Abstract
Mycetoma is a unique neglected tropical disease which is endemic in what is known as the “mycetoma belt”. The disease has many devastating impacts on patients and communities in endemic area and is characterised by massive deformity, destruction and disability. Mycetoma is commonly seen in the foot and hand and less frequent in other parts of the body. Mycetoma of the head and neck is a rarity and is associated with high morbidity and even mortality if not treated early. In this communication we report on 49 patients with head and neck mycetoma followed up at the Mycetoma Research Centre in Khartoum. Most of the reported patients had actinomycetoma and the majority were young adult males from mycetoma endemic areas in the Sudan. Most of them were students, farmers and workers. Prior to presentation the majority had long disease duration and the cause was multifactorial. Advanced disease with massive lesion, deformity and disability was the common presentation. There was no obvious history of local trauma, familial tendency or other predisposing factor identified in this group of patients. MRI and CT scan were the most accurate diagnostic tools to determine the disease extent. The treatment outcome was rather poor and characterised by low cure rate, poor outcome and high follows-up dropout. Such a gloomy outcome calls for structured and objective health education programs.
Author Summary
Although head and neck mycetoma is a rare disease entity yet it is a dreadful disease for the patient, the family and the treating physician. It is potentially fatal and the most challenging to treat. The current study highlighted that, most patients were young adult males, from rural areas of the Sudan and of low socioeconomic status. The lack of medical and health facilities, financial constrains and compounded by poor health education in endemic areas meant that most of the studied patients presented late with advanced disease. The diagnosis of mycetoma in the studied population was confirmed by several imaging techniques; MRI, CT scan and radiography and tissue diagnosis by histopathology and cytology techniques. The treatment outcome was rather unsatisfactory. The cure rate was low, the dropout rate was high and the disease was associated with high morbidity. Structured and objective health education programmes in the endemic areas is important to encourage patients to seek medical advice early in the course of the disease particularly that there is no clear preventive or control measurement in mycetoma.
Introduction
Mycetoma is one of the neglected tropical diseases, characterised by massive deformity, disability and can be fatal if not managed properly and timely [1–3]. It is a chronic, specific, granulomatous, progressive subcutaneous inflammatory disease that spreads to involve the skin, deep structures and bones [4,5]. The disease is caused by true fungi or by certain bacteria and hence it is usually classified into eumycetoma and actinomycetoma, respectively [6,7]. Madurella mycetomatis is the commonest eumycetoma causative agent while Streptomyces somaliensis and Nocardiae are the common causative organisms for actinomycetoma [8–10]. Mycetoma has a definite geographic distribution and it is endemic in what is known as the “Mycetoma Belt” that includes Sudan, Senegal, Somalia, South India, South America and Mexico; however, it is reported in many other countries [11–17]. The infection usually progresses slowly over many years and it is commonly painless and that may contribute to the late presentation of many patients [2,18]. The painless subcutaneous mass, multiple sinuses and discharge with grains is distinctive of this infection [1] Young adult males in the age range 20–40 years are more frequently affected [2,4]. Farmers, workers and students are affected most but no occupation is exempted [2,5].
The diagnosis of mycetoma is tedious and several tools are required to reach a proper diagnosis. These tools include imaging techniques such as radiography, ultrasonography, CT, MRI [19–21], molecular techniques such as PCR [22], serodiagnosis as ELISA, CIE [23, 24] as well the classical grain culture and histopathological diagnosis [23]. Although the disease can be diagnosed clinically this is not accurate and can be misleading.
Early lesions are amenable to medical and surgical treatment with good prognosis [24,25]. Generally, actinomycetoma responds to medical treatment in the form of combined antibiotics while eumycetoma requires both antifungal and surgical excision [26,27]. Late and advanced disease is difficult to treat, has poor prognosis and is associated with high recurrence and amputation rates [28]. Currently there is no preventive or control measurements as the route of infection, susceptibility and resistance to the infection are still an enigma and hence health education is essential to avoid the disease and its high morbidity and complications. Mycetoma of the head and neck region is a rarity, patients commonly present with massive lesions and is associated with poor prognosis and can be fatal. In this communication, the Mycetoma Research Centre of the University of Khartoum experience of managing 49 patients with mycetoma of the head and neck region is presented.
