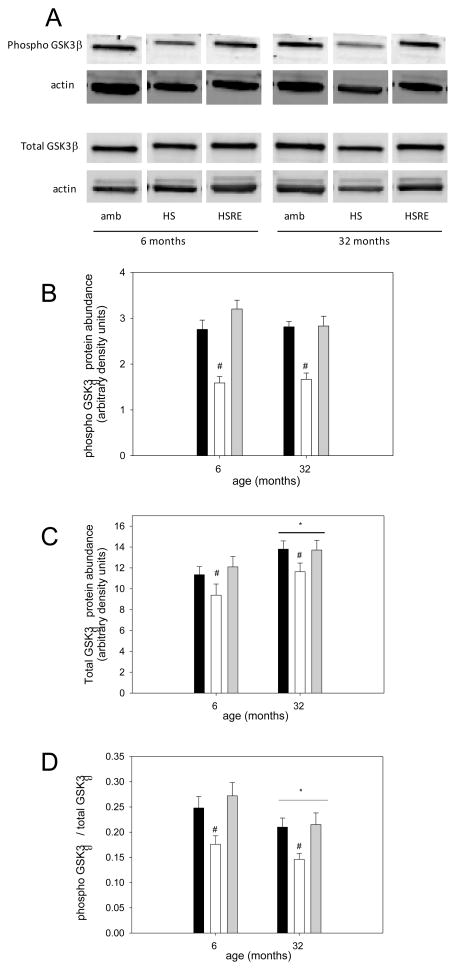
The Akt/mTOR pathway is a key regulator of muscle growth (Bodine et al., 2001a) and therefore we investigated components of this pathway. pAkt was not changed in response to hind limb suspension, but was higher in soleus muscle of rats after two weeks of reloading in both young and old (Figure 3A,B). Total Akt was not changed at all under any loading condition in young or old (Figure 3A,C), resulting in an increased ratio of pAkt to total Akt in soleus muscle of reloaded rats at both ages. Therefore, no difference in the response to loading was observed for Akt between young and old. We then investigated the abundance of the downstream marker of mTOR activation, p70s6k. There was a main effect for p-p70s6k to be elevated in aged rats, but no changes were observed with different loading conditions in either young or old (Figure 4A, B); similarly, total p70s6k and the ratio of p-p70s6k to total p70s6k was not changed at either age under any of the loading conditions (Figure 4A,C,D). Another downstream target of Akt involved in hypertrophy and atrophy regulation, is glycogen synthase kinase -3β (GSK3β), which is inactivated when phosphorylated by Akt (Glass, 2005; Verhees et al., 2011; Vyas et al., 2002). Indeed, GSK3β phosphorylation is decreased with atrophy induced by unloading at both ages and recovers with reloading. Additionally, the total abundance of GSK3β responds similarly to different loading conditions at both ages, while its shows a main effect for an increase at old age. Therefore, the ratio of p-GSK3β to total GSK3β is decreased with unloading and restored with reloading at both ages. These data indicate that GSK3β likely is involved in the muscle growth response to changes in loading conditions, but there is no difference between ages in the response.
Figure 3. Akt response to reloading is similar in young adult and old rats.
Representative bands for Western analysis of phospho(Ser473)-Akt, total Akt, and actin (used as loading control) are shown for soleus muscle of ambulatory (amb), hind limb suspended (HS) and reloaded (HSRE) rats at 6 and 32 months (A). Quantification of Western analysis is depicted for phospho- Akt (B), total Akt (B) and the ratio of phospho to total Akt (C) for ambulatory (black bars), hind limb suspended (HS, white bars), and reloaded (HSRE, grey bars) rats at 6 and 32 months (n=8–10). Values are means ± SE; * indicates a main effect for HSRE difference from HS and ambulatory, and # indicates a main effect for HSRE from HS; p<0.05.
Figure 4. p70s6k is not changed in response to disuse and re-ambulation.
Representative bands for Western analysis of phospho(Thr389)-p70s6k, total p70s6k and actin (used as loading control) are shown for soleus muscle of ambulatory (amb), hind limb suspended (HS) and reloaded (HSRE) rats at 6 and 32 months (A). Quantification of Western analysis is depicted for phospho-p70s6k (B), total p706sk (B) and the ratio of phospho to total p70s6k (C) for ambulatory (black bars), hind limb suspended (HS, white bars), and reloaded (HSRE, grey bars) rats at 6 and 32 months (n=8–10). Values are means ± SE; ^ indicates a main effect for age; p<0.05.
Protein degradation markers
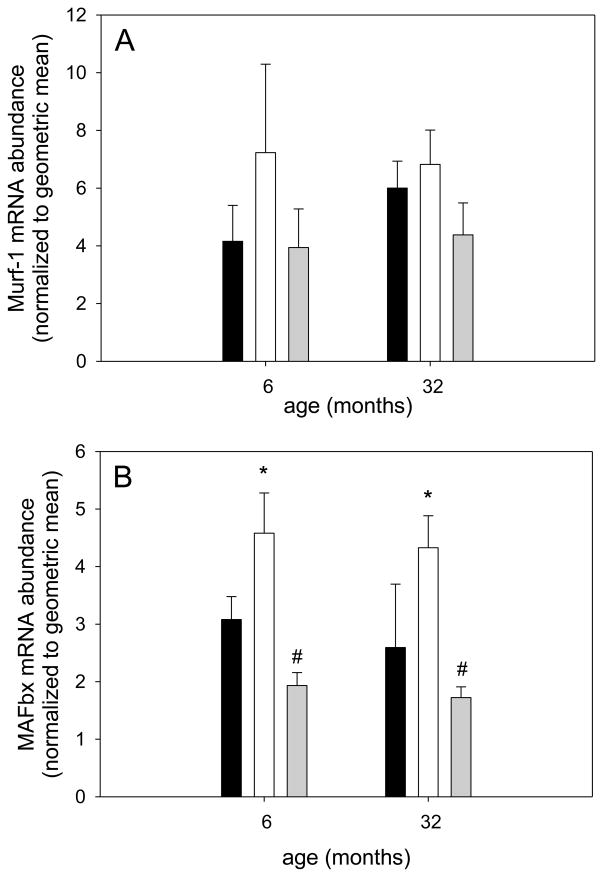
Since we did not observe age-related differences in protein synthesis markers, we investigated the abundance of protein degradation markers MAFbx (atrogin-1) and Murf-1 in response to different loading conditions in young and old rats (Bodine et al., 2001b; Foletta et al., 2011). We found that Murf-1 mRNA abundance did not chance with different loading conditions at either age (Figure 6A). However, MAFbx gene expression was increased with atrophy in response to unloading in soleus muscle of young and old rats and was restored to ambulatory levels with reloading at both ages (Figure 6B). Even though Murf-1 and MAFbx protein generally follow the mRNA levels closely, it is acknowledged that differences in protein levels between the groups may not be the same.
Figure 6. Protein degradation markers MAFbx and Murf-1 are similarly affected by reloading at different ages.
Soleus muscle mRNA abundance for Murf-1 (A) and Mafbx (B) for ambulatory (black bars), hind limb suspended (white bars) and reloaded (grey bars) rats at 6 and 32 months is shown (n=8–10). Values are means ± SE; * indicates a main effect for HS different from ambulatory and # indicates a main effect for HSRE different from HS; p<0.05.
