Abstract
Dimethylarginine dimethylaminohydrolase (DDAH) degrades (ADMA) which inhibits nitric oxide synthase (NOS). Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcriptional factor that binds to antioxidant response elements (ARE) and transcribes many antioxidant genes. Since the promoters of the human DDAH-1 and -2, eNOS and PPAR- γ genes contain 2–3 putative AREs, we hypothesized that they were regulated by Nrf2/ARE. Incubation of human renal glomerular endothelial cells (HRGECs) with the Nrf2 activator tert-butylhydroquinone (tBHQ) (20 µmol∙l−1) significantly (P<0.05) increased NO and activities of NOS and DDAH and decreased ADMA. It upregulated genes for hemoxygenase -1, eNOS, DDAH-1 and -2 and PPAR-γ and partitioned Nrf2 into the nucleus. Knockdown of Nrf2 abolished these effects. Nrf2 bound to one ARE on the DDAH-1 and -2 and PPAR-γ promoters but not to the eNOS promoter. An increased eNOS and phosphorylated eNOS (P-eNOSser-1177) expression with tBHQ was prevented by knockdown of PPAR-γ. Expression of Nrf2 was reduced by knockdown of PPAR-γ whereas PPAR-γ was reduced by knockdown of Nrf2, thereby demonstrating 2-way positive interactions. Thus, Nrf2 transcribes the HO-1 and other genes to reduce reactive oxygen species, and DDAH-1 and -2 to reduce ADMA and PPAR-γ to increase eNOS and its phosphorylation and activity thereby coordinating three pathways that enhance endothelial NO generation.
Keywords: asymmetrical dimethylarginine, endothelial dysfunction, tert-butylhydroquinone, antioxidant response element, cytoprotective genes
Introduction
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a redox-sensitive transcription factor that transcribes many antioxidant genes through a cis–acting antioxidant response element (ARE). Cytoplamic Nrf2 is bound to its inhibitor Kelch-like ECH-associated protein (Keap)-1. Specific cystein residues on Keap 1 can be oxidized during oxidative stress causing a conformational change that prevents ubiquitination and releases newly synthesized Nrf2 [1]. Other stimuli which can activate the pathway independent of oxidative stress [2], include bardoxolone methyl, naturally occurring substances such as rosveritrol or sulforaphane [3] or the small molecular weight substance tert-butylhydroxyquinone (tBHQ). tBHQ dissociates Nrf2-Keap1, stabilizes Nrf2 and phosphorylates it at Serine-40 Nrf2-PS40 via phosphatidylinositol 3-kinase (PI3K)/Akt [4, 5]. After dissassociation from Keap-1, Nrf2 and translocates it into the nucleus [6] where it dimerises with other transcription factors, notably mafmusculoaponeurotic fibrosarcoma oncogene homolog [7]. Specific binding to AREs initiates transcription of a plethora of cytoprotective genes including NAD(P)H quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), glutathione S-transferase-1 (GST-1), peroxisome proliferator activated receptor gamma (PPAR-γ), copper/zinc superoxide dismutase ( SOD1) and manganese superoxide dismutase (SOD2)[8].
Nitric oxide (NO) produced by eNOS in the vascular endothelium reduces vascular tone, mediates endothelium dependent relaxation and protects glomeruli from sclerosis and damage [9, 10]. eNOS activity is tightly regulated by expression, phosphorylation and availability of cofactors, and is inhibited by asymmetric dimethylarginine (ADMA) [11, 12] . Reactive oxygen species (ROS) enhance NO degredation and uncouple eNOS to redirect the enzyme to generate ROS [13]. ADMA is metabolized by two isoforms of dimethylarginine dimethylaminohydrolase (DDAH) to inactive products [11].
eNOS in the endothelium of the renal microvessels and glomeruli preserves glomerular function [14]. However, the effects of Nrf2 in human renal glomerular endothelial cells (HRGECs) has not been studied. tBHQ was selected as a potent activator of Nrf2 [15]. Since we detected 2 or 3 putative AREs in promoter regions of the human genes for eNOS, DDAH-1 and-2 and PPAR-γ, we tested the hypothesis that activation of Nrf2 by tBHQ regulated DDAH/ eNOS/ PPAR-γ transcription and thereby the metabolism of ADMA and the generation of NO. Primary cultures of HRGECs were selected since recent studies have shown that activation of Nrf2 with bardoxolene methyl increases the glomerular filtration rate in patients with diabetic nephropathy [16]. Moreover, single nucleotide polymorphisms of eNOS increase the risk of advanced diabetic nephropathy in type I diabetes mellitus [17]. Since ROS, endothelial dysfunction and ADMA predict cardiovascular disease (CVD) and progression of chronic kidney disease (CKD), interventions that may prevent or reverse these are attractive novel therapeutic targets [18–20].
Methods
Cell culture and proliferation of human renal glomerular endothelial cells (HRGECs)
Primary cultures were purchased from ScienCell Research Laboratories (Carlsbad, CA) and studied from passage 2 to 5 where eNOS protein expression was maintained (Supplement Figure S1). HRGEC proliferation was assessed from mitochondrial succinate-tetrazolium reductase using premixed WST-1 cell proliferation reagent (Clontech Lab.Mountain View, CA). (See Data Supplement).
Knockdown of Nrf2
HRGECS were transiently transfected with Nrf2 specific siRNA or control, non- targeted, siRNA (Santa Cruz Biotechnology,Inc. Santa cruz, CA).( See Data Supplement).
RNA isolation and real time quantitative RT-PCR
Real-time quantitative PCR was as described [21] (see Data Supplement).
Western blotting for protein in whole cell lysate or cell fractions
Immunoblotting assay was performed as described [21]. Nuclear and cytoplasmic fractions were prepared using the Cell Fraction System (BioVision Inc., Milpitas, CA) (See Data Supplement).