Materials and Methods
This descriptive, cross-sectional hospital based study was conducted at the Mycetoma Research Centre (MRC), University of Khartoum, Khartoum, Sudan. The study included 49 patients with confirmed head and neck mycetoma seen in the period January 1991 and October 2014. The diagnosis of mycetoma was confirmed by careful interview, meticulous clinical examinations and standard investigations.
The investigations included fine needle aspiration for cytology (FNA), histopathological examination of surgical biopsies using different staining techniques and grains culture in various media. The common sero-diagnostic test used was counter-immuno-electrophoresis. Different imaging techniques were used and that included radiography of the affected sites in at least in two views: anterio-posterior and lateral, lesion ultrasound examination, and in some patients MRI and CT scan. The electronic patients’ notes were carefully and meticulously reviewed.
Ethical Statement
The study ethical clearance was obtained from Soba University Hospital Ethical Committee, it waived the need for consent.
Statistical Analysis
Statistical analysis was conducted using SPSS computer programme. Data was summarized as percentages for categorical variables and mean ± standard error of the mean (SEM) and median for continuous variables.
Results
The 49 studied patients with confirmed head & neck mycetoma constituted 0.76% of the total MRC patient population seen during the study duration. In the present study, 33 patients (67.3%) had actinomycetoma and 16 (32.7%) had eumycetoma. There were 39 males (79.6%) and 10 females (20.4%). Their ages ranged between 9 and 67 years with a mean age of 27.9 ± 14.7 years. 31(63.3%) of the patients were under 40 years-old at presentation and 23(46.9%) were in the age group 1–20 years. Only five patients (10.2%) were more than 50 years of age at presentation.
In this study, there were 14 students (28.6%), 11 workers (22.4%) and 10 farmers (20.4%). Due to prolonged illness and disability, seven patients (14.3%) were unemployed. There were five housewives (10.2%) in this population.
The majority of the patients, 27(55.1%) were from central Sudan; AL Jazeera State 10(20.4%) and Sinnar State 7(14.3%). There were eight patients (16.3%) from Kordofan States, seven patients (14.3%) from Khartoum State, five patients (10.2%) from Kassala State, four patients (8.1%) form Darfour States and three patients (6.1%) from the White Nile State.
The disease duration at presentation ranged between one and 40 years with a mean duration of 11.23 ± 19.7 years. The majority of the patients 33(67.3%) had mycetoma for less than 10 year and 16(32.7%) of them had the disease for less than one year. Only three patients (6.1%) had the disease for more than 30 years.
Thirty patients (61.2%) had history of discharge contained grains and the colour of the grains was yellow (38.8%), black (24.5%) or white (8.2%). Pain at the mycetoma site, was not a frequent symptom among the study population; documented in only 11 patients (22.4%). Only fourteen patients (28.6%), recalled history of local trauma at the mycetoma site and three patients could not recall such a history. Concomitant other illness was documented in only two patients (4.1%). Four patients (8.2%) had family history of mycetoma.
The majority of patients, 36(73.5%) had recurrent disease and underwent previous surgical excisions; 23 patients (46.9%) had one surgical excision, five patients (10.2%) underwent two surgical excisions, six patients (12.2%) had three previous surgical excisions while two patients (4.1%) had more than three surgical excisions. The type of anaesthesia used ranged between general (41.8%) and local (58.2%).
Different parts of the head and neck were involved which included the frontal (n = 12), occipital (n = 5), parietal (n = 1) and temporal region (n = 1). Multiple skull bones involvement was documented in 12 patients, (Table 1). Five patients had combined frontal and parietal and/ or temporal bone involvement. Two patients had combined occipito-temporo-parietal bones affection. One patient had massive sphenoid, ethmoid, maxillary, nasal bones, anterior cranial fossa, temporal, frontal and occipital bones and supra-orbital areas. One patient had infra-temporal fossa mycetoma extending to the nasopharynx involvement. Two patients had base of the skull and occipital mycetoma with cervical region extension. The orbit was involved in two patients. The upper eye lid, buccal cavity and cheek were affected in one each. Ten patients (20%) had cervical mycetoma. Four patients (4%) had intracranial lesions (Figs. 1, 2, 3).
