Abstract
We investigated the role of urban coyote feeding ecology in the transmission of Echinococcus multilocularis, the causative agent of Alveolar Echinococcosis in humans. As coyotes can play a main role in the maintenance of this zoonotic parasite within North American urban settings, such study can ultimately aid disease risk management. Between June 2012 and June 2013, we collected 251 coyote feces and conducted trapping of small mammals (n = 971) in five parks in the city of Calgary, Alberta, Canada. We investigated E. multilocularis epidemiology by assessing seasonal variations of coyote diet and the selective consumption of different rodent intermediate host species. Furthermore, accounting for small mammal digestibility and coyote defecation rates we estimated the number of small mammal preys ingested by coyote and consequently, coyote encounter rates with the parasite. Dominant food items included small mammals, fruit and vegetation, although hare and deer were seasonally relevant. The lowest frequency of occurrence per scat of small mammals was recorded in winter (39.4 %), when consumption of deer was highest (36.4 %). However, highest encounter rates (number of infected hosts predated/season) with E. multilocularis (95% CI: 1.0 - 22.4), combined with the lack of predation on non-competent small mammal species, suggest that winter is the critical season for transmission and control of this parasite. Within the small mammal assemblage, voles (Microtus pennsylvanicus and Myodes gapperi) were the selected preys of urban coyotes and likely played a key role for the maintenance of the urban sylvatic life-cycle of E. multilocularis in Calgary.
Introduction
The feeding ecology of wild species is traditionally investigated to assess predator-prey relations and dietary selectivity [1–3], habitat requirements [1–3], causes of decline [4] and human-related conflicts [5], overall aiming to identify conservation policies and inform management strategies. Much less frequently, animal feeding ecology is analyzed to shed light on the transmission of those parasites that depend on the predator-prey relationship between definitive and intermediate hosts [6].
Among trophically-transmitted parasites, Echinococcus multilocularis offers an interesting and complex system that may shed light on important ecological and epidemiological processes. This parasitic cestode is widely distributed in the Northern Hemisphere [7] and mainly infects wild canids such as foxes (Vulpes spp.) and coyotes (Canis latrans) as definitive hosts, and more than 40 species of small mammals (mainly Arvicolidae, Cricetidae and Muridae, [8]) as intermediate hosts [9]. Importantly, the parasite is the causative agent of Alveolar Echinococcosis in humans, currently considered among the most serious zoonotic diseases of the Northern hemisphere (case fatality rate >90% if untreated [8]). Typically, infections in humans follow accidental ingestion of parasite eggs through contaminated soil and/or food, or through contact with infected dogs [10]. Despite a primarily sylvatic life-cycle, E. multilocularis can circulate and be maintained within urban habitats [8, 10]: here, given the high risk of zoonotic transmission, understanding parasite ecology becomes crucial for disease prevention and risk management.
In the Northern-central region of North America (13 US states and the four Canadian western provinces [11–13]), meadow voles (Microtus pennsylvanicus) and deer mice (Peromyscus maniculatus) are abundant throughout the parasite range and are traditionally considered the most important intermediate hosts [8, 14]. Furthermore, the competence of southern red-backed vole (Myodes gapperi) for E. multilocularis has been only recently demonstrated [8], and the geographic distribution of the species [15] suggests that this host could be relevant for the transmission of the parasite in Canada. However, local parasite prevalence in small mammals alone cannot illuminate E. multilocularis transmission unless combined with information on prey selection of the definitive host [16].
Several studies have explored coyote feeding ecology in natural [17], suburban [18–20] and urban areas [21]. Although the relevance of small mammals in coyote diet has been widely documented [22, 23], no research has ever specifically explored the feeding ecology of this opportunistic predator in relation to the transmission of E. multilocularis. Such a study is particularly relevant in North American urban habitats, where coyotes can be responsible for the maintenance of the urban sylvatic life-cycle of this parasite [19, 21–24]. In particular, data available for Calgary (AB, Canada) would suggest that coyotes are highly more abundant than red foxes inside the city limits, and can thus can act as the main local wild definitive host [13]. To date, studies of coyote diet in urban habitats mainly aimed to investigate the usage of human-associated food sources and inform management of coyote-human conflict [25], whereas aspects of disease ecology are still unexplored.
Herein, we investigated the role of definitive host feeding ecology on E. multilocularis transmission in urban habitats, using coyotes as the focal species. Specifically, we aimed to i) assess seasonal variations of E. multilocularis infection in coyotes in relation to their diet; ii) determine the impact of coyote selective consumption of small mammal preys on E. multilocularis transmission; and iii) evaluate how these processes affect parasite transmission through changes in encounter rate of coyotes with E. multilocularis.
Material and Methods
Ethic statement
Small mammals were captured in the field with lethal traps (Woodstream Museum Special Traps). If necessary, animals were euthanized through cervical dislocation by trained operators. The animal use protocol was approved by the Animal Care Committee of the Faculty of Veterinary Medicine, University of Calgary (protocol number: AC12–0037). Field permit was granted by the City of Calgary and Alberta Government (Provincial Parks).
Study area and sample collection
The study was conducted in the following five parks and natural areas of the City of Calgary (51°5′N, 114°5′W), Alberta, Canada: Nose Hill Park (NHP), Bowmont (BM), Weaselhead Natural Environment Areas (WSH), Southland Lowlands (SL) and Fish Creek Provincial Park (FCPP) (for details and map, see [23, 26]). Habitat types available in Calgary are primarily represented by grassland, although two major rivers, several creeks and water bodies provide a large amount of riparian habitat, often encompassed in city parks, natural areas and golf courses [13].
Between June 2012 and June 2013, coyote feces were collected on a ten-day sampling schedule following methodologies and protocols previously described [27]. After collection, feces were weighed and stored at -80°C for 72 hrs to inactivate Echinococcus spp. eggs [13, 28] and protect operators from potential exposure. Samples were then stored at -20°C until analysis.
In the same study sites, the relative abundance of small mammals was estimated through lethal trapping sessions conducted within a broader investigation on the prevalence of E. multilocularis in intermediate hosts [29]. Small mammals were captured monthly with Woodstream Museum Special traps deployed along 1–2 ha rectangular grids and with a sampling effort of 200 traps per three nights (for details, see [13]). Relative abundance of the species was assumed to be reflected in their effective capture rate [13], controlling for the number of misfires (number of animal captured divided by the number of active traps, multiplied by 100%), with the exception of the northern pocket gopher (Thomomys talpoides). For this species, capture rate was not considered a reliable indicator of relative availability in the study area, given its fossorial activity [30] and the type of traps used, and was consequently removed from the analysis of small mammal abundance. Small mammal species that were captured only occasionally (i.e., <10 occurrences) and were not encountered in coyote diet, were also removed from the analysis.
