Abstract
In mosquitoes, the maternally inherited bacterial Wolbachia induce a form of embryonic lethality called cytoplasmic incompatibility (CI). This property can be used to reduce the density of mosquito field populations through inundative releases of incompatible males in order to sterilize females (Incompatible Insect Technique, or IIT, strategy). We have previously constructed the LR[wPip(Is)] line representing a good candidate for controlling field populations of the Culex quinquefasciatus mosquito in the islands of the south-western Indian Ocean. The main purpose of the present study was to fill the gap between laboratory experiments and field implementation, i.e. assessing mating competitiveness of these incompatible males under semi-field conditions. In a first set of experiments, we analyzed crossing relationships between LR[wPip(Is)] males and La Réunion field females collected as larvae in 19 distinct localities throughout the island. This investigation revealed total embryonic mortality, confirming the strong sterilizing capacity of LR[wPip(Is)] males. Subsequently, mating competitiveness of LR[wPip(Is)] males was assessed under semi-field conditions in the presence of field males and females from La Réunion. Confrontations were carried out in April and December using different ratios of LR[wPip(Is)] to field males. The results indicated that the LR[wPip(Is)] males successfully compete with field males in mating with field females, displaying even higher competitiveness than field males in April. Our results support the implementation of small-scale field tests in order to assess the feasibility of IIT against Cx. quinquefasciatus in the islands of southwestern Indian Ocean where this mosquito species is a proven competent vector for human pathogens.
Introduction
Mosquito-borne diseases such as malaria, dengue, chikungunya and Rift valley fever are among the leading causes of mortality and morbidity in humans. In the absence of effective vaccines, the control of mosquito natural populations is one of the few available strategies for limiting pathogen transmission to humans. Considerable efforts have been made in order to control mosquito natural populations notably through the use of insecticides providing outstanding results among which the eradication of malaria in several countries after World war II [1]. However, the recurrent selection of resistant individuals in response to frequent and often suboptimal implementation of insecticides together with the potential negative effects of insecticides on non-targeted organisms are a problem of clearly growing concern [2, 3]. More recently, the development of a number of insecticide free strategies has emerged with the aim of providing alternative or at least complementary tools for the control of vector populations. Among these strategies, the use of the endosymbiotic bacteria Wolbachia has been focusing increasing attention and is currently under development in several countries worldwide [4–7].
Wolbachia are maternally inherited bacteria widespread in filarial nematodes and arthropods [8]. These bacteria also infect some mosquito species of medical importance such as the members of the Culex pipiens complex [9, 10] and the Asian tiger mosquito Aedes albopictus [11]. In mosquitoes, Wolbachia induce a form of embryonic mortality called cytoplasmic incompatibility (CI) [12]. This phenomenon results from sperm-egg incompatibility that occurs when Wolbachia-infected males mate with uninfected females or with females infected with an incompatible Wolbachia strain. Cytoplasmic incompatibility provides this endosymbiont with strong invasive properties in field populations [13, 14]. Cytoplasmic incompatibility can be either bidirectional when the death of embryos is observed in both reciprocal crosses, or unidirectional when one cross is incompatible while the reciprocal cross is viable. In addition to the CI phenotype, previous studies have shown that Wolbachia infections may inhibit the development of pathogens in mosquitoes [15–19]. Inhibition of pathogens replication and strong invasive capability make Wolbachia a promising tool for the control of pathogens transmission through mosquito population replacement [20]. Alternatively, Wolbachia-induced CI can be exploited to reduce mosquito population densities via a derivative of the sterile insect technique (SIT) called the incompatible insect technique (IIT). This last strategy relies on the inundative releases of incompatible males, which are able to copulate and thereby sterilize females in the wild [21–23]. Incompatible insect technique was first deployed in 1967 in Burma as a measure against the filariasis vector Cx. quinquefasciatus, demonstrating the ability of Wolbachia-infected insects to eliminate local mosquito populations [24]. More recently, encouraging results have also been obtained in field assays against the Polynesian tiger mosquito Aedes polynesiensis in the South pacific islands [6] as well as in laboratory experiments targeting the medfly Ceratitis capitata [23], the mosquitoes Cx. quinquefasciatus [4] and Ae. albopictus [5].
Culex pipiens complex mosquitoes- whose main subspecies are Culex quinquefasciatus and Culex pipiens, ubiquitous in tropical and temperate regions, respectively [25, 26], are naturally infected by Wolbachia strains (wPip) that belong to a unique clade of the B supergroup [9, 27–29]. However, wPip strains display a high genetic polymorphism at a small evolutionary scale and five wPip groups (referred to as wPip-I to V) are currently recognized [29, 30]. Interestingly, very low Wolbachia diversity was found in natural populations of Cx. quinquefasciatus in the five investigated islands of south-western Indian Ocean (SWIO), namely La Réunion Island, Madagascar, Mayotte, Mauritius and Grande Glorieuse: all identified Wolbachia strains belonged to the wPip-I group [4, 31]; thus suggesting that a single incompatible Wolbachia may be used for the control of all regional populations. Laboratory crossing experiments identified the Wolbachia wPip(Is) strain (from the wPip-IV group) as a good candidate for IIT in the islands of the SWIO. This Wolbachia strain was further introgressed into the nuclear background of Cx. quinquefasciatus mosquitoes from La Réunion island leading to the LR[wPip(Is)] line conferring total embryonic lethality in crosses between LR[wPip(Is)] males and field females sampled at a single site on each of the five investigated SWIO islands [4]. Confrontations carried out in small cages under laboratory conditions also revealed a good mating competitiveness of LR[wPip(Is)] males with La Réunion field males and demonstrated that a population crash could be reached in competition experiments involving 1:5 to 1:10 La Réunion:LR[wPip(Is)] males ratios [4].
In this study, we performed tests under semi-field conditions in order to assess the feasibility of IIT against Cx. quinquefasciatus in the islands of SWIO, where this species is considered as the main vector for lymphatic filarial and Rift Valley Fever virus [32], and exhibits high levels of insecticides resistance [33, 34]. As the efficacy of IIT may be impaired by the co-circulation of several distinct CI phenotypes in natural populations [31, 35], we first examined CI properties of the LR[wPip(Is)] line in reciprocal crosses involving field mosquitoes sampled as larvae in 19 distinct localities on La Réunion Island. We then assessed incompatible males’ competitiveness under semi field conditions, an investigation that is required prior to open field release since these conditions are more closely related to mosquitoes’ natural habitat. Indeed, laboratory conditions are homogeneous in terms of temperature, relative humidity and light intensity; while field conditions are highly dynamic and diverse, which could affect LR[wPip(Is)] males competitiveness. Overall, this study provides data that can be used for operational implementation of IIT against Cx. quinquefasciatus in SWIO islands.