Autophagy markers
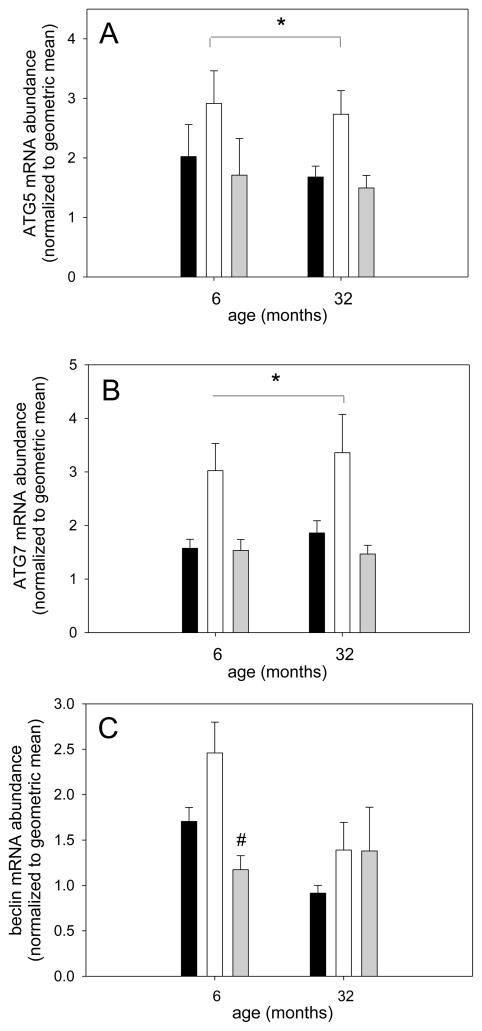
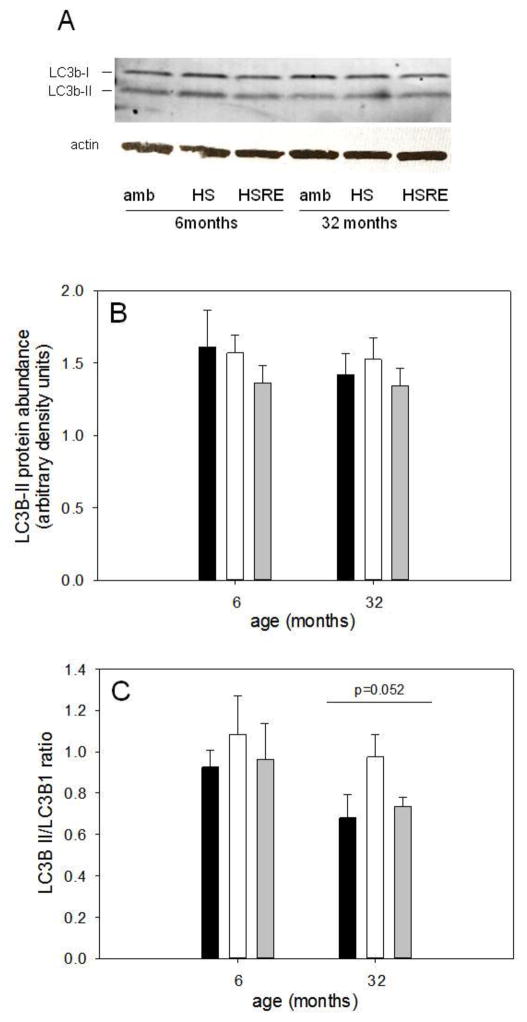
Autophagy is a process that recently has been proposed to be involved in skeletal muscle growth regulation although its exact role is not clear; inhibition of autophagy through the deletion of autophagy-related (Atg) gene-7 induces muscle atrophy while it has also been shown that autophagy contributes to atrophy (Banerjee and Guttridge, 2012; Masiero et al., 2009; Zhao et al., 2007). We therefore determined the gene expression of three autophagy-related genes: beclin (Atg6) which contributes to early phagosome formation, and Atg5 and -7, which are involved in phagosome elongation (Ravikumar et al., 2010). Atg5 and Atg7 showed very similar responses to differences in loading in soleus muscle from young and old rats (Figures 7A and B respectively); both genes were upregulated with unloading indicating that they might be involved in the atrophy response, but returned to levels similar to ambulatory in both ages. Beclin gene expression was regulated similarly by loading in soleus muscle from young, but not aged rats (Figure 7C). Since protein abundance does not always mimic mRNA levels and to assess autophagic flux, we further measured the protein abundance of LC3; an increase in LC3b-II or the ratio of LC3b-II over LC3b-I indicates an increase in autophagic flux (Mizushima et al., 2010). We found total levels of LC3b-II were not different between the groups in either young or old (Figure 8B) and the overall autophagic flux, as measured by the LC3b-II/LC3b-I ratio, was trending lower in the aged (p=0.052), but showed no difference between the groups at either age (Figure 8C).
Figure 7. mRNA abundance of markers for autophagy show similar responses to different loading conditions in young adult and old rats.
Soleus muscle mRNA abundance for ATG5 (A), ATG7 (B) and beclin (C) for ambulatory (black bars), hind limb suspended (white bars) and reloaded (grey bars) rats at 6 and 32 months is shown (n=8–10). Values are means ± SE; * indicates a main effect for HS different from other groups and # indicates difference from HS in young; p<0.05.
Figure 8. Marker of autophagic flux is lower with age, but not different with loading.
Representative bands for Western analysis of LC3b-I (top band), LC3b-II (bottom band) and actin (used as loading control) are shown for soleus muscle of ambulatory (amb), hind limb suspended (HS) and reloaded (HSRE) rats at 6 and 32 months of age (A). Quantification of Western analysis for LC3b-II (B) and the ratio of LC3b-II to LC3b-I (C) is depicted for ambulatory (amb, black bars), hind limb suspended (HS, white bars), and reloaded (HSRE, grey bars) rats at 6 and 32 months of age (n=8–10). Values are means ± SE; p=0.052 indicates a trend for main effect for age.