Coyote diet analysis
After collection, a subsample (4–6 g) of feces was removed and saved for parasitological and molecular analysis [31]. The remaining portion of the fecal sample was then weighed, hand-washed, and macroscopic fragments isolated using a 500 μm mesh sieve [13] and left to dry at room temperature.
Coyote diet was analyzed following the point-frame method [32]: undigested macro-components were mixed and evenly spread on a glass tray (18x28cm, 15x20cm or 11x16.5cm, depending on the amount of material) with an underlying grid of 50 points equally spaced to allow for systematic sampling [33]. Food items were identified by three trained operators, whose reliability and concordance were previously tested. In particular, operators were evaluated on their ability to correctly identify small mammals through a blind test [34] based on 11 hair samples belonging to the following 8 species: meadow vole, southern red-backed vole (Myodes gapperi), house mouse (Mus musculus), muskrat (Ondatra zibethicus), deer mouse, shrews (Sorex spp.), northern pocket gopher and western jumping mouse (Zapus princeps). Prey remains were microscopically identified using mammalian hair and skull keys [34]. An archive of locally collected mammalian hair, as well as the zoological collection of the Department of Biological Sciences at the University of Calgary, were used as additional supporting reference for the identification of hairs, bones and skulls. Presence of fruit and vegetation was assessed through the recovery of seeds, berries and leaves [35–37], although plants were not identified taxonomically. Similarly, occurrence of reptile, bird and invertebrate remains was recorded on the basis of diagnostic items (i.e., scales, feathers, exoskeleton), but taxa within each category were not classified. Deer (Odocoileus spp.) and hares (Lepus spp.) were identified at the genus level. Ground squirrels (genus Urocitellus and Ictidomys) and tree squirrels (genus Sciurus) were identified at the species level but grouped together as Sciuridae.
Overall coyote diet was quantified using the frequency of occurrence per scat (i.e., percentage of scats containing a given food item) [38], as well as the frequency of occurrence per item (i.e., percentage of the number of occurrences of a given food item of the total number of occurrences of all food items), which estimates the relative importance of each food item in the animal diet [39]. Items accounting for less than 2% of the scat volume, as estimated by the proportion of grid points occupied by each food item [40], were excluded from the analysis [34].
Small mammal consumption and encounter rate with E. multilocularis
Samples containing remains of small mammals were further analyzed to estimate the number of individual preys associated with each coyote scat. First, for each fecal sample, hairs of small mammal species were meticulously separated by hand upon morphological identification, and their total amount weighed at four decimal precision with an Ohaus PA214 scale. Mass of prey hairs in the sample analyzed was extrapolated to the total fecal mass. Undigested prey hair mass was then converted to ingested biomass according to an existing predictive model of prey (house mouse Mus musculus) consumed by coyotes [41]. Specifically, we applied the average digestibility recorded along sets of feeding trials differing in the number of preys fed per meal (i.e., 10–50) and meal composition (i.e., with or without ground meat filler) [42]. For the purpose of this study, the indigestibility coefficient reported for house mouse hair (0.0252) was considered representative of vole and mouse-size preys (i.e., genus Microtus, Myodes, Peromyscus, Zapus, Sorex, and Thomomys talpoides) [42]. Finally, the number of preys per coyote scat was estimated dividing the total biomass ingested by the mean mass recorded for each small mammal species in our study area [43].
For each species of small mammal occurring in the diet of coyote, the total number of preys ingested per coyote per season was then estimated as
where NPrey feces is the mean number of ingested small mammal preys estimated per coyote feces; rdej is the coyote mean daily defecation rate (0.79 scats/day) observed in feeding tests of coyotes, and reported as independent of consumed biomass [13]; d is the number of days in each three-months season (summer: June-August; fall: September-November; winter: December-February; spring: March-May). For each season, we calculated the proportion (0 to 1) of intermediate hosts within the total number of ingested small mammals using the formula
where IHs and NIHs are, respectively, the number of intermediate and non-intermediate hosts ingested per individual coyote [44]. For the purposes of this study, we considered as intermediate hosts all the species of small mammals reported to be competent for E. multilocularis in North America and found to be present in the study area, including deer mouse, meadow vole, sourthern-red backed vole and house mouse [13].
Finally, seasonal encounter rate (mean, 95% CI) of coyotes with E. multilocularis was estimated as
where N IHPrey i is the total number of ingested preys of the i intermediate host species, and p IHi is the parasite prevalence (mean, 95% CI) in the i intermediate host species as observed in the study area during the same time period [15, 45, 46]. In detail, prevalence of E. multilocularis (95% CI) in intermediate hosts was considered as follows. Meadow vole: summer (0–0.007), fall (0.009–0.017), winter (0.034–0.06), spring (0–0.027); deer mouse: summer (0.009–0.017), fall (0–0.005), winter (0.031–0.062), spring (0–0.026); southern red-backed vole: summer (0–0.028), fall (0.021–0.043), winter (0–0.5) [13]. As prevalence of southern red-backed voles was unknown for spring, the species was not considered when estimating the encounter rate with the parasite in that specific season.
Statistical analysis
Differences in frequency of occurrence per scat of food items across seasons were tested by Fisher’s exact Chi-square test; multiple comparisons across seasons were taken into account using Bonferroni correction to the type I error threshold [13]. Variations in the capture rate of different small mammal species were tested by Kruskal-Wallis test for k independent samples. For each species, differences of relative abundance across seasons were tested by Friedman test for paired samples. For pairwise comparisons, we used Mann-Whitney U-tests for independent samples or the Wilcoxon test for paired ones and applied the Bonferroni correction to the type I error threshold [47]. Following ln-transformation and normalization of data, seasonal variations in the relative abundance of small mammals were tested with a one-way ANOVA and Fisher’s least significant difference (LSD) post-hoc test. Seasonal variations in the proportion of intermediate hosts within the total number of ingested small mammals were tested using the Pearson’s Chi-square test. To test for selective consumption of small mammal species by coyotes, we used Fisher's exact Chi-square [48] calculating, for each species, the proportion of individual preys ingested by coyotes (observed) and comparing it to the proportion of animals captured in the field (expected). Ivlev's electivity index [49] was calculated to measure the degree of coyote selection (positive or negative) for small mammal species. Means and Standard Errors (± SEM) are reported throughout the text, unless otherwise specified. All the analyses were run on SPSS version 20.0 (IBM Corporation, USA).
Results
Coyote diet
A total of 251 coyote fecal samples were collected in the five sites (NHP, n = 37; BM, n = 71; WSH, n = 51; SL, n = 38; FCPP, n = 54) and submitted to diet analysis. Among vertebrates, small mammals had the highest frequency of occurrence per scat overall (57.1%) and in each season, followed by hares (20.3%), deer (17.5%), Sciuridae (15.1%) and birds (14.3%). Red fox remains were found in coyote feces in summer and spring, with a total frequency of occurrence of 4.7%. Other species that were detected in less than 5% of the coyote feces included muskrat, porcupine, cat and domestic dog (Table 1). Remains of cattle and skunk were identified in one single occasion (<0.5%; data not shown).
