Abstract
Leishmania protozoan parasites (Trypanosomatidae family) are the causative agents of cutaneous, mucocutaneous and visceral leishmaniasis worldwide. While these diseases are associated with significant morbidity and mortality, there are few adequate treatments available. Sterol 14alpha-demethylase (CYP51) in the parasite sterol biosynthesis pathway has been the focus of considerable interest as a novel drug target in Leishmania. However, its essentiality in Leishmania donovani has yet to be determined. Here, we use a dual biological and pharmacological approach to demonstrate that CYP51 is indispensable in L. donovani. We show via a facilitated knockout approach that chromosomal CYP51 genes can only be knocked out in the presence of episomal complementation and that this episome cannot be lost from the parasite even under negative selection. In addition, we treated wild-type L. donovani and CYP51-deficient strains with 4-aminopyridyl-based inhibitors designed specifically for Trypanosoma cruzi CYP51. While potency was lower than in T. cruzi, these inhibitors had increased efficacy in parasites lacking a CYP51 allele compared to complemented parasites, indicating inhibition of parasite growth via a CYP51-specific mechanism and confirming essentiality of CYP51 in L. donovani. Overall, these results provide support for further development of CYP51 inhibitors for the treatment of visceral leishmaniasis.
Author Summary
Visceral leishmaniasis is the second most lethal parasitic infection after malaria. Other forms of leishmaniasis also cause significant morbidity. However, there are few treatments available, and many cause severe side effects or are associated with the development of resistance. A key difference between mammalian cells and Leishmania parasites is the type of sterol in their membranes: while mammalian cell membranes contain cholesterol, Leishmania parasites use ergosterol. There has therefore been considerable interest in developing inhibitors of sterol biosynthesis pathways to target Leishmania parasites. Sterol 14alpha-demethylase (CYP51) is one of the enzymes in the sterol biosynthesis pathway, and the target of significant drug development research in Leishmania. Here we use a double approach to determine whether this gene is essential in Leishmania donovani, the causative agent of visceral leishmaniasis. We demonstrate via gene knockout and drug targeting approaches that loss or inhibition of CYP51 inhibits L. donovani growth. These results validate CYP51 as a drug target in L. donovani and support further work to develop CYP51-directed therapies for visceral leishmaniasis.
Introduction
Leishmania are vector-borne protozoan parasites. They have a digenetic lifecycle; promastigotes are transmitted by the sandfly vector to the mammalian host, where they are taken up by phagocytic cells and differentiate into the amastigote stage within the macrophage phagolysososme. Amastigotes proliferate within the phagolysosome and can be taken up by a sandfly during a subsequent bloodmeal. Within the sandfly gut, amastigotes then differentiate into promastigotes, thereby completing the parasite lifecycle [1].
Leishmania parasites cause a range of disease manifestations: cutaneous leishmaniasis in which lesions develop at the site of the sandfly bite, mucocutaneous leishmaniasis with destruction of the mucosal tissues in the nose, mouth and throat, and visceral leishmaniasis in which parasites disseminate to the liver, bone marrow and spleen. Visceral leishmaniasis is the most lethal form of the disease. It is associated with high fever, hepatosplenomegaly and pancytopenia [1]. The infecting species of Leishmania is the major determinant of disease manifestation; parasites from the Leishmania donovani species complex are the main causes of visceral leishmaniasis, while other species, including the Leishmania major species complex, cause cutaneous manifestations [2,3].
Leishmania parasites are distributed across tropical and subtropical regions of the world. 350 million people live in endemic areas and are at risk of developing the disease, with 12 million people currently infected [4]. Overall, there are 1.6 million new cases per year [5], associated with a disease burden of 3.3 million DALYs and over 50,000 deaths per year [6], making leishmaniasis the second most lethal parasitic infection after malaria [5]. However, treatment options are limited; while recent progress has been made with the development of single-dose amphotericin B therapy in India [7], this treatment regimen was not effective in East Africa [8]. All other drugs require long treatment regimens; toxicity and drug resistance are also significant concerns [9].
Cell membrane sterols regulate membrane fluidity and contribute to the organization of membrane domains. Unlike mammalian cells, but similar to fungi, Leishmania and Trypanosoma parasite cell membranes contain ergosterol and ergosterol-like sterols rather than cholesterol. Sterols are generated from acetyl-CoA via a multistep metabolic pathway. The first three steps, catalyzed by acetoacetyl-CoA thiolase, HMG-CoA synthase and HMG-CoA reductase, lead to the generation of mevalonate. Mevalonate is the substrate of the isoprenoid pathway that generates farnesyl diphosphate. Squalene synthase then produces squalene from two farnesyl diphosphate molecules. Squalene is oxidized by squalene oxidase, and the resulting product cyclized to lanosterol. Sterol 14alpha-demethylase (CYP51, LdBPK_111100.1) catalyses the removal of a 14alpha-methyl group from lanosterol [10,11]. The L. infantum CYP51 enzyme has broad substrate specificity, with the ability to demethylate obtusifoliol, C4-norlanosterol and 14α-methylzymosterol, in addition to lanosterol, although with a preference for the first two substrates [12]. The following steps differ between ergosterol and cholesterol biosynthesis, with variations in the reaction intermediates and enzymes involved depending on species [13]. One of these key latter steps in ergosterol biosynthesis is the methylation of C24 via sterol 24-methyltransferase, leading to the formation of fecosterol, episterol or 5-dehydroepisterol depending on the substrate [14].
Azole antifungals have been investigated for treatment of Leishmania infections, but with large variations in efficacy between species [15]. The first experiments on azole sensitivity in visceral Leishmania species showed efficacy of ketoconazole [16] and oxiconazole [17] on intracellular amastigotes and of ketoconazole on extracellular promastigotes [18]. Posaconazole [19] and ketoconazole [20] were also effective in mouse models of visceral leishmaniasis, albeit less so than amphotericin B or pentavalent antimonial compounds currently used for visceral leishmaniasis treatments. Azoles have also been extensively tested on cutaneous Leishmania species (see for instance [21,22,23,24] for early work on these parasites). Given the importance of CYP51 as a drug target and the severity of disease caused by L. donovani, we investigated the essentiality of L. donovani CYP51 by biological and pharmacological methods.
Materials and Methods
Ethics statement
All vertebrate animal studies were performed in accordance with the USDA Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the University of California San Francisco Institutional Animal Care and Use Committee (protocol AN087316). Euthanasia was performed by carbon dioxide inhalation followed by cervical dislocation.
Cell culture and infection
L. donovani 1S/Cl2D promastigotes were maintained at 26°C in M199 medium (Sigma) supplemented with 10% heat inactivated fetal bovine serum (FBS, Sigma), 25 mM HEPES, penicillin, streptomycin, adenosine, glutamine, hemin, and folic acid at pH 7.2. Axenic amastigote differentiation was performed as described in [25]: promastigotes were resuspended in amastigote media (M199 medium supplemented with 25% FBS, streptomycin, penicillin, succinic acid, adenine, glycerol, L-proline and folic acid, at pH 5.5) at a cell density of 5x106 cells/mL and transferred to 37°C, 5% CO2.