Medium nitrite, cellular NO and NOS and DDAH activities
Nitrite was measured using Griess reagent and NO generation from 4-amino-5-methylamino- 2’, 7’-difluorofluorescein diacetate (DAF-FM DA) fluorescence. NOS activity was measured from conversion of [3H] arginine to [3H] citrulline and DDAH activity from conversion of [14C]-ADMA to [14C]-citrulline as described [21](see Data Supplement).
Medium ADMA
Medium ADMA was determined by capillary zone electrophoresis (see Data Supplement).
Chromatin immunoprecipitation assay (ChIP)
HRGECs were studied using EpiTect ChIP One Day Kit (SABiosciences, Frederick, MD)(see Data Supplement ) after 24 hour incubation with 20µmol∙l−1 tBHQ or vehicle.
Statistical analysis
Results are expressed as mean ± SEM with n=number of separate experiments. Each study was conducted in triplicate on 3 or more separate frozen vials of cells purchased at different times. Differences between experimental groups were compared by 2x2 ANOVA with interaction. Where appropriate, post-hoc testing was by Bonferroni t test. P < 0.05 was considered significant.
Results
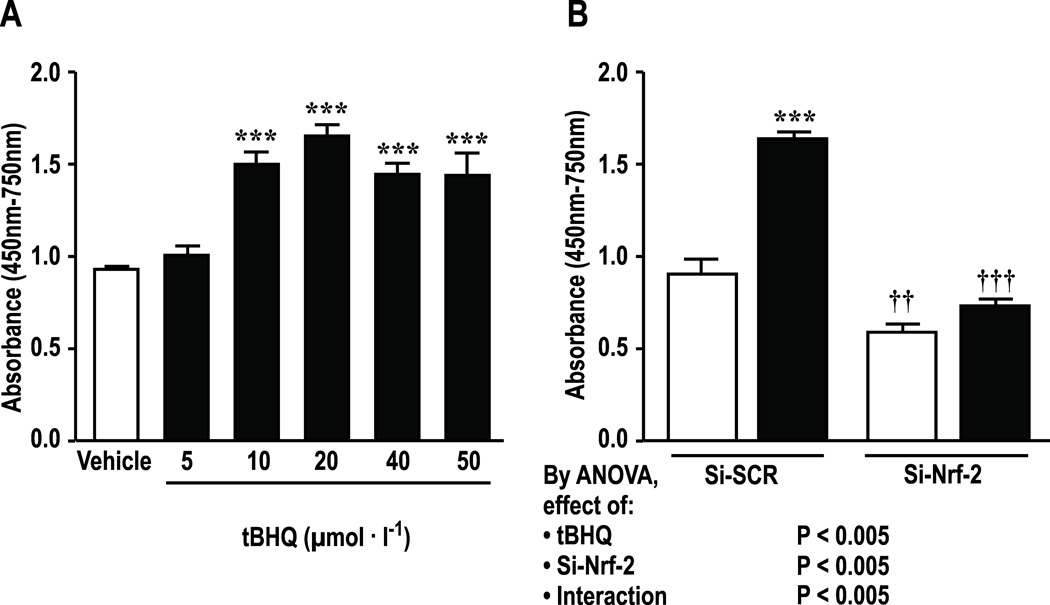
Incubation of HRGECs for 24 hours with tBHQ enhanced cell proliferation maximally at 20 µmol∙l−1 which was therefore selected for study (Figure 1 A). Knockdown of Nrf2 reduced basal and tBHQ-stimulated proliferation (figure 1B).
Figure 1.
