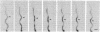Abstract
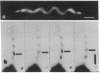
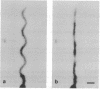
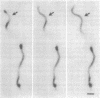
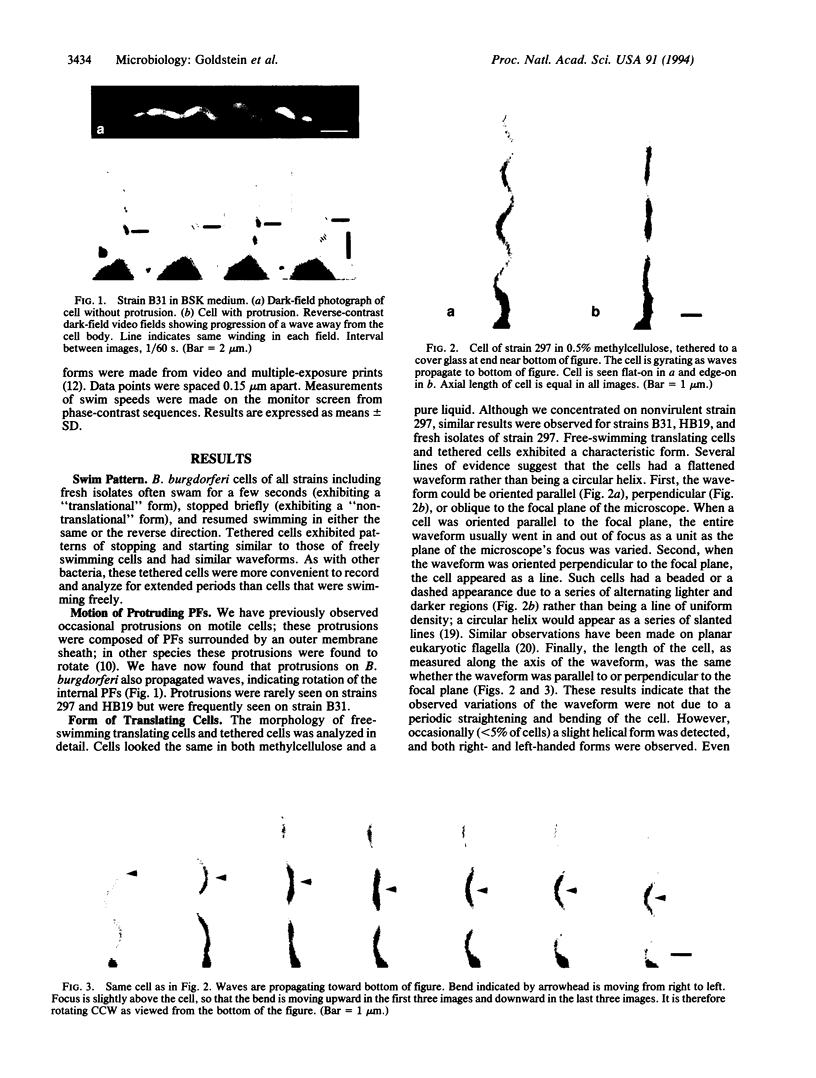
Borrelia burgdorferi is a motile spirochete with multiple internal periplasmic flagella (PFs) attached near each end of the cell cylinder; these PFs overlap in the cell center. We analyzed the shape and motion of wild type and PF-deficient mutants using both photomicrography and video microscopy. We found that swimming cells resembled the dynamic movements of eukaryotic flagella. In contrast to helically shaped spirochetes, which propagate spiral waves, translating B. burgdorferi swam with a planar waveform with occasional axial twists; waves had a peak-to-peak amplitude of 0.85 micron and a wavelength of 3.19 microns. Planar waves began full-sized at the anterior end and propagated toward the back end of the cell. Concomitantly, these waves gyrated counter-clockwise as viewed from the posterior end along the cell axis. In nontranslating cells, wave propagation ceased. Either the waveform of nontranslating cells resembled the translating form, or the cells became markedly contorted. Cells of the PF-deficient mutant isolated by Sadziene et al. [Sadziene, A., Thomas, D. D., Bundoc, V. G., Holt, S. C. & Barbour, A. G. (1991) J. Clin. Invest. 88, 82-92] were found to be relatively straight. The results suggest that the shape of B. burgdorferi is dictated by interactions between the cell body and the PFs. In addition, the PFs from opposite ends of the cell are believed to interact with one another so that during the markedly distorted nontranslational form, the PFs from opposite ends rotate in opposing directions around one another, causing the cell to bend.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. F., Magnarelli L. A., Burgdorfer W., Barbour A. G. Spirochetes in Ixodes dammini and mammals from Connecticut. Am J Trop Med Hyg. 1983 Jul;32(4):818–824. doi: 10.4269/ajtmh.1983.32.818. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Anderson R. A. Bacteria swim by rotating their flagellar filaments. Nature. 1973 Oct 19;245(5425):380–382. doi: 10.1038/245380a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Turner L. Movement of microorganisms in viscous environments. Nature. 1979 Mar 22;278(5702):349–351. doi: 10.1038/278349a0. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Non-sinusoidal bending waves of sperm flagella. J Exp Biol. 1965 Aug;43(1):155–169. doi: 10.1242/jeb.43.1.155. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J., Wright L. Bending Waves of the Posterior Flagellum of Ceratium. Science. 1963 Nov 29;142(3596):1169–1170. doi: 10.1126/science.142.3596.1169. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Charon N. W., Daughtry G. R., McCuskey R. S., Franz G. N. Microcinematographic analysis of tethered Leptospira illini. J Bacteriol. 1984 Dec;160(3):1067–1073. doi: 10.1128/jb.160.3.1067-1073.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Goldstein S. F., Block S. M., Curci K., Ruby J. D., Kreiling J. A., Limberger R. J. Morphology and dynamics of protruding spirochete periplasmic flagella. J Bacteriol. 1992 Feb;174(3):832–840. doi: 10.1128/jb.174.3.832-840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Goldstein S. F., Curci K., Limberger R. J. The bent-end morphology of Treponema phagedenis is associated with short, left-handed, periplasmic flagella. J Bacteriol. 1991 Aug;173(15):4820–4826. doi: 10.1128/jb.173.15.4820-4826.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Greenberg E. P., Koopman M. B., Limberger R. J. Spirochete chemotaxis, motility, and the structure of the spirochetal periplasmic flagella. Res Microbiol. 1992 Jul-Aug;143(6):597–603. doi: 10.1016/0923-2508(92)90117-7. [DOI] [PubMed] [Google Scholar]