Methods
Mosquito collections
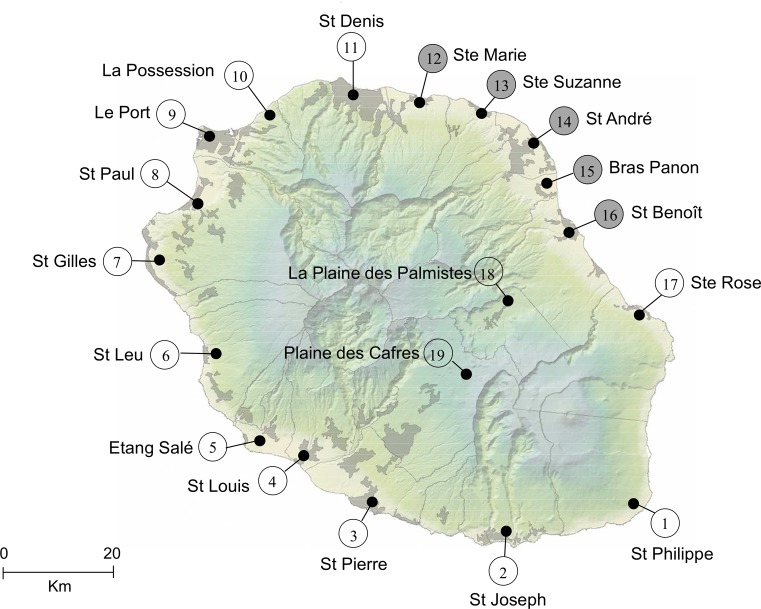
Culex quinquefasciatus larvae and pupae were collected in breeding sites in 19 localities of La Réunion Island in March, April, May and June 2012; and in March, April, November and December 2013 (Fig. 1). Mosquitoes were reared in the laboratory until emergence and adults were used in crossing experiments. Several hundred field-caught four instar larvae (L4) and pupae were sampled from five arbitrarily chosen localities (Ste Marie, #12; Ste Suzanne, #13; St André, #14; Bras Panon, #15 and St Benoît, #16; Fig. 1) and mosquitoes from the five populations were mixed in order to provide the field mosquitoes required for the semi-field tests. The previously described incompatible LR[wPip(Is)] line, harboring the sterilizing wPip(Is) strain together with a nuclear background of La Réunion mosquitoes [4] was used for the production of incompatible males.
Fig 1. Sample sites of Culex quinquefasciatus field populations in La Réunion Island.
Localities in grey correspond to those sites where mosquitoes were sampled for semi-field tests.
Mosquitoes were maintained in the laboratory under conditions at 25 ± 2°C and 75 ± 2% relative humidity and a LD 12:12 h photoperiod. Larvae were reared at a density of approximately 500 larvae per tray (30×40 cm) containing 1 liter of water and were fed ad libitum with a mixture of rabbit and fish-food whilst adults were fed with 10% sucrose solution [w/v].
Ethics statement
None of the samples used in this study were collected in protected areas and the Cx. quinquefasciatus mosquito is not considered as an endangered or protected species. So, no specific permission was required to collect mosquito larvae and pupae in public areas.
Crossing experiments
Reciprocal crosses were performed in the laboratory between the LR[wPip(Is)] line and field mosquitoes from 19 localities of La Réunion island (Fig. 1). Crosses were carried out in cages (30×30×30 cm) with 100–200 virgin females and an equivalent number of virgin males. All individuals were 2–5 days old (age was assessed from the emergence of adults; day 0 = emergence). Females were allowed to blood feed 5 days after caging and egg rafts (with 50–250 eggs per raft) were collected and stored individually at 25°C ± 2°C until hatching. Hatching rates (HR) were scored 72 h after egg rafts’ collection and all unhatched egg rafts were checked for embryonic development following a procedure described by Duron & Weill [36]. All unfertilized (i.e. unembryonated) egg rafts were removed from the analyses.
Semi-field experiments
Field cages design
Semi-field tests were conducted on La Réunion Island within the CIRAD research institute located in La Bretagne, Saint Denis (20°90′55''S 55°49′78''E). Two set of experiments were performed: the first one in April 2013, and the second in December 2013, corresponding to the end and beginning of the warm humid season, respectively. Four field cages (180×150×150 cm) were installed and covered with gardening tents (300×300×245 cm) in order to prevent accidental escapes/invasions of mosquitoes and to protect the cages from rain (S1 Fig.). The site was partly covered with trees belonging to four species: Inga laurina, Pongamia pinnata, Senna siamea and Tabebuia pallida, providing partial protection from direct sunlight and wind. Within each field cage, a wooden table was used as resting area for mosquitoes. The bottom of table legs was placed into cups filled with water in order to prevent ants from reaching sucrose solution contained in plastic cups, which were placed on top of the table. Temperature and relative humidity were monitored using a Hobo data logger (U23–001, Pro v2) placed inside the cages. In the course of the experiments, temperatures were between 23.35°C and 26.85°C in April (mean 24.93 ± 0.16°C) and between 24.13°C and 28.18°C in December (mean 26.06 ± 0.14°C); and the values of relative humidity were between 63.48% and 80.62% in April (mean 73.70 ± 0.59%) and between 64.61% and 89.90% in December (mean 73.73 ± 0.96%) (S2 Fig.).
Mating competitiveness experiments
To be as close as possible to an operational phase of IIT, experiments were performed using field males and females that emerged from field collected larvae and/or pupae from five localities (Ste Marie, #12; Ste Suzanne, #13; St André, #14; Bras Panon, #15 and St Benoît, #16), whilst LR[wPip(Is)] males were obtained from the mosquito line reared in standardized laboratory conditions since 2010 (i.e. during ~60 generations assuming 12 generations per year). Males and females were held in separate laboratory cages (30×30×30 cm) before being transported to the field cages where males were released before females. Only one-day-old virgin adult mosquitoes were used in the experiments. Two hundred field females were mixed with each of the four following ratios of field to LR[wPip(Is)] males: 1:0 (N = 200 males), 1:1 (N = 400 males), 1:5 (N = 1200 males) and 0:1 (N = 200 males). A total of 15 trials were performed (S1 Table). These included: (a) two trials for the 1:0 and 0:1 ratios (in April), (b) six trials for the 1:1 ratio (two in April and four in December), and (c) five trials for the 1:5 ratio (two in April and three in December). For each trial, cages were randomly assigned to different locations to avoid a potential bias due to environmental variations in cage locations.