THP-1 macrophages were maintained in RMPI 1640 media supplemented with 5% FBS 1% penicillin-streptomycin at 37°C, 5% CO2. For Leishmania infection, THP-1 cells were treated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) for 48 h and then infected with stationary phase promastigotes. Cells were then fixed with 4% formaldehyde and stained with 4′,6′-diamidino-2-phenylindole (DAPI). Images were obtained with an automated InCell 2000 automated imaging system (G.E. Healthcare) and parasite levels determined using IN CELL developer 1.9 software (see S1 Methods), leading to determination of cell boundaries and counting of parasite inside the boundary but outside the nucleus in an automated fashion.
Female BALB/c mice (17–20 g, 6 per group) were purchased from Simonsen Laboratories and maintained in the animal care facility under pathogen-free conditions. Mice were infected intravenously via the tail vein with 5x107 stationary phase promastigotes and sacrificed 28 days post- infection. Liver parasite burden was determined by direct counting of amastigotes on Diff-Quick stained liver impressions and calculated as Leishman-Donovan Units (LDU): number of amastigotes per 1000 cell nuclei × liver weight (g).
Generation of transfected L. donovani lines
All sequences were retrieved from TriTrypDB [26]. 3′ L. donovani CYP51 flanking sequences was amplified by PCR from parasite genomic DNA (LdBPK_111100.1, primers 1 and 2), digested with SpeI and XbaI, and ligated into the XbaI site of vectors pGEM-PAC and pGEM-Hyg. 5′ CYP51 flanking region was amplified with primers 3 and 4, digested with SpeI XbaI, and ligated into the SpeI site of vectors already containing the CYP51 3′ flanking region. Knockout cassettes were then liberated by restriction enzyme digestion with SpeI XbaI. The CYP51 coding region was amplified by PCR from genomic DNA (primers 5 and 6), digested with BglII and ligated into the BglII site of the pXNG4 vector [27]. In all cases, constructs were verified by diagnostic digest and sequencing.
Transfection was performed as described in [28] by electroporation in cytomix transfection buffer (120mM KCl, 0,15mM CaCl2, 10mM K2HPO4, 25mM HEPES, 2mM EDTA, 2mM MgCl2) using a BioRad Gene Pulser Xcell, delivering two pulses at 1500 V and 25 μF. Parasites were transfected first with the hygromycin knockout cassette; HKO clonal lines were selected with 100 μg/ml hygromycin (Invitrogen), then transfected with the empty pXNG4 vector or the pXNG4 vector encoding CYP51, thereby generating the HKO + C and HKO + CYP lines, respectively. Double transfectants were maintained with a combination of hygromycin and 100 μg/ml nourseothricin (GoldBio). HKO + C or HKO + CYP lines were transfected with the puromycin knockout cassette and clonal HKO + C + PAC and HKO + CYP + PAC lines isolated by limiting dilution under selection with hygromycin, nourseothricin and 20 μg/ml puromycin (Sigma). Correct targeting of CYP51 genes was verified by PCR using primers 7 (in CYP51 5′UTR) and 8 (in hygromycin resistance gene) or 9 (in puromycin resistance gene). Persistence of CYP51 genes in double resistant, uncomplemented strains and loss of chromosomal CYP51 in complemented strains were verified by PCR (primers 10 + 11 and primers 12 + 13, respectively). Primer 12 is upstream of CYP51, outside of the knockout cassette, and primer 13 anneals within the CYP51 gene.
Ganciclovir selection
50 μg/mL ganciclovir (Invivogen) was added to the parasite cultures. qPCR on extracted DNA to monitor pXNG4 loss and flow cytometry analysis to assess GFP levels were performed weekly (see below). Results shown represent the average of two independent selection experiments on a total of seven independent clonal lines.
Quantitative PCR
DNA was extracted as described previously [29]. qPCR reactions containing 100 ng of parasite DNA in Lightcycler 480 Sybr green I Master mix (Roche) were run on a Stratagene Mx3005P RT-PCR thermocycler using the following thermal profile: initial denaturation at 95°C for 10 min, then 40 cycles of denaturation at 95°C for 10 s, annealing at 57°C for 20 s and extension at 72°C for 20 s. Melting curve analysis and agarose gel electrophoresis were used to confirm correct PCR product formation. Chromosomal CYP51 (primers 14 and 15), total CYP51 (primers 16 and 17), and pXNG4 (primers 18 and 19) relative levels were determined by qPCR using the 2-ΔΔCt method [30], normalizing to serine acetyltransferase (SAT, primers 20 and 21) or cystathionine beta-synthase (CBS, primers 22 and 23), previously shown to be present in only two copies in L. donovani [31].
Flow cytometry
Analyses were performed on a BDFACSDiva LSRII flow cytometer in HTS mode. Cells were stained with 5 μM propidium iodide (PI, Sigma). Quadrant gates were set used PI-stained wild-type parasites (GFP-negative) and percentage of GFP+ PI- cells determined using FloJo X software (Tree Star Inc).
SDS PAGE and Western blot
1x107 parasites were lysed in 1x LDS buffer (Invitrogen) and separated using NuPage bis-tris precast polyacrylamide gels (Invitrogen). Proteins were transferred to a PVDF membrane (BioRad). Western blot was performed as described in [32]. Affinity-purified anti-CYP51 antibodies (Genescript) and anti-GAPDH antibody (from Paul Michels, Université catholique de Louvain, Bruxelles) were used at 1:5,000 dilution. The secondary antibody was a 1:5,000 dilution of peroxidase-conjugated anti-rabbit IgG antibody (GE Healthcare). All proteins were visualized using SuperSignal West Pico Chemoluminescent Substrate (Thermo Scientific). Proteins expression levels were quantified with Image J program, normalizing CYP51 levels to GAPDH levels.
Sterol GC-MS
Sterol extraction was performed as described in [33]. Briefly, the parasite cell pellet was resuspended in chloroform-methanol solution (2:1 ratio), then dried under nitrogen gas, followed by overnight treatment with chloroform. The organic phase was then washed with water and dried under nitrogen. The dried pellet was resuspended in chloroform-methanol (9:1 ratio), and washed again with water. Acetonitrile was added to the samples, washing steps were repeated and solvents evaporated under nitrogen.
Extracted sterols were then derivatized by resuspending the dried residue in 25 μL hexanes and 75 μL BSTFA (Sigma-Aldrich, St. Louis MO) for 2 hr at 37°C to generate the trimethylsilyl (TMS) sterols. TMS-derivatized sterols were analyzed using gas chromatography-mass spectrometry (GC-MS) on an Agilent HP 6850 GC coupled to a mass selective detector (Agilent MSD 5973) operating at 70 eV in electron impact mode. The sterols were separated using a DB5-MS analytical column (30 m x 0.25 mm inner diameter, 0.25-μm film thickness, Agilent) with a temperature profile that begins at 200°C for 1 min, increases by 15°C/min up to 300°C, and holds at 300°C for 20 min. The inlet and detector temperatures were held at 200 and 250°C, respectively. The MSD was set to scan the range 50–750 m/z for sterol profiling. Selected Ion Monitoring (SIM) was used for ergosterol quantification by using the same GC temperature profile but assaying for fragment ions specific to ergosterol that elute at the same time window as ergosterol standard: m/z 468.4, 378.4, 363.4, 337.4, and 253.1 We prepared an 8-point standard curve of ergosterol using serial dilution over a concentration range of 9 pmol to 1.2 nmoles. The area under the curve in the SIM assay was then compared to standard samples to calculate ergosterol concentrations.