- Chwang A. T., Winet H., Wu T. Y. A theoretical mechanism of spirochete locomotion. J Mechanochem Cell Motil. 1974;3(1):69–76. [PubMed] [Google Scholar]
- Cox C. D. Shape of Treponema pallidum. J Bacteriol. 1972 Feb;109(2):943–944. doi: 10.1128/jb.109.2.943-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLAMATER E. D., WIGGALL R. H., HAANES M. Studies on the life cycle of spirochetes; the life cycle of the Nichols pathogenic Treponema pallidum in the rabbit testis as seen by phase contrast microscopy. J Exp Med. 1950 Sep;92(3):239–246. doi: 10.1084/jem.92.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosnaugh K., Greenberg E. P. Motility and chemotaxis of Spirochaeta aurantia: computer-assisted motion analysis. J Bacteriol. 1988 Apr;170(4):1768–1774. doi: 10.1128/jb.170.4.1768-1774.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. Flagellar movement and adenosine triphosphatase activity in sea urchin sperm extracted with triton X-100. J Cell Biol. 1972 Jul;54(1):75–97. doi: 10.1083/jcb.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. F. Asymmetric waveforms in echinoderm sperm flagella. J Exp Biol. 1977 Dec;71:157–170. doi: 10.1242/jeb.71.1.157. [DOI] [PubMed] [Google Scholar]
- Goldstein S. F., Charon N. W. Motility of the spirochete Leptospira. Cell Motil Cytoskeleton. 1988;9(2):101–110. doi: 10.1002/cm.970090202. [DOI] [PubMed] [Google Scholar]
- Goldstein S. F., Charon N. W. Multiple-exposure photographic analysis of a motile spirochete. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4895–4899. doi: 10.1073/pnas.87.13.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Relationship between cell coiling and motility of spirochetes in viscous environments. J Bacteriol. 1977 Sep;131(3):960–969. doi: 10.1128/jb.131.3.960-969.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovind-Hougen K. Ultrastructure of spirochetes isolated from Ixodes ricinus and Ixodes dammini. Yale J Biol Med. 1984 Jul-Aug;57(4):543–548. [PMC free article] [PubMed] [Google Scholar]
- Ishijima S., Mohri H. A quantitative description of flagellar movement in golden hamster spermatozoa. J Exp Biol. 1985 Jan;114:463–475. doi: 10.1242/jeb.114.1.463. [DOI] [PubMed] [Google Scholar]
- Ishijima S., Witman G. B. Flagellar movement of intact and demembranated, reactivated ram spermatozoa. Cell Motil Cytoskeleton. 1987;8(4):375–391. doi: 10.1002/cm.970080410. [DOI] [PubMed] [Google Scholar]
- Kimsey R. B., Spielman A. Motility of Lyme disease spirochetes in fluids as viscous as the extracellular matrix. J Infect Dis. 1990 Nov;162(5):1205–1208. doi: 10.1093/infdis/162.5.1205. [DOI] [PubMed] [Google Scholar]
- SEQUEIRA P. J. The morphology of Treponema pallidum. Lancet. 1956 Oct 13;271(6946):749–749. doi: 10.1016/s0140-6736(56)90959-x. [DOI] [PubMed] [Google Scholar]
- Sadziene A., Thomas D. D., Bundoc V. G., Holt S. C., Barbour A. G. A flagella-less mutant of Borrelia burgdorferi. Structural, molecular, and in vitro functional characterization. J Clin Invest. 1991 Jul;88(1):82–92. doi: 10.1172/JCI115308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Kamiya R., Asakura S. Left-handed to right-handed helix conversion in Salmonella flagella. Nature. 1975 Mar 27;254(5498):332–334. doi: 10.1038/254332a0. [DOI] [PubMed] [Google Scholar]
- Stepan D. E., Johnson R. C. Helical conformation of Treponema pallidum (Nichols strain), Treponema paraluis-cuniculi, Treponema denticola, Borrelia turicatae, and unidentified oral spirochetes. Infect Immun. 1981 May;32(2):937–940. doi: 10.1128/iai.32.2.937-940.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K. E., Gibbons I. R. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3092–3096. doi: 10.1073/pnas.68.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]