Abstract
There is a demand for effective training methods that encourage exercise adherence during advancing age, particularly in sedentary populations. This study examined the effects of high-intensity interval training (HIIT) exercise on health-related quality of life (HRQL), aerobic fitness and motivation to exercise in ageing men. Participants consisted of males who were either lifelong sedentary (SED; N = 25; age 63 ± 5 years) or lifelong exercisers (LEX; N = 19; aged 61 ± 5 years). and HRQL were measured at three phases: baseline (Phase A), week seven (Phase B) and week 13 (Phase C). Motivation to exercise was measured at baseline and week 13. was significantly higher in LEX (39.2 ± 5.6 ml kg min−1) compared to SED (27.2 ± 5.2 ml kg min−1) and increased in both groups from Phase A to C (SED 4.6 ± 3.2 ml kg min−1, 95 % CI 3.1 – 6.0; LEX 4.9 ± 3.4 ml kg min−1, 95 % CI 3.1–6.6) Physical functioning (97 ± 4 LEX; 93 ± 7 SED) and general health (70 ± 11 LEX; 78 ± 11 SED) were significantly higher in LEX but increased only in the SED group from Phase A to C (physical functioning 17 ± 18, 95 % CI 9–26, general health 14 ± 14, 95 % CI 8–21). Exercise motives related to social recognition (2.4 ± 1.2 LEX; 1.5 ± 1.0 SED), affiliation (2.7 ± 1.0 LEX; 1.6 ± 1.2 SED) and competition (3.3 ± 1.3 LEX; 2.2 ± 1.1) were significantly higher in LEX yet weight management motives were significantly higher in SED (2.9 ± 1.1 LEX; 4.3 ± 0.5 SED). The study provides preliminary evidence that low-volume HIIT increases perceptions of HRQL, exercise motives and aerobic capacity in older adults, to varying degrees, in both SED and LEX groups.
Keywords: Ageing men, High-intensity interval training, Health-related quality of life, Motivation, Exercise
Introduction
Current physical activity guidelines for older adults aged 65 years or older recommend at least 150 min of moderate-intensity aerobic activity per week and muscle strengthening exercises on at least 2 days per week (Department of Health 2011). However, data from the Health England Survey in 2012 (Craig and Mindell 2012) indicated that only 58 and 52 % of men and women, respectively, aged 65–74 years old and 44 and 20 % of men and women, respectively, aged 75–84 years were achieving the recommended amounts of physical activity for health. The benefits of regular exercise are widely acknowledged. Older adults can achieve specific benefits as exercise may offset the rapid decline in muscle mass, aerobic capacity (Chrysohoou et al. 2014) and cognitive function (Hogan 2005; Snowden et al. 2011), as well as enhancing psychological well-being and quality of life (McAuley et al. 2006; Penedo and Dahn 2005). Despite these benefits, many older adults remain sedentary and few achieve the recommended levels of physical activity needed to accrue these health benefits.
For many older adults, the ageing process centres on a perceived concurrent loss of control (Bandura 1997) and decline in quality of life. Quality of life is defined by the World Health Organisation as, “an individual’s perception of their position in life in the context of culture and value systems in which they live, and in relation to their goals, expectations, standards and concerns” (p. 1). Health-related quality of life (HRQL) is a multi-dimensional construct that has been used to determine and/or evaluate the impact of illness and treatment. Domain measures of HRQL extend beyond the presence or absence of illness to that of complete physical, mental and social well-being and general health perceptions. Health-related quality of life is often considered as a secondary outcome measure in exercise intervention research. However, independent of physiological improvements, ensuring an individual’s perceptions of their HRQL, are positive and maintenance of HRQL during ageing is of primary importance.