Mosquitoes released in field cages were recaptured five days following releases. Surviving mosquitoes were collected using a mouth aspirator at the end of each confrontation. Males and females were then placed into separate laboratory cages (30×30×30 cm) and brought back to the laboratory. Males were immediately stored in 70% EtOH and surviving mosquitoes were next counted and genotyped (see below); while females were blood fed in the laboratory and sugar fed until oviposition. Egg rafts were then collected and analysed as described above. Mating competitiveness of LR[wPip(Is)] males was assessed by comparing the observed and expected frequencies of infertile egg rafts assuming random mating.
Measure of males’ survival
Survivorship can be measured by analyzing surviving males from the field cage at the end of the confrontation (e.g. see [37]) or by counting daily survival for a single category of males in individual field cages placed in the semi field setup (e.g. see [38]). Here, we used both strategies to compare the survival of field and LR[wPip(Is)] males. For males recaptured from cage confrontations, we measured the survival rate by counting surviving males when only one type of males was present in cages (i.e. in the 1:0 and 0:1 control ratios) and by genotyping Wolbachia infecting each male’s category to determine the ratio of field vs. LR[wPip(Is)] surviving males at the end of confrontations. Due to the high number of males released in the 1:5 ratio (N = 1200), this analysis was restricted to individuals from trials with the 1:1 ratio. The discrimination of Wolbachia strains infecting field males (carrying wPip-I group strains) and LR[wPip(Is)] males (infected with a wPip-IV group strain) was performed through the genotyping of the Wolbachia ank2 marker, an ankyrin domain encoding gene which allows distinguishing wPip-I and wPip-IV groups on the basis of the size of the PCR amplified fragments (313 bp and 511 bp fragments for groups I and IV, respectively [29, 39]). Estimation of daily survival of males was performed for the experiments conducted in December 2013. Freshly-emerged field (N = 220) and LR[wPip(Is)] (N = 177) virgin males were introduced into separated laboratory cages (45×45×45 cm) and were transported in the semi-field setup. Males were fed with 10% sucrose solution and dead mosquitoes were checked twice a day. Survival data were fitted to the Cox proportional hazards models (coxph, survival package [40]) and a ratio for each type of males was estimated as their instantaneous risk of death relative to each other. These analyses were performed using R software [41].
Data analysis
In semi-field experiments, the deviation between observed proportions of infertile egg rafts and expected proportions was tested against the null hypothesis of no deviation, as expected under random mating and equal males’ mating competitiveness hypotheses, with a Wilcoxon rank sum test. A generalized linear model (GLM) with binomial error was calibrated to test the effect of the male ratio and the period of testing on the proportion of infertile egg rafts. Male survival was compared across types (field vs. LR[wPip(Is)]) with Kruskal-Wallis tests. All statistical analyses were performed using the R software [41].
Results
Stability of CI and sterilizing capacity induced by LR[wPip(Is)] males in La Réunion island
As several studies have shown that CI is a very dynamic process in the Cx. pipiens complex [31, 35, 42], we performed extensive crossing experiments between the LR[wPip(Is)] line and field Cx. quinquefasciatus specimens sampled in 19 localities in La Réunion Island (Fig. 1) in order to confirm CI properties of the LR[wPip(Is)] line over a wide range of natural populations. Full compatibility (HR >90%) was found in control crosses between La Réunion field males and females as well as in the cross between LR[wPip(Is)] males and females (S2 Table).
All crosses between LR[wPip(Is)] males and field females from the 19 localities were incompatible and displayed total embryonic mortality (N = 1452 egg rafts, Table 1). While confirming the strong sterilizing capacity of LR[wPip(Is)] males towards field females sampled throughout La Réunion Island, this result also shows a temporal stability of CI intensity considering that the LR[wPip(Is)] line has been maintained in the laboratory for over four years.
Table 1. Reciprocal crosses between the LR[wPip(Is)] line and field mosquitoes from 19 localities in La Réunion.
| Crosses | Phenotype of egg rafts (n) | Outcomes | ||
|---|---|---|---|---|
| N | Infertile | Fertile | ||
| ♂ LR[wPip(Is)] × ♀ St Philippe (#1) | 35 | 100% (35) | 0% (0) | BCI > UCI |
| ♂ St Philippe (#1) × ♀ LR[wPip(Is)] | 20 | 80% (16) | 20% (4) | |
| ♂ LR[wPip(Is)] × ♀ St Joseph (#2) | 28 | 100% (28) | 0% (0) | BCI > UCI |
| ♂ St Joseph (#2) × ♀ LR[wPip(Is)] | 14 | 73% (11) | 27% (3) | |
| ♂ LR[wPip(Is)] × ♀ St Pierre (#3) | 73 | 100% (73) | 0% (0) | BCI > UCI |
| ♂ St Pierre (#3) × ♀ LR[wPip(Is)] | 92 | 78% (72) | 22% (20) | |