Activation of Nrf-2 by tBHQ enhances proliferation of HRGECs. A, dose response for incubation with tBHQ over 24hr. B, effect of si-RNA to Nrf-2, compared to scrambled si-RNA. Si-SCR, scrambled si-RNA; Si-Nrf-2, Nrf-2-siRNA; open boxes, vehicle, solid boxes; tBHQ (20 µmol∙l−1 in Panel B for 24 hr). Mean± SEM values (n=6 per group). Compared to vehicle; ***P < 0.005. Compared to si-SCR: ††, P<0.01; †††, P<0.005
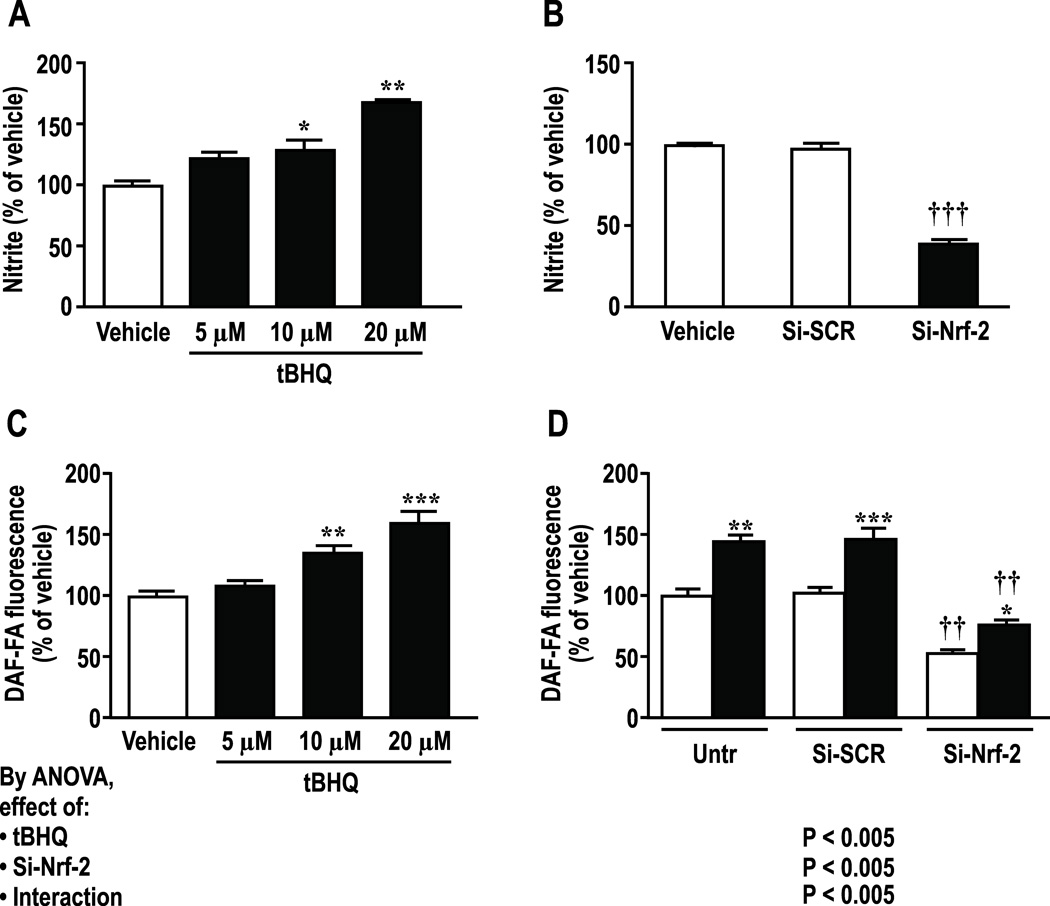
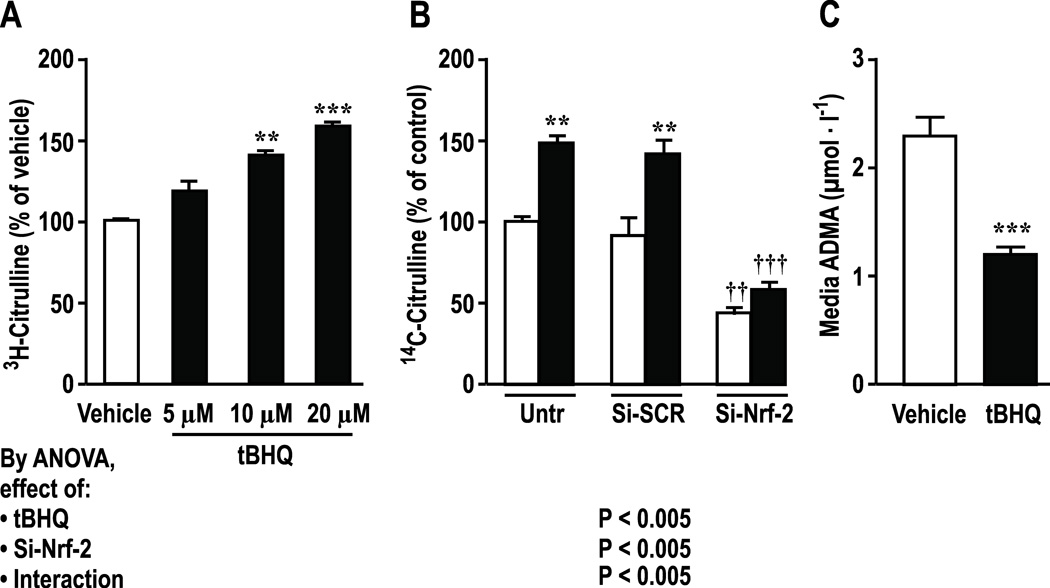
tBHQ (10–20 µmol∙l−1) increased (P<0.01) medium nitrite and intracellular NO by circa >60% which were prevented by knockdown of Nrf2 (Figure 2). Effects were maximal at 24hr (Supplemental Figure S2) which was selected for study. Incubation of HRGECs with tBHQ increased activity of NOS by 58±4% (P <0.005, figure 3A) and DDAH by 47±9% (P < 0.01, figure 3B) whereas knockdown of Nrf2 reduced basal and tBHQ-stimulated DDAH activity and almost halved medium ADMA from 2.3±0.16 to 1.2 ± 0.02 µmol∙l−1 (figure 3 C, P < 0.001).
Figure 2.
Effects of tBHQ or si-RNA to Nrf-2 vs scrambled siRNA medium nitrite and intracellular NO activity over 24 hours. A and C, dose response; B and D effect of si-RNA to Nrf-2 vs. scrambled si-RNA in HRGECs. Si-SCR, scrambled si-RNA; Si-Nrf-2, Nrf-2-siRNA; open boxes, vehicle, solid boxes, tBHQ (20 µmol∙l−1 in Panels B and D for 24hr). Mean ± SEM (n=6 per group). Compared to vehicle: *P < 0.05; **P < 0.01 and ***P < 0.005. Compared to si-SCR: ††, P<0.01; †††, P<0.005
Figure 3.