Previous research has demonstrated that various types of exercise training (e.g. aerobic, strength, functional strength and flexibility) increase perceptions of HRQL in older adults (King et al. 2000; Reid et al. 2010; Rejeski and Mihalko 2001). However, a minority of studies have examined HRQL in response to high-intensity, interval-based activities, with positive findings evident in ageing heart failure patients (Chrysohoou et al. 2014). Physically active older adults tend to consistently favour lower-intensity activities such as walking, golf and gardening, yet older adults have been reported to find exercise participation overly time-consuming (Chao et al. 2000) and this could be considered an important barrier for those older adults who are not regularly active. Lifestyle habits and perceived barriers to exercise are often deep-rooted in older adults and altering their motivation to exercise and ensuring long-term maintenance of behaviour change is a significant challenge. Consequently, there is a need to consider alternative methods to encourage and motivate older adults, in particular those who are currently sedentary, to adopt and maintain exercise during advancing ageing.
High-intensity interval training (HIIT) has recently re-emerged as a viable method of improving cardiovascular health amongst a variety of young and adult populations. HIIT is characterised by brief, intermittent bursts of vigorous exercise, interspersed by periods of rest or low intensity recovery (Gibala et al. 2012). There is an emergent body of evidence that endorses HIIT as an effective alternative to traditional endurance training that can yield improvements in both cardiorespiratory fitness and variety of health outcomes. Previous (Pollock, 1977) and more recent supporting evidence has demonstrated that a single HIIT exercise session, performed once per week, can improve in young adults (Matsuo et al. 2014; Nakahara et al. 2014) and a recent meta-analysis of low-volume HIIT acknowledged the young age of participants enrolled to such studies and further proposed that studies using older participants were required to clarify the effects of low-volume HIIT in older participants (Weston et al. 2014b).
Further to this, our research group has recently demonstrated, using a parallel-randomised crossover trial, that older men (aged ~62 years) required 5 days for recovery of peak leg power output (PPO), following a single session of HIIT compared with younger (aged ~22 years) counterparts (Herbert et al. 2015b) who achieved complete recovery within 3 days. This study of Herbert and colleagues provides the first evidence that HIIT protocols utilising standard frequencies (3 × session wk−1) are likely to be overly fatiguing, thus poorly tolerated amongst older cohorts, and supports the requirement for low-frequency high-intensity interval exercise training (LfHIIT) in older individuals to employ an extended recovery protocol.
With this in mind, the aim of the present study was to examine the effects of HIIT exercise on HRQL, motivation to exercise and aerobic fitness in ageing men. It was hypothesised that HIIT exercise would improve HRQL, aerobic fitness and motivations to exercise in lifelong sedentary ageing men (SED) compared with a positive control group comprising age-matched lifelong exercisers (LEX). A secondary aim was to determine the impact of a greatly reduced relative volume of exercise work on HRQL, maximal aerobic capacity and motivation to exercise in LEX participants. It was hypothesised that low-volume HIIT would maintain aerobic fitness in LEX.
Methods
Participants
Forty four male participants over the age of 55 years were recruited to the study and allocated to either: (1) SED (N = 25; age 63 ± 5 years), men who did not participate in any formal exercise programme and had not done so for a minimum of 30 year or (2) LEX (N = 19; aged 61 ± 5 years), men who were highly active regular exercisers and who exercised for an average 281 ± 144 min per week. Twelve LEX participants were current active Masters national competitors in sports including triathlon, athletics, sprint cycling and racquet sports.
Participants were male volunteers who had responded to recruitment posters placed in leisure centres, medical surgeries, public houses, coffee shops and newsagents in the Carmarthen district of South Wales, UK. Participants were met individually for an informal explanation of the study objectives and supplied with a participant information sheet. As a condition to study enrolment, general medical practitioners (GPs) for each potential participant were contacted and provided with a copy of the study design, protocols and intended exercise programmes. GPs were invited to contact the lead authors with any query relating to the study and were further required to provide a written letter of approval for their patient to enrol to the study. Participants were withdrawn if, in the opinion of their GP, risks to their health were present. This could include a history of myocardial infarction, angina, stroke and chronic pulmonary disease. Consequently, three of the original 47 participants were advised to withdraw under GP advice. The remaining 44 participants completed a physical activity readiness questionnaire (PAR-Q) and provided written informed consent, which was approved by the institution’s research ethics committee, and enrolled for baseline measurements. Power calculation for the present study was based on previously published data regarding changes in aerobic capacity in older but otherwise healthy men (Hepple et al. 1997). G*Power V3 was used to calculate the sample size required for a single-tailed within-group comparison (alpha set to 0.05 and power set to 0.95). This resulted in a required sample size of 17 participants per group. The within groups effect size from Phase A to Phase C was 0.97.