Ergosterol biosynthesis inhibitor assay
Amphotericin B, ketoconazole and voriconazole were purchased from Sigma. All other CYP51 inhibitors were synthesized in-house (see supplementary methods and [34,35,36]). Stationary phase promastigotes (8x105/mL) were treated for 72 h with two-fold dilution of inhibitors in 384 well plate format. Resazurin (0.025 mg/mL, Santa Cruz) was added for 5 h, cells were fixed, and fluorescence measured at 490 nm excitation and 595 nm emission wavelengths. Data was normalized to the amphotericin B positive control and DMSO vehicle negative control for each plate, and EC50 values calculated using Collaborative Drug Discovery Vault software. T. cruzi cell-based activity was determined by high content screening in triplicate, as previously described [36].
Results
Leishmania donovani tolerate modulations in CYP51 levels
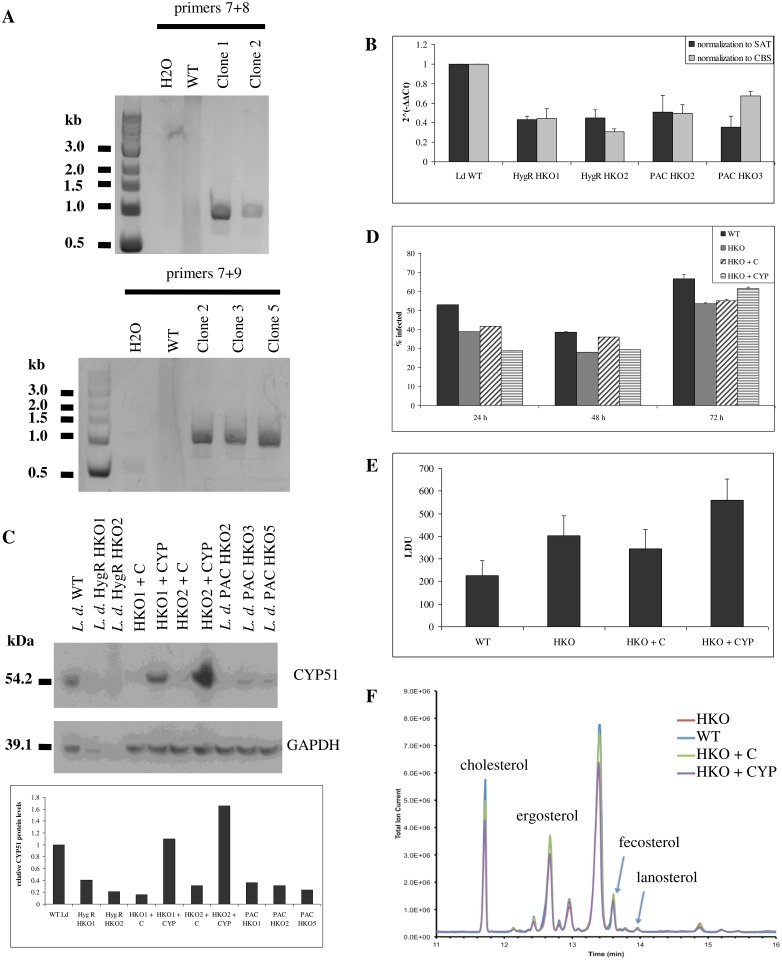
We generated half knockout L. donovani parasites (HKO strains) in which a single CYP51 allele was replaced with either a puromycin or hygromycin resistance marker (Fig. 1A; see Fig. 2B for the knockout approach). CYP51 is located on chromosome 11, which is disomic in reference L. donovani genomes [37], but trisomic in some clinical L. donovani isolates [31]. In addition, the Leishmania genome contains many direct and indirect repeats that can promote extrachromosomal element formation under drug pressure or for essential genes [31,38]. Prior to targeting another CYP51 allele, we therefore verified CYP51 copy number in parasites transfected with the first knockout cassette, resistant to either hygromycin (HygR HKO strains) or puromycin (PAC HKO strains). HKO strains contained half of the CYP51 DNA content found in wild-type, indicating loss of one out of two alleles (Fig. 1B). Furthermore, CYP51 protein levels were decreased two to five fold in half knockout strains. Complementation with an episomal CYP51 gene restored protein expression to levels comparable to wild-type L. donovani (Fig. 1C).
Fig 1. Modulation of CYP51 levels in L. donovani.
A, Replacement of a CYP51 allele by homologous recombination. Correct targeting of the knockout cassettes was verified by PCR using one primer within the knockout cassette and one upstream of CYP51 (primers 7 and 8 (hygromycin), top or 7 and 9 (puromycin), bottom). B, qPCR quantification of chromosomal CYP51 levels, normalized to SAT or to CBS and to wild-type levels. C, CYP51 protein levels in half knockout and complemented strains. CYP51 and GAPDH were detected by Western blot (top) and quantified by densitometry (bottom) D, In vitro infectivity of half knockout and complemented strains. THP1 macrophages were infected at a 10:1 parasite to macrophage ratio. Cells were fixed and stained with DAPI 24, 48 and 72 h post-infection, and macrophage infection levels were determined by automated high-throughput imaging and parasite detection. E, In vivo infectivity of half knockout and complemented strains. BALB/c mice were infected intravenously. Liver parasite burden (Leishman-Donovan Units, LDU) was determined 28 days post-infection by counting stained liver impressions. F, Sterol profiling by GC-MS.
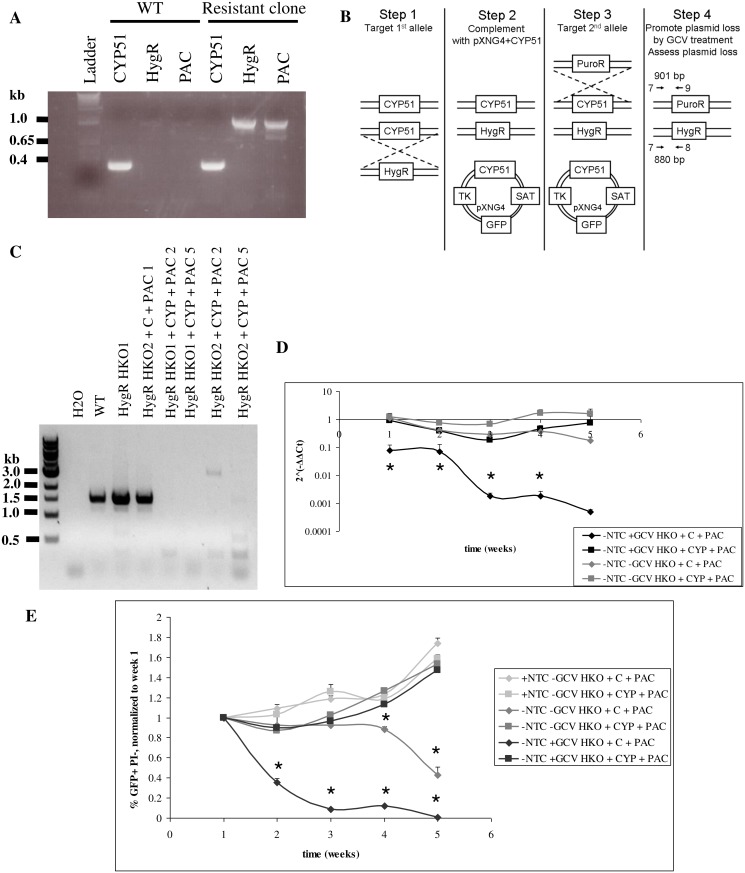
Fig 2. Essentiality of CYP51 in L. donovani.