Apoptosis-related processes
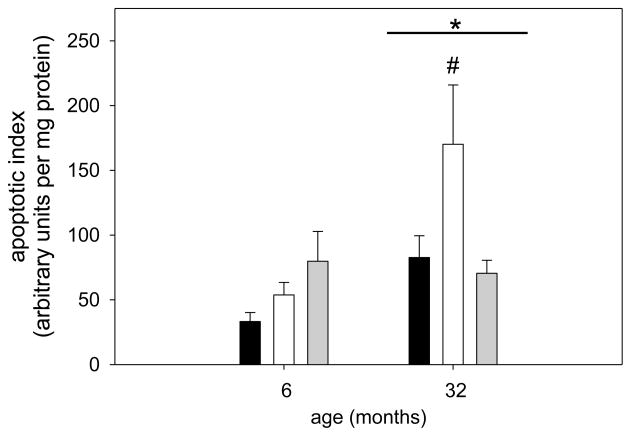
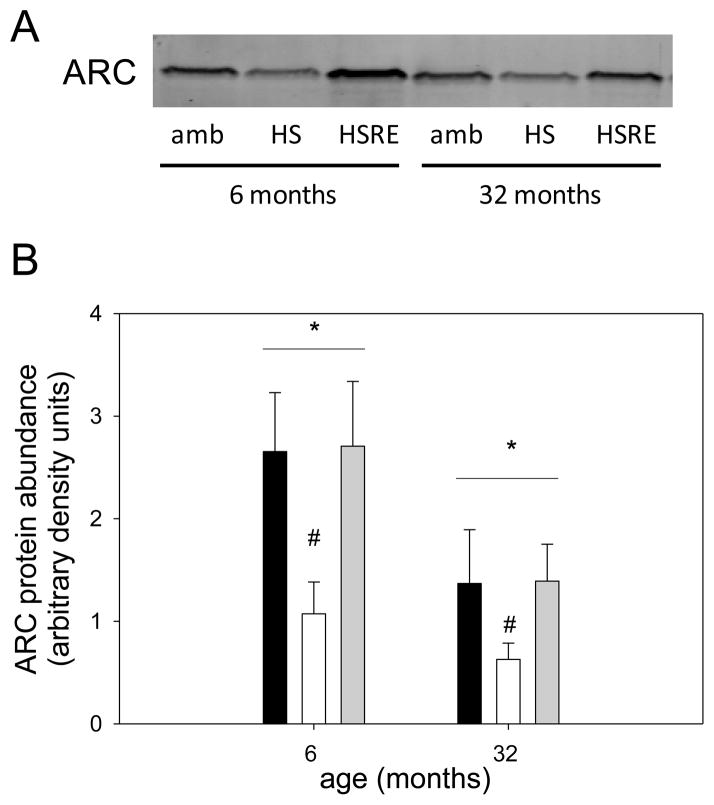
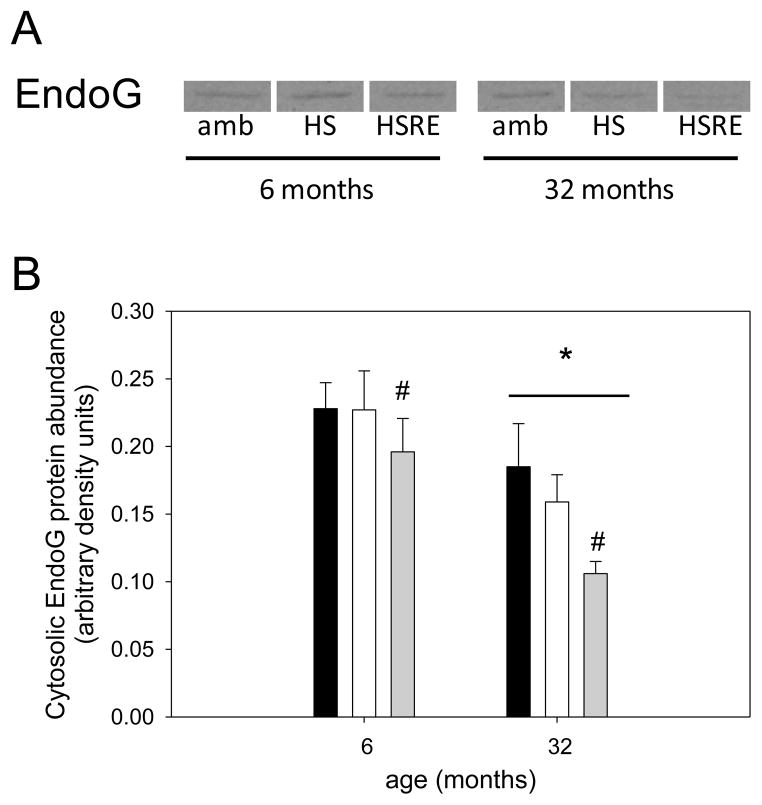
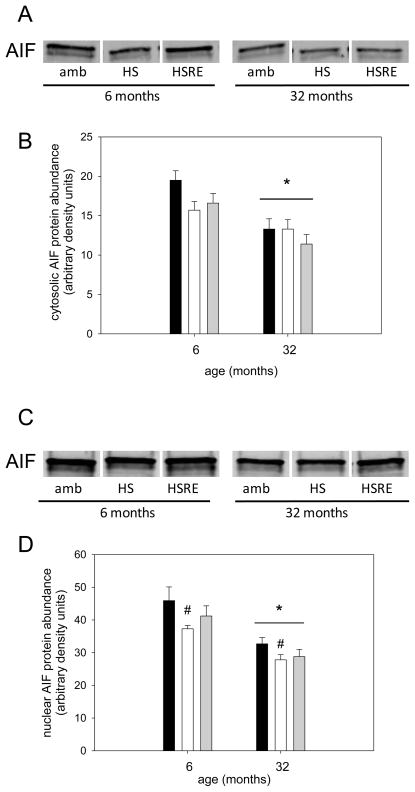
Apoptosis is a process that has been postulated to be involved in skeletal muscle atrophy (Chopard et al., 2009; Marzetti et al., 2010) although the concept of myonuclear loss through apoptotic nuclear death has recently been challenged particularly in young muscles (Bruusgaard et al., 2012). In this study we investigated the potential involvement of apoptotic-related pathways with regard to changes in loading conditions. For apoptotic index, a measure of total apoptosis, a main effect was observed for age, indicating that aged muscle in general shows a higher level of DNA fragmentation (Figure 9). In addition, only in aged soleus muscle, the apoptotic index was elevated with hind limb suspension, but returned to ambulatory levels upon reloading (Figure 9). To investigate underlying mechanisms for this increase in apoptosis in aged muscle particularly in combination with atrophy we first investigated the involvement of caspases, the main proteolytic enzymes involved in apoptosis in mononucleated cells. Surprisingly, caspase-3, -8, and -9 were not changed with unloading or reloading at either age, indicating that these proteins are likely not involved in the response to atrophy and regrowth (Table 1). We further investigated ARC, a muscle specific inhibitor of apoptosis (Koseki et al., 1998), and found that abundance of this protein was significantly decreased with unloading, but returned to ambulatory levels upon reloading in both ages (Figure 10A&B). Moreover, there was a main effect for age such that ARC levels were lower in aged soleus muscle in all groups, which may account for the overall increase in apoptosis in aged muscle (Figure 9 & 10B). It has previously been shown that EndoG may be a specific inducer of apoptosis in skeletal muscle, particularly in the aged (Dupont-Versteegden et al., 2006; Leeuwenburgh et al., 2005). Cytosolic EndoG was decreased in the aged muscle (main effect for age) and was decreased in reloaded soleus muscle (Figure 11), however we were unable to detect EndoG in the nuclear fraction of our samples. Another caspase-independent inducer of apoptosis is AIF, and we determined its protein abundance in nuclear and cytosolic fractions. Cytosolic AIF showed a decrease with age (main effect), but no change with different loading conditions at either age (Figure 12). Nuclear AIF was also decreased with age (main effect) and showed a decrease with hind limb suspension at both ages (Figure 12). These data indicate that AIF is most likely not involved in the increase in apoptosis with aging or hind limb suspension in the aged.
Figure 9. Apoptosis is increased in soleus muscle of aged rats with disuse.
Apoptotic index as a measure of DNA fragmentation of soleus muscle from ambulatory (amb, black bars), hind limb suspended (HS, white bars) and reloaded (HSRE, grey bars) rats at 6 and 32 months is shown (n=8–10). Values are means ± SE; * indicates a main effect for age and # indicates difference from ambulatory and HSRE in old; p<0.05.
Table 1.
Caspase activities from soleus muscle of young adult and old rats
| caspase-3 | caspase-8 | caspase-9 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amb | HS | HSRE | Amb | HS | HSRE | Amb | HS | HSRE | |
| 6 months | 775 ± 35 | 799 ± 49 | 930 ± 40 | 1480 ± 78 | 1489 ± 91 | 1673 ± 56 | 258 ± 22 | 274 ± 26 | 293 ± 11 |
| 32 months | 898 ± 44 | 784 ± 52 | 876 ± 77 | 1698 ± 85 | 1455 ± 103 | 1603 ± 119 | 307 ± 29 | 270 ± 29 | 285 ± 33 |
caspase activities in nM AMC per hour per μg protein
Amb: ambulatory; HS: hind limb suspended; HSRE: reloaded
Values are means ± SE
Figure 10. ARC protein abundance is decreased with atrophy in response to age and hind limb suspension.
Representative bands for Western analysis of ARC are shown for soleus muscle of ambulatory (amb), hind limb suspended (HS) and reloaded (HSRE) rats at 6 and 32 months of age (A). Ponceau S was used as a loading control (not shown). Quantification of Western analysis is depicted (B) for ambulatory (black bars), hind limb suspended (HS, white bars), and reloaded (HSRE, grey bars) rats at 6 and 32 months of age (n=8–10). Values are means ± SE; * indicates a main effect for age, and # indicates a main effect for HS; p<0.05.
Figure 11. Cytosolic EndoG is decreased with age and reloading.