Study design
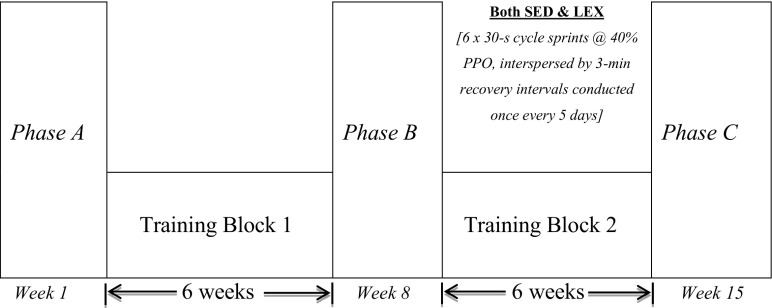
The study employed a prospective cohort intervention design with LEX group acting as a positive control. Upon enrolment to the study, two briefing sessions were conducted in which all study requirements were explained and preceded familiarisation to equipment and procedures. As SED participants were unaccustomed to exercise and the effects of HIIT exercise in sedentary ageing men is largely unknown, prudence dictated that the SED group should undertake 6 weeks of supervised progressive conditioning exercise (training block 1) whilst LEX maintained their normal exercise training (Fig. 1). SED and LEX participants kept a weekly log detailing exercise achievements, which was documented and confirmed using telemetric heart rate data (Polar, Kempele, Finland) at the end of each week. The study consisted of three data collection phases (Phases A, B and C), separated by two distinct training blocks (training blocks 1 and 2). During Phase A, LEX completed on average 290 +/− 128 min wk−1 of structured exercise training each week (range 180–550 min wk−1), whilst SED achieved the American College of Sports Medicine (ACSM) guidelines of 150 min wk−1 of supervised exercise from weeks 3 to 6 inclusively. In each measurement phase, data were obtained 5 days following the last exercise session at the same time of day, where possible.
Fig. 1.
Schematic depicting study design incorporating three testing phases (A, B and C) of two distinct training blocks for lifelong sedentary (SED) and lifelong exercising (LEX) groups
Measures
Psychological measures
Health-related quality of life and exercise motivation questionnaires were completed by the SED group at measurement Phases A, B and C and by the LEX group at A and C. Health-related quality of life was assessed using the Medical Outcomes Survey Short Form-36 (MOS SF-36) questionnaire (Ware and Sherbourne 1992) which has shown to be a reliable and valid criterion measure of HRQL in numerous populations, including older adults (Acree et al. 2006; Marsh et al. 2009). The MOS SF-36 questionnaire has 36 questions that are scored to measure eight domains of HRQL pertaining to both physical and mental health. The domains of physical functioning, role limitations due to physical health (role-physical), bodily pain and general health relate to the physical component of HRQL. Domains of vitality, social functioning, role limitations due to emotional health (role-emotional) and mental health relate to the mental component of HRQL. Each domain was scored using a scale ranging between 0 and 100, with higher scores indicating higher quality of life. Internal consistency for the eight domains of the MOS SF-36 was good with Cronbach’s alphas ranging between 0.65 and 0.89.
Exercise motives were assessed using the Exercise Motives Inventory-II (Markland and Ingledew 1997), which has been shown to be a reliable and valid measure of motives for exercising in a range of population samples, including older adults (Dacey et al. 2008). The inventory has 51 questions examining exercise motives across 14 subscales: Affiliation, Appearance, Challenge, Competition, Enjoyment, Health Pressures, Ill-Health Avoidance, Nimbleness, Positive Health, Revitalisation, Social Recognition, Strength and Endurance, Stress Management and Weight Management. Each subscale comprises of three or four items. Scores for each subscale are calculated from the mean of item scores. Internal consistency for the 14 subscales was good, with Cronbach’s alphas ranging between 0.63 and 0.91.