Effects of tBHQ and si-RNA to Nrf-2 vs scrambeled siRNA on NOS activity (A), DDAH activity (B), and ADMA levels (C) in supernatants of HRGECs. Untr, untransfected control; Si-SCR, scrambled si-RNA; Si-Nrf-2, Nrf-2-siRNA; open boxes, vehicle, solid boxes, tBHQ (20 µmol∙l−1 in Panels B and C). Mean ± SEM (n=6 per group). Compared to vehicle: **P < 0.01 and ***P < 0.005. Compared to si-SCR: ††, P<0.01; †††, P<0.005
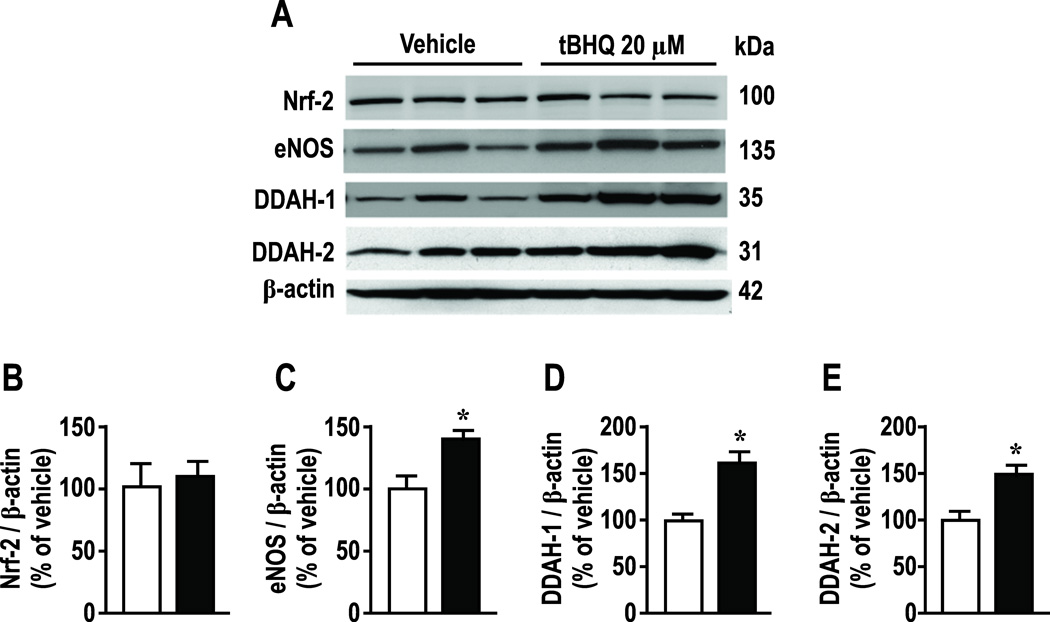
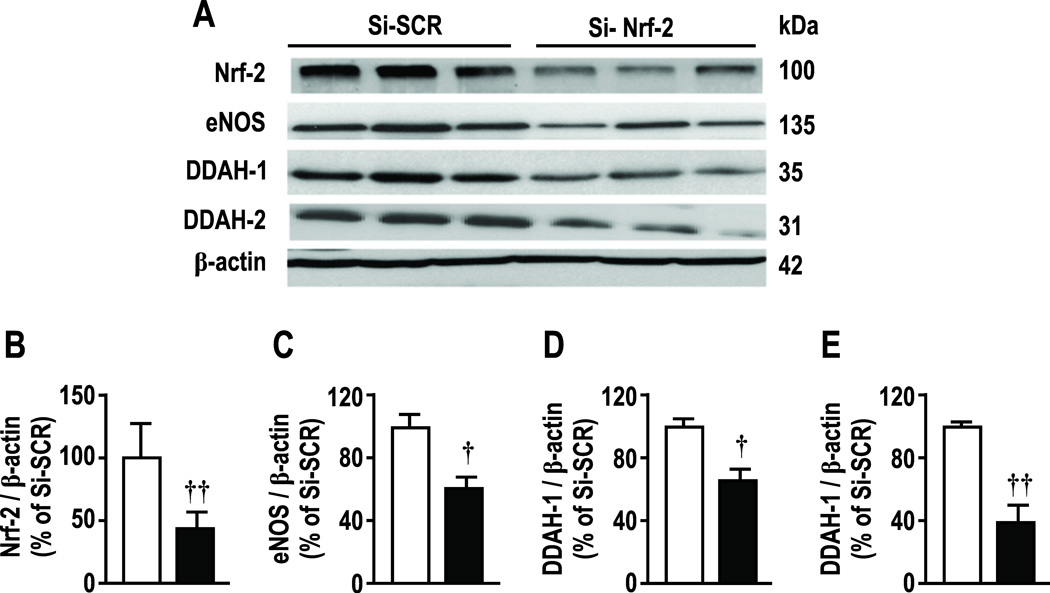
As anticipated, tBHQ (20 µmol∙l−1) did not affect mRNA expression for Nrf2 [4, 5] but upregulated eNOS by 79 ± 9%, DDAH-1 by 129 ± 10% and DDAH-2 by 119 ± 25% (Supplemental Figure S3; all P < 0.005 ). All effects were prevented by knockdown of Nrf2. tBHQ did not change protein expression for Nrf2 but, similar to mRNA, increased eNOS protein by 43± 17%, DDAH-1 by 62 ± 21% and for DDAH-2 by 48 ± 18% (Figure 4; all P < 0.05). Nrf2 reduced basal protein expression of Nrf2 by 55%, and reduced basal expression of eNOS, DDAH-1 and -2 (figure 5). Figures 4 and 5 report Nrf2 immunoblots at 100 kDa corresponding to the poly-ubiquitanted form but another band was deleted at 58 kDa. Analysis of both yielded the same conclusions. Variable molecular weights for Nrf2 have been reported [22–24].
Figure 4.
Effects of tBHQ on protein expressions for Nrf-2, eNOS, DDAH-1 and DDAH-2 in HRGECs. Open boxes, vehicle, solid boxes, tBHQ (20 µmol∙l−1 for 24hr). Mean ± SEM (n=6 per group). Compared to vehicle: *, P<0.05.
Figure 5.
Effects of transient transfection of Nrf-2 specific siRNA, compared to scrambled siRNA, on protein expressions of Nrf-2, eNOS, DDAH-1 and DDAH-2 in HRGECs. Si-SCR, scrambled si-RNA; Si-Nrf-2, Nrf-2-siRNA. Mean ± SEM (n=6 per group). Compared to si-SCR: *, P<0.05, **, P<0.01.