A, Persistence of CYP51 gene even after correct targeting of two knockout cassettes into the CYP51 locus. Correct targeting of the knockout cassettes was verified by PCR using one primer within the knockout cassette and one upstream of CYP51 (primers 7 and 8 (hygromycin) or 7 and 9 (puromycin)). CYP51 was amplified with primers within the coding region (primers 10 and 11). B, Targeting strategy. Primers 7 and 8 or 7 and 9 and expected PCR product sizes to confirm correct targeting of chromosomal CYP51 alleles are indicated by arrows (step 4). C, Loss of chromosomal CYP51 in the presence of episomal CYP51 complementation but not in strains complemented with the empty vector. PCR was performed using primers 12+13. D, pXNG4 loss monitored by qPCR during GCV selection or in the absence of either positive or negative selection (untreated samples). pXNG4 Ct values were normalized to SAT levels and to samples under positive selection (treated with nourseothricn (NTC) in the absence of GCV). E, GFP positive, propidium iodide (PI) negative levels were monitored by flow cytometry. Percentage of GFP+ PI- cells were normalized to the levels for week 1. *, p<0.05 compared to HKO+CYP+PAC.
Given the importance of ergosterol biosynthesis in trypanosomatid parasites, we assessed the impact of this loss of CYP51 expression before proceeding to targeting of the second CYP51 allele. In vivo and in vitro infectivity was comparable between strains (Fig. 1D, Fig. 1E, S1 Fig); any differences between wild-type and transfected strains were not due to changes in CYP51 levels since infectivity of HKO, HKO+C and HKO+CYP was comparable. These strains also all had comparable sterol profiles (Fig. 1F) and ergosterol levels (Table 1).
Table 1. Ergosterol levels in half knockout strains.
| Stage | Strain | Ergosterol levels (pmol) a |
|---|---|---|
| Promastigotes | Wild-type | 7236.7 |
| HKO | 5167.3 | |
| HKO + C | 7535.2 | |
| HKO + CYP | 5887.9 | |
| Axenic amastigotes | Wild-type | 1374.7 |
| HKO | 1102.8 | |
| HKO + C | 904.4 | |
| HKO + CYP | 983.3 | |
| Intracellular amastigotes | Wild-type | 135.2 |
| HKO | 165.3 | |
| HKO + C | 462.0 | |
| HKO + CYP | 327.7 |
a Normalized to host cholesterol levels for intracellular amastigotes
Essentiality of CYP51: Biological approach
Since L. donovani parasites were able to tolerate over two-fold reductions in CYP51 protein levels with no apparent effects on parasite phenotype, we then proceeded to targeting the second CYP51 allele. However, while we obtained correct targeting of CYP51 with hygromycin and puromycin resistance markers, double drug-resistant parasites still retained CYP51, despite multiple targeting attempts (Fig. 2A). We therefore used a facilitated knockout approach (Fig. 2B) [27,39,40], targeting the second CYP51 allele in the presence of episomal CYP51 complementation. As a control, we targeted this second allele in parasites transfected with the empty pXNG4 vector [27] (S2 Fig.). We obtained complete loss of chromosomal CYP51 genes only in the presence of episomal CYP51; genomic CYP51 was retained in the parasites transfected with the empty plasmid, supporting essentiality of CYP51 (Fig. 2C).
The pXNG4 vector used for complementation also encodes a green fluorescence protein gene (GFP) and a herpes virus thymidine kinase gene; cells that contain the plasmid are sensitive to treatment with ganciclovir (GCV). We therefore performed negative selection against the pXNG4 vector by treating transfected parasites with GCV. Plasmid persistence during GCV treatment was monitored by qPCR and by flow cytometry for GFP. The pXNG4 plasmid was lost much faster from parasites transfected with the empty vector (retain chromosomal CYP51) than from parasites transfected with the vector encoding CYP51 (only source of CYP51), indicating selection for CYP51 persistence (Fig. 2D, 2E). One clonal line (HKO1 + CYP + PAC2) showed greater loss of pXNG4 plasmid, but still retained CYP51 DNA levels comparable to half knockout strains, with 2^(-ΔΔCt) values of 0.6, leading to ergosterol levels similar to wild-type, even after 7 weeks of GCV selection (S4 Fig). Overall, these results support essentiality of CYP51 in L. donovani.
Essentiality of CYP51: Pharmacological approach
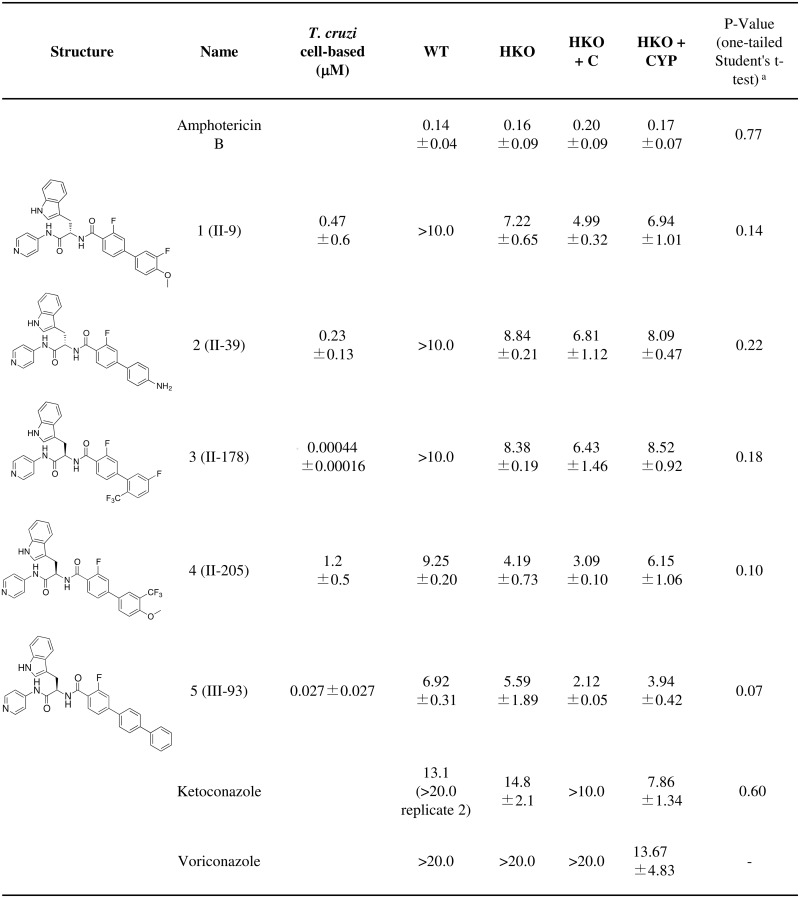
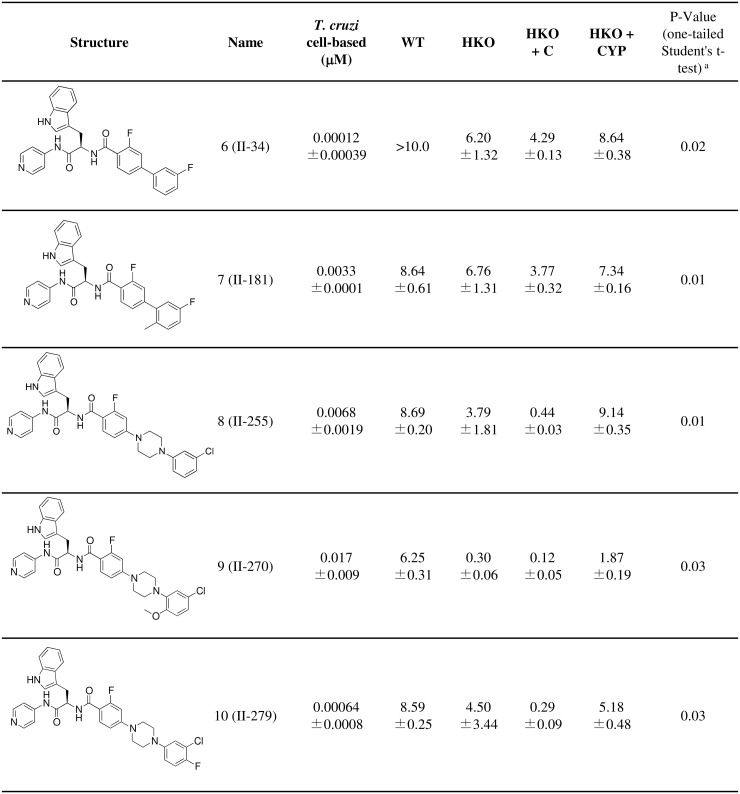
The persistence of CYP51-encoding pXNG4 plasmids even under GCV negative selection indicates that CYP51 is essential in L. donovani. Pharmacological inhibition of CYP51 should therefore lead to parasite growth arrest and death. The 4-aminopyridyl-based compound series of CYP51 inhibitors was derived from an initial hit in target-based high-throughput screening, followed by hit-to-lead optimization using structure-activity relationships (SAR), structure-property relationships (SPR), and biological and structural evaluation for T. cruzi CYP51 [34,35,36,41,42,43,44]. We tested 205 compounds from this series on wild-type intracellular L. donovani amastigotes by high content assay. Fifty-four compounds with over 60% activity at 10 μM were then used for dose-response experiments on wild-type L. donovani promastigotes and strains in which we modulated CYP51 expression (HKO, HKO+C and HKO+CYP). Representative compounds with the highest activity on promastigotes are shown in Fig. 3, Fig. 4. Activity on intracellular amastigotes is shown in S3 Table. No clear difference in EC50 values were observed between strains with ketoconazole and voriconazole controls, possibly due to their lower activity on L. donovani. In-house compounds were more potent in this assay than the commercial antifungal azoles. Overall, HKO+CYP strains were less sensitive to these 4-aminopyridyl-based inhibitors compared to HKO+C, indicating that these compounds inhibit Leishmania growth via a CYP51-mediated mechanism. This confirms that targeting CYP51 pharmacologically promotes inhibition of parasite growth, further supporting essentiality of CYP51 in L. donovani metabolism.