Laboratory measures
On assessment Phases A, B and C, participants arrived in the exercise physiology laboratory between the hours of 07:00–09:00 a.m., having abstained from any strenuous activity for a minimum of 48 h. Participants were instructed to arrive in a hydrated state having abstained from caffeine and alcohol consumption for 36 h. Participants were reminded to maintain standardised conditions prior to each assessment phase.
Determination of peak power output
Peak power output (PPO) was determined using a 6-s peak power test (PPO-6 s) on an air-braked cycle ergometer (Wattbike Ltd., Nottingham, UK), which we have recently shown to be a valid measure of PPO generated during 30 s Wingate test on a Monark 818E cycle ergometer (Herbert et al. 2015a). Prior to conducting each test, the Wattbike cycle ergometer was calibrated according to the manufacturers’ guidelines. Saddle height was adjusted relative to the crank position with participant’s knee joint at almost full extension (approx. 170–180°), and the foot was secured to a pedal with clips. Each test was preceded with a 5-min warm-up on the Wattbike set at resistance level 8, corresponding to a Borg-derived rating of perceived exertion (RPE) 11–13 (light to somewhat hard) incorporating two acceleration phases of ~3 s commencing after 90 and 180 s followed by a 5-min recovery. The PPO-6 s employed a seated stationary start with dominant leg initiating the first down-stroke. The air braking resistance was set to level 10, and magnetic resistance set to level 1 (equating to 1045 W at 130 rpm and approximately 90–100 W increases for every further 5-rpm increase in cadence). The test was initiated following a 5-s countdown followed by a firm verbal command. The completion of the test was indicated with another verbal command. PPO data were subsequently used to determine the starting cadence during the individual tests (further detailed below) and establishing the intensity of individual HIIT intervals during training block 2 (further detailed below).
Determination of aerobic capacity
Aerobic capacity was determined using open circuit spirometry using a Cortex II Metalyser 3B-R2 (Cortex, Biophysik, Leipzig, Germany). Expiratory airflow was achieved using a volume transducer (Triple V® turbine, digital) connected to an oxygen (O2) analyser. Expired gases were analysed for O2 with electrochemical cells and for carbon dioxide (CO2) output with an infrared analyser. Prior to each test, the Metalyser was calibrated according to the manufacturers’ guidelines. After a 60-min warm-up period, the CO2 and O2 sensors were calibrated against room air and to a reference gas of known composition (5 % CO2, 15 % O2 and 80 % N2). Volume measurement was calibrated by five inspiratory and expiratory strokes using a 3-litre pump. Five minutes of warm-up exercise preceded a ramped protocol until volitional exhaustion. Participant performance on peak power test dictated the cadence (either 70, 75, 80 or 85 rpm) to be maintained throughout the test. Participants warmed up on resistance setting level 1 (75 rpm = 100 W) at the cadence they would use in the test, which was conducted using a modified Storer test (Storer et al. 1990), which estimates oxygen uptake using body mass, age and peak power output (PPO). Work rate was increased each minute by raising the damper setting by one (equating to 18 W) until volitional exhaustion was achieved. Based on prior pilot study, the test was expected to elicit in 10 ± 2 min. Oxygen uptake , carbon dioxide production , respiratory exchange ratio (RER) and ventilation were displayed continuously. Heart rate (HR) was recorded every 5 s using short-range telemetry (Polar T31, Kempele, Finland). Coefficient of variation (CV) for the determination of HRmax in our laboratory is <1.4 %. Participants indicated perceived exertion using the Borg (1973) scale, which was recorded during the last 10 s of each 1-min stage. Fingertip blood lactate (BLa−1) samples were collected into a portable automated lactate analyser (Lactate Pro, Arkray, Inc., Kyoto, Japan) within 45 s and again 5 min following the termination of the test. Breath by breath data were sampled and transferred to a PC for real-time display. The recorded data was saved to the internal database (Metasoft version 3.7.0) until analysis. Coefficient of variation (CV) for the determination of in our laboratory is <3.0 %. was confirmed when participants achieved a minimum of any four of the following criteria: plateau, respiratory exchange ratio at >1.10, peak heart rate within 10 beats of age-predicted maximum and blood lactate above 8 mmol/L−1, final rating of perceived exertion >18 on Borg Scale.