Table 1. Frequency of occurrence per scat (Scat) and frequency of occurrence per item (Occur) of food items encountered in coyote feces collected in urban Calgary, AB, between June 2012 and June 2013.
N indicate sample size (number of feces analyzed).
| Summer (n = 86) | Autumn (n = 69) | Winter (n = 33) | Spring (n = 63) | Overall (n = 251) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scat | Occur | Scat | Occur | Scat | Occur | Scat | Occur | Scat | Occur | |
| Vertebrates | ||||||||||
| Small mammals | 65.1 | 16.3 | 50.7 | 17.2 | 39.4 | 17.1 | 62.5 | 23.0 | 57.1 | 18.0 |
| Hare (Lepus spp.) | 11.6 | 2.9 | 17.4 | 5.9 | 27.3 | 11.8 | 32.8 | 12.1 | 20.6 | 6.5 |
| Deer (Odocoileus spp.) | 17.4 | 4.4 | 20.3 | 6.9 | 36.4 | 15.8 | 4.7 | 1.7 | 17.5 | 5.5 |
| Birds | 27.9 | 7.0 | 11.6 | 3.9 | 9.1 | 3.9 | 1.6 | 0.6 | 14.3 | 4.5 |
| Sciuridae | 30.2 | 7.6 | 8.7 | 2.9 | 6.1 | 2.6 | 6.2 | 2.3 | 15.1 | 4.8 |
| Muskrat | 11.6 | 2.9 | - | - | - | - | 6.2 | 2.3 | 5.6 | 1.8 |
| Porcupine | 4.6 | 1.2 | 4.3 | 1.5 | 12.1 | 5.3 | 1.6 | 2.6 | 4.8 | 1.5 |
| Cat | 3.5 | 0.9 | 2.9 | 1.0 | - | - | - | - | 2.0 | 0.6 |
| Red fox | 4.7 | 1.2 | - | - | - | - | 1.6 | 0.6 | 2.0 | 0.6 |
| Dog | 1.2 | 0.3 | - | - | 3.0 | 1.3 | - | - | 0.8 | 0.3 |
| Invertebrates | 5.8 | 1.5 | 14.5 | 4.7 | 3.0 | 1.3 | 3.1 | 1.1 | 7.1 | 2.3 |
| Vegetation | 97.7 | 24.4 | 82.6 | 27.9 | 27.3 | 11.8 | 84.4 | 31.0 | 81.0 | 25.6 |
| Fruit | 57.0 | 14.2 | 37.7 | 21.1 | 42.4 | 18.4 | 42.2 | 15.5 | 52.8 | 16.7 |
| Anthropogenic | 18.6 | 4.7 | 7.2 | 2.5 | 15.1 | 6.6 | 1.6 | 0.6 | 10.7 | 3.4 |
Small mammals, hares and deer represented the key preys of coyotes, overall accounting for 30% of the total food items (range: 23.6%, summer—44.7%, winter) (Table 1). Frequency of occurrence per scat of small mammals varied across seasons (X 2 = 8.290; df = 3; p = 0.039), with a maximum in summer (65.1%) and a minimum in winter (39.4%), although no differences among seasons were detected once the type I error threshold was corrected to account for multiple comparisons (Bonferroni’s correction, α’(0.05; 6) = 0.0083). An opposite trend was observed for deer, for which the frequency of occurrence per scat also varied across seasons (X 2 Exact = 16.224; df = 3; p = 0.001), reaching its peak in winter (36.4%) and the minimum in spring (4.7%) (winter vs. spring, X 2 Exact = 16.540; df = 1; all the other comparisons not significant). Occurrence of hare showed a similar seasonal trend (X 2 Exact = 11.263; df = 3; p = 0.009), with the lowest frequency observed in summer (11.6%) and the highest in spring (32.8%) (summer vs. spring, X 2 Exact = 10.044; df = 1; p = 0.002; all the other comparisons, p >0.0083).
Vegetation (25.6%) and fruit (16.7%) were relevant components of coyote diet in terms of frequency of occurrence per item (Table 1), although vegetation was often present only in small amounts (46.5% of the cases with ≤10% volume).
Small mammal assemblage and relative abundance
A total of 971 small mammals were captured over 30,200 trap-nights, for an overall capture rate of 5.61% when accounting for trap misfires. Small mammal species included meadow vole (n = 267), southern red-backed vole (n = 71), deer mouse (n = 305), western jumping mouse (n = 32) and shrews (n = 296). Relative abundance (effective capture rate, in %) of small mammals varied across species (Kruskal-Wallis test, X 2 = 70.583; df = 3; p<0.001). Overall, highest relative abundance was recorded for deer mouse (1.53±0.29; median = 1.03; range = 0–10.7), followed by shrews (1.37±0.26; median = 0.72; range = 0–9.7), meadow vole (1.27±0.37; median = 0.28; range = 0–14.4), southern red-backed vole (0.61±0.38; median = 0; range = 0–16.7) and western jumping mouse (0.24±0.12; median = 0; range = 0–5.0). No statistical difference (Mann-Whitney test with Bonferroni corrections for multiple comparisons, p>0.005) was observed between the relative abundance of meadow vole, deer mouse and shrews, as well as between southern red-backed vole and western jumping mouse (all the other comparisons, p<0.001). Overall, small mammal relative abundance varied across seasons (F3, 43 = 5.226; p = 0.004), with a maximum during summer (6.84±1.47) and fall (8.39±3.12) and a minimum during winter (2.13±0.33) and spring (1.49±0.29) (Fisher’s LSD post-hoc: summer vs. winter, p = 0.026; summer vs. spring, p = 0.001; fall vs. spring, p = 0.004; all the other comparisons not significant).