Table 1. Table showing the head and neck distribution of mycetoma among the studied population.
| Site | No. | % |
|---|---|---|
| Frontal region | 12 | 24.5 |
| Occipital region | 5 | 10.2 |
| Temporal region | 1 | 2.0 |
| Parietal region | 1 | 2.0 |
| Cheek | 2 | 4.0 |
| Orbit | 2 | 4.0 |
| Buccal cavity | 1 | 2.0 |
| Upper eye lid | 1 | 2.0 |
| Fronto-parietal regions | 2 | 4.0 |
| Fronto-temporo-parietal regions | 2 | 4.0 |
| Fronto-temporal regions | 1 | 2.0 |
| Frontal bone and supra-ocular region with parietal extension | 1 | 2.0 |
| Occipito-parietal regions | 1 | 2.0 |
| Occipito-temporo-parietal regions | 1 | 2.0 |
| Sphenoid, ethmoid, maxillary, nasal bones, anterior cranial fossa, with temporal, frontal and occipital regions and supra-orbital areas | 1 | 2.0 |
| Infra-temporal fossa extending to the naso-pharynx | 1 | 2.0 |
| Skull base and neck involvement | 1 | 2.0 |
| Occipital with upper cervical bone involvement | 1 | 2.0 |
| Neck only | 10 | 20.4 |
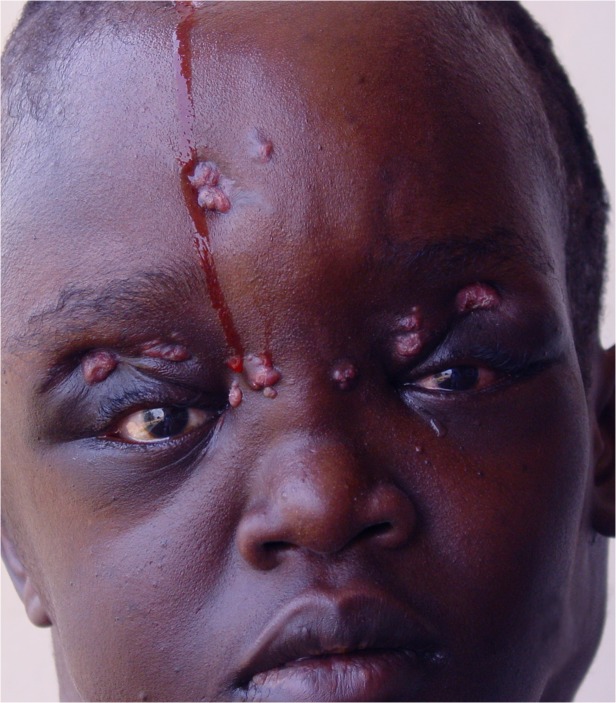
Fig 1. A: Showing massive head actinomycetoma with severe bilateral proptosis.
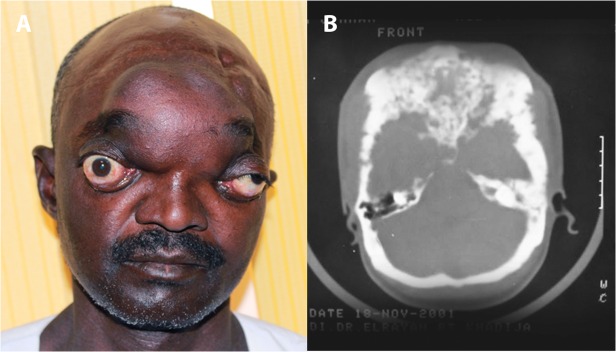
This patient had vision loss in the right eye, and loss of hearing in both ears due massive intra-cranial involvement. B: Skull CT scan showing massive intracranial actinomycetoma involvement with bones destruction. The patient agreed to show his photos for publication purpose.