Training block 1: preconditioning exercise
The SED participants completed training block 1 which comprised 6 weeks personalised and supervised exercise designed to achieve the ACSM recommended guidelines of moderate-intensity physical activity (150 min · week−1, ≥30 min · day−1 on ≥5 days · week−1). Light to moderate exercise was advised for the first 2 weeks reaching 130–150 min by week three. Participants were instructed on the use of a Polar FT1 HR monitor (Polar, Kempele, Finland) which enabled self-monitoring and recording of exercise time, average and peak HR. The training target for SED participants was to achieve an average HR reserve (HRR) approximating 55 % for the first 2 weeks, increasing to 60 % of HRR for the subsequent 2 weeks and 60–65 % HRR for the final 2 weeks. During the final 2 weeks, participants were encouraged to include some vigorous bursts of exercise during their exercise sessions. The mode of training was optional, and included walking, walking/jogging, jogging and cycling which was adapted to suit the participants’ current physiological and physical status.
Training block 2: high-intensity interval training
The rationale for extended recovery between high-intensity interval training (HIIT) sessions during training block 2 is supported by our recent work where 3- and 5-day recovery strategies from single HIIT sessions (6 × 30 s at 50 % PPO) in active older participants compared with active younger counterparts identified that 5 days of recovery was required for recovery of PPO from HIIT amongst the older cohort (Herbert et al. 2015b). Both groups completed training block 2 which consisted of HIIT sessions performed once every 5 days for 6 weeks (9 sessions) with each session consisting of 6 × 30 s sprints at 40 % of PPO determined during familiarisation. HIIT sessions were performed on a Wattbike Pro cycle ergometer (Wattbike Ltd., Nottingham, UK) which was interspersed with 3-min active recovery intervals against a low (0–50 W) resistance and self-selected speed. HIIT sessions were conducted in groups of between four and six participants. The HIIT sessions were the sole exercise performed by both SED and LEX groups during this training block period and immediately preceded Phase C. Two participants missed three sessions due to vacation. However, both completed improvised training sessions, which included six repetitions of 30 s duration of high-intensity cycle ergometry, interspersed with 3 min of active recovery. To allow for comparison with other literature, training intensities were compared with power achieved at . In the majority of cases, 40 % of PPO was greater than power at in both SED and LEX; in four cases (1 SED; 3 LEX), it exceeded 90 % of power at (92; 92; 96; 98 %, respectively). In SED, mean training intensity equated to 141 ± 27 % of power at , whilst in LEX, mean training intensity equated to 126 ± 22 % of power output at , confirming that training doses were commensurate with LV-HIIT.
Statistical analysis
Data are reported as mean ± s.d. Differences in and the separate constructs of the EMI-2 and SF-36 questionnaires were analysed using a two-factor mixed-model repeated measures ANOVA to examine the effects of training status (SED vs. LEX), and HIIT (Phase A vs. Phase C) and the interaction between the two. Given the likely difference in outcome measures between the groups at Phase A, these data were also analysed using a univariate ANCOVA model (Vickers and Altman 2001). For each variable, the delta values (pre-post differences) were entered as the dependent variable, the Phase A values as the covariate and the training status (SED vs. LEX) as the fixed factor. A separate one-factor within-subjects repeated measures ANOVA was used to determine the differences in and the separate constructs of the EMI-2 and SF-36 questionnaires between Phases A, B and C in the SED group. Post-hoc analysis was completed using Bonferroni multiple comparisons. The null hypothesis was rejected when P < 0.05. Confidence intervals (95 % CI) and effect sizes (Cohen’s d or ή 2) are included together with P values, where appropriate. Cohen’s d effect sizes were interpreted as: small effect = 0.20–0.49, medium effect = 0.50–0.79 and large effect ≥0.80. ή 2 effect sizes were interpreted as: small effect = 0.02–0.12, medium effect = 0.13–0.25 and large effect ≥0.26. All statistical procedures were completed using SPSS for windows, version 19.0.