| ♂ LR[wPip(Is)] × ♀ St Louis (#4) | 77 | 100% (77) | 0% (0) | BCI > UCI |
| ♂ St Louis (#4) × ♀ LR[wPip(Is)] | 63 | 59% (37) | 41% (26) | |
| ♂ LR[wPip(Is)] × ♀ Etang Salé (#5) | 106 | 100% (106) | 0% (0) | BCI > UCI |
| ♂ Etang Salé (#5) × ♀ LR[wPip(Is)] | 58 | 79% (46) | 21% (12) | |
| ♂ LR[wPip(Is)] × ♀ St Leu (#6) | 53 | 100% (53) | 0% (0) | BCI > UCI |
| ♂ St Leu (#6) × ♀ LR[wPip(Is)] | 102 | 98% (100) | 2% (2) | |
| ♂ LR[wPip(Is)] × ♀ St Gilles (#7) | 102 | 100% (102) | 0% (0) | BCI |
| ♂ St Gilles (#7) × ♀ LR[wPip(Is)] | 77 | 100% (77) | 0% (0) | |
| ♂ LR[wPip(Is)] × ♀ St Paul (#8) | 84 | 100% (84) | 0% (0) | BCI > UCI |
| ♂ St Paul (#8) × ♀ LR[wPip(Is)] | 100 | 97% (97) | 3% (3) | |
| ♂ LR[wPip(Is)] × ♀ Le Port (#9) | 58 | 100% (58) | 0% (0) | BCI > UCI |
| ♂ Le Port (#9) × ♀ LR[wPip(Is)] | 77 | 68% (52) | 32% (25) | |
| ♂ LR[wPip(Is)]× ♀ La Possession (#10) | 74 | 100% (74) | 0% (0) | BCI |
| ♂ La Possession (#10) × ♀ LR[wPip(Is)] | 109 | 100% (109) | 0% (0) | |
| ♂ LR[wPip(Is)] × ♀ St Denis (#11) | 98 | 100% (98) | 0% (0) | BCI > UCI |
| ♂ St Denis (#11) × ♀ LR[wPip(Is)] | 97 | 87% (84) | 13% (13) | |
| ♂ LR[wPip(Is)] × ♀ Ste Marie (#12) | 78 | 100% (78) | 0% (0) | BCI < UCI* |
| ♂ Ste Marie (#12) × ♀ LR[wPip(Is)] | 95 | 26% (25) | 74% (70) | |
| ♂ LR[wPip(Is)] × ♀ Ste Suzanne (#13) | 82 | 100% (82) | 0% (0) | BCI > UCI |
| ♂ Ste Suzanne (#13) × ♀ LR[wPip(Is)] | 94 | 57% (54) | 43% (40) | |
| ♂ LR[wPip(Is)] × ♀ St André (#14) | 45 | 100% (45) | 0% (0) | BCI > UCI |
| ♂ St André (#14) × ♀ LR[wPip(Is)] | 42 | 93% (39) | 7% (3) | |
| ♂ LR[wPip(Is)] × ♀ Bras Panon (#15) | 113 | 100 (113) | 0% (0) | BCI > UCI |
| ♂ Bras Panon (#15) × ♀ LR[wPip(Is)] | 104 | 94% (98) | 6% (6) | |
| ♂ LR[wPip(Is)] × ♀ St Benoît (#16) | 87 | 100% (87) | 0% (0) | BCI > UCI |
| ♂ St Benoît (#16) × ♀ LR[wPip(Is)] | 134 | 90% (121) | 10% (13) | |
| ♂ LR[wPip(Is)] × ♀ Ste Rose (#17) | 93 | 100% (93) | 0% (0) | BCI < UCI* |
| ♂ Ste Rose (#17) × ♀ LR[wPip(Is)] | 118 | 19% (22) | 81% (96) | |
| ♂ LR[wPip(Is)] × ♀ La Plaine des Palmistes (#18) | 79 | 100% (79) | 0% (0) | BCI > UCI |
| ♂ La Plaine des Palmistes (#18) × ♀ LR[wPip(Is)] | 69 | 72% (50) | 28% (19) | |
| ♂ LR[wPip(Is)] × ♀ La Plaine des Cafres (#19) | 87 | 100% (87) | 0% (0) | BCI < UCI* |
| ♂ La Plaine des Cafres (#19) × ♀ LR[wPip(Is)] | 61 | 49% (30) | 51% (31) | |
| Total ♂ LR[wPip(Is)] × ♀ La Réunion | 1452 | 100% (1452) | 0% (0) | BCI > UCI |
| Total ♂ La Réunion × ♀ LR[wPip(Is)] | 1526 | 75% (1140) | 25% (386) | |
For each cross, the percentage of infertile egg rafts (all hatching rate (HR) = 0%) and fertile egg rafts (HR >90%) are reported. Outcomes correspond to the combination of reciprocal crosses between the LR[wPip(Is)] line and field mosquitoes from each of the 19 localities. N = total number of egg rafts collected for each cross; BCI = bidirectionally incompatible crosses and UCI = unidirectionally incompatible crosses. *, localities where the percentage of UCI was higher than that of BCI. Localities are numbered as in Fig. 1.
In contrast to LR[wPip(Is)] males, crosses between LR[wPip(Is)] females and field males from the 19 localities produced polymorphic phenotypes with both infertile (HR = 0%) and fertile egg rafts (HR >90%) depending on crosses (Table 1). For instance when LR[wPip(Is)] females were crossed with males from La Possession (#10), only infertile egg rafts were observed (N = 74); most egg rafts were infertile with males from St Denis (#11) [87% (N = 84/97)] whilst the frequency of infertile egg rafts was the lowest with males from Ste Rose (#17) [19% (N = 22/118)].
When considering crosses of the LR[wPip(Is)] males and females with La Réunion field mosquitoes, two CI patterns were thus observed: bidirectional CI (or BCI i.e. only infertile egg rafts occurring in both reciprocal crosses between LR[wPip(Is)] and field mosquitoes from a locality) and unidirectional CI (or UCI i.e. infertile egg rafts found in the cross between LR[wPip(Is)] males and field females, and fertile egg rafts in the reciprocal cross). BCI was the most frequent CI pattern, occurring in all examined localities, with a frequency depending on localities (Table 1). Strict BCI was observed in two localities (in St Gilles, #7 and La Possession, #10). The frequency of BCI was higher than that of UCI in 14 localities (for instance in St Philippe, #1 and St Leu, #6) whilst the frequency of BCI was the lowest in three localities (Ste Marie, #12; Ste Rose, #17 and Plaine des Cafres, #19; Table 1).
Performances of LR[wPip(Is)] males in field cages
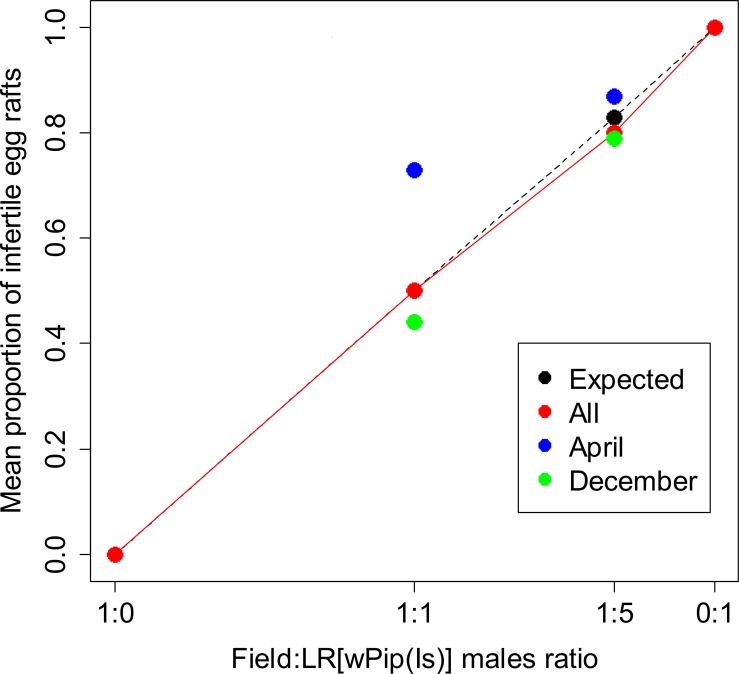
As expected, all egg rafts from cages with only field males and females (1:0 ratio) were fertile (HR >90%) and full sterility (HR = 0%) occurred when only LR[wPip(Is)] males were present with field females (0:1 ratio) (Fig. 2 and S1 Table). Experimental cages with distinct ratios of field to LR[wPip(Is)] males (i.e. in 1:1 and 1:5 ratios) produced both fertile and infertile egg rafts. We never observed egg rafts with intermediate hatching rate, all egg rafts being either fully fertile or infertile.