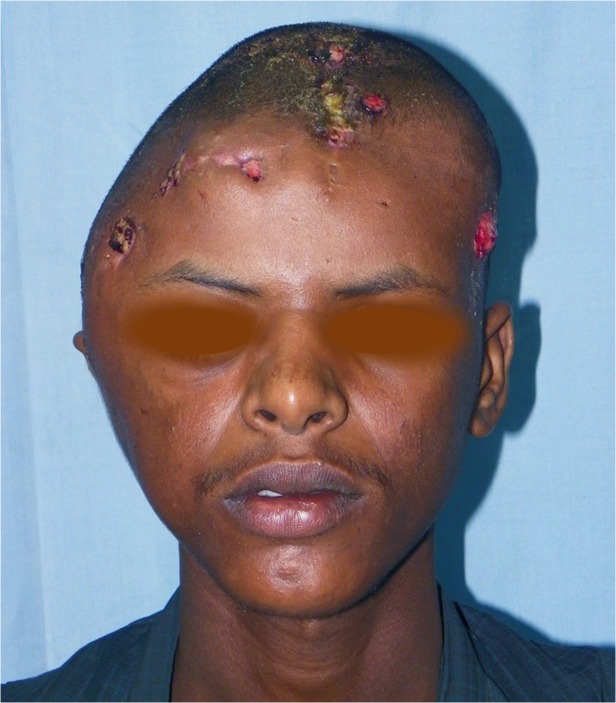
Fig 2. Showing massive head actinomycetoma with involvement of the different parts of the skull with multiple sinuses and discharge.
The patient agreed to show her photos for publication purpose.
Fig 3. Showing massive head actinomycetoma with multiple sinuses.
The patient agreed to show his photos for publication purpose.
The mycetoma lesions were classified according to their sizes into small (less than 5 cm), moderate lesion (5–10 cm) and massive lesion (>10cm). The study showed that, 20 patients (40.8%) had massive lesions at presentation while 12 patients (24.5%) had small lesions. At presentation, 30 patients (61.2%) had lesions with sinuses; they were active in 15 patients (30.6%), healed in six patients (12.2%) and nine patients (18.3%) had both active and healed sinuses.
Grains discharged through the sinuses were not detected on clinical examination in 37 patients (75.5%) while in 12 patients (24.5%) grains were detected. Local hyper-hydrosis at and around the mycetoma lesion was detected in one patient (2%).
Regional lymph nodes enlargement was detected in six patients (12.2%). Dilated tortuous veins proximal to the mycetoma lesions were not detected in the present. One patient presented with massive intracranial eumycetoma with minimal skin and subcutaneous affection.
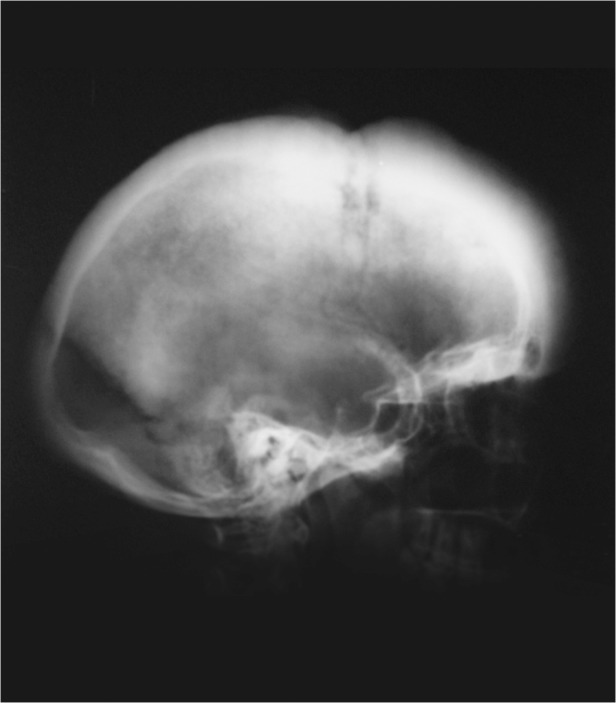
At presentation 30 patients had skull and cervical X-Ray examination in at least two views and that showed normal findings in eight patients (16.3%), soft tissue mass in 10(20.4%), periosteal reaction in one patient (2%), bone destruction in five patients (10.2%) and in six patients (12.2%) a combination of these findings were detected (Fig. 4).
Fig 4. Skull X-ray showing massive thickening of the skull tables and generalised osteoblastic changes.
Ultrasound examination of the mycetoma lesion was performed in 11 patients (22.4%). This showed evidence of eumycetoma in five patients (10.2%), actinomycetoma in four patients (8.2%) while in two patients (4.1%) no diagnosis was established.