Results
Participants
Of the 44 participants enrolled to the study, five withdrew (3 SED, 2 LEX), during the first 2 weeks of training block 1. Reasons for withdrawal were: two for personal reasons that would impact commitment to the study, one due to arthritic discomfort, one due to lower back discomfort and one due to arrhythmia that was later confirmed to be asymptomatic vascular disease. The remaining 39 (22 SED, 17 LEX) participants completed training blocks 1 and 2, without accession.
Aerobic capacity
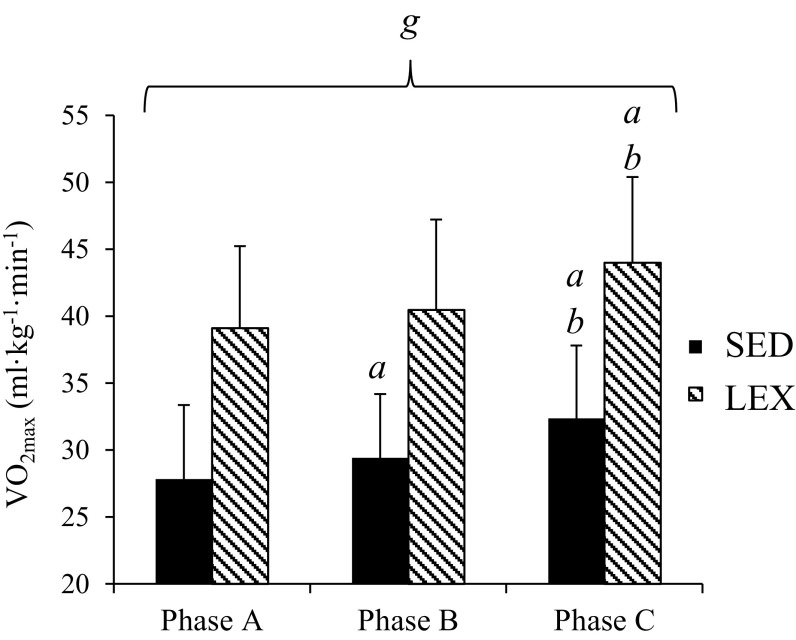
The two-way repeated measures ANOVA revealed a significant difference in between groups (P < 0.001) and a significant main effect of measurement phase (P < 0.001). There was no interaction between groups and measurement phase (P = 0.858). Participants in the LEX group had significantly higher values of than the SED group at all phases (Fig. 2). The ANCOVA established that differences in between groups at Phase A did not have a significant effect on the response to training. In the SED group, there was a medium increase in between Phase A to Phase B (1.6 ± 2.6 ml · kg−1 · min−1, P = 0.03, 95 % CI 0.1–3.1, d = 0.60) and a large increase from Phase B to Phase C (3.0 ± 2.8 ml · kg−1 · min−1, P < 0.001, 95 % CI 1.4–4.5, d = 1.06). There was no difference in between Phase A and Phase B in LEX (P = 0.38) but was significantly higher at Phase C compared to both Phase A (4.9 ± 3.4 ml · kg−1 · min−1, P < 0.001, 95 % CI 2.7–7.1, d = 1.43) and Phase B (3.5 ± 4.0 ml · kg−1 · min−1, P < 0.01, 95 % CI 1.0–6.1, d = 0.90).
Fig. 2.