Fig 3. Susceptibility of half knockout strains to CYP51 inhibitors (EC50, μM): compounds with no significant differences between HKO + C and HKO + CYP strains.
Values ± standard error are shown. a p-values are for comparison between HKO+C and HKO+CYP.
Fig 4. Susceptibility of half knockout strains to CYP51 inhibitors (EC50, μM): compounds with decreased activity on HKO + CYP strains.
Values ± standard error are shown. a p-values are for comparison between HKO+C and HKO+CYP.
Discussion
CYP51 (sterol 14alpha-demethylase) belongs to the large cytochrome P450 enzyme family, which contains over 20,000 members. While there is significant variation at the sequence level, CYP51 is highly conserved across eukaroytes at the structural level [45]. However, small variations between species and strains can lead to significant variations in sensitivity to CYP51 inhibitors. Indeed, a single amino acid change in CYP51 in T. cruzi Y strain compared to Tulahuen strain was associated with significant decrease in sensitivity to two CYP51 inhibitors, at concentrations that caused 100% inhibition of the Tulahuen enzyme [46].
In vitro side-by-side comparison of azole efficacy on promastigotes between Leishmania species provides conflicting results: two studies observed increased susceptibility of six different L. donovani strains to ketoconazole and itraconazole compared to six different L. major strains [14,18], while other studies on different L. donovani and L. major strains indicated that L. donovani is more resistant to ketoconazole [47] and posaconazole [19] than L. major. In a separate study, intracellular L. donovani amastigotes were more sensitive to ketoconazole than amastigotes from cutaneous leishmaniasis patients [16]. L. major promastigotes were also insensitive to our 4-aminopyridyl-based compound series of CYP51 inhibitors, even with a longer exposure to the compounds (S2 Table). With regards to clinical trials, azoles have shown large variations in clinical efficacy between Leishmania species, from no effect to almost 90% efficacy [15], although the majority of these studies have focused on cutaneous leishmaniasis. While there is a single case report of successful posaconazole use to treat cutaneous leishmaniasis caused by L. donovani infantum [48], to our knowledge there has been no clinical trial of azoles for visceral leishmaniasis. Persistent Leishmania growth in the presence of azoles has been tied to tolerance to 14-methyl sterol accumulation in parasite membranes [14,18,49] as well as increased exogenous cholesterol incorporation [18].
Recent work indicated that CYP51 appears to be dispensable in L. major, albeit at a high fitness cost [14]. In contrast, given (1) our inability to fully knockout chromosomal CYP51 unless we provide an extrachromosomal episomal source of CYP51, (2) the persistence of this CYP51 episome during negative selection under conditions in which it is the only source of CYP51, and (3) the CYP51-specific growth inhibition of 4-aminopyridyl-based non-azole CYP51 inhibitors in L. donovani, our results support essentiality of CYP51 in L. donovani. While extrachromosomal episomal-encoded CYP51 could not fully complement the knockout phenotype, it was indeed active, given its ability to substitute for chromosomal CYP51 and to increase resistance to the 4-aminopyridyl-based non-azole CYP51 inhibitors which directly target the CYP51 active site [13,34,36].
L. donovani and L. major CYP51 are overall very similar. Comparing the L. major and L. donovani CYP51 protein sequences highlights two amino acid substitutions in β helices 1–1 and 1–2 and a single amino acid insertion at the C-terminal in L. donovani compared to L. major (S5 Fig). This suggests that other mechanisms may be responsible for the observed differences in CYP51 essentiality between L. major and L. donovani. Indeed, squalene synthase, which catalyzes the first committed step in ergosterol biosynthesis, has been involved in resistance to itraconazole [50]; differences in sensitivity to some squalene synthase inhibitors were observed between L. major and L. donovani [51]. Likewise, there were differences in sensitivity to sterol 24-methyltransferase inhibitors between L. major and L. donovani [52]. Finally, the activity of L. donovani 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase) was 50-fold higher than the activity of the L. major enzyme [53]. HMG-CoA reductase catalyzes the third step of sterol synthesis from acetyl-CoA and is the rate-limiting step in human sterol biosynthesis [54]. Finally, another member of the cytochrome P450 family, CYP5122A1 (LdBPK_270090.1), also modulates ergosterol levels in L. donovani [55]; its expression or activity could be altered in CYP51-deficient L. major, to complement for loss of CYP51.