Small mammal consumption and encounter rate with E. multilocularis
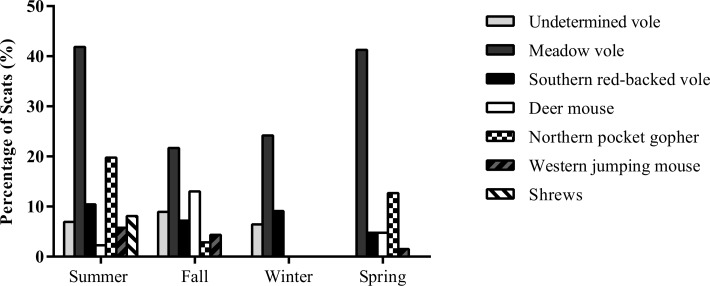
According to the frequency of occurrence per scat, consumption of meadow vole (33.9%) was significantly higher than any other species of small mammals (southern red-backed vole = 8.0%, X 2 Exact = 50.880, df = 1, p<0.001; deer mouse = 5.6%, X 2 Exact = 63.428, df = 1, p<0.001; northern pocket gopher = 10.8%, X 2 Exact = 38.661, df = 1, p<0.001; western jumping mouse = 3.6%, X 2 Exact = 75.604, df = 1, p<0.001; shrews = 2.8%, X 2 Exact = 80.969, df = 1, p< 0.001) (Fig. 1). Meadow vole ranked as the most consumed small mammal also according to the mean number of animals ingested per coyote feces (0.90±0.13; median = 0, range = 0–12.44), which was significantly higher than all the other species (southern red-backed vole = 0.39± 0.14; median = 0, range = 0–25.39; U = 23597.5, df = 1, p<0.001; deer mouse = 0.17 ± 0.07; median = 0, range = 0–16.54; U = 22700.5, df = 1, p<0.001; northern pocket gopher = 0.15 ± 0.04; median = 0, range = 0–3.78; U = 23960.5, df = 1, p<0.001; western jumping mouse = 0.08±0.03; median = 0, range = 0–5.84; U = 22097.0, df = 1, p<0.001; shrews = 0.02±0.02; median = 0, range = 0–4.60; U = 21484.000, df = 1, p<0.001).
Fig 1. Seasonal variations in the consumption of small mammal species by coyotes, expressed as the percentage of scats containing a given food items (i.e., frequency of occurrence per scat), in five sites in urban Calgary, AB, Canada, between June 2012 and June 2013.
Compared to their relative availability, vole species were overall consumed significantly more than expected (meadow vole, X 2 Exact = 65.288, df = 1, p<0.001; southern red-backed vole, X 2 Exact = 71.301, df = 1, p<0.001), whereas deer mouse (X 2 Exact = 82.390, df = 1, p<0.001) and shrews (X 2 Exact = 161.512, df = 1, p<0.001) were consumed significantly less than expected (Table 2). Positive selection of voles (M. pennsylvanicus and M. gapperi) was exhibited in every season with the exception of the fall, during which both species were consumed in proportion of their availability. Deer mouse was preyed less than expected in summer (X 2 Exact = 64.099, df = 1, p<0.001), winter (X 2 Exact = 11.745, df = 1, p<0.001) and spring (X 2 Exact = 47.685, df = 1, p<0.001), but consumed significantly more than expected during fall (X 2 Exact = 5.527, df = 1, p = 0.023). Shrews were negatively selected during every season (summer, X 2 Exact = 27.748, df = 1, p<0.001; fall, X 2 Exact = 36.515, df = 1, p<0.001; winter, X 2 Exact = 25.686, df = 1, p<0.001; spring, X 2 Exact = 75.892, df = 1, p<0.001), whereas western jumping mouse was consumed according to its availability with the exception of a significantly higher consumption during fall (X 2 Exact = 32.897, df = 1, p<0.001) (Table 2).
Table 2. Selective foraging of coyotes on small mammal species in urban Calgary, AB, Canada, between June 2012 and June 2013.
| Summer | Fall | Winter | Spring | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Ivlev | p | Ivlev | p | Ivlev | p | Ivlev | p | Ivlev | p |
| Microtus pennsylvanicus | 0.31 | < 0.001 | 0.07 | 0.686 | 0.30 | 0.043 | 0.27 | 0.001 | 0.28 | < 0.001 |
| Myodes gapperi | 0.59 | < 0.001 | 0.18 | 0.258 | 0.97 | < 0.001 | 1.00 b | < 0.001 | 0.53 | < 0.001 |
| Peromyscus maniculatus | -0.99 | < 0.001 | 0.42 | 0.023 | -1.00 | < 0.001 | -0.82 | < 0.001 | -0.54 | < 0.001 |
| Zapus princeps | -0.19 | 0.298 | 0.95 | < 0.001 | - a | - | 1.00 b | 0.559 | 0.14 | 0.296 |
| Sorex sp. | -0.73 | < 0.001 | -1.00 | < 0.001 | -1.00 | < 0.001 | -1.00 | < 0.001 | -0.93 | < 0.001 |
Ivlev's electivity index is calculated for each species using the proportion of individuals ingested (used) and the proportion of animals captured in the field (available); p-values indicate the statistical significance of differences between observed and expected small mammal consumption (Fisher's exact Chi-square test).
a species not captured in the field and not found in feces
b species not captured in the field, but found in feces
Based on the defecation rate applied in this study, we estimated a mean of 116.7±24.2 small mammals consumed per individual coyote per 3-month season, ranging from a maximum of 178.7 during spring to a minimum of 70.6 in winter. Most of the predated small mammals were competent species for E. multilocularis, resulting in a mean of 101.7±19.8 intermediate hosts ingested per season, and ranging from a maximum of 157.3 in spring to a minimum of 70.6 in winter (Table 3).
Table 3. Seasonal and annual estimates of small mammals, overall and infected intermediate hosts (IHs) of Echinococcus multilocularis ingested by individual coyotes in Calgary, AB, Canada, between June 2012 and June 2013.