Mean ± s.d. maximal aerobic capacity in sedentary (SED) participants and lifelong exercisers (LEX) at Phase A (baseline), Phase B (post-conditioning training in untrained and post-maintenance of training in LEX) and Phase C (post-high-intensity exercise training in both groups). a denotes significant increase from Phase A (P < 0.05), b denotes significant increase from Phase B and g denotes significant difference between the SED and LEX groups
Health-related quality of life (SF-36)
Between and within subject effects
Descriptive statistics from the eight constructs of the SF-36 questionnaire are presented in Table 1. The two-way repeated measures ANOVA revealed a significant main effect for group and an interaction between group and measurement phase in the “physical functioning” (P < 0.001 and P = 0.002, respectively) and “general health” (P = 0.046 and P = 0.01, respectively) constructs. There was a significant main effect of measurement phase for all constructs (P < 0.05) with the exception of “role limitations due to emotional problems” (P = 0.166). The differences between groups at each phase are reported in Table 1.
Table 1.
Descriptive statistics for the eight constructs of the SF-36 questionnaire in sedentary (SED) and lifelong exercising (LEX) groups at each phase of the exercise intervention
| SED group | LEX group | ||||
|---|---|---|---|---|---|
| Phase A | Phase B | Phase C | Phase A | Phase C | |
| Physical functioning | 75 ± 15c | 83 ± 11 | 93 ± 7a,b,c | 94 ± 4c | 97 ± 4c |
| Role limitations due to physical health | 91 ± 17 | 95 ± 16 | 100 ± 0a | 94 ± 14 | 97 ± 8 |
| Role limitations due to emotional problems | 96 ± 11 | 100 ± 0 | 100 ± 0 | 93 ± 18 | 96 ± 11 |
| Energy/fatigue | 68 ± 16 | 74 ± 10a | 80 ± 10a | 74 ± 14 | 79 ± 8 |
| Emotional well-being | 79 ± 14 | 84 ± 11a | 91 ± 7a,b | 85 ± 10 | 88 ± 6 |
| Social functioning | 91 ± 12 | 95 ± 10a | 97 ± 9 | 94 ± 14 | 99 ± 4 |
| Pain | 74 ± 23 | 76 ± 21 | 90 ± 11a,b | 79 ± 18 | 91 ± 10a |
| General health | 67 ± 12c | 70 ± 11a | 81 ± 8a,b | 77 ± 12c | 78 ± 11 |
SF-36 questionnaires were only completed by the SED group at Phase B. Data is presented as mean ± standard deviation
aSignificant increase from Phase A
bSignificant increase from Phase B
cSignificant difference between SED and LEX groups
Differences between measurement phases
In the SED group, there was a medium increase in “energy/fatigue” (5.3 ± 9.4, P = 0.025, 95 % CI 0.8–9.8, d = 0.56), “social functioning” (3.9 ± 7.3, P = 0.03, 95 % CI 0.4–7.5, d = 0.54) and “general health” (2.6 ± 5.4, P = 0.047, 95 % CI 0.1–5.2, d = 0.50) from Phase A to Phase B and a large increase in “emotional well-being” (5.2 ± 6.1, P = 0.001, 95 % CI 2.3–8.2, d = 0.86). Furthermore, “physical functioning” (9.7 ± 13.4, P = 0.005, 95 % CI 3.3–16.2, d = 0.73), “emotional well-being” (6.3 ± 12.7, P = 0.043, 95 % CI 0.2–12.4, d = 0.50) and “pain” (13.8 ± 23.3, P = 0.019, 95 % CI 2.6–25.1, d = 0.59) all increased to a medium extent from Phase B to Phase C. There was also a large increase in the “general health” score (11.6 ± 12.1, P = 0.001, 95 % CI 5.7–17.4, d = 0.95). In the SED group, there were significant increases in all SF-36 constructs from Phase A to Phase C (P < 0.05) with the exception of “role limitations due to emotional problems” (P = 0.163) and “social functioning” (P = 0.132). In the LEX group, there was a medium increase in the “pain” construct from Phase A to Phase C (11.5 ± 22.7, P = 0.045, 95 % CI 0.3–22.8, d = 0.51) with no change in the other constructs. The ANCOVA revealed that differences in “role limitations due to physical health”, “energy”, “social functioning”, “pain” and “general health” between groups at Phase A had a significant effect (P < 0.05) on the change in these constructs following training. However, there were no differences in the change to these constructs from A to C between SED and LEX when baseline scores were corrected for all (P > 0.110).