Representative bands for Western analysis of cytosolic EndoG are shown for soleus muscle of ambulatory (amb), hind limb suspended (HS) and reloaded (HSRE) rats at 6 and 32 months of age (A). Ponceau S was used as a loading control (not shown). Quantification of Western analysis is depicted (B) for ambulatory (black bars), hind limb suspended (HS, white bars), and reloaded (HSRE, grey bars) rats at 6 and 32 months of age (n=8–10). Values are means ± SE; * indicates a main effect for age, and # indicates a main effect for HSRE, different from other groups; p<0.05.
Figure 12. Cytosolic and nuclear AIF protein abundance is decreased with age.
Representative bands for Western analysis of cytosolic (A) and nuclear (C) AIF are shown for soleus muscle of ambulatory (amb), hind limb suspended (HS) and reloaded (HSRE) rats at 6 and 32 months of age. Ponceau S was used as a loading control (not shown). Quantification of Western analysis for cytosolic (B) and nuclear (D) AIF is depicted for ambulatory (black bars), hind limb suspended (HS, white bars), and reloaded (HSRE, grey bars) rats at 6 and 32 months of age (n=8–10). Values are means ± SE; * indicates a main effect for age, and # indicates a main effect for HS different from ambulatory; p<0.05.
Immune cell abundance and inflammatory serum markers
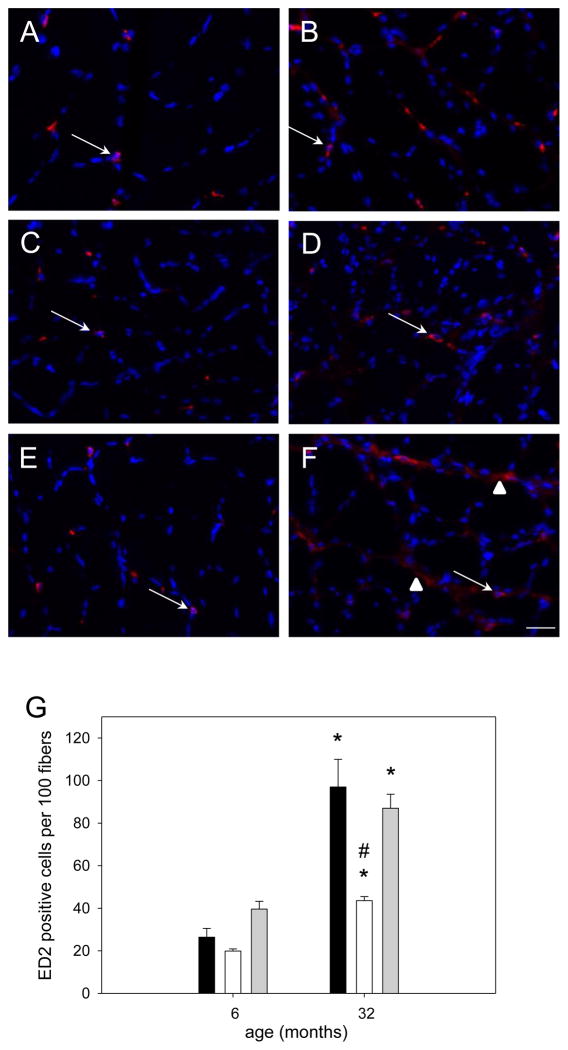
Inflammatory markers are elevated with aging and are associated with a decrease in muscle mass and strength (Ibebunjo et al., 2013; Schaap et al., 2009). In addition, it has been suggested that defective macrophage function may contribute to the blunted hypertrophy of aged muscle in response to resistance exercise (Przybyla et al., 2006). Therefore we determined the abundance of immune cells (neutrophils and macrophages) in soleus muscles of young and old rats in response to unloading and reloading. We did not detect any neutrophils or pro-inflammatory ED1 (also called M1) macrophages, but found that anti-inflammatory ED2 (also called M2) macrophages were present in soleus muscle from young and old rats. In muscles from young rats we found that the staining of ED2 macrophages in all groups presented as a very classical ringing around the nucleus of the macrophage (arrows in Figure 13A,C and E); however in aged soleus muscle ED2 staining quite often was seen as a smear surrounding a nucleus which was mostly present in muscle from reloaded rats (arrowhead in Figure 13F) in addition to the classical staining (arrows in Figure 13B, D and F). Quantification showed that in all groups there was an increased number of ED2 positive cells in soleus muscle from old rats (Figure 13G). Also, no difference in the number of ED2 macrophages was observed between the groups in young rats while in old rats ED2 positive cell number decreased with unloading while it recovered to ambulatory levels with reloading. To see whether the changes macrophages in the muscle were related to changes in inflammatory cytokines in the blood we measured serum TNFα, IL-6, and RANTES. No differences were observed in serum TNFα levels with age or between groups (Table 2). However, IL-6 levels were elevated in aged animals independent of group and RANTES was decreased in reloaded rats independent of age (Table 2). This indicates that the immune cell response in muscle from aged rats is different than in young rats, but that this response is not correlated with changes in pro-inflammatory cytokines in the serum.
Figure 13. Macrophage (ED2) abundance in dysregulated in soleus muscle of aged rats.
Representative cross sections immunoreacted for ED2 (CD163) of soleus muscle from 6 (A,C,E) or 32 (B,D,F) month old ambulatory (A,B), hind limb suspended (C,D), or reloaded (E,F) rats. CD163 is stained in red and nuclei are stained in blue (DAPI); arrows indicate regular staining and arrow heads point to diffuse CD163 positive staining. Bar in F represents 25μm for all pictures. Quantification of ED2 positive staining (G) in soleus muscle from ambulatory (amb, black bars), hind limb suspended (HS, white bars) and reloaded (HSRE, grey bars) rats at 6 and 32 months is shown (n=8–10). Values are means ± SE; * indicates a significant difference from young and # indicates a significant difference from ambulatory and HSRE in old; p<0.05.
Table 2.
TNFα, IL-6 and RANTES serum values for young adult and old rats
| TNFα (pg/ml) | IL-6 (pg/ml) | RANTES (pg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amb | HS | HSRE | Amb | HS | HSRE | Amb | HS | HSRE | |
| 6 months | 13.1 ± 1.1 | 15.5 ± 2.1 | 12.5 ± 1.0 | 78.4 ± 13.3 | 121.4 ± 27.0 | 198.9 ± 78.5 | 3851 ± 441 | 3357 ± 281 | 3159 ± 293 # |
| 32 months | 12.6 ± 1.3 | 14.0 ± 1.0 | 15.6 ± 2.4 | 382.8 ± 131.5 * | 436.2 ± 76.8 * | 808.8 ± 251.3 * | 5381 ± 990 | 3646 ± 542 | 3216 ± 354 # |
Amb: ambulatory; HS: hind limb suspended; HSRE: reloaded
indicates a main effect for age;
indicates main effect for difference between Amb and HSRE
Values are means ± SE
DISCUSSION
In this study, we demonstrated that changes associated with regrowth in most of the investigated cellular pathways were very similar between young and aged rats, despite the inability of muscle from aged rats to recover muscle size lost during atrophy. This result is similar to the lack of differences in translational signaling with overload hypertrophy despite an attenuated growth response (Chale-Rush et al., 2009). We hypothesized that muscles from aged rats would show a blunted recovery of muscle size with regrowth in pathways responsible for the balance between protein synthesis and degradation, but failed to prove this hypothesis for most pathways at the time point measured, even though there were some differences in aged muscle in general which may have influenced the regrowth response.