| Season | Summer | Fall | Winter | Spring | Total |
|---|---|---|---|---|---|
| Small mammals ingested per feces | 1.8 | 1.2 | 1.0 | 2.5 | 1.6 |
| IHs ingested per feces a | 1.4 | 1.1 | 1.0 | 2.2 | 1.4 |
| N small mammals ingested b | 130.0 | 87.4 | 70.6 | 178.7 | 466.7 |
| N IHs ingested b | 102.5 | 76.4 | 70.6 | 157.3 | 406.8 |
| N infected IHs ingested (95% CI) c | 0.002 (0.002–1.397) | 0.509 (0.496–1.175) | 1.052 (0.994–22.443) | 0 (0–3.383) | 1.563 (1.492–28.398) |
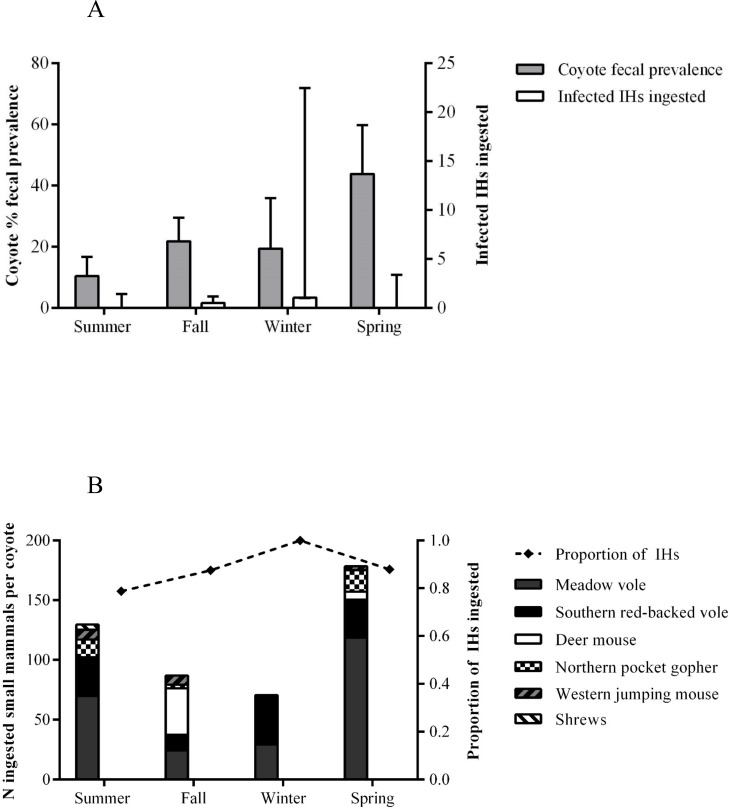
The proportion of intermediate hosts predated varied across seasons (X 2 Exact = 23.646, df = 3, p<0.001), with that in winter (1.0) being significantly higher than values observed in summer (0.79; X 2 = 17.767, df = 1, p<0.001), fall (0.87; X 2 = 9.649, df = 1, p = 0.003) and spring (0.88; X 2 = 9.148, df = 1, p = 0.001). Meadow voles accounted for the majority of ingested intermediate hosts, with a mean of 60.6±21.9 individuals preyed by coyote per season, and ranging from a minimum of 24.4 in fall to a maximum of 118.8 in spring. Southern red-backed vole was the second most recurrent prey species (mean = 29.6±5.9), followed by deer mouse (11.5±9.3), northern pocket gopher (9.1±4.4), western jumping mouse (4.8±2.0) and shrew (1.1±2.2) (Fig. 2B).
Fig 2. Seasonal variations in coyote fecal prevalence (mean, 95% CI) [13] and the number of infected intermediate hosts (IHs; mean, 95% CI) estimated to be ingested by individual coyote in five sites in urban Calgary, AB, Canada, between June 2012 and June 2013 (A); number of small mammals and proportion of intermediate hosts (IHs/total number of small mammals) estimated to be ingested by individual coyote in five sites in urban Calgary, AB, Canada, between June 2012 and June 2013 (B).
Given the prevalence of E. multilocularis in intermediate hosts in the study area, the encounter rate with the parasite was highest in winter, with an estimate mean of 1.05 (95% CI: 0.99–22.44) infected intermediate hosts ingested per coyote, and lowest in spring (mean = 0; 95% CI: 0–3.38; Table 3).
Discussion
Our results suggest that small mammals, fruit and vegetation constituted the bulk of urban coyote diet in the study area, although hare and deer were seasonally very relevant. Within the small mammal assemblage, voles were the selected preys of coyotes and likely played an important role in the transmission of E. multilocularis in urban Calgary during our study. Despite a relatively lower consumption of small mammals in winter, the encounter rate of coyote with the parasite was at a maximum during this season, when the number of infected intermediate hosts ingested was estimated to be highest.
Seasonal variations in coyote diet and encounter with E. multilocularis
In urban Calgary, small mammals were the most frequent prey found in coyote feces in all seasons. For most of the year (summer-winter), their frequency of occurrence in diet reflected their relative availability, as previously documented in prairies [52] and boreal forests [24]. However, this trend was not confirmed for spring, during which we observed a high occurrence of small mammals in coyote diet despite a low capture rate in the field. Due to abundant snow cover, trapping success of small mammals in winter and spring is likely influenced not only by their relative abundance, but also by their activity in the subnivean space [53]. A reduction of the snow cover depth and hardness during spring could expose small mammals to higher risk of predation by coyotes [54, 55] without necessarily increasing their activity above the snowpack and the success of snap-trapping sessions, thus explaining the discrepancy observed.
Interestingly, lower occurrence of small mammals during winter was concurrent with higher consumption of deer. In this season, deer (juveniles in particular) are more exposed to the risk of coyote predation [56], as snow cover and low forage abundance/quality can hinder animal movements and negatively affect their body condition [57]. An increased access of coyotes to alternative (and abundant) food sources could thus concur to explain the decrement in the consumption of small mammals observed in winter. The interdependence between the consumption of small mammals and the availability of deer (i.e., prey switch) has been previously suggested for coyotes [58–60], and could affect the transmission ecology of E. multilocularis in areas where this canid is the parasite’s main definitive host. Nonetheless, despite a relatively lower number of small mammals predated, encounter rate of coyotes with E. multilocularis was highest in winter, given the peak in parasite prevalence reported for intermediate hosts in this season [57, 61, 62]. Importantly, composition of local small mammal assemblages can further shape spatial patterns of infection with E. multilocularis in definitive hosts [13], and our data suggest that such a dilution effect (sensu [13]) may be significant in summer but absent in winter, when only competent intermediate host species are consumed (Fig. 2B). These observations are consistent with the higher parasite prevalence documented in coyote feces during spring [63] (Fig. 2A), once the patency period in the canid host (30–90 days post-infection, [13]) is taken into account, further emphasizing the relevance of winter for E. multilocularis transmission [64].
Our estimates of parasite encounter rate, however, are conservative and need to be interpreted as the minimum number of intermediate hosts ingested per season. Although likely not applicable in urban habitats, higher predation rates on small mammals (and higher defecation rates) were reported for coyotes in Yellowstone National Park [13, 65]. Further research, possibly combining field observation with feeding trials, would allow refining correcting factors for small mammal digestibility and coyote defecation rates. Similarly, larger sample size, multiannual data series and indices of availability for other prey species (i.e., deer, hare) would aid in a better understanding of seasonal variations in coyote diet and parasite encounter rates.
Coyote diet and E. multilocularis transmission ecology
Among small mammals, meadow vole and southern red-backed vole were the selected preys of coyotes in the study area. Considering their competence for the parasite [56], they are likely playing a key role in the transmission of E. multilocularis in Calgary. In particular the meadow vole, given its high abundance, is likely very important for the maintenance of the sylvatic life-cycle of E. multilocularis in this urban landscape. On the other hand, the southern red-backed vole could be locally highly relevant in relation to suitable wood patches [8, 15], that are in Calgary less abundant and more heterogeneously distributed than grasslands. This species is likely very important in winter, when its contribution to coyote diet and parasite transmission are higher. Although the deer mouse is a locally abundant competent host [66], its under-representation in coyote diet would suggest that it is unlikely to be a key species for the infection of coyotes in urban Calgary.