Fig 2. Assessment of mating competitiveness of LR[wPip(Is)] males under semi-field conditions.
In each confrontation, 200 field females were mixed with each of the four following field to LR[wPip(Is)] males ratios: 1:0 (200 field males), 1:1 (200 field males and 200 LR[wPip(Is)] males), 1:5 (200 field males and 1000 LR[wPip(Is)] males) and 0:1 (200 LR[wPip(Is)] males). The trials of the 1:0 and 0:1 ratios were performed in April; two trials for the 1:1 ratio were performed in April and four in December; whilst for the 1:5 ratio two trials were performed in April and three in December. Expected frequency of infertile egg rafts (in black) was calculated assuming equal competitiveness of LR[wPip(Is)] and field males. Total embryonic mortality (HR = 0%) was noted in all infertile egg rafts.
Under random mating and equal males’ mating competitiveness hypotheses, equilibrated field to LR[wPip(Is)] males ratio (1:1 ratio) should produce 50% infertile egg rafts whilst 83.3% (5/6) of infertile egg rafts are expected in the 1:5 field to LR[wPip(Is)] males ratio. Observed frequencies of infertile egg rafts overall showed no significant deviation from theoretical expectations (Wilcox rank-sum's test: V = 49, P = 0.46); the mean frequency of infertile egg rafts produced by field females were 50% (N = 158 out of 313 egg rafts) and 80% (N = 148 out of 184 egg rafts) for the 1:1 and 1:5 ratios, respectively (Fig. 2 and S1 Table). The analysis of mating competitiveness according to experimental period (i.e. April and December) revealed higher frequencies of infertile egg rafts than expected in April compared to December where less infertile egg rafts were observed for both 1:1 and 1:5 ratios (Fig. 2). The frequencies of infertile egg rafts observed in the 1:1 ratio appeared higher than the expected frequency in all tests performed in April, this difference being significant in one of the two trials (Binomial exact test, P = 0.002 and P = 0.06 for the trials 1 and 2, respectively). In contrast to the results obtained in April, frequencies of infertile egg rafts observed in December were not significantly different from the expected frequency for three out of four trials. When the 1:5 ratio was implemented, no significant difference was noted between observed and expected frequencies of infertile egg rafts in April (Binomial exact test, all P > 0.3). However, in December, the observed frequencies of infertile egg rafts were either significantly higher (replication 3, Binomial exact test, P = 0.01) or significantly lower than expected (Binomial exact test, P = 0.01 and P = 0.03 for replications 4 and 5, respectively); however, significance did not resist the multiple Hommel’s sequential Bonferroni correction. A GLM model was then performed to test the effect of the experimental period (two-levels variable i.e. April and December) and ratio on the proportion of infertile egg rafts. Both variables had a significant effect (P < 0.0001).
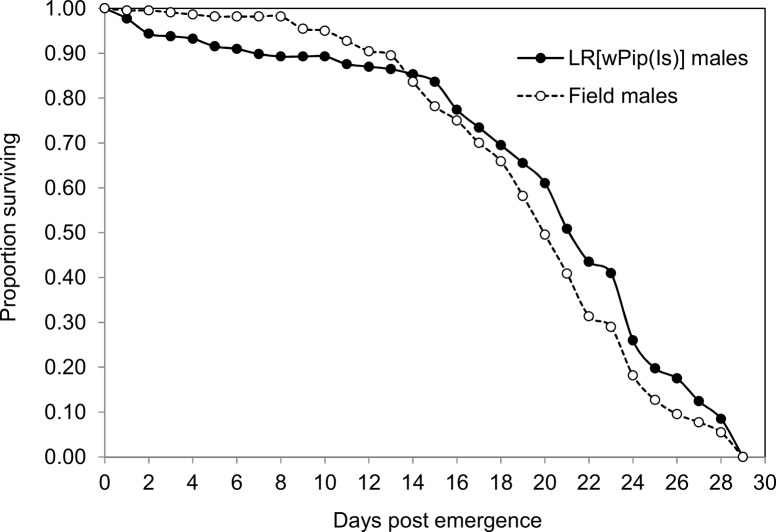
Since males’ survival may affect mating competitiveness and thus sterilizing programs, we compared the survival of LR[wPip(Is)] and field males. The overall survival of LR[wPip(Is)] males recaptured from cages at the end of confrontations was not significantly different from that of field males, when considering all replicates or only the 1:1 ratio replicates (Kruskal-Wallis test, all: chi-squared = 0.176, df = 1, P = 0.67, 1:1 ratio: chi-squared = 0.10, df = 1, P = 0.75; Table 2). Moreover, no significant effect of the experimental period on males survival was noted (Kruskal-Wallis test, chi-squared = 3.57, df = 1, P = 0.06). Daily survival of field males (N = 220) and LR[wPip(Is)] males (N = 177) was also investigated by counting dead males placed in separated cages in the semi-field setup. The survival of field males was higher than that of LR[wPip(Is)] during the first 13 days of monitoring, whilst LR[wPip(Is)] males survival was the highest from day 13 to day 28 (Fig. 3). However, when comparing the overall survival of both male categories, no significant difference was observed (χ2 = 2.78, P = 0.09, Fig. 3).
Table 2. Survival of LR[wPip(Is)] and field males under semi-field conditions.
| field♂:LR[wPip(Is)]♂ ratio | Period | Replicate | field♂ | LR[wPip(Is)]♂ | ||||
|---|---|---|---|---|---|---|---|---|
| Released | Recaptured | Mean recaptured ± SE | Released | Recaptured | Mean recaptured ± SE | |||
| 1:0 | April | 1 | 200 | 26 | 12.5 ± 0.3 | 0 | - | - |
| April | 2 | 200 | 24 | 0 | - | - | ||
| 0:1 | April | 1 | 0 | - | - | 200 | 107 | 57 ± 3.5 |
| April | 2 | 0 | - | - | 200 | 121 | ||
| 1:1 | April | 1 | 200 | 65 | 49.2 ± 10 | 200 | 146 | 46.7 ± 17.3 |
| April | 2 | 200 | 12 | 200 | 4 | |||
| December | 3 | 200 | 70 | 200 | 79 | |||
| December | 4 | 200 | 150 | 200 | 115 | |||
| December | 5 | 200 | 136 | 200 | 100 | |||
| December | 6 | 200 | 158 | 200 | 116 | |||
| Total | 1600 | 641 | 40 ± 8.9 | 1600 | 788 | 49.2 ± 5 | ||
Each cage was set up by mixing 200 field females with different field to LR[wPip(Is)] males ratios. Mosquitoes released in field cages were recaptured five days following releases. The survival was estimated by counting or by genotyping recaptured males after confrontations. All tests were performed in 2013.