Protein synthesis-related mechanisms
IGF-1 is an important regulator of muscle mass and can inhibit muscle atrophy as well as induce hypertrophy (for review (Clemmons, 2009)). Therefore, we investigated IGF-1 serum levels and found it was indeed decreased with atrophy in both young and old, and recovered only in the young animals with regrowth indicating that it potentially plays a role in the blunted regrowth response of muscle. In addition, IGFBP-3 showed a similar pattern such that it recovered to pre-suspension levels in young, but not aged rats; this is of interest since IGFBP-3 is the most abundant binding protein and also has been shown to attenuate muscle atrophy when in complex with IGF-1 (Spangenburg et al., 2003; Yamada and Lee, 2009; Zdanowicz and Teichberg, 2003). IGF-1 dependent intracellular pathways have been studied extensively and been shown to be involved in regulating skeletal muscle mass (for review (Goodman et al., 2011). Interestingly, p-Akt levels were actually higher in muscles from rats undergoing regrowth independent of age, but p-p70s6k was not changed with the loading conditions. It is likely that the phosphorylation of these proteins was changed at some point in time after reloading as it has been shown that different signaling proteins exhibit distinct time courses during muscle recovery and Akt is one of the fastest proteins to respond to reloading (Childs et al., 2003). Therefore, some changes in phosphorylation status may have been missed at the time point we investigated the muscles in this study. In one of the few papers where the recovery of these proteins was compared, p-p70s6k peaked at 5 days after recovery and was higher in the young than the aged at that time point (Morris et al., 2004). Similar to results in that paper however, was the fact that p-p70s6k in our study was also elevated in aged, indicating that this pathway is potentially already elevated in the aged and is not as readily activated upon reloading.
GSK3β has been shown to be involved in the regrowth response and has been implicated to be a modulator of the late regrowth response because of its consistent phosphorylation late during the process (Childs et al., 2003; Morris et al., 2004; van der Velden et al., 2007). We found that phosphorylation of GSK3β was decreased during atrophy and recovered to similar extent in young and old muscle. Since one of the upstream kinases capable of phosphorylating GSK3β is Akt, the increased levels of p-Akt during the reloading phase may be responsible for the sustained recovery of GSK3β in both young and aged muscle. This is different from the data in Morris et al (2004) which did not implicate Akt as the upstream kinase for GSK3β, but this may be due to fact that in their study GSK3β did not decrease with immobilization which was induced by casting instead of hind limb suspension. Interestingly, even though no difference in the response to loading and unloading was found between the age groups, there was a main effect of age on the total GSK3β levels, showing an increased abundance in muscles from old rats resulting in a decreased ratio of phospho-to-total GSK3β. This suggests that in aged muscle it will take a higher level of kinase activity to phosphorylate and inhibit total GSK3β to enhance muscle growth and this may explain the blunted regrowth response; this possibility requires further investigation.
Protein degradation-related mechanisms
The attenuation of the muscle regrowth response in aged rats could also be related to differences in protein degradation pathways. Previous research indeed indicated that blunted recovery of muscle size in aged rats after starvation was associated with a lack of inhibition of proteolysis during refeeding, despite a similar return of protein synthesis to control values in young and old rats (Mosoni et al., 1999). Two protein degradation pathways are responsible for the majority of muscle atrophy during inactivity: the ubiquitin-proteasome and autophagy-lysosomal systems (Sandri, 2013). Mafbx and Murf-1 are the most widely accepted markers for protein degradation through the proteasomal pathway (Bodine and Baehr, 2014) and in our study the gene expression of these two E3 ubiquitin ligases was almost identical between muscles from young and aged rats. Both E3 ubiquitin ligases are known to respond rapidly after the onset of atrophy and return to their baseline values within 10–14 days (Bodine and Baehr, 2014; Bodine et al., 2001b), which is the case here for Murf-1 in particular. The fact that Mafbx was still elevated after 14 days of HS indicates that it may respond somewhat slower than Murf-1 to changes in loading, but the data indicate that both genes respond similarly in young and aged rats. It has recently been shown that Mafbx and Murf-1 are elevated early after the onset of recovery of muscle mass after casting (Slimani et al., 2012), potentially as mediators of remodeling during this phase; it is possible that the response to this early remodeling is different in muscles of young and aged rats leading to a blunted growth response in the aged. This possibility needs further investigation.
The autophagy-lysosome system has recently received increased attention in the muscle community, because of its activation under a number of atrophy-inducing stimuli (for review see (Sandri, 2013)). While it is involved in the breakdown of muscle protein and could thereby contribute to muscle wasting, it is also necessary for normal muscle function. Disrupting autophagy related genes, such as Atg7, leads to muscle atrophy and weakness (Masiero et al., 2009) and impaired autophagy has been postulated to contribute to sarcopenia (McMullen et al., 2009; Wohlgemuth et al., 2007). Therefore, changes in autophagy are important to consider under different growth conditions to investigate its potential involvement in differential muscle size changes. To our knowledge, no studies have investigated age-related differences in the expression of autophagy-associated genes during regrowth after atrophy. However, a study in human muscle showed that changes in the autophagy pathway in response to resistance exercise were similar between young and old, even though the aged had increased levels of Atg7 and beclin (Atg6) overall (Fry et al., 2013). In our study the changes in Atg5 and Atg7 were identical in soleus muscles from young and aged rats, but the recovery of beclin in the young was not present in the aged. Since beclin is involved in the formation of autophagosomal membranes, which is an early step in the process of autophagy (Ravikumar et al., 2010), this may indicate a continued elevation in initiation of autophagy during regrowth in the aged. Slimani et al (2012) showed an elevation in beclin early in the recovery after atrophy by casting in the tibialis anterior muscle which exhibited attenuated regrowth compared to other muscles in the same animal. Therefore, it is possible that an inability to turn off autophagy initiation in the aged soleus muscle contributes to a blunted regrowth response. However, when we measured autophagic flux by assessing the LC3b-II/LC3b-I ratio we did not see any changes in autophagy with the different loading conditions, even though there was a tendency for autophagy to be decreased with age, as has been shown previously in different muscles (McMullen et al., 2009; Wohlgemuth et al., 2007). This indicates that autophagic pathways, at this time point, are likely not responsible for the difference in muscle recovery with age.
Apoptosis and inflammation-related processes
Apoptosis in muscle is an interesting as well as controversial pathway. We and others have shown that DNA fragmentation and apoptosis-related pathways are elevated in muscles undergoing atrophy in response to aging and disuse and might contribute to the decrease in myonuclear number with inactivity induced by hind limb suspension and spinal cord injury (Dupont-Versteegden et al., 1999; Leeuwenburgh et al., 2005; Oishi et al., 2008; Siu et al., 2005b; Siu et al., 2005a). However, others have not observed similar changes in myonuclear number and increases in apoptotic processes in muscle fibers (Bruusgaard et al., 2012; Bruusgaard and Gundersen, 2008) and therefore the role of nuclear apoptosis has been challenged (Gundersen and Bruusgaard, 2008). Regardless of the role of apoptosis in potential myonuclear loss, it is clear that activation of apoptotic pathways, such as caspases, contributes to atrophy since inhibition of apoptosis-related processes through pharmacological or genetic means inhibits the loss of muscle mass (Hao et al., 2011; Siu and Alway, 2006); nonetheless, whether apoptosis is elevated in response to atrophy or directly contributes to muscle loss is not clear. In this study, we investigated the changes in some of these apoptosis-related pathways. Interestingly, caspase-3, -8 and -9 activities were not changed with age or disuse even though the apoptotic index was different in the aged with HS and reloading; similar results were found by Siu et al. (Siu et al., 2005b), but reloading was not investigated in that study. It is possible that, similar to protein synthesis and degradation data discussed above, changes in these proteins occurred early during the atrophy or regrowth process, and were missed at the time points investigated in the current study. However, these data also indicate that interventions geared towards these proteins may not be effective, since the changes in caspase may be transient while apoptosis is still elevated at a later time point. Apoptosis in aged muscle in general and with disuse and reloading in particular, was correlated with concurrent changes in the apoptosis inhibitor ARC; this protein may in the aged in particular be involved in allowing apoptosis to become elevated although the precise mechanisms through which this occurs is unclear. We have previously suggested that apoptosis in muscle may proceed through caspase-independent mechanisms, mainly because of the multinucleated nature of skeletal muscle (Dupont-Versteegden, 2005). EndoG and AIF are proteins which translocate to the nucleus upon induction of apoptosis and induce DNA fragmentation without the activation of caspases. Indeed, it has been shown that EndoG and AIF may be involved mainly in the aged during atrophy, since their release and accumulation was elevated (Leeuwenburgh et al., 2005; Siu et al., 2005b). A current study also shows EndoG present in higher abundance in nuclei from aged human muscle indicating that it may be involved in sarcopenia itself (Gouspillou et al., 2014). However, in the current study levels of EndoG and AIF were actually decreased in aged muscle, which is similar to data observed in quail in which anti-apoptotic proteins were increased while pro-apoptotic processes were decreased in the aged and it was suggested that aged muscle has a compensatory ‘apoptosis-breaking’ response which may be temporary and is ineffective (Siu et al., 2005a). The decrease in cytosolic EndoG with reloading in young and old indicates that the response to regrowth at this level is not changed with age; unfortunately, we were unable to accurately determine the nuclear EndoG with the currently available antibodies for immunoblotting. The decrease in nuclear AIF in both young and aged muscle with HS is puzzling, but similar to an observation in which nuclear AIF was decreased with unloading (Siu et al., 2005a), and indicates that this protein is likely not involved in atrophy in the soleus muscle or plays a role other than inducing apoptosis in skeletal muscle.