Microtine multi-annual population fluctuations are considered major drivers of E. multilocularis transmission intensity [8]. In particular, the relevance of Microtus species (i.e. M. arvalis) for the maintenance of the parasite life-cycle has been recently demonstrated also at large scales [17] where red foxes are the main (and often only) wild definitive host [67], although several other intermediate host species (i.e., Myodes sp., Ellobius sp., Ochotona sp., Eospalax sp., Cricetulus sp.) can be locally important, depending on ecosystem characteristics [11, 68]. While our data emphasize the importance of considering the whole small mammal assemblage [69, 70], as the relevance of intermediate hosts can vary depending on asynchronous population fluctuations of different species [13], it is necessary to further investigate the interaction between availability of suitable habitats (i.e., ROMPA [17, 69]) and population dynamics of meadow voles in urban habitats, as well as their effect on E. multilocularis transmission. Integrated with existing knowledge on parasite ecology and epidemiology, such information could help prevent and manage potential disease outbreaks [71].
Finally, our study suggests that coyotes may predate upon red foxes, although occasionally. Such information was not reported in earlier investigations on coyote diet in our study area [72], possibly also due to its rare occurrence. The suppression of red fox populations by coyotes has been documented in North America [38], and it has been hypothesized as the mechanism explaining the low abundance of foxes in urban Calgary [73, 74]. By shaping the composition of the definitive host community and the density of highly susceptible host species such as foxes, the existence of such a direct competition might have consequences on the transmission dynamics of E. multilocularis. However, our data are not able to tell whether fox is consumed by coyotes as a consequence of predation, direct (interference) competition, or scavenging. Further research is needed to quantify interference competition between coyote and red fox, and assess the relative role of these species (as well as of domestic dogs) in the circulation of E. multilocularis in Calgary urban and rural habitats.
Conclusions
In this study we estimated, for the first time, the seasonal encounter rate of a canid host with E. multilocularis infected intermediate hosts, thus offering a quantitative framework for further epidemiological studies. Our data shows that winter is the most important season for parasite transmission, due to higher encounter rates when coyotes predate upon susceptible intermediate hosts, and the lack of dilution offered by non-competent small mammal species. Furthermore, we provided evidence that within the small mammal assemblage, the meadow vole Microtus pennsylvanicus is likely playing a key role in the maintenance of the urban sylvatic life-cycle of E. multilocularis in Calgary, along with the southern red-backed vole (Myodes gapperi) which may be locally very important. Long-term studies of population dynamics of these species, in response to changes in habitat availability and land use, should be integrated in future research on E. multilocularis transmission in urban habitats to inform disease risk management.
Acknowledgments
We thank Sultana Majid, Holly Shaw, Heather Gordon, Megan Hart and Abraham Munene for contributing to coyote diet analysis and field trapping of small mammals. We are grateful to Jack Millar, for precious advice and for providing small mammal traps. We thank Jeroen De Buck for laboratory support.
Data Availability
The following data are available from Figshare: Overall coyote diet (http://dx.doi.org/10.6084/m9.figshare.1309944); and small mammal undigested items (http://dx.doi.org/10.6084/m9.figshare.1309943).
Funding Statement
This work has been supported by the following funders: Canadian Institutes of Health Research (RT#: 736488; http://www.cihr-irsc.gc.ca/e/193.html), Institute for Public Health (RT#: 10002391; http://obrieniph.ucalgary.ca/), and Calgary Parks (RT: 10001048; http://www.calgary.ca/CSPS/Parks/Pages/home.aspx). SL was supported by studentships from the University of Calgary, Department of Ecosystem & Public Health (University of Calgary) and Alberta Innovates Health Solutions (RT#: 10006087; http://www.aihealthsolutions.ca/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Farrell LE, Roman J, Sunquist ME. Dietary separation of sympatric carnivores identified by molecular analysis of scats. Mol Ecol. 2000;9(10):1583–1590. [DOI] [PubMed] [Google Scholar]
- 2. Hayward MW, O'Brien J, Hofmeyr M, Kerley GI. Prey preferences of the African wild dog Lycaon pictus (Canidae: Carnivora): ecological requirements for conservation. J Mammal. 2006;87(6):1122–1131. [Google Scholar]
- 3. Bagchi S, Mishra C, Bhatnagar YV. Conflicts between traditional pastoralism and conservation of Himalayan ibex (Capra sibirica) in the Trans-Himalayan mountains. Anim Conserv. 2004;7(2):121–128. [Google Scholar]
- 4. Loucks CJ, Zhi L, Dinerstein E, Dajun W, Dali F, Hao W. The giant pandas of the Qinling Mountains, China: a case study in designing conservation landscapes for elevational migrants. Conserv Biol. 2003;17(2):558–565. [Google Scholar]
- 5. Ramakrishnan U, Coss RG, Pelkey NW. Tiger decline caused by the reduction of large ungulate prey: evidence from a study of leopard diets in southern India. Biol Cons. 1999;89(2):113–120. [Google Scholar]
- 6. Bagchi S, Mishra C. Living with large carnivores: predation on livestock by the snow leopard (Uncia uncia). J Zool. 2006;268(3):217–224. [Google Scholar]
- 7. Lafferty KD. The evolution of trophic transmission. Parasitol Today. 1999;15(3):111–115. [DOI] [PubMed] [Google Scholar]
- 8. Eckert J, Gemmell MA, Meslin F- X, Pawłowski ZS. WHO/OIE manual on Echinococcosis in humans and animals: a public health problem of global concern. Paris, France: World Organisation for Animal Health; 2001. [Google Scholar]
- 9. Vuitton DA, Zhou H, Bresson-Hadni S, Wang Q, Piarroux M, Raoul F, et al. Epidemiology of alveolar echinococcosis with particular reference to China and Europe. Parasitology. 2003;127 Suppl:S87–107. [PubMed] [Google Scholar]