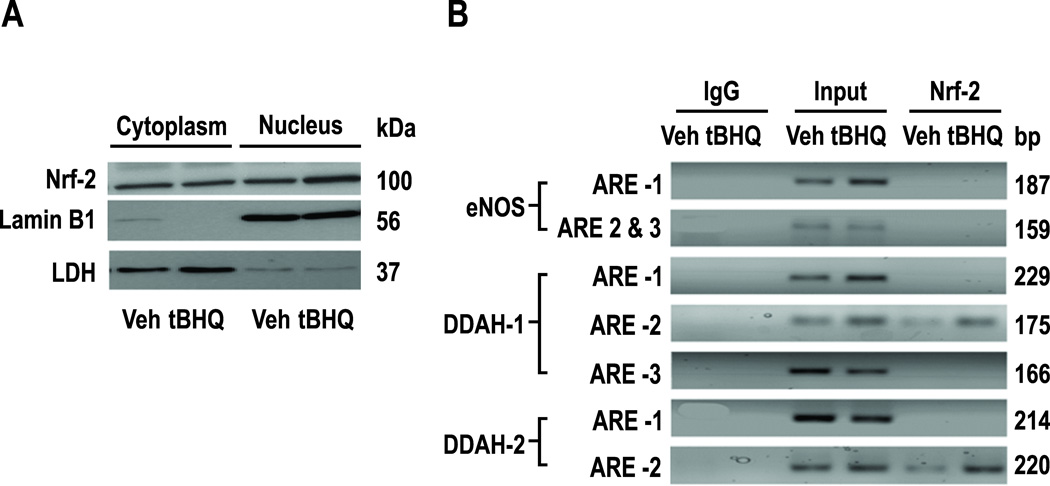
tBHQ stabilizes Nrf2 and translocates it to the nucleus. Cells treated for 24hr with tBHQ (20 µmol∙l−1) had increased Nrf2 protein in the nucleus, but not the cytoplasm (figure 6A). Lamin B-1 was the nuclear marker and LDH the cytoplasmic marker. There are 3, 3, 2 putative AREs on the promoters of eNOS, DDAH-1 and -2 located upstream (−1/−3000bp) of individual transcriptional start sites (TGACnnnGC or GCTGAGnnn) (See supplemental table S1) and functional AREs on the promoter of PPAR-γ [25]. ChiP assays from primary HRGECs (See supplemental table S2) demonstrated protein/DNA complexes that were immunoprecipitated by Nrf2 antibody and enriched by incubation with tBHQ covering ARE-2 for DDAH-1 (−1016/−1008) and ARE2 for DDAH-2 (−1152/−1143) but no nuclear binding to the eNOS promoter (figure 6B).
Figure 6.
Effects of tBHQ on translocation of Nrf-2 to the nucleus in HRGECs. Panel A, cytoplasmic and nuclear expressions of Nrf-2 and enhancement of nuclear expression by tBHQ (20 µmol∙l−1). Panel B, chip assays showing Nrf-2 binding to ARE-2 on promoters for DDAH-1 and -2 and enhancement by tBHQ (20 µmol∙l−1), but no nuclear binding, to AREs on eNOS.
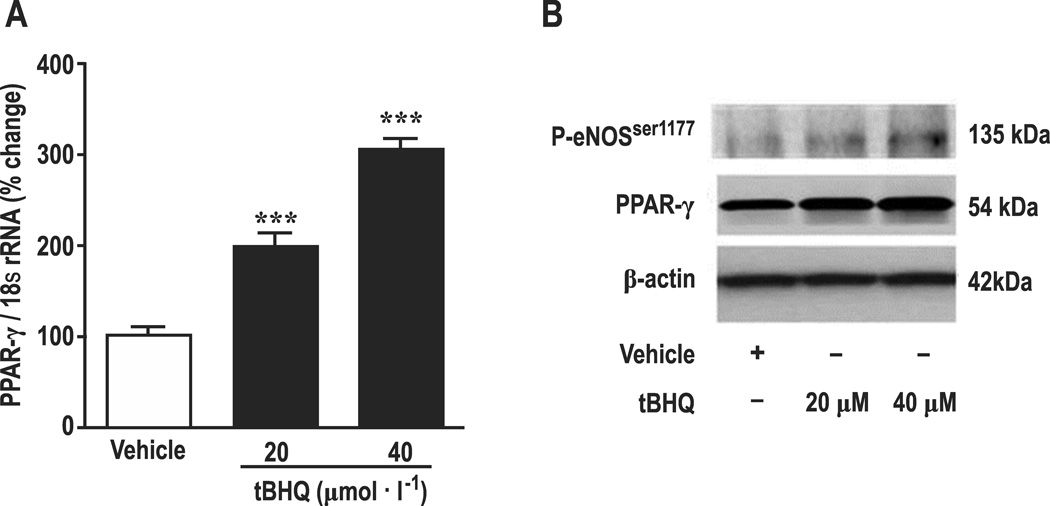
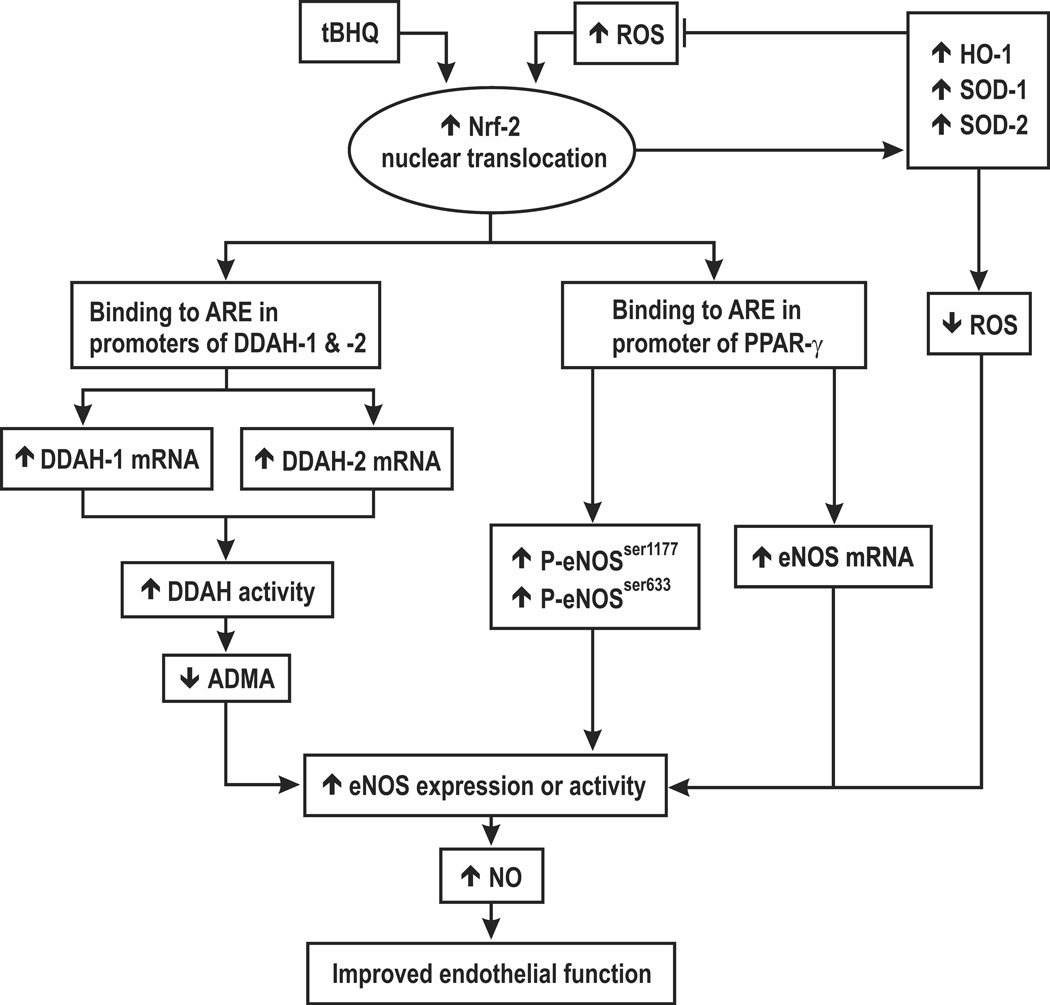
Since PPAR-γ increases eNOS expression and phosphorylation [26] and Nrf2 binds to AREs on the PPAR-γ promoter [25], we considered that PPAR-γ might mediate the effects of tBHQ on eNOS. Incubation of HRGECs for 24 hours with tBHQ (20 and 40 µmol∙l−1 ) increased PPAR-γ mRNA by 202 ± 12% (Figure 7A) with parallel increases in the protein expression for PPAR-γ and phosphorylated eNOS at amino acid residue Ser 1177(P-eNOSser-1177 ,Fig 7B). tBHQ significantly increased nuclear protein accumulation for Nrf2, Nrf2-ps 40 (phosphorylated at Ser-40 of Nrf2) [4, 5] and the ratio of Nrf2-ps 40 to Nrf2 (Supplemental Figure S4). Knockdown of Nrf2 reduced basal and tBHQ- stimulated mRNA for PPAR-γ (Supplemental Figure S5). Knockdown of PPAR-γ reduced basal levels of Nrf2, and reduced tBHQ-induced increases in gene expression for eNOS, DDAH-1 and DDAH-2 (Supplemental Figure S6) and led to parallel reductions in eNOS and p-eNOSser-1177. However, the increase in expression of DDAH-1 or -2 persisted after PPAR-γ knockdown (Supplemental Figure S7). Finally, tBHQ greatly increased the gene expression for HO-1 and NQO1 (Supplemental Figure S8). These pathways are summarized in Figure 8.
Figure 7.
Effects of tBHQ on mRNA expressions for PPAR-γ (Panel A), protein expression for p-eNOSser1177, and PPAR-γ (Panel B) in HRGECs. Open boxes, vehicle, closed boxes, tBHQ. Compared to vehicle in A: ***, P<0.005. Blot band images shown in B are representatives from 3 independent experiments.