Recently the involvement of inflammation and the presence of macrophages in skeletal muscle have become apparent as modulators of skeletal muscle mass (Koh and Pizza, 2009). In particular, the finding that anti-inflammatory macrophages express IGF-1 and are protective of muscle size during atrophy is of interest in our study (Dumont and Frenette, 2010). Muscle reloading after atrophy has been shown to be associated with early damage and a transient inflammatory response (Oishi et al., 2008; St Pierre and Tidball, 1994; Tidball and Wehling-Henricks, 2007) and this inflammatory response seems critical for full recovery as a decrease in macrophages or IL-6 inhibits regrowth (Tidball and Wehling-Henricks, 2007; Washington et al., 2011). This inflammatory response, however, is an early and transient response, but we nonetheless assessed the presence of macrophages and neutrophils. Neutrophils and ED1-expressing macrophages were not detected in soleus muscles in our study which is consistent with reports that these are rare in non-injured muscle (Krippendorf and Riley, 1993) and transient upon injury (Tidball and Villalta, 2010). However, ED2 expressing macrophages reside in normal skeletal muscle, are presumed anti-inflammatory, and in our study were elevated in muscles of aged rats independent of loading status. In addition, these cells responded to loading in aged muscles only such that they decreased with atrophy, but recovered with reloading. We suggest that the ED2+ cells are increased in aged muscles as a compensatory response to a higher inflammatory environment, and their decrease with disuse may contribute to a more inflammatory state thereby contributing to atrophy. In aged animals serum levels of TNFα were not different in aged animals, but IL-6 was elevated in aged regardless of treatment; IL-6 is considered a pro-inflammatory cytokine, unless produced by exercising muscle (Pratesi et al., 2013), and likely contributes to the pro-inflammatory environment in the aged muscles. However, the fact that the number of ED2+-cells recovers upon reloading indicates that the muscles in aged animals are capable of responding to the new loading environment and therefore there is no indication that a dysregulation of macrophages contributes directly to the decreased regrowth response. This point is enforced by the fact that RANTES, which is inhibitory to muscle regeneration (Kohno et al., 2011) and elevated in response to repetitive movements which induce inflammation (Barbe et al., 2008), was decreased with reloading in both young and aged muscle, indicating that the animals of different ages respond in similar fashion to reloading. Whether the increase in anti-inflammatory macrophages in aged muscle plays a role in sarcopenia itself remains to be determined.
It should also be noted that there is always the possibility that the impaired regrowth response in the aged is caused by a lower level of physical activity of the aged rats during the reloading period, which was not measured in the current study. This would imply that there is a smaller growth stimulus in the aged than the young potentially causing the blunted regrowth. Of interest is that the muscles of young rats have also not completely recovered to ambulatory control levels and it is plausible that the young and old animals both are still regaining muscle mass, but at a different rate and therefore it might take the aged animals longer to recover, which may be due to their decreased activity in the reloading period. This possibility would lend itself well to therapeutic interventions during the recovery period and would be of clinical importance.
In summary, the impaired skeletal muscle regrowth after atrophy in aged animals is not associated with a general lack of response to changes in loading conditions. In particular, changes in markers of the protein synthesis and degradation pathways were very similar between young and old, indicating that old muscles are capable of responding, but may need a longer time period or increased levels of physical activity to recover.
Figure 5. Recovery of GSK3β is similar in young and old rats.
Representative bands for Western analysis of phospho(Ser9)-GSK3β, total GSK3β, and actin (used as loading control) are shown for soleus muscle of ambulatory (amb), hind limb suspended (HS) and reloaded (HSRE) rats at 6 and 32 months (A). Quantification of Western analysis is depicted for phospho- GSK3β (B), total GSK3β (B) and the ratio of phospho to total GSK3β (C) for ambulatory (black bars), hind limb suspended (HS, white bars), and reloaded (HSRE, grey bars) rats at 6 and 32 months (n=8–10). Values are means ± SE; * indicates a main effect for age and # indicates a main effect for HS; p<0.05.
Highlights.
Muscles of young and aged rats were investigated during regrowth after atrophy
Soleus muscle regrowth was blunted in aged rats
Age-related differences existed for protein synthesis markers and immune cells
Protein synthesis and degradation markers showed similar changes at different ages
Changes in signaling pathways do not explain the blunted regrowth response in aged
Acknowledgments
We would like to thank Ilja Zeilstra for technical assistance. This work was funded through NIH grant AG028925.