- 10. Craig PS, Rogan MT, Allan JC. Detection, screening and community epidemiology of taeniid cestode zoonoses: cystic echinococcosis, alveolar echinococcosis and neurocysticercosis. Adv Parasitol. 1996;38:169–250. [DOI] [PubMed] [Google Scholar]
- 11. Deplazes P, Hagglin D, Gloor S, Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis . Trends Parasitol. 2004;20(2):77–84. [DOI] [PubMed] [Google Scholar]
- 12. Tsukada H, Morishima Y, Nonaka N, Oku Y, Kamiya M. Preliminary study of the role of red foxes in Echinococcus multilocularis transmission in the urban area of Sapporo, Japan. Parasitology. 2000;120(4):423–428. [DOI] [PubMed] [Google Scholar]
- 13. Liccioli S, Kutz SJ, Ruckstuhl KE, Massolo A. Spatial heterogeneity and temporal variations in Echinococcus multilocularis infections in wild hosts in a North American urban setting. Int J Parasitol. 2014;44(7):457–465. 10.1016/j.ijpara.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 14. Gesy K, Hill JE, Schwantje H, Liccioli S, Jenkins EJ. Establishment of a European-type strain of Echinococcus multilocularis in Canadian wildlife. Parasitology. 2013;140(9):1133–7. 10.1017/S0031182013000607 [DOI] [PubMed] [Google Scholar]
- 15. Liccioli S, Duignan PJ, Lejeune M, Deunk J, Majid S, Massolo A. A new intermediate host for Echinococcus multilocularis: the southern red-backed vole (Myodes gapperi) in urban landscape in Calgary, Canada. Parasitol Int. 2013;62(4):355–357. 10.1016/j.parint.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 16. Jung TS, Runck AM, Nagorsen DW, Slough BG, Powell T. First records of the Southern red-backed vole, Myodes gapperi, in the Yukon. Can Field Nat. 2006;120(3):331–334. [Google Scholar]
- 17. Giraudoux P, Craig PS, Delattre P, Bao G, Bartholomot B, Harraga S, et al. Interactions between landscape changes and host communities can regulate Echinococcus multilocularis transmission. Parasitology. 2003;127:S121–S31. [PubMed] [Google Scholar]
- 18. Gese EM, Grothe S. Analysis of coyote predation on deer and elk during winter in Yellowstone National Park, Wyoming. Am Midl Nat. 1995;133(1):36–43. [Google Scholar]
- 19. Gese EM, Ruff RL, Crabtree RL. Intrinsic and extrinsic factors influencing coyote predation of small mammals in Yellowstone National Park. Can J Zool. 1996;74(5):784–97. [Google Scholar]
- 20. Thurber JM, Peterson RO, Woolington JD, Vucetich JA. Coyote coexistence with wolves on the Kenai Peninsula, Alaska. Can J Zool. 1992;70(12):2494–2498. [Google Scholar]
- 21. Grigione MM, Burman P, Clavio S, Harper SJ, Manning D, Sarno RJ. Diet of Florida coyotes in a protected wildland and suburban habitat. Urban Ecos. 2011;14(4):655–663. [Google Scholar]
- 22. Quinn T. Coyote (Canis latrans) food habits in three urban habitat types of western Washington. Northwest Sci. 1997;71(1):1–5. [Google Scholar]
- 23. Morey PS, Gese EM, Gehrt S. Spatial and temporal variation in the diet of coyotes in the Chicago metropolitan area. Am Midl Nat. 2007;158(1):147–161. [Google Scholar]
- 24. Litvaitis JA, Shaw JH. Coyote movements, habitat use, and food habits in southwestern Oklahoma. J Wildl Manage. 1980:62–68. [Google Scholar]
- 25. Catalano S, Lejeune M, Liccioli S, Verocai GG, Gesy KM, Jenkins EJ, et al. Echinococcus multilocularis in urban coyotes, Alberta, Canada. Emerg Infect Dis. 2012;18(10):1625–1628. 10.3201/eid.1810.120119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lukasik VM, Alexander SM. Spatial and temporal variation of coyote (Canis latrans) diet in Calgary, Alberta. Cities Env 2012;4(1):8. [Google Scholar]
- 27. Foley J. Calgary's natural parks: yours to explore. 1st ed Calgary, Alta.: Calgary Field Naturalists' Society; 2006. [Google Scholar]
- 28. Liccioli S, Catalano S, Kutz SJ, Lejeune M, Verocai GG, Duignan PJ, et al. Gastrointestinal parasites of coyotes (Canis latrans) in the metropolitan area of Calgary, Alberta, Canada. Can J Zool. 2012;90(8):1023–1030. [Google Scholar]
- 29. Veit P, Bilger B, Schad V, Schaefer J, Frank W, Lucius R. Influence of environmental factors on the infectivity of Echinococcus multilocularis eggs. Parasitology. 1995;110(1):79–86. [DOI] [PubMed] [Google Scholar]
- 30. Village A, Myhill D. Estimating small mammal abundance for predator studies: snap-trapping versus sign indices. J Zool. 1990;222(4):681–689. [Google Scholar]
- 31. Engeman RM, Campbell DL, Evans J. A comparison of 2 activity measures for Northern pocket gophers. Wildlife Soc B. 1993;21(1):70–73. [Google Scholar]
- 32. Reynolds JC, Aebischer NJ. Comparison and quantification of carnivore diet by fecal analysis—a critique, with recommendations, based on a study of the fox Vulpes vulpes . Mammal Rev. 1991;21(3):97–122. [Google Scholar]
- 33. Chamrad AD, Box TW. A point frame for sampling rumen contents. J Wildl Manage. 1964;28(3):473–477. [Google Scholar]
- 34. Ciucci P, Boitanti L, Pelliccioni ER, Rocco M, Guy I. A comparison of scat-analysis methods to assess the diet of the wolf Canis lupus . Wildlife Biol. 1996;2(1):37–48. [Google Scholar]
- 35. Adorjan A, Kolenosky G. A manual for the identification of hairs of selected Ontario mammals. Research Branch, Ontario Dept. of Lands and Forests; (Toronto: ); 1969. [Google Scholar]
- 36. Moore TD, Spence LE, Dugnolle CE, Hepworth WG. Identification of the dorsal guard hairs of some mammals of Wyoming. Cheyenne: Wyoming Game and Fish Dept.; 1997. [Google Scholar]
- 37. Jones JK, Manning RW. Illustrated key to skulls of genera of North American land mammals. Lubbock, Tex.: Texas Tech University Press; 1992. [Google Scholar]
- 38. Lukasik VM, Alexander SM. Spatial and temporal variation of coyote (Canis latrans) diet in Calgary, Alberta. Cities Env. 2012;4(1):8. [Google Scholar]
- 39. Klare U, Kamler JF, Macdonald DW. A comparison and critique of different scat-analysis methods for determining carnivore diet. Mammal Rev. 2011;41(4):294–312. [Google Scholar]
- 40. Loveridge AJ, Macdonald DW. Niche separation in sympatric jackals (Canis mesomelas and Canis adustus). J Zool. 2003;259:143–53. [Google Scholar]
- 41. Prugh LR. Coyote prey selection and community stability during a decline in food supply. Oikos. 2005;110(2):253–264. [Google Scholar]