Figure 8.
Schematic hypothesis for roles of Nrf-2 in preserving endothelial function during activation by tBHQ or reactive oxygen species.
Discussion
Activation of Nrf2 transcriptionally upregulates many oxidant defense pathways [8]. We confirmed robust upregulation by tBHQ of the mRNA expression for HO-1 and NQO-1.
The main new findings are that activation of AREs in HRGECs by tBHQ promotes cell proliferation, NOS activity and NO generation, increases DDAH activity, reduces ADMA and increases the expression of eNOS, DDAH-1 and -2 and PPAR-γ mRNAs and proteins. These effects were prevented by knockdown of Nrf2. While a ChiP assay identified one ARE site that bound Nrf2 and was enriched by incubation with tBHQ in both DDAH-1 and DDAH-2 gene promoters and PPAR-γ contains another [25], no binding site was detected on the eNOS promoter. Rather, the increased expression of eNOS protein with tBHQ was related to increased PPAR-γ expression since it was prevented by knockdown of PPAR- γ. Furthermore, tBHQ increased eNOS phosphorylation at serine 1177 which is a post-translational activation pathway. Thus, Nrf-2 activation may improve endothelial function by three distinct pathways (figure 8). First, it promotes gene expression for antioxidant molecules that counter the effects of ROS to impair endothelial function and uncouple eNOS. Second, it interacts with specific AREs on the DDAH-1 and -2 genes whose products enhance the degradation of ADMA which thereby lessen inhibition of eNOS. Third, tBHQ interacts with specific AREs on the PPAR-γ genes to enhance eNOS expression, phosphorylation and activity.
DDAH-2 metabolizes ADMA in blood vessels and maintains endothelial function whereas DDAH-1 regulates plasma levels of ADMA [11, 27] that predict cardiovascular events and death in high risk groups and the rate of loss of function in CKD [11, 18, 28]. The transcriptional regulation of DDAH expression is not well understood. Our study is one of few that have defined a pathway that can decrease ADMA [23, 29] and increase PPAR-γ and eNOS expressions and activity [25].
PPAR-γ agonists upregulate Nrf2 [25, 30]. Interestingly, we found that activation of Nrf2 by tBHQ increased the mRNA and protein expression for PPAR-γ whereas these were reduced by knockdown of Nrf2. Moreover, knockdown of PPAR-γ reduced the mRNA expression for Nrf2. This indicates a tight, positive, two-way reinforcing transcriptional interaction between PPAR-γ and Nrf2 that may improve endothelial function [31].