Beyond differences in CYP51 and sterol biosynthetic pathways between L. major and L. donovani, additional factors could also contribute to this observed difference in CYP51 essentiality. Indeed, Xu et al showed that L. major CYP51 is involved in protection against heat shock [14]. L. major is considerably more sensitive to heat shock than L. donovani, but the mechanism of resistance to heat shock differs between these species, with the L. donovani-specific A2 protein family a key contributor to L. donovani survival during heat stress [25,32]. Likewise, gp63 and lipophosphoglycan levels were altered in CYP51-deficient L. major [14]. Lipophosphoglycan is structurally different between L. major and L. donovani [56], and gp63 from members of the L. donovani species complex is less active than L. major gp63 [57].
Overall, our results support further investigation of CYP51 inhibitors for the treatment of visceral leishmaniasis. While recent clinical trial results using posaconazole for the treatment of Chagas disease were disappointing [58], the enhanced potency we observed in L. donovani for 4-aminopyridyl-based non-azole inhibitors of CYP51 compared to ketoconazole and voriconazole supports the development of novel inhibitor scaffolds, potentially using our 4-aminopyridyl inhibitor series as a starting point. Given the lower efficacy of these inhibitors on Leishmania compared to T. cruzi, efforts should be made through further medicinal chemistry to optimize both pharmacodynamic and pharmacokimetic properties of these compounds for activity against Leishmania. In particular, efficacy was much lower for intracellular wild-type L. donovani amastigotes compared to our transfected promastigote strains (S3 Table), possibly due to additional constraints with regards to drug uptake into the host cell and into the parasite-containing acidic phagolysosome. Finally, this work and the work of others indicate that CYP51-targeted therapies may not be suitable to treat all Leishmania species. This highlights the importance of considering variations between species and strains early during the drug development process.
Supporting Information
(DOC)
Reagents and conditions: (a) Arylboronic acid, 5 mol% Pd2(dba)3, 10 mol% PCy3, 2M K3PO4, dioxane, 100°C (microwave), 1h, ca. 90% (b) 1-(aryl)piperazine, Pd(OAc)2, P(o-tolyl)3, Cs2CO3, toluene, 50°C, 48 h, ca. 70% (c) 10% NaOH (aq), MeOH/THF (1/1), 60°C, 3 h, ca. 95% (d) 11, 12, 13, 14, 15, 16, 17, 18b, 19b, and 20b (as appropriate), PyBOP, HOBt, Et3N, CH2Cl2, 23°C, 1h, ca. 50%.
(PPT)
THP1 macrophages were infected at a 10:1 parasite to macrophage ratio. Cells were fixed and stained with DAPI 24, 48 and 72 h post-infection, and parasite numbers per infected cell were determined by automated high-throughput imaging and parasite detection
(PPT)
Correct targeting of the hygromycin (A) and puromycin (B) resistance knockout cassettes was verified by PCR using one primer upstream of CYP51 and one specific to the resistance marker (primers 7 and 8 (hygromycin) or 7 and 9 (puromycin)). HKO1 + CYP + PAC clones 2, 4, 5; HKO2 + C + PAC clones 1, 2 and 3; HKO2 + CYP + PAC clones 1, 2, 4 and 5 have correct targeting of both knockout cassettes.
(PPT)
Parasites were treated with NTC (positive selection), GCV (negative selection) or left untreated (-NTC-GCV) for five weeks. One representative cell line is shown for HKO + C + PAC and for HKO + CYP + PAC. A, Quadrant analysis. Numbers indicate the percentage of cells in each quadrant. B, Representative GFP histogram plots of PI-negative cells. Wild-type parasites (dotted line) serve as the non-fluorescent cutoff reference. Black, NTC treatment (positive selection). Grey, GCV treatment (negative selection).
(PPT)
CYP51 persistence was assessed by qPCR (A) and Western blot (B) following seven weeks of GCV selection. Sterol profiles and ergosterol levels were determined by GC-MS (C). Chol., cholesterol. Erg, ergosterol.
(PPT)
A, Clustal Omega alignment. β 1–1 and 1–2 helices are positioned as in [12]. B, Secondary structure alignment. 3-D models of L. major and L. donovani CYP51 were generated using the I-TASSER server. The top scoring models were overlaid using UCSF Chimera. Red, L. donovani. Blue, L. major.
(PPT)
(XLS)
(DOC)
(DOC)
Acknowledgments
We wish to thank Dr Stephen Beverley, Washington University in St. Louis, for providing the pXNG4 plasmid, Dr Julio Urbina, Venezuelan Institute for Scientific Research (IVIC), for fruitful discussions and suggestions, Dr Claudia Calvet for the anti-CYP51 antibody, Dr Paul Michels, Université catholique de Louvain, Bruxelles for the anti-GAPDH antibody, and Brian Suzuki for help with the intravenous infections. The authors also thank the Ortiz de Montellano laboratory at UCSF for use of the GC-MS instrument.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
LIM acknowledges receiving a postdoctoral fellowship from the Fonds de Recherche Santé-Québec (29361, http://www.frqs.gouv.qc.ca/en/index.shtml). This work was supported in part by NIH RO1 grant AI095437 (to LMP and WRR, http://www.nih.gov/) and by the European Union Seventh Framework Programme (602773-KINDRED to JHM, http://cordis.europa.eu/fp7/home_en.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Murray HW, Berman JD, Davies CR, Saravia NG (2005) Advances in leishmaniasis. Lancet 366: 1561–1577. [DOI] [PubMed] [Google Scholar]
- 2. McCall LI, McKerrow JH (2014) Determinants of disease phenotype in trypanosomatid parasites. Trends in Parasitology 30: 342–349. 10.1016/j.pt.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 3. McCall LI, Zhang WW, Matlashewski G (2013) Determinants for the development of visceral leishmaniasis disease. PLoS Pathog 9: e1003053 10.1371/journal.ppat.1003053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desjeux P (2004) Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27: 305–318. [DOI] [PubMed] [Google Scholar]
- 5. Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, et al. (2012) Leishmaniasis Worldwide and Global Estimates of Its Incidence. Plos One 7: e35671 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hotez PJ, Alvarado M, Basanez MG, Bolliger I, Bourne R, et al. (2014) The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis 8: e2865 10.1371/journal.pntd.0002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW (2010) Single-Dose Liposomal Amphotericin B for Visceral Leishmaniasis in India. New England Journal of Medicine 362: 504–512. 10.1056/NEJMoa0903627 [DOI] [PubMed] [Google Scholar]