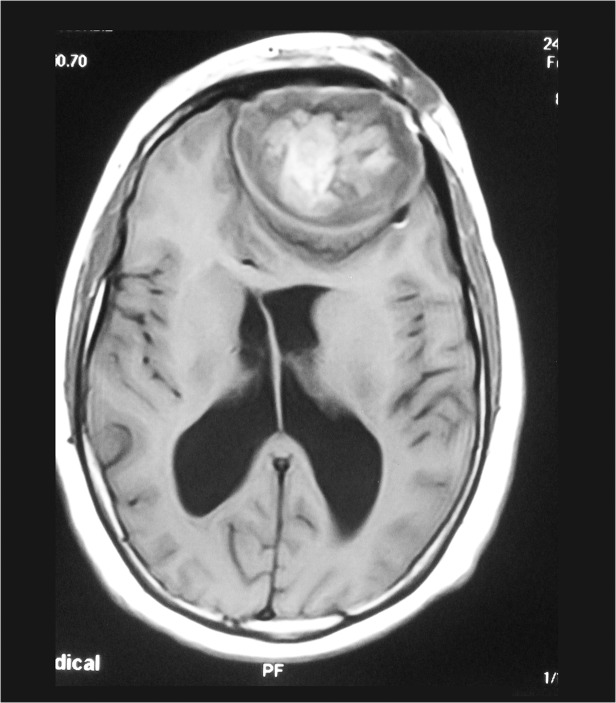
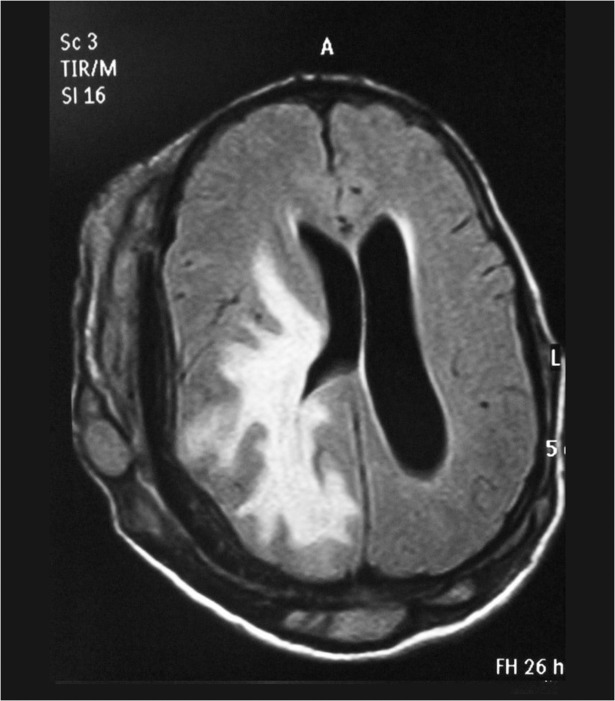
Most of the patients had MRI examination and it showed the skin, subcutaneous, skull and intracranial disease spread with the typical dot-in-circle sign in most of them, (Figs. 5, 6).
Fig 5. Cranial MRI showing eumycetoma involving the skin, subcutaneous tissue and destruction of the frontal bone and with well encapsulated intracranial lesion.
Fig 6. Cranial MRI showing generalised skin, subcutaneous tissue and bone involvement with massive intracranial spread.
FNA for cytology was performed in 21 patients to confirm the diagnosis and it showed evidence of Actinomadura madurae in eight patients (16.3%), M. mycetomatis in six patients (12.2%), Streptomyces somaliensis in four patients (8.1%) and in three patients (6.1%) no grains were not detected.
In this series, 24 patients (49%) had histopathological examinations of surgical biopsies. The diagnosis of M. mycetomatis was established in nine patients (18.4%), Streptomyces somaliensis in 11 patients (22.4%) and Actinomadura madurae in three patients (6.1%). In one patient (2%) no diagnosis was established due to grains absence and the diagnosis was established by FNA.
For actinomycetoma a combination of antimicrobial agents was given and that included streptomycin sulphate and dapsone, or streptomycin and trimethoprim-sulfamethoxazole. More recently, trimethoprim-sulfamethoxazole 8/40 mg/kg/day in cycles for 5 weeks and amikacin 15 mg/kg/day in a divided dose every 12 hours for 3 weeks were administered. The two week interval of amikacin in the five-week cycle is used for renal and audiometric monitoring.
For eumycetoma several antifungal agents combined with various surgical excisions were performed. The common antifungal agents used were ketoconazole and Itraconazole.
All patients were offered follow up appointments but due to various reasons 14 patients (28.5%) were subsequently lost for follow up, five patients (10.2%) were completely cured, and 30 patients (61.2%) had partial cure.