Fig 3. Survival curves of field males (N = 220; dotted line) and LR[wPip(Is)] males (N = 177; solid line) in the semi-field setup.
Discussion
The main purpose of this study was the examination of mating competitiveness of LR[wPip(Is)] males under semi-field conditions. However, before performing tests in field cages, we first confirmed the sterilizing capacity of LR[wPip(Is)] males through extensive crossing experiments with field females sampled in 19 localities in La Réunion Island. Indeed, due to the previously reported rapid evolution of CI phenotypes in the Cx. pipiens complex [31, 35, 42] extensive crossing experiments are required in order to: (i) assess sterilizing capacity of incompatible males towards an exhaustive number of field populations and (ii) control the stability of CI intensity over time.
Crosses between LR[wPip(Is)] males and La Réunion field females showed that there was no alteration of sterilizing capacities of incompatible LR[wPip(Is)] males in spite of four years of laboratory maintenance. However, crosses between LR[wPip(Is)] females and field males revealed two phenotypes (compatibility and incompatibility) leading to both uni-and bidirectional CI phenotypes. This result complements previously reported data obtained with La Réunion mosquitoes sampled in a single locality St Denis (#11) in 2010 [4] and confirms that distinct crossing types do coexist within Cx. quinquefasciatus field populations in La Réunion Island [31]. These phenotypes may result from multiple Wolbachia infections, although they have never been evidenced in Cx. pipiens complex mosquitoes [27–29, 31]. In addition, there is no effect of Wolbachia density or nuclear background in the expression of CI in Cx. pipiens [31], in contrast to other host species such as Drosophila [43] or Ae. albopictus [44, 45]. The difference in crossing types of La Réunion mosquitoes have actually been previously proposed to be controlled by different Wolbachia infections, harbouring distinct mod and resc factors although these remain to be characterized [31, 35].
In addition to the sterilizing capacity of LR[wPip(Is)] males, the success of an IIT vector control strategy will also depend on the ability of the released males to compete with indigenous wild males. So, intermediate tests under semi-field conditions are necessary to identify potential problems before proceeding to field implementation [46]. LR[wPip(Is)] males exhibit mating competitiveness that is indistinguishable from field collected Cx. quinquefasciatus males, confirming the results observed under laboratory conditions showing comparable competitiveness of LR[wPip(Is)] and La Réunion males [4]. An unexpected better competitiveness of LR[wPip(Is)] males as compared to field males was observed in April (corresponding to the end of the warm humid season); whilst in December (i.e. at beginning of warm humid season), LR[wPip(Is)] males displayed a mating competitiveness mostly comparable to that of wild males. Several factors including environmental conditions may affect mosquito quality and thus mating competitiveness of wild males in April. Larval rearing conditions such as density and food availability are known to modulate mosquito’s fitness [47–49]. The LR[wPip(Is)] larvae were bred under low-crowding conditions and fed ad libitum, whilst larvae collected in natural breeding sites and used in the confrontations may have faced more challenging conditions. While LR[wPip(Is)] line was reared under controlled conditions of the insectary, crowding and food availability faced by wild mosquitoes surely vary along the year but were not controlled in our experimental setup.
Finally, while CI and mating performances of incompatible males represent key parameters for implementing IIT, factors related to females such as mating choice of wild type females and accidental releases of females from the incompatible line can also affect the success of IIT. In this study, no evidence of mating choice was noted for field females, thus confirming random mating between Cx. pipiens mosquitoes infected by incompatible Wolbachia strains as previously reported [4, 24, 50–52]. For the accidental release of females, bidirectional CI between the incompatible line and specimens in target populations is expected to lower the risk of population replacement since incompatible females will be sterilized by field males. Data reported herein show that bidirectional CI is not the only CI phenotype observed in crosses between the LR[wPip(Is)] line and La Réunion field mosquitoes; unidirectional CI also occurs and must be taken into consideration. Altogether, our data set encourages the development of an effective sexing method strictly producing LR[wPip(Is)] males by any means (biological, genetic or transgenic, see [53–55]) in order to facilitate the operational up scaling of IIT together with minimizing the accidental release of LR[wPip(Is)] females that would impair the success of such an attractive vector control strategy. Moreover, IIT as other sterile-male systems should be accompanied by regular molecular monitoring of field-caught mosquitoes in order to detect any impact of released mosquitoes on the evolution of natural populations. Although several studies have highlighted the potential of Wolbachia to inhibit pathogen replication [15–19]; Wolbachia can also increase pathogen replication as observed with both artificial [56] and natural [57] infections. So, vector competence analyses of LR[wPip(Is)] females for pathogens of medical importance in the region such as lymphatic filarial and Rift Valley Fever virus are needed to fully investigate the impact of any accidental release of LR[wPip(Is)] females in the field.
Conclusion
Tests carried out in field cages in La Réunion Island confirm the stability of sterilizing performances of LR[wPip(Is)] males and evidenced a good competitiveness of LR[wPip(Is)] males compared to wild type males. In addition, crossing experiments confirm the coexistence of distinct CI phenotypes on several natural populations on the island, with limited but still not negligible expression of unidirectional CI. These data should be considered in future inundative releases as accidental releases of LR[wPip(Is)] females without a proper monitoring of Wolbachia dynamics in natural populations might compromise the success of IIT.