Footnotes
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Rosenberg IH. Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Am J Clin Nutr. 1989;50:1231–1233. [PubMed] [Google Scholar]
- Bassey EJ, Fiatarone MA, O’Neill EA, Kelley M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci. 1992;82:321–327. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- Laukkanen P, Heikkinen E, Kauppinen M. Muscle strength and mobility as predictors of survival in 75–84-year-old people. Age Ageing. 1995;24:468–73. doi: 10.1093/ageing/24.6.468. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–65. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Sakari-Rantala R, Leveille S, Simonsick EM, Ling S, Fried LP. Disability, physical activity, and muscle strength in older women: the women’s health and aging study. Arch Phys Med Rehabil. 1999;80:130–135. doi: 10.1016/s0003-9993(99)90109-0. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55:M168–73. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- Szulc P. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. Am J Clin Nutr. 2010;91:1227–1236. doi: 10.3945/ajcn.2009.28256. [DOI] [PubMed] [Google Scholar]
- Hebuterne X, Schneider S, Peroux JL, Rampal P. Effects of refeeding by cyclic enteral nutrition on body composition: comparative study of elderly and younger patients. Clin Nutr. 1997;16:283–9. doi: 10.1016/s0261-5614(97)80013-1. [DOI] [PubMed] [Google Scholar]
- Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ortenblad N, Kjaer M, Suetta C. Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J Appl Physiol. 2010;109:1628–34. doi: 10.1152/japplphysiol.00637.2010. [DOI] [PubMed] [Google Scholar]
- Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol. 2009;107:1172–80. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13:34–9. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Taillandier D, Savary I, Attaix D, Grizard J. Sensitivity and protein turnover response to glucocorticoids are different in skeletal muscle from adult and old rats. Lack of regulation of the ubiquitin-proteasome proteolytic pathway in aging. J Clin Invest. 1995;96:2113–9. doi: 10.1172/JCI118264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Jackson JR, Wang Y, Edens N, Pereira SL, Alway SE. beta-Hydroxy-beta-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am J Physiol Regul Integr Comp Physiol. 2011;301:R701–15. doi: 10.1152/ajpregu.00840.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magne H, Savary-Auzeloux I, Vazeille E, Claustre A, Attaix D, Anne L, Veronique SL, Philippe G, Dardevet D, Combaret L. Lack of muscle recovery after immobilization in old rats does not result from a defect in normalization of the ubiquitin-proteasome and the caspase-dependent apoptotic pathways. J Physiol. 2011;589:511–24. doi: 10.1113/jphysiol.2010.201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosoni L, Malmezat T, Valluy MC, Houlier ML, Attaix D, Mirand PP. Lower recovery of muscle protein lost during starvation in old rats despite a stimulation of protein synthesis. Am J Physiol. 1999;277:E608–16. doi: 10.1152/ajpendo.1999.277.4.E608. [DOI] [PubMed] [Google Scholar]
- Zarzhevsky N, Carmeli E, Fuchs D, Coleman R, Stein H, Reznick AZ. Recovery of muscles of old rats after hindlimb immobilisation by external fixation is impaired compared with those of young rats. Exp Gerontol. 2001a;36:125–40. doi: 10.1016/s0531-5565(00)00189-3. [DOI] [PubMed] [Google Scholar]
- Zarzhevsky N, Menashe O, Carmeli E, Stein H, Reznick AZ. Capacity for recovery and possible mechanisms in immobilization atrophy of young and old animals. Ann N Y Acad Sci. 2001b;928:212–25. doi: 10.1111/j.1749-6632.2001.tb05651.x. [DOI] [PubMed] [Google Scholar]
- Gallegly JC, Turesky NA, Strotman BA, Gurley CM, Peterson CA, Dupont-Versteegden EE. Satellite cell regulation of muscle mass is altered at old age. J Appl Physiol. 2004;97:1082–1090. doi: 10.1152/japplphysiol.00006.2004. [DOI] [PubMed] [Google Scholar]
- Chale-Rush A, Morris EP, Kendall TL, Brooks NE, Fielding RA. Effects of chronic overload on muscle hypertrophy and mTOR signaling in young adult and aged rats. J Gerontol A Biol Sci Med Sci. 2009;64:1232–9. doi: 10.1093/gerona/glp146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degens H, Alway SE. Skeletal muscle function and hypertrophy are diminished in old age. Muscle Nerve. 2003;27:339–47. doi: 10.1002/mus.10314. [DOI] [PubMed] [Google Scholar]
- Thomson DM, Gordon SE. Impaired overload-induced muscle growth is associated with diminished translational signalling in aged rat fast-twitch skeletal muscle. J Physiol. 2006;574:291–305. doi: 10.1113/jphysiol.2006.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funai K, Parkington JD, Carambula S, Fielding RA. Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTor signaling in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1080–6. doi: 10.1152/ajpregu.00277.2005. [DOI] [PubMed] [Google Scholar]
- Haddad F, Adams GR. Aging-sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol (1985) 2006;100:1188–203. doi: 10.1152/japplphysiol.01227.2005. [DOI] [PubMed] [Google Scholar]
- Hwee DT, Bodine SC. Age-related deficit in load-induced skeletal muscle growth. J Gerontol A Biol Sci Med Sci. 2009;64:618–28. doi: 10.1093/gerona/glp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger TA, Mateja RD, Chin ER, Andrews JL, Esser KA. Aging does not alter the mechanosensitivity of the p38, p70S6k, and JNK2 signaling pathways in skeletal muscle. J Appl Physiol (1985) 2005;98:1562–6. doi: 10.1152/japplphysiol.00870.2004. [DOI] [PubMed] [Google Scholar]
- Suetta C, Frandsen U, Mackey AL, Jensen L, Hvid LG, Bayer ML, Petersson SJ, Schroder HD, Andersen JL, Aagaard P, Schjerling P, Kjaer M. Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J Physiol. 2013;591:3789–804. doi: 10.1113/jphysiol.2013.257121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RT, Spangenburg EE, Booth FW. Responsiveness of cell signaling pathways during the failed 15-day regrowth of aged skeletal muscle. J Appl Physiol (1985) 2004;96:398–404. doi: 10.1152/japplphysiol.00454.2003. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Abraha T, Childs TE, Pattison JS, Booth FW. Skeletal muscle IGF-binding protein-3 and -5 expressions are age, muscle, and load dependent. Am J Physiol Endocrinol Metab. 2003;284:E340–50. doi: 10.1152/ajpendo.00253.2002. [DOI] [PubMed] [Google Scholar]
- Lipman R, Chrisp E, Hazzard D, Bronson R. Pathologic characterization of Brown Norway, Brown Norway X Fischer 344, and Fischer 344 X Brown Norway rats with relation to age. Journal of Gerontology: Biological Sciences. 1996;51A:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Strotman BA, Gurley CM, Gaddy D, Knox M, Fluckey JD, Peterson CA. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1730–40. doi: 10.1152/ajpregu.00176.2006. [DOI] [PubMed] [Google Scholar]
- Hofer T, Marzetti E, Xu J, Seo AY, Gulec S, Knutson MD, Leeuwenburgh C, Dupont-Versteegden EE. Increased iron content and RNA oxidative damage in skeletal muscle with aging and disuse atrophy. Experimental Gerontology. 2008;43:563–570. doi: 10.1016/j.exger.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahunty KM, Shultz KL, Gronowicz GA, Koczon-Jaremko B, Adamo ML, Horton LG, Lorenzo J, Donahue LR, Ackert-Bicknell C, Kream BE, Beamer WG, Rosen CJ. Congenic mice provide in vivo evidence for a genetic locus that modulates serum insulin-like growth factor-I and bone acquisition. Endocrinology. 2006;147:3915–23. doi: 10.1210/en.2006-0277. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, Campbell KS, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Satellite Cell Depletion does not Inhibit Adult Skeletal Muscle Re-Growth Following Unloading-Induced Atrophy. Am J Physiol Cell Physiol. 2012 doi: 10.1152/ajpcell.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen CA, Ferry AL, Gamboa JL, Andrade FH, Dupont-Versteegden EE. Age-related changes of cell death pathways in rat extraocular muscle. Exp Gerontol. 2009;44:420–5. doi: 10.1016/j.exger.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1288–96. doi: 10.1152/ajpregu.00576.2004. [DOI] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol. 2005a;288:C338–49. doi: 10.1152/ajpcell.00239.2004. [DOI] [PubMed] [Google Scholar]
- Xiao R, Ferry AL, Dupont-Versteegden EE. Cell death-resistance of differentiated myotubes is associated with enhanced anti-apoptotic mechanisms compared to myoblasts. Apoptosis. 2011;16:221–34. doi: 10.1007/s10495-010-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Nagarajan R, Beggs ML, Bearden ED, Simpson PM, Peterson CA. Identification of cold-shock protein RBM3 as a possible regulator of skeletal muscle size through expression profiling. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1263–73. doi: 10.1152/ajpregu.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol Metab. 2009;20:349–56. doi: 10.1016/j.tem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Yamada PM, Lee KW. Perspectives in mammalian IGFBP-3 biology: local vs. systemic action. Am J Physiol Cell Physiol. 2009;296:C954–76. doi: 10.1152/ajpcell.00598.2008. [DOI] [PubMed] [Google Scholar]