Discussion
The incidence of mycetoma of the head and neck region is infrequent. Review of the medical literature revealed only few reports on mycetoma in this site [10,29–31], and although Sudan is considered the mycetoma homeland, only few reports on head and neck mycetoma were reported. Lynch in 1964, reported on 1860 mycetoma patients and of these only 18 patients (0.96%) had head and neck mycetoma [15]. Mahgoub in 1977 reported an incidence of 3% of head and neck mycetoma [32]. In 1986, Gumaa and her associates reported on 15 out 400 patients with mycetoma (3.75%) involving the head and neck region. This communication is in line with the fact that, mycetoma at this region is a rarity.
In agreement with the previously reported series, actinomycetoma was the prevalent type of mycetoma in our series and the explanation for this prevalence remains unclear [10,29]. It is possible that the actinomycetes are resilient and able to survive in the extra-paedal areas more than eumyceteces.
Males were predominantly affected in our series and this is in accordance with previous reports from the Sudan [10,11,29]. Again the explanation for this is unclear; however there is suggestion that sex hormones play a role in this predominance [33]. The majority of the reported patients were young adults with a mean age of 27.9 ± 14.7 years and this is a typical age in mycetoma patients [4,10,34]. Students were affected most, and this may be explained by the fact that, young age groups of patients contract the disease more. The study showed that 44.8% of the affected patients were farmers and workers. This is an important finding as the nature of their work puts them in direct contact with the soil on a daily basis and it has been postulated that the soil harbours the causative organisms and these patients are constantly exposed to minor injuries which facilitate the traumatic subcutaneous inoculation of the organisms.
The mean disease duration at presentation among the affected study population is quite long. This may be explained by the painless nature of the disease, the lack of health education, low socio-economic status of the affected patients and lack of medical and health facilities in the endemic regions.
The clinical presentation of patients in this series was typical and in agreement with other reports [1,2,35]. It started gradually at the subcutaneous tissue and progressed to affect the deep structures. It was painless in the majority of patients and that may be an important contributory factor for the late presentation in most patients.
The study showed that 73.5% of the patients had multiple surgical excisions and recurrence and most of them had surgery performed under local anaesthesia. It is well known that incomplete surgical excision performed under local anaesthesia is the major factor leading in recurrence.
At presentation almost half of the patients had massive lesions which is caused by their late presentation and the fact that, most of them had actinomycetoma which is known to be aggressive and can invade the deep structures and bone at an early disease stage [10].
Different skull parts were affected in our series, however, the frontal and occipital parts were affected most. The explanation for this is unclear however these parts are more prone to direct trauma and hence local inoculation of the causative organisms. Rare sites were encountered and this included the eye.
One patient presented with massive intracranial eumycetoma with minimal skin and subcutaneous involvement, again the explanation is unclear but deep inoculation of the infection may provide some explanation.
In the past, the disease extends in the head and neck area was assessed clinically and radiologically by skull and cervical X-rays or by cerebral angiography which is invasive and with many complications. Currently, the use of the MRI and CT scans provided an accurate assessment with minimal complications. Mycetoma has characteristic MRI features which are diagnostic. The MRI can delineate the involvement of the skin, subcutaneous, muscles and bones accurately and can grade the disease and help in planning patients’ management [21].
The present series showed poor treatment outcome, only five patients were cured and this is in line with previous reports [28–30,36]. This low cure rate necessitates the need for more efficient and safe novel drugs for the treatment of mycetoma. The dropout rate (28.5%) in our series is high. The reasons for the high dropout rate are multifactorial and to mention but a few, the patients’ dissatisfaction due to the high cost and the prolonged treatment duration which is commonly more than one year duration, the drug side effects and complications, the patients low socio-economic status, the lack of health education and difficulty to reach the MRC, particularly during rainy seasons. All these can contribute to the poor treatment outcome.
In conclusion, mycetoma of the head and neck region is a serious medical and health problem, is associated with serious complications, low cure rate and high follow-up dropout rate. The route of infection, susceptibility and resistance in mycetoma remains poorly understood and this is compounded by the lack of preventive and control measures. Hence health education may be the only tool to reduce the disease morbidity and mortality.
Supporting Information
The patients agreed to show their photos for publication purpose.