Supporting Information
(PDF)
(PDF)
(DOC)
(DOC)
Acknowledgments
We are grateful to Stanislas Zafihita, Frederic Jean and Rudy Mevizou for technical assistance; Marina Beral and Claire Bernard for their help in statistical analyses; Nicole Pasteur, for helpful comments on the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Celestine M. Atyame Post-Doctoral position was funded by the European Union's Seventh Framework Programme ([FP7/2007-2013]) under grant agreement n°263958 (RUN-Emerge project). This investigation had received the joint financial supports from the French Ministry of Health and the European Regional Development Fund (FEDER-Reunion) under the Convergence 2007-2013 Programme. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Enayati A, Hemingway J. Malaria management: past, present, and future. Annu Rev Entomol. 2010; 55: 569–91. 10.1146/annurev-ento-112408-085423 [DOI] [PubMed] [Google Scholar]
- 2. Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000; 45: 371–391. [DOI] [PubMed] [Google Scholar]
- 3. Rivero A, Vézilier J, Weill M, Read AF, Gandon S. Insecticide control of vector-borne diseases: when is insecticide resistance a problem? PLoS Pathog. 2010; 6(8): e1001000 10.1371/journal.ppat.1001000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atyame CM, Pasteur N, Dumas E, Tortosa P, Tantely M, Pocquet N, et al. Cytoplasmic incompatibility as a means to control Culex pipiens quinquefasciatus mosquito in the islands of the southwestern Indian Ocean. PLoS Negl Trop Dis. 2011; 5: e1440 10.1371/journal.pntd.0001440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moretti R, Calvitti M. Male mating performance and cytoplasmic incompatibility in a wPip Wolbachia trans-infected line of Aedes albopictus (Stegomyia albopicta). Med Vet Entomol. 2012; 27(4): 377–386. 10.1111/j.1365-2915.2012.01061.x [DOI] [PubMed] [Google Scholar]
- 6. O'Connor L, Plichart C, Cheong Sang A, Brelsfoard CL, Bossin HC, Dobson SL. Open release of male mosquitoes infected with a Wolbachia biopesticide: field performance and infection containment. PLoS Negl Trop Dis. 2012; 6(11): e1797 10.1371/journal.pntd.0001797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bourtzis K, Dobson SL, Xi Z, Rasgon JL, Calvitti M, Moreira LA, et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014; 132: 150–163 [DOI] [PubMed] [Google Scholar]
- 8. Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nature Rev Microbiol. 2008; 6: 741–51. 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- 9. Rasgon JL, Scott TW. Wolbachia and cytoplasmic incompatibility in the California Culex pipiens mosquito species complex: parameter estimates and infection dynamics in natural populations. Genetics. 2003; 165: 2029–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yen JH, Barr AR. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature. 1971; 232: 657–658. [DOI] [PubMed] [Google Scholar]
- 11. Kittayapong P, Baimai V, O'Neill SL. Field prevalence of Wolbachia in the mosquito vector Aedes albopictus . Am J Trop Med Hyg. 2002; 66(1): 108–11. [DOI] [PubMed] [Google Scholar]
- 12. Hoffmann AA. Entomology: incompatible mosquitoes. Nature. 2005; 436: 189 [DOI] [PubMed] [Google Scholar]
- 13. Kriesner P, Hoffmann AA, Lee SF, Turelli M, Weeks AR. Rapid sequential spread of two Wolbachia variants in Drosophila simulans . PLoS Pathog. 2013; 9(9): e1003607 10.1371/journal.ppat.1003607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turelli M, Hoffmann AA. Rapid spread of an inherited incompatibility factor in California Drosophila . Nature. 1991; 353: 440–442. [DOI] [PubMed] [Google Scholar]
- 15. Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X, et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 2013; 340: 748–751. 10.1126/science.1236192 [DOI] [PubMed] [Google Scholar]
- 16. Blagrove MS, Arias-Goeta C, Failloux AB, Sinkins SP. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus . Proc Natl Acad Sci USA. 2012; 109: 255–60. 10.1073/pnas.1112021108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kambris Z, Blagborough AM, Pinto SB, Blagrove MS, Godfray HC, Sinden RE, et al. Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles gambiae . PLoS Pathog. 2010; 6(10): e1001143 10.1371/journal.ppat.1001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu GJ, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009; 139: 1268–1278. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 19. Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. A non-virulent Wolbachia infection blocks dengue transmission and rapidly invades Aedes aegypti populations. Nature. 2011; 476: 450–455. 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- 20. Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011; 476: 454–457. 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- 21. Apostolaki A, Livadaras I, Saridaki A, Chrysargyris A, Savakis C, Bourtzis K. Transinfection of the olive fruit fly Bactrocera oleae with Wolbachia: towards a symbiont-based population control strategy. J Appl Entomol. 2011; 135: 546–553. [Google Scholar]
- 22. Bourtzis K. Wolbachia-based technologies for insect pest population control. Adv Exp Med Biol. 2008; 627: 104–13. 10.1007/978-0-387-78225-6_9 [DOI] [PubMed] [Google Scholar]
- 23. Zabalou S, Riegler M, Theodorakopoulou M, Stauffer C, Savakis C, Bourtzis K. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc Natl Acad Sci USA. 2004; 101: 15042–15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature. 1967; 216: 383–384. [DOI] [PubMed] [Google Scholar]
- 25. Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, et al. Emerging vectors in the Culex pipiens complex. Science. 2004; 303: 1535–1538. [DOI] [PubMed] [Google Scholar]
- 26. Harbach R. Culex pipiens: species versus species complex—taxonomic history and perspective. J Am Mosq Control Assoc. 2012; 28: 10–23. [DOI] [PubMed] [Google Scholar]
- 27. Duron O, Lagnel J, Raymond M, Bourtzis K, Fort P, Weill M. Transposable element polymorphism of Wolbachia in the mosquito Culex pipiens: evidence of genetic diversity, superinfection and recombination. Mol Ecol. 2005; 14: 1561–1573. [DOI] [PubMed] [Google Scholar]
- 28. Duron O, Fort P, Weill M. Hypervariable prophage WO sequences describe an unexpected high number of Wolbachia variants in the mosquito Culex pipiens . Proc R Soc Lond Ser B. 2006; 273: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Atyame CM, Delsuc F, Pasteur N, Weill M, Duron O. Diversification of Wolbachia endosymbiont in the Culex pipiens mosquito. Mol Biol Evol. 2011; 28(10): 2761–2772. 10.1093/molbev/msr083 [DOI] [PubMed] [Google Scholar]