We acknowledge some limitations. First, we did not study the time course of Nrf2 nuclear binding after tBHQ incubation. Rather, we selected 24 hours for all studies since this was maximal for eNOS activation. Second, although we demonstrated specific nuclear binding of Nrf2 to the ARE-2 of DDAH-1 and -2, further studies of promoter transcriptional activity and ARE site mutation are required to definitely confirm its role in DDAH-1 and -2 gene transcription.
In conclusion, the established antioxidant actions of Nrf2 were complemented by upregulation of NO generation by increased NOS activity and ADMA metabolism by increased DDAH activity (figure 8). Thus Nrf2 can provide a coordinated regulation of the ROS/DDAH/PPAR-γ /eNOS pathways to protect endothelial function.
Perspectives
Increased ROS and ADMA have been implicated in early cardiovascular and renal disease [11, 32, 33]. The Nrf2 activator bardoxolone methyl given to patients with type 2 diabetes and CKD increased their eGFR by about 33% [16, 34], consistent with increased glomerular endothelial NO generation. However, this potentially beneficial effect was offset by an unexplained increase in congestive heart failure [16]. Nevertheless, NO has multiple beneficial effects in blood vessels, the cardiovascular system, and the kidneys. NO generation is determined by NOS activity which in turn is increased by NOS expression that is promoted by DDAH [27]. Phosphorylation of eNOS at specific sites also increases NOS activity by ADMA and by uncoupling of the enzyme by ROS [13]. Remarkably, Nrf2 activation can promote NO activity by favorably influencing all of these pathways. Our study is the first to identify a strategy with a single drug (tBHQ) that not only reduces ROS, but enhances NO generation and PPAR-γ and reduces ADMA. Accordingly, drugs that activate Nrf2 could have widespread beneficial effects on the cardiovascular and renal systems, although the experience with bardoxolone methyl provides a cautionary note.
Novelty and Significance.
What is New?
Activation of Nrf2 by tBHQ increases NO and PPAR-γ and decreases ADMA in human glomerular endothelial cells.
tBHQ increases nuclear binding of Nrf2 that increases expression of DDAH-1 and 2 and PPAR-γ .
eNOS and its phosphorlation with tBHQ are mediated by PPAR-γ transcription.
There is one functional ARE site on DDAH-1and-2 promoters and one on PPAR- γ but none on eNOS.
What Is Relevant?
Activation of Nrf2 promotes NO activity by multiple pathways (reduced ROS, increased eNOS transcription and phosphorylation and increased metabolism of ADMA)
This provides a potential unique pathway to garner the many cardiovascular and renal benefits of enhanced NO
Summary
An activated Nrf2 may promote endothelial function by a coordinated effect to limit oxidative stress and enhance the DDAH/ PPAR-γ /eNOS pathways.
Acknowledgments
Sources of Funding
This study was supported by research grants from the National Institute of Health NIDDK (DK-036079 and DK-049870) and from the NHLBI (HL-68686) and by funds from the George E. Schreiner Chair of Nephrology, the Georgetown University Hypertension, Kidney and Vascular Research Centre and the Smith - Kogard Family Foundation. Z.L. was supported by a Nephrology Research Training Grant from the NIDDK (T32-DK-059274).
Footnotes
Disclosure Statement: None
References
- 1.Cazanave SC, Wang X, Zhou H, Rahmani M, Grant S, Durrant DE, Klaassen CD, Yamamoto M, Sanyal AJ. Degradation of Keap1 activates BH3-only proteins Bim and PUMA during hepatocyte lipoapoptosis. Cell Death Differ. 2014;21:1303–1312. doi: 10.1038/cdd.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J, Williams D, Walter GA, Thompson WE, Sidell N. Estrogen increases Nrf2 activity through activation of the PI3K pathway in MCF-7 breast cancer cells. Exp Cell Res. 2014;328:351–360. doi: 10.1016/j.yexcr.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Yoon HY, Kang NI, Lee HK, Jang KY, Park JW, Park BH. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem Pharmacol. 2008;75:2214–2223. doi: 10.1016/j.bcp.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Gharavi N, Haggarty S, El-Kadi AO. Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr Drug Metab. 2007;8:1–7. doi: 10.2174/138920007779315035. [DOI] [PubMed] [Google Scholar]
- 5.Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc Natl Acad Sci U S A. 2000;97:12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 7.Kimura M, Yamamoto T, Zhang J, Itoh K, Kyo M, Kamiya T, Aburatani H, Katsuoka F, Kurokawa H, Tanaka T, Motohashi H, Yamamoto M. Molecular basis distinguishing the DNA binding profile of Nrf2-Maf heterodimer from that of Maf homodimer. J Biol Chem. 2007;282:33681–33690. doi: 10.1074/jbc.M706863200. [DOI] [PubMed] [Google Scholar]
- 8.Hur W, Gray NS. Small molecule modulators of antioxidant response pathway. CurrOpinChemBiol. 2011;15:162–173. doi: 10.1016/j.cbpa.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Gilkeson GS, Mashmoushi AK, Ruiz P, Caza TN, Perl A, Oates JC. Endothelial nitric oxide synthase reduces crescentic and necrotic glomerular lesions, reactive oxygen production, and MCP1 production in murine lupus nephritis. PLoS One. 2013;8:e64650. doi: 10.1371/journal.pone.0064650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuen DA, Stead BE, Zhang Y, White KE, Kabir MG, Thai K, Advani SL, Connelly KA, Takano T, Zhu L, Cox AJ, Kelly DJ, Gibson IW, Takahashi T, Harris RC, Advani A. eNOS deficiency predisposes podocytes to injury in diabetes. J Am Soc Nephrol. 2012;23:1810–1823. doi: 10.1681/ASN.2011121170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilcox CS. Asymmetric dimethylarginine and reactive oxygen species: unwelcome twin visitors to the cardiovascular and kidney disease tables. Hypertension. 2012;59:375–381. doi: 10.1161/HYPERTENSIONAHA.111.187310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Gill P, Chabrashvili T, Onozato ML, Raggio J, Mendonca M, Dennehy K, Li M, Modlinger P, Leiper J, P V, Adler O, Leone A, Tojo A, Welch WJ, Wilcox CS. Isoform-specific regulation by N(G),N(G)-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ Res. 2007;101:627–635. doi: 10.1161/CIRCRESAHA.107.158915. [DOI] [PubMed] [Google Scholar]