- 8. Khalil EA, Weldegebreal T, Younis BM, Omollo R, Musa AM, et al. (2014) Safety and Efficacy of Single Dose versus Multiple Doses of AmBisome(R) for Treatment of Visceral Leishmaniasis in Eastern Africa: A Randomised Trial. PLoS Negl Trop Dis 8: e2613 10.1371/journal.pntd.0002613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monge-Maillo B, Lopez-Velez R (2013) Therapeutic options for visceral leishmaniasis. Drugs 73: 1863–1888. 10.1007/s40265-013-0133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lepesheva GI, Waterman MR (2011) Sterol 14alpha-demethylase (CYP51) as a therapeutic target for human trypanosomiasis and leishmaniasis. Current topics in medicinal chemistry 11: 2060–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Souza W, Rodrigues JC (2009) Sterol Biosynthesis Pathway as Target for Anti-trypanosomatid Drugs. Interdisciplinary perspectives on infectious diseases 2009: 642502 10.1155/2009/642502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hargrove TY, Wawrzak Z, Liu J, Nes WD, Waterman MR, et al. (2011) Substrate preferences and catalytic parameters determined by structural characteristics of sterol 14alpha-demethylase (CYP51) from Leishmania infantum. J Biol Chem 286: 26838–26848. 10.1074/jbc.M111.237099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi JY, Podust LM, Roush WR (2014) Drug Strategies Targeting CYP51 in Neglected Tropical Diseases. Chemical Reviews. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu W, Hsu F-F, Baykal E, Huang J, Zhang K (2014) Sterol Biosynthesis Is Required for Heat Resistance but Not Extracellular Survival in Leishmania. PLoS Pathog 10: e1004427 10.1371/journal.ppat.1004427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, et al. (2007) Cutaneous leishmaniasis. The Lancet infectious diseases 7: 581–596. [DOI] [PubMed] [Google Scholar]
- 16. Berman JD (1982) In vitro susceptibility of antimony-resistant Leishmania to alternative drugs. The Journal of infectious diseases 145: 279 [DOI] [PubMed] [Google Scholar]
- 17. Gebre-Hiwot A, Frommel D (1993) The in-vitro anti-leishmanial activity of inhibitors of ergosterol biosynthesis. The Journal of antimicrobial chemotherapy 32: 837–842. [DOI] [PubMed] [Google Scholar]
- 18. Beach DH, Goad LJ, Holz GG Jr. (1988) Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Molecular and Biochemical Parasitology 31: 149–162. [DOI] [PubMed] [Google Scholar]
- 19. Al-Abdely HM, Graybill JR, Loebenberg D, Melby PC (1999) Efficacy of the triazole SCH 56592 against Leishmania amazonensis and Leishmania donovani in experimental murine cutaneous and visceral leishmaniases. Antimicrobial Agents and Chemotherapy 43: 2910–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gangneux JP, Dullin M, Sulahian A, Garin YJ, Derouin F (1999) Experimental evaluation of second-line oral treatments of visceral leishmaniasis caused by Leishmania infantum. Antimicrobial Agents and Chemotherapy 43: 172–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hart DT, Lauwers WJ, Willemsens G, Vanden Bossche H, Opperdoes FR (1989) Perturbation of sterol biosynthesis by itraconazole and ketoconazole in Leishmania mexicana mexicana infected macrophages. Molecular and Biochemical Parasitology 33: 123–134. [DOI] [PubMed] [Google Scholar]
- 22. Berman JD, Goad LJ, Beach DH, Holz GG (1986) Effects of Ketoconazole on Sterol Biosynthesis by Leishmania-Mexicana Mexicana Amastigotes in Murine Macrophage Tumor-Cells. Molecular and Biochemical Parasitology 20: 85–92. [DOI] [PubMed] [Google Scholar]
- 23. Berman JD, Gallalee JV (1987) In vitro antileishmanial activity of inhibitors of steroid biosynthesis and combinations of antileishmanial agents. The Journal of parasitology 73: 671–673. [PubMed] [Google Scholar]
- 24. Goad LJ, Holz GG Jr., Beach DH (1985) Sterols of ketoconazole-inhibited Leishmania mexicana mexicana promastigotes. Molecular and Biochemical Parasitology 15: 257–279. [DOI] [PubMed] [Google Scholar]
- 25. McCall LI, Matlashewski G (2012) Involvement of the Leishmania donovani virulence factor A2 in protection against heat and oxidative stress. Exp Parasitol 132: 109–115. 10.1016/j.exppara.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 26. Aslett M, Aurrecoechea C, Berriman M, Brestelli J, Brunk BP, et al. (2010) TriTrypDB: a functional genomic resource for the Trypanosomatidae. Nucleic Acids Research 38: D457–D462. 10.1093/nar/gkp851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murta SM, Vickers TJ, Scott DA, Beverley SM (2009) Methylene tetrahydrofolate dehydrogenase/cyclohydrolase and the synthesis of 10-CHO-THF are essential in Leishmania major. Mol Microbiol 71: 1386–1401. 10.1111/j.1365-2958.2009.06610.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robinson KA, Beverley SM (2003) Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Molecular and Biochemical Parasitology 128: 217–228. [DOI] [PubMed] [Google Scholar]
- 29. Medinaacosta E, Cross GAM (1993) Rapid Isolation of DNA from Trypanosomatid Protozoa Using a Simple Mini-Prep Procedure. Molecular and Biochemical Parasitology 59: 327–329. [DOI] [PubMed] [Google Scholar]
- 30. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 31. Downing T, Imamura H, Decuypere S, Clark TG, Coombs GH, et al. (2011) Whole genome sequencing of multiple Leishmania donovani clinical isolates provides insights into population structure and mechanisms of drug resistance. Genome Research 21: 2143–2156. 10.1101/gr.123430.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCall LI, Matlashewski G (2010) Localization and induction of the A2 virulence factor in Leishmania: evidence that A2 is a stress response protein. Mol Microbiol 77: 518–530. 10.1111/j.1365-2958.2010.07229.x [DOI] [PubMed] [Google Scholar]
- 33. Gunatilleke SS, Calvet CM, Johnston JB, Chen CK, Erenburg G, et al. (2012) Diverse inhibitor chemotypes targeting Trypanosoma cruzi CYP51. PLoS Negl Trop Dis 6: e1736 10.1371/journal.pntd.0001736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi JY, Calvet CM, Vieira DF, Gunatilleke SS, Cameron MD, et al. (2014) R-Configuration of 4-Aminopyridyl-Based Inhibitors of CYP51 Confers Superior Efficacy Against Trypanosoma cruzi. Acs Medicinal Chemistry Letters 5: 434–439. 10.1021/ml500010m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choi JY, Calvet CM, Gunatilleke SS, Ruiz C, Cameron MD, et al. (2013) Rational development of 4-aminopyridyl-based inhibitors targeting Trypanosoma cruzi CYP51 as anti-chagas agents. Journal of Medicinal Chemistry 56: 7651–7668. 10.1021/jm401067s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calvet CM, Vieira DF, Choi JY, Kellar D, Cameron MD, et al. (2014) 4-Aminopyridyl-based CYP51 inhibitors as anti-Trypanosoma cruzi drug leads with improved pharmacokinetic profile and in vivo potency. Journal of Medicinal Chemistry 57: 6989–7005. 10.1021/jm500448u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rogers MB, Hilley JD, Dickens NJ, Wilkes J, Bates PA, et al. (2011) Chromosome and gene copy number variation allow major structural change between species and strains of Leishmania. Genome Research 21: 2129–2142. 10.1101/gr.122945.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ubeda JM, Raymond F, Mukherjee A, Plourde M, Gingras H, et al. (2014) Genome-wide stochastic adaptive DNA amplification at direct and inverted DNA repeats in the parasite leishmania. PLoS biology 12: e1001868 10.1371/journal.pbio.1001868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dacher M, Morales MA, Pescher P, Leclercq O, Rachidi N, et al. (2014) Probing druggability and biological function of essential proteins in Leishmania combining facilitated null mutant and plasmid shuffle analyses. Mol Microbiol. [DOI] [PubMed] [Google Scholar]