- 42. Kelly BT, Garton EO. Effects of prey size, meal size, meal composition, and daily frequency of feeding on the recovery of rodent remains from carnivore seats. Can J Zool. 1997;75(11):1811–1817. [Google Scholar]
- 43. Kelly BT. Carnivore scat analysis: an evaluation of existing techniques and the development of predictive models of prey consumed: College of Graduate Studies, University of Idaho; 1991. [Google Scholar]
- 44. Monroy-Vilchis O, Frieven C. Dejection and expulsion rates of coyotes (Canis latrans) in captivity. Southwest Nat. 2006;51(2):272–276. [Google Scholar]
- 45. Holmes JC, Mahrt JL, Samuel WM. The occurrence of Echinococcus multilocularis Leuckart, 1863 in Alberta. Can J Zool. 1971;49(4):575–576. [DOI] [PubMed] [Google Scholar]
- 46. Leiby PD, Carney WP, Woods CE. Studies on sylvatic echinococcosis. 3. Host occurrence and geographic distribution of Echinococcus multilocularis in the north central United States. J Parasitol. 1970;56(6):1141–1150. [PubMed] [Google Scholar]
- 47. Sokal RR, Rohlf FJ. Biometry The principles and practice of statistics in biological research. 3 ed New York, USA: W.H. Freeman & C.; 1995. [Google Scholar]
- 48. Curtin F, Schulz P. Multiple correlations and Bonferroni's correction. Biol Psychiatry. 1998;44(8):775–777. [DOI] [PubMed] [Google Scholar]
- 49. Byers CR, Steinhorst RK, Krausman PR. Clarification of a technique for analysis of utilization-availability data. J Wildl Manage. 1984;48(3):1050–1053. [Google Scholar]
- 50. Ivlev VS. Experimental ecology of the feeding of fishes. New Haven,: Yale University Press; 1961. [Google Scholar]
- 51. Nellis CH, Keith LB. Population dynamics of coyotes in central Alberta, 1964–68. J Wildl Manage. 1976:389–399. [Google Scholar]
- 52. Pruitt WJ. Snow and small animals In: Merritt J, editor. Winter ecology of small mammals. Pittsburgh: Special Publication of Carnegie Museum of Natural History; 1984. p. 1–8. [Google Scholar]
- 53. Schwingel H, Norment C. Use of hair tubes to detect small-mammal winter activity in a northern forest habitat. Northeast Nat. 2010;17(4):531–540. [Google Scholar]
- 54. Gese EM, Ruff RL, Crabtree RL. Intrinsic and extrinsic factors influencing coyote predation of small mammals in Yellowstone National Park. Can J Zool. 1996;74(5):784–797. [Google Scholar]
- 55. Lingle S. Coyote predation and habitat segregation of white-tailed deer and mule deer. Ecology (Washington D C). 2002;83(7):2037–2048. [Google Scholar]
- 56. DelGiudice GD, Riggs MR, Joly P, Pan W. Winter severity, survival, and cause-specific mortality of female white-tailed deer in north-central Minnesota. J Wildl Manage. 2002:698–717. [Google Scholar]
- 57. Nelson ME, Mech LD. Relationship between snow depth and gray wolf predation on white-tailed deer. J Wildl Manage. 1986;50(3):471–4. [Google Scholar]
- 58. Van Deelen TR, Campa H III, Haufler JB, Thompson PD. Mortality patterns of white-tailed deer in Michigan's Upper Peninsula. J Wildl Manage. 1997:903–910. [Google Scholar]
- 59. Bowen WD. Variation in coyote Canis latrans social organization the influence of prey size. Can J Zool. 1981;59(4):639–652. [Google Scholar]
- 60. Hamlin KL, Riley SJ, Pyrah D, Dood AR, Mackie RJ. Relationships among mule deer fawn mortality, coyotes, and alternate prey species during summer. J Wildl Manage. 1984;48(2):489–499. [Google Scholar]
- 61. Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecology Letters. 2006;9(4):485–98. [DOI] [PubMed] [Google Scholar]
- 62. Kapel CMO, Torgerson PR, Thompson RCA, Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int J Parasitol. 2006;36(1):79–86. [DOI] [PubMed] [Google Scholar]
- 63. Burlet P, Deplazes P, Hegglin D. Age, season and spatio-temporal factors affecting the prevalence of Echinococcus multilocularis and Taenia taeniaeformis in Arvicola terrestris . Parasite Vector. 2011;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bondrup-Nielsen S. Demography of Clethrionomys gapperi in different habitats. Can J Zool. 1987;65(2):277–283. [Google Scholar]
- 65. Guerra D, Hegglin D, Bacciarini L, Schnyder M, Deplazes P. Stability of the southern European border of Echinococcus multilocularis in the Alps: evidence that Microtus arvalis is a limiting factor. Parasitology. 2014:1–10. [DOI] [PubMed] [Google Scholar]
- 66. Hegglin D, Bontadina F, Contesse P, Gloor S, Deplazes P. Plasticity of predation behaviour as a putative driving force for parasite life-cycle dynamics: the case of urban foxes and Echinococcus multilocularis tapeworm. Funct Ecol. 2007;21(3):552–560. [Google Scholar]
- 67. Giraudoux P, Raoul F, Afonso E, Ziadinov I, Yang Y, Li L, et al. Transmission ecosystems of Echinococcus multilocularis in China and Central Asia. Parasitology. 2013;140(13):1655–1666. 10.1017/S0031182013000644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saitoh T, Takahashi K. The role of vole populations in prevalence of the parasite (Echinococcus multilocularis) in foxes. Res Popul Ecol. 1998;40(1):97–105. [Google Scholar]
- 69. Lidicker WZ Jr. A food web/landscape interaction model for microtine rodent density cycles. Oikos. 2000;91(3):435–445. [Google Scholar]
- 70. Viel JF, Giraudoux P, Abrial V, Bresson-Hadni S. Water vole (Arvicola terrestris scherman) density as risk factor for human alveolar echinococcosis. Am J Trop Med Hyg 1999;61(4):559–565. [DOI] [PubMed] [Google Scholar]
- 71. Levi T, Wilmers CC. Wolves-coyotes-foxes: a cascade among carnivores. Ecology. 2012;93(4):921–929. [DOI] [PubMed] [Google Scholar]
- 72. Harrison DJ, Bissonette JA, Sherburne JA. Spatial relationships between coyotes and red foxes in Eastern Maine. J Wildl Manage. 1989;53(1):181–185. [Google Scholar]
- 73. Hnatiuk JM. First occurrence of Echinococcus multilocularis Leuckart, 1863 in Microtus pennsylvanicus in Saskatchewan. Can J Zool. 1966;44(3):493-. [DOI] [PubMed] [Google Scholar]
- 74. Lee C-F. Larval Echinococcus multilocularis Leuckart, 1863 in the southern interlake area in Manitoba. Can J Zool. 1969;47(4):733–734. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following data are available from Figshare: Overall coyote diet (http://dx.doi.org/10.6084/m9.figshare.1309944); and small mammal undigested items (http://dx.doi.org/10.6084/m9.figshare.1309943).