- 13.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller V, Tain YL, Croker B, Baylis C. Chronic nitric oxide deficiency and progression of kidney disease after renal mass reduction in the C57Bl6 mouse. Am J Nephrol. 2010;32:575–580. doi: 10.1159/000322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khodagholi F, Tusi SK. Stabilization of Nrf2 by tBHQ prevents LPS-induced apoptosis in differentiated PC12 cells. Mol Cell Biochem. 2011;354:97–112. doi: 10.1007/s11010-011-0809-2. [DOI] [PubMed] [Google Scholar]
- 16.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanchi A, Moczulski DK, Hanna LS, Wantman M, Warram JH, Krolewski AS. Risk of advanced diabetic nephropathy in type 1 diabetes is associated with endothelial nitric oxide synthase gene polymorphism. Kidney Int. 2000;57:405–413. doi: 10.1046/j.1523-1755.2000.00860.x. [DOI] [PubMed] [Google Scholar]
- 18.Aucella F, Maas R, Vigilante M, Tripepi G, Schwedhelm E, Margaglione M, Gesualdo L, Boeger R, Zoccali C. Methylarginines and mortality in patients with end stage renal disease: a prospective cohort study. Atherosclerosis. 2009;207:541–545. doi: 10.1016/j.atherosclerosis.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Zoccali C. The endothelium as a target in renal diseases. J Nephrol. 2007;20(Suppl 12):S39–S44. [PubMed] [Google Scholar]
- 20.Drummond GR, Sobey CG. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol Metab. 2014;25:452–463. doi: 10.1016/j.tem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Luo Z, Teerlink T, Griendling K, Aslam S, Welch WJ, Wilcox CS. Angiotensin II and NADPH oxidase increase ADMA in vascular smooth muscle cells. Hypertension. 2010;56:498–504. doi: 10.1161/HYPERTENSIONAHA.110.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau A, Tian W, Whitman SA, Zhang DD. The predicted molecular weight of Nrf2: it is what it is not. Antioxid Redox Signal. 2013;18:91–93. doi: 10.1089/ars.2012.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pendyala S, Moitra J, Kalari S, Kleeberger SR, Zhao Y, Reddy SP, Garcia JG, Natarajan V. Nrf2 regulates hyperoxia-induced Nox4 expression in human lung endothelium: identification of functional antioxidant response elements on the Nox4 promoter. Free RadicBiol Med. 2011;50:1749–1759. doi: 10.1016/j.freeradbiomed.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou Y, Xue P, Bai Y, Liu D, Woods CG, Yarborough K, Fu J, Zhang Q, Sun G, Collins S, Chan JY, Yamamoto M, Andersen ME, Pi J. Nuclear factor erythroid-derived factor 2-related factor 2 regulates transcription of CCAAT/enhancer-binding protein beta during adipogenesis. Free Radic Biol Med. 2012;52:462–472. doi: 10.1016/j.freeradbiomed.2011.10.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho HY, Gladwell W, Wang X, Chorley B, Bell D, Reddy SP, Kleeberger SR. Nrf2-regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice. Am J Respir Crit Care Med. 2010;182:170–182. doi: 10.1164/rccm.200907-1047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balakumar P, Kathuria S. Submaximal PPARgamma activation and endothelial dysfunction: new perspectives for the management of cardiovascular disorders. Br J Pharmacol. 2012;166:1981–1992. doi: 10.1111/j.1476-5381.2012.01938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Gill PS, Chabrashvili T, Onozato ML, Raggio J, Mendonca M, Dennehy K, Li M, Modlinger P, Leiper J, Vallance P, Adler O, Leone A, Tojo A, Welch WJ, Wilcox CS. Isoform-specific regulation by N(G),N(G)-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ Res. 2007;101:627–635. doi: 10.1161/CIRCRESAHA.107.158915. [DOI] [PubMed] [Google Scholar]
- 28.Zoccali C, Maas R, Cutrupi S, Pizzini P, Finocchiaro P, Cambareri F, Panuccio V, Martorano C, Schulze F, Enia G, Tripepi G, Boger R. Asymmetric dimethyl-arginine (ADMA) response to inflammation in acute infections. Nephrol Dial Transplant. 2007;22:801–806. doi: 10.1093/ndt/gfl719. [DOI] [PubMed] [Google Scholar]
- 29.Teerlink T, Luo Z, Palm F, Wilcox CS. Cellular ADMA: regulation and action. Pharmacol Res. 2009;60:448–460. doi: 10.1016/j.phrs.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan L, Zhang H, Zhang Q, Woods CG, Chen Y, Xue P, Dong J, Tokar EJ, Xu Y, Hou Y, Fu J, Yarborough K, Wang A, Qu W, Waalkes MP, Andersen ME, Pi J. Regulatory role of KEAP1 and NRF2 in PPARgamma expression and chemoresistance in human non-small-cell lung carcinoma cells. Free Radic Biol Med. 2012;53:758–768. doi: 10.1016/j.freeradbiomed.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polvani S, Tarocchi M, Galli A. PPARgamma and Oxidative Stress: Con(beta) Catenating NRF2 and FOXO. PPAR Res. 2012;2012:641087. doi: 10.1155/2012/641087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Strandgaard S, Borresen ML, Luo Z, Connors S, Yan Q, Wilcox CS. Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am J Kid Dis. 2008;51:184–191. doi: 10.1053/j.ajkd.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Wang D, Strandgaard S, Iversen J, Wilcox CS. Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am J Physiol Regul Integr Comp Physiol. 2009;296:R195–R200. doi: 10.1152/ajpregu.90506.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. NEnglJMed. 2011;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]