- 40. Norris-Mullins B, VanderKolk K, Vacchina P, Joyce MV, Morales MA (2014) LmaPA2G4, a Homolog of Human Ebp1, Is an Essential Gene and Inhibits Cell Proliferation in L. major. PLoS Negl Trop Dis 8: e2646 10.1371/journal.pntd.0002646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Podust LM, von Kries JP, Eddine AN, Kim Y, Yermalitskaya LV, et al. (2007) Small-molecule scaffolds for CYP51 inhibitors identified by high-throughput screening and defined by X-ray crystallography. Antimicrobial Agents and Chemotherapy 51: 3915–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vieira DF, Choi JY, Roush WR, Podust LM (2014) Expanding the binding envelope of CYP51 inhibitors targeting Trypanosoma cruzi with 4-Aminopyridyl-based sulfonamide derivatives. Chembiochem: a European journal of chemical biology 15: 1111–1120. 10.1002/cbic.201402027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen CK, Doyle PS, Yermalitskaya LV, Mackey ZB, Ang KK, et al. (2009) Trypanosoma cruzi CYP51 inhibitor derived from a Mycobacterium tuberculosis screen hit. PLoS Negl Trop Dis 3: e372 10.1371/journal.pntd.0000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doyle PS, Chen CK, Johnston JB, Hopkins SD, Leung SS, et al. (2010) A nonazole CYP51 inhibitor cures Chagas′ disease in a mouse model of acute infection. Antimicrobial Agents and Chemotherapy 54: 2480–2488. 10.1128/AAC.00281-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lepesheva GI, Waterman MR (2011) Structural basis for conservation in the CYP51 family. Biochimica Et Biophysica Acta 1814: 88–93. 10.1016/j.bbapap.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cherkesova TS, Hargrove TY, Vanrell MC, Ges I, Usanov SA, et al. (2014) Sequence variation in CYP51A from the Y strain of Trypanosoma cruzi alters its sensitivity to inhibition. Febs Letters 588: 3878–3885. 10.1016/j.febslet.2014.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kulkarni MM, Reddy N, Gude T, McGwire BS (2013) Voriconazole suppresses the growth of Leishmania species in vitro. Parasitology Research 112: 2095–2099. 10.1007/s00436-013-3274-x [DOI] [PubMed] [Google Scholar]
- 48. Paniz Mondolfi AE, Stavropoulos C, Gelanew T, Loucas E, Perez Alvarez AM, et al. (2011) Successful treatment of Old World cutaneous leishmaniasis caused by Leishmania infantum with posaconazole. Antimicrobial Agents and Chemotherapy 55: 1774–1776. 10.1128/AAC.01498-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rangel H, Dagger F, Hernandez A, Liendo A, Urbina JA (1996) Naturally azole-resistant Leishmania braziliensis promastigotes are rendered susceptible in the presence of terbinafine: comparative study with azole-susceptible Leishmania mexicana promastigotes. Antimicrobial Agents and Chemotherapy 40: 2785–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cotrim PC, Garrity LK, Beverley SM (1999) Isolation of genes mediating resistance to inhibitors of nucleoside and ergosterol metabolism in Leishmania by overexpression/selection. J Biol Chem 274: 37723–37730. [DOI] [PubMed] [Google Scholar]
- 51. Lorente SO, Gomez R, Jimenez C, Cammerer S, Yardley V, et al. (2005) Biphenylquinuclidines as inhibitors of squalene synthase and growth of parasitic protozoa. Bioorganic & Medicinal Chemistry 13: 3519–3529. [DOI] [PubMed] [Google Scholar]
- 52. Lorente SO, Rodrigues JCF, Jimenez CJ, Joyce-Menekse M, Rodrigues C, et al. (2004) Novel azasterols as potential agents for treatment of leishmaniasis and trypanosomiasis. Antimicrobial Agents and Chemotherapy 48: 2937–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dinesh N, Pallerla DS, Kaur PK, Kishore Babu N, Singh S (2014) Exploring Leishmania donovani 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) as a potential drug target by biochemical, biophysical and inhibition studies. Microbial Pathogenesis 66: 14–23. 10.1016/j.micpath.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 54. Friesen JA, Rodwell VW (2004) The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome biology 5: 248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Verma S, Mehta A, Shaha C (2011) CYP5122A1, a novel cytochrome P450 is essential for survival of Leishmania donovani. Plos One 6: e25273 10.1371/journal.pone.0025273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kamhawi S, Modi GB, Pimenta PFP, Rowton E, Sacks DL (2000) The vectorial competence of Phlebotomus sergenti is specific for Leishmania tropica and is controlled by species-specific, lipophosphoglycan-mediated midgut attachment. Parasitology 121: 25–33. [DOI] [PubMed] [Google Scholar]
- 57. Tzinia AK, Soteriadou KP (1991) Substrate-dependent pH optima of gp63 purified from seven strains of Leishmania. Molecular and Biochemical Parasitology 47: 83–89. [DOI] [PubMed] [Google Scholar]
- 58. Molina I, Gomez i Prat J, Salvador F, Trevino B, Sulleiro E, et al. (2014) Randomized trial of posaconazole and benznidazole for chronic Chagas′ disease. The New England journal of medicine 370: 1899–1908. 10.1056/NEJMoa1313122 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Reagents and conditions: (a) Arylboronic acid, 5 mol% Pd2(dba)3, 10 mol% PCy3, 2M K3PO4, dioxane, 100°C (microwave), 1h, ca. 90% (b) 1-(aryl)piperazine, Pd(OAc)2, P(o-tolyl)3, Cs2CO3, toluene, 50°C, 48 h, ca. 70% (c) 10% NaOH (aq), MeOH/THF (1/1), 60°C, 3 h, ca. 95% (d) 11, 12, 13, 14, 15, 16, 17, 18b, 19b, and 20b (as appropriate), PyBOP, HOBt, Et3N, CH2Cl2, 23°C, 1h, ca. 50%.
(PPT)
THP1 macrophages were infected at a 10:1 parasite to macrophage ratio. Cells were fixed and stained with DAPI 24, 48 and 72 h post-infection, and parasite numbers per infected cell were determined by automated high-throughput imaging and parasite detection
(PPT)
Correct targeting of the hygromycin (A) and puromycin (B) resistance knockout cassettes was verified by PCR using one primer upstream of CYP51 and one specific to the resistance marker (primers 7 and 8 (hygromycin) or 7 and 9 (puromycin)). HKO1 + CYP + PAC clones 2, 4, 5; HKO2 + C + PAC clones 1, 2 and 3; HKO2 + CYP + PAC clones 1, 2, 4 and 5 have correct targeting of both knockout cassettes.
(PPT)
Parasites were treated with NTC (positive selection), GCV (negative selection) or left untreated (-NTC-GCV) for five weeks. One representative cell line is shown for HKO + C + PAC and for HKO + CYP + PAC. A, Quadrant analysis. Numbers indicate the percentage of cells in each quadrant. B, Representative GFP histogram plots of PI-negative cells. Wild-type parasites (dotted line) serve as the non-fluorescent cutoff reference. Black, NTC treatment (positive selection). Grey, GCV treatment (negative selection).
(PPT)
CYP51 persistence was assessed by qPCR (A) and Western blot (B) following seven weeks of GCV selection. Sterol profiles and ergosterol levels were determined by GC-MS (C). Chol., cholesterol. Erg, ergosterol.
(PPT)
A, Clustal Omega alignment. β 1–1 and 1–2 helices are positioned as in [12]. B, Secondary structure alignment. 3-D models of L. major and L. donovani CYP51 were generated using the I-TASSER server. The top scoring models were overlaid using UCSF Chimera. Red, L. donovani. Blue, L. major.
(PPT)
(XLS)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.