- Zdanowicz MM, Teichberg S. Effects of insulin-like growth factor-1/binding protein-3 complex on muscle atrophy in rats. Exp Biol Med (Maywood) 2003;228:891–7. doi: 10.1177/153537020322800804. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology. 2001a;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–84. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Verhees KJ, Schols AM, Kelders MC, Op den Kamp CM, van der Velden J, Langen RC. Glycogen synthase kinase 3 β is required for the induction of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2011;301:C995–C1007. doi: 10.1152/ajpcell.00520.2010. [DOI] [PubMed] [Google Scholar]
- Vyas DR, Spangenburg EE, Abraha TW, Childs TE, Booth FW. GSK-3beta negatively regulates skeletal myotube hypertrophy. Am J Physiol Cell Physiol. 2002;283:C545–51. doi: 10.1152/ajpcell.00049.2002. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001b;294:1704–8. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Foletta VC, White LJ, Larsen AE, Leger B, Russell AP. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflugers Arch. 2011;461:325–35. doi: 10.1007/s00424-010-0919-9. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Guttridge DC. Mechanisms for maintaining muscle. Curr Opin Support Palliat Care. 2012;6:451–6. doi: 10.1097/SPC.0b013e328359b681. [DOI] [PubMed] [Google Scholar]
- Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–15. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–83. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopard A, Hillock S, Jasmin BJ. Molecular events and signalling pathways involved in skeletal muscle disuse-induced atrophy and the impact of countermeasures. J Cell Mol Med. 2009;13:3032–50. doi: 10.1111/j.1582-4934.2009.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, Bernabei R, Leeuwenburgh C. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta. 2010;1800:235–244. doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard JC, Egner IM, Larsen TK, Dupre-Aucouturier S, Desplanches D, Gundersen K. No change in myonuclear number during muscle unloading and reloading. J Appl Physiol. 2012;113:290–6. doi: 10.1152/japplphysiol.00436.2012. [DOI] [PubMed] [Google Scholar]
- Koseki T, Inohara N, Chen S, Nunez G. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci U S A. 1998;95:5156–60. doi: 10.1073/pnas.95.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibebunjo C, Chick JM, Kendall T, Eash JK, Li C, Zhang Y, Vickers C, Wu Z, Clarke BA, Shi J, Cruz J, Fournier B, Brachat S, Gutzwiller S, Ma Q, Markovits J, Broome M, Steinkrauss M, Skuba E, Galarneau JR, Gygi SP, Glass DJ. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol. 2013;33:194–212. doi: 10.1128/MCB.01036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–9. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla B, Gurley C, Harvey JF, Bearden E, Kortebein P, Evans WJ, Sullivan DH, Peterson CA, Dennis RA. Aging alters macrophage properties in human skeletal muscle both at rest and in response to acute resistance exercise. Exp Gerontol. 2006;41:320–7. doi: 10.1016/j.exger.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011;23:1896–906. doi: 10.1016/j.cellsig.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs TE, Spangenburg EE, Vyas DR, Booth FW. Temporal alterations in protein signaling cascades during recovery from muscle atrophy. Am J Physiol Cell Physiol. 2003;285:C391–8. doi: 10.1152/ajpcell.00478.2002. [DOI] [PubMed] [Google Scholar]
- van der Velden JL, Langen RC, Kelders MC, Willems J, Wouters EF, Janssen-Heininger YM, Schols AM. Myogenic differentiation during regrowth of atrophied skeletal muscle is associated with inactivation of GSK-3beta. Am J Physiol Cell Physiol. 2007;292:C1636–44. doi: 10.1152/ajpcell.00504.2006. [DOI] [PubMed] [Google Scholar]
- Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013;45:2121–9. doi: 10.1016/j.biocel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307:E469–84. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimani L, Micol D, Amat J, Delcros G, Meunier B, Taillandier D, Polge C, Bechet D, Dardevet D, Picard B, Attaix D, Listrat A, Combaret L. The worsening of tibialis anterior muscle atrophy during recovery post-immobilization correlates with enhanced connective tissue area, proteolysis, and apoptosis. Am J Physiol Endocrinol Metab. 2012;303:E1335–47. doi: 10.1152/ajpendo.00379.2012. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn WA., Jr Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–92. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Volpi E, Rasmussen BB. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci. 2013;68:599–607. doi: 10.1093/gerona/gls209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Murphy RJ, Houle JD, Gurley CM, Peterson CA. Activated satellite cells fail to restore myonuclear number in spinal cord transected and exercised rats. Am J Physiol. 1999;277:C589–97. doi: 10.1152/ajpcell.1999.277.3.C589. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Ogata T, Yamamoto KI, Terada M, Ohira T, Ohira Y, Taniguchi K, Roy RR. Cellular adaptations in soleus muscle during recovery after hindlimb unloading. Acta Physiol (Oxf) 2008;192:381–95. doi: 10.1111/j.1748-1716.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Alway SE. Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am J Physiol Regul Integr Comp Physiol. 2005b;289:R1015–R1026. doi: 10.1152/ajpregu.00198.2005. [DOI] [PubMed] [Google Scholar]
- Bruusgaard JC, Gundersen K. In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. J Clin Invest. 2008;118:1450–7. doi: 10.1172/JCI34022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen K, Bruusgaard JC. Nuclear domains during muscle atrophy: nuclei lost or paradigm lost? J Physiol. 2008;586:2675–81. doi: 10.1113/jphysiol.2008.154369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Alway SE. Deficiency of the Bax gene attenuates denervation-induced apoptosis. Apoptosis. 2006;11:967–81. doi: 10.1007/s10495-006-6315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont-Versteegden EE. Apoptosis in muscle atrophy: relevance to sarcopenia. Experimental Gerontology. 2005;40:473–481. doi: 10.1016/j.exger.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Gouspillou G, Sgarioto N, Kapchinsky S, Purves-Smith F, Norris B, Pion CH, Barbat-Artigas S, Lemieux F, Taivassalo T, Morais JA, Aubertin-Leheudre M, Hepple RT. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J. 2014;28:1621–33. doi: 10.1096/fj.13-242750. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Pizza FX. Do inflammatory cells influence skeletal muscle hypertrophy? Front Biosci (Elite Ed) 2009;1:60–71. doi: 10.2741/E7. [DOI] [PubMed] [Google Scholar]
- Dumont N, Frenette J. Macrophages protect against muscle atrophy and promote muscle recovery in vivo and in vitro: a mechanism partly dependent on the insulin-like growth factor-1 signaling molecule. Am J Pathol. 2010;176:2228–35. doi: 10.2353/ajpath.2010.090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pierre BA, Tidball JG. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J Appl Physiol. 1994;77:290–7. doi: 10.1152/jappl.1994.77.1.290. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578:327–36. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington TA, White JP, Davis JM, Wilson LB, Lowe LL, Sato S, Carson JA. Skeletal muscle mass recovery from atrophy in IL-6 knockout mice. Acta Physiol (Oxf) 2011;202:657–69. doi: 10.1111/j.1748-1716.2011.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krippendorf BB, Riley DA. Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle Nerve. 1993;16:99–108. doi: 10.1002/mus.880160116. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–87. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratesi A, Tarantini F, Di Bari M. Skeletal muscle: an endocrine organ. Clin Cases Miner Bone Metab. 2013;10:11–4. doi: 10.11138/ccmbm/2013.10.1.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno S, Ueji T, Abe T, Nakao R, Hirasaka K, Oarada M, Harada-Sukeno A, Ohno A, Higashibata A, Mukai R, Terao J, Okumura Y, Nikawa T. Rantes secreted from macrophages disturbs skeletal muscle regeneration after cardiotoxin injection in Cbl-b-deficient mice. Muscle Nerve. 2011;43:223–9. doi: 10.1002/mus.21829. [DOI] [PubMed] [Google Scholar]
- Barbe MF, Elliott MB, Abdelmagid SM, Amin M, Popoff SN, Safadi FF, Barr AE. Serum and tissue cytokines and chemokines increase with repetitive upper extremity tasks. J Orthop Res. 2008;26:1320–6. doi: 10.1002/jor.20674. [DOI] [PubMed] [Google Scholar]