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Fahal AH, Hassan MA (1992) Mycetoma. Br J Surg 79: 1138–1141. [DOI] [PubMed] [Google Scholar]
- 2. Fahal AH (2004) Mycetoma: a thorn in the flesh. Trans R Soc Trop Med Hyg 98: 3–11. [DOI] [PubMed] [Google Scholar]
- 3. Ahmed AO, van Leeuwen W, Fahal A, van de Sande W, Verbrugh H, et al. (2004) Mycetoma caused by Madurella mycetomatis: a neglected infectious burden. Lancet Infect Dis 4: 566–574. [DOI] [PubMed] [Google Scholar]
- 4. Fahal AH, Shaheen S, Jones DH (2014) The orthopaedic aspects of mycetoma. Bone Joint J 96-B: 420–425. 10.1302/0301-620X.96B3.31421 [DOI] [PubMed] [Google Scholar]
- 5. van Belkum A, Fahal A, van de Sande WW (2013) Mycetoma caused by Madurella mycetomatis: a completely neglected medico-social dilemma. Adv Exp Med Biol 764: 179–189. [DOI] [PubMed] [Google Scholar]
- 6. van de Sande WW, Maghoub el S, Fahal AH, Goodfellow M, Welsh O, et al. (2014) The mycetoma knowledge gap: identification of research priorities. PLoS Negl Trop Dis 8: e2667 10.1371/journal.pntd.0002667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van de Sande WW (2013) Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis 7: e2550 10.1371/journal.pntd.0002550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Estrada R, Chavez-Lopez G, Estrada-Chavez G, Lopez-Martinez R, Welsh O (2012) Eumycetoma. Clin Dermatol 30: 389–396. 10.1016/j.clindermatol.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 9. de Hoog GS, van Diepeningen AD, Mahgoub el S, van de Sande WW (2012) New species of Madurella, causative agents of black-grain mycetoma. J Clin Microbiol 50: 988–994. 10.1128/JCM.05477-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lopez-Martinez R, Mendez-Tovar LJ, Bonifaz A, Arenas R, Mayorga J, et al. (2013) [Update on the epidemiology of mycetoma in Mexico. A review of 3933 cases]. Gac Med Mex 149: 586–592. [PubMed] [Google Scholar]
- 11. Lopez Martinez R, Mendez Tovar LJ, Lavalle P, Welsh O, Saul A, et al. (1992) [Epidemiology of mycetoma in Mexico: study of 2105 cases]. Gac Med Mex 128: 477–481. [PubMed] [Google Scholar]
- 12. Padhi S, Uppin SG, Uppin MS, Umabala P, Challa S, et al. (2010) Mycetoma in South India: retrospective analysis of 13 cases and description of two cases caused by unusual pathogens: Neoscytalidium dimidiatum and Aspergillus flavus. Int J Dermatol 49: 1289–1296. 10.1111/j.1365-4632.2010.04610.x [DOI] [PubMed] [Google Scholar]
- 13. Zarei Mahmoudabadi A, Zarrin M (2008) Mycetomas in Iran: a review article. Mycopathologia 165: 135–141. [DOI] [PubMed] [Google Scholar]
- 14. Lichon V, Khachemoune A (2006) Mycetoma: a review. Am J Clin Dermatol 7: 315–321. [DOI] [PubMed] [Google Scholar]
- 15. Lynch JB (1964) Mycetoma in the Sudan. Ann R Coll Surg Engl 35: 319–340. [PMC free article] [PubMed] [Google Scholar]
- 16. Destombes P, Mariat F, Rosati L, Segretain G (1977) [Mycetoma in Somalia—results of a survey done from 1959 to 1964]. Acta Trop 34: 355–373. [PubMed] [Google Scholar]
- 17. Erbakan N, Or AN, Unal M, Palali Z (1973) A review of mycetomas in Turkey. Mycopathol Mycol Appl 51: 105–113. [DOI] [PubMed] [Google Scholar]
- 18. Fahal A, Mahgoub el S, El Hassan AM, Abdel-Rahman ME, Alshambaty Y, et al. (2014) A new model for management of mycetoma in the Sudan. PLoS Negl Trop Dis 8: e3271 10.1371/journal.pntd.0003271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abd El Bagi ME (2003) New radiographic classification of bone involvement in pedal mycetoma. AJR Am J Roentgenol 180: 665–668. [DOI] [PubMed] [Google Scholar]