- 30. Dumas E, Atyame CM, Milesi P, Fonseca DM, Shaikevich EV, Unal S, et al. Population structure of Wolbachia and cytoplasmic introgression in a complex of mosquito species. BMC Evol Biol. 2013; 13(1): 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Atyame CM, Duron O, Tortosa P, Pasteur N, Fort P, Weill M. Multiple Wolbachia determinants control the evolution of cytoplasmic incompatibilities in Culex pipiens mosquito populations. Mol Ecol. 2011; 20: 286–298. 10.1111/j.1365-294X.2010.04937.x [DOI] [PubMed] [Google Scholar]
- 32. Balenghien T, Cardinale E, Chevalier V, Elissa N, Failloux AB, Jean Jose Nipomichene TN, et al. Towards a better understanding of Rift Valley fever epidemiology in the south-west of the Indian Ocean. Vet Res. 2013; 44(78): 2–10. 10.1186/1297-9716-44-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pocquet N, Milesi P, Makoundou P, Unal S, Zumbo B, Atyame C, et al. Multiple insecticide resistances in the disease vector Culex p. quinquefasciatus from Western Indian Ocean. Plos ONE. 2013; 8(10): e77855 10.1371/journal.pone.0077855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tantely ML, Tortosa P, Alout H, Berticat C, Berthomieu A, Rutee A, et al. Insecticide resistance in Culex pipiens quinquefasciatus and Aedes albopictus mosquitoes from La Reunion Island. Insect Biochem Mol Biol. 2010; 40(4): 317–24. 10.1016/j.ibmb.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 35. Atyame CM, Labbé P, Dumas E, Milesi P, Charlat S, Fort P, et al. Wolbachia divergence and the evolution of cytoplasmic incompatibility in Culex pipiens . Plos ONE. 2014; 9(1): e87336 10.1371/journal.pone.0087336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duron O, Weill M. Wolbachia infection influences the development of Culex pipiens embryo in incompatible crosses. Heredity. 2006; 96: 493–500. [DOI] [PubMed] [Google Scholar]
- 37. Chambers EW, Hapairai L, Peel BA, Bossin H, Dobson SL. Male mating competitiveness of a Wolbachia-introgressed Aedes polynesiensis strain under semi-field conditions. PLoS Negl Trop Dis. 2011; 5(8): e1271 10.1371/journal.pntd.0001271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oliva CF, Maier MJ, Gilles J, Jacquet M, Lemperiere G, Quilici S, et al. Effects of irradiation, presence of females, and sugar supply on the longevity of sterile males Aedes albopictus (Skuse) under semi-field conditions on Reunion Island. Acta Trop. 2013; 125(3): 287–93. 10.1016/j.actatropica.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 39. Duron O, Boureux A, Echaubard P, Berthomieu A, Berticat C, Fort P, et al. Variability and expression of ankyrin domain genes in Wolbachia variants infecting the mosquito Culex pipiens . J Bacteriol. 2007; 189: 4442–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crawley M. The R Book. John Wiley & Sons Ltd, Chichester, UK; 2007. [Google Scholar]
- 41. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: 2013; ISBN 3–900051–07–0, URL http://www.R-project.org/. 10.3758/s13428-013-0330-5 [DOI] [Google Scholar]
- 42. Duron O, Bernard J, Atyame CM, Dumas E, Weill M. Rapid evolution of Wolbachia incompatibility types. Proc R Soc Lond Ser B. 2012; 279(1746): 4473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clark ME, Veneti Z, Bourtzis K, Karr TL. Wolbachia distribution and cytoplasmic incompatibility during sperm development: the cyst as the basic cellular unit of CI expression. Mech Dev. 2003; 120: 185–198. [DOI] [PubMed] [Google Scholar]
- 44. Tortosa P, Charlat S, Labbé P, Dehecq J- S, Barré H, Weill M. Wolbachia age-sex-specific density in Aedes albopictus: a host evolutionary response to cytoplasmic incompatibility? PLoS ONE. 2010; 5: e9700 10.1371/journal.pone.0009700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Calvitti M, Moretti R, Skidmore AR, Dobson SL. Wolbachia strain wPip yields a pattern of cytoplasmic incompatibility enhancing a Wolbachia-based suppression strategy against the disease vector Aedes albopictus . Parasit Vectors. 2012; 5: 254 10.1186/1756-3305-5-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Facchinelli L, Valerio L, Ramsey JM, Gould F, Walsh RK, Bond G, et al. Field cage studies and progressive evaluation of genetically-engineered mosquitoes. PLoS Negl Trop Dis. 2013; 7(1): e2001 10.1371/journal.pntd.0002001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alto BW, Muturi EJ, Lampman RL. Effects of nutrition and density in Culex pipiens . Med Vet Entomol. 2012; 26(4): 396–406. 10.1111/j.1365-2915.2012.01010.x [DOI] [PubMed] [Google Scholar]
- 48. Jannat KN, Roitberg BD. Effects of larval density and feeding rates on larval life history traits in Anopheles gambiae s.s. (Diptera: Culicidae). J Vector Ecol. 2013; 38(1): 120–126. 10.1111/j.1948-7134.2013.12017.x [DOI] [PubMed] [Google Scholar]
- 49. Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J Med Entomol. 1998; 35(5): 639–645. [DOI] [PubMed] [Google Scholar]
- 50. Barr AR. Cytoplasmic incompatibility in natural populations of a mosquito, Culex pipiens L. Nature. 1980; 283: 71–2. [DOI] [PubMed] [Google Scholar]
- 51. Curtis CF, Brooks GD, Ansari MA, Grover KK, Krishnamurthy BS, Rajagopalan PK, et al. A field trial on control of Culex quinquefasciatus by release of males of a strain integrating cytoplasmic incompatibility and a translocation. Entomol Exp Appl. 1982; 31: 181–190. [Google Scholar]
- 52. Duron O, Raymond M, Weill M. Many compatible Wolbachia strains coexist within natural populations of Culex pipiens mosquito. Heredity. 2011; 106(6): 986–993. 10.1038/hdy.2010.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Papathanos PA, Bossin H, Benedict MQ, Catteruccia F, Malcolm CA, Alphey L, et al. Sex separation strategies: past experience and new approaches. Malar J. 2009; 8(s2): S5 10.1186/1475-2875-8-S2-S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zabalou S, Apostolaki A, Livadaras I, Franz G, Robinson AS, Savakis C, et al. Incompatible insect technique: incompatible males from a Ceratitis capitata genetic sexing strain. Entomol Exp Appl. 2009; 132: 232–240. [Google Scholar]
- 55. Gilles JR, Schetelig MF, Scolari F, Marec F, Capurro ML, Franz G, et al. Towards mosquito sterile insect technique programmes: exploring genetic, molecular, mechanical and behavioural methods of sex separation in mosquitoes. Acta Trop. 2014; 132:178–187. [DOI] [PubMed] [Google Scholar]
- 56. Dodson BL, Hughes GL, Paul O, Matacchiero AC, Kramer LD, Rasgon JL, et al. Wolbachia Enhances West Nile Virus (WNV) Infection in the Mosquito Culex tarsalis . PLoS Negl Trop Dis. 2014; 8(7): e2965 10.1371/journal.pntd.0002965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zele F, Berthomieu A, Weill M, Duron O, Rivero A. Wolbachia increases susceptibility to Plasmodium infection in a natural system. Proc R Soc Lond Ser B. 2014; 281(1779): 20132837 10.1098/rspb.2013.2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.