- 20. Abd El-Bagi ME, Fahal AH (2009) Mycetoma revisited. Incidence of various radiographic signs. Saudi Med J 30: 529–533. [PubMed] [Google Scholar]
- 21. El Shamy ME, Fahal AH, Shakir MY, Homeida MM (2012) New MRI grading system for the diagnosis and management of mycetoma. Trans R Soc Trop Med Hyg 106: 738–742. 10.1016/j.trstmh.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 22. Ahmed AO, Mukhtar MM, Kools-Sijmons M, Fahal AH, de Hoog S, et al. (1999) Development of a species-specific PCR-restriction fragment length polymorphism analysis procedure for identification of Madurella mycetomatis. J Clin Microbiol 37: 3175–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fahal AH, el Toum EA, el Hassan AM, Mahgoub ES, Gumaa SA (1995) The host tissue reaction to Madurella mycetomatis: new classification. J Med Vet Mycol 33: 15–17. [PubMed] [Google Scholar]
- 24. Welsh O, Al-Abdely HM, Salinas-Carmona MC, Fahal AH (2014) Mycetoma Medical Therapy. PLoS Negl Trop Dis 8: e3218 10.1371/journal.pntd.0003218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Welsh O (1991) Mycetoma. Current concepts in treatment. Int J Dermatol 30: 387–398. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed AA, van de Sande WW, Fahal A, Bakker-Woudenberg I, Verbrugh H, et al. (2007) Management of mycetoma: major challenge in tropical mycoses with limited international recognition. Curr Opin Infect Dis 20: 146–151. [DOI] [PubMed] [Google Scholar]
- 27. Fahal AH, Rahman IA, El-Hassan AM, Rahman ME, Zijlstra EE (2011) The safety and efficacy of itraconazole for the treatment of patients with eumycetoma due to Madurella mycetomatis. Trans R Soc Trop Med Hyg 105: 127–132. 10.1016/j.trstmh.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 28. Zein HA, Fahal AH, Mahgoub el S, El Hassan TA, Abdel-Rahman ME (2012) Predictors of cure, amputation and follow-up dropout among patients with mycetoma seen at the Mycetoma Research Centre, University of Khartoum, Sudan. Trans R Soc Trop Med Hyg 106: 639–644. 10.1016/j.trstmh.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 29. Gumaa SA, Mahgoub ES, el Sid MA (1986) Mycetoma of the head and neck. Am J Trop Med Hyg 35: 594–600. [DOI] [PubMed] [Google Scholar]
- 30. Gumaa SA, Satir AA, Shehata AH, Mahgoub ES (1975) Tumor of the mandible caused by Madurella mycetomil. Am J Trop Med Hyg 24: 471–474. [DOI] [PubMed] [Google Scholar]
- 31. Joshi A, Acharya S, Anehosur VS, Tayaar AS, Gopalkrishnan K (2014) Oral eumycetoma of infancy: a rare presentation and a brief review. J Craniomaxillofac Surg 42: 35–40. 10.1016/j.jcms.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 32. Mahgoub ES (1977) Mycoses of the Sudan. Trans R Soc Trop Med Hyg 71: 184–188. [DOI] [PubMed] [Google Scholar]
- 33. van de Sande WW, Fahal A, Tavakol M, van Belkum A (2010) Polymorphisms in catechol-O-methyltransferase and cytochrome p450 subfamily 19 genes predispose towards Madurella mycetomatis-induced mycetoma susceptibility. Med Mycol 48: 959–968. 10.3109/13693781003636680 [DOI] [PubMed] [Google Scholar]
- 34. Fahal AH, Sabaa AH (2010) Mycetoma in children in Sudan. Trans R Soc Trop Med Hyg 104: 117–121. 10.1016/j.trstmh.2009.07.016 [DOI] [PubMed] [Google Scholar]
- 35. Dieng MT, Sy MH, Diop BM, Niang SO, Ndiaye B (2003) [Mycetoma: 130 cases]. Ann Dermatol Venereol 130: 16–19. [PubMed] [Google Scholar]
- 36. Elbeshir EI, Idris AM, Gumaa SA, Mahgoub ES (1987) Actinomycetoma of the mandibular region causing ankylosis. Br J Oral Maxillofac Surg 25: 256–260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The patients agreed to show their photos for publication purpose.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.