Exercise motivation inventory
Between and within subject effects
Descriptive statistics from the 14 constructs of the exercise motivation inventory (EMI) questionnaire are presented in Table 2. The two-way repeated measures ANOVA revealed a significant main effect for group in the “enjoyment” (P < 0.001), “challenge” (P < 0.001), “social recognition” (P = 0.001), “affiliation” (P = 0.001), “competition” (P < 0.001) and “weight management” (P = 0.002) constructs. There was a significant main effect of measurement phase for all constructs (P < 0.05) with the exception of “health pressures” (P = 0.214), “ill health avoidance” (P = 0.282) and “nimbleness” (P = 0.239). There was a significant interaction between group and measurement phase for all constructs (P < 0.05) with the exception of “social recognition” (P = 0.967), “affiliation” (P = 0.562), “competition” (P = 0.209) and “health pressures” (P = 0.679). The differences between groups at each phase are reported in Table 2.
Table 2.
Descriptive statistics for the fourteen constructs of the EMI-2 questionnaire in sedentary (SED) and lifelong exercising (LEX) groups at Phase A and Phase C of the exercise intervention
| SED group | LEX group | |||
|---|---|---|---|---|
| Phase A | Phase C | Phase A | Phase C | |
| Stress management | 1.9 ± 1.2 | 3.8 ± 0.9a | 2.5 ± 1.4 | 2.8 ± 1.2a |
| Revitalisation | 3.0 ± 1.0 | 4.5 ± 0.6a | 3.9 ± 0.8 | 4.1 ± 0.6 |
| Enjoyment | 3.0 ± 0.7b | 4.4 ± 0.4a | 4.2 ± 0.8b | 4.3 ± 0.4 |
| Challenge | 2.1 ± 0.9b | 3.3 ± 0.8a | 3.4 ± 0.8b | 3.6 ± 0.8 |
| Social recognition | 0.8 ± 0.7b | 1.5 ± 1.0a,b | 2.0 ± 1.3b | 2.4 ± 1.2a,b |
| Affiliation | 1.2 ± 0.9b | 1.6 ± 1.2b | 2.2 ± 1.4b | 2.7 ± 1.0b |
| Competition | 1.4 ± 1.1b | 2.2 ± 1.1a,b | 3.1 ± 1.4b | 3.3 ± 1.3b |
| Health pressures | 1.0 ± 0.9 | 1.4 ± 1.4 | 1.4 ± 1.3 | 1.5 ± 1.2 |
| Ill-health avoidance | 4.1 ± 0.6 | 4.6 ± 0.4 | 4.4 ± 0.7 | 4.1 ± 0.9 |
| Positive health | 4.2 ± 0.8 | 4.7 ± 0.3 | 4.6 ± 0.5 | 4.5 ± 0.5 |
| Weight management | 3.2 ± 0.8 | 4.3 ± 0.5a,b | 3.0 ± 1.1 | 2.9 ± 1.1b |
| Appearance | 1.9 ± 1.1 | 3.7 ± 0.7a | 2.8 ± 1.1 | 2.8 ± 1.0 |
| Strength and endurance | 3.2 ± 0.9 | 4.3 ± 0.4a | 3.8 ± 0.7 | 4.0 ± 0.5 |
| Nimbleness | 4.0 ± 0.9 | 4.5 ± 0.5 | 4.3 ± 1.0 | 4.0 ± 1.0 |
Data is presented as mean ± standard deviation
aSignificant increase from Phase A
bSignificant difference between SED and LEX groups
Differences between measurement phases
In the SED group, there were no changes in “affiliation”, “health pressures”, “ill-health avoidance”, “positive health” or “nimbleness” (all P > 0.05) from Phase A to Phase C. There were medium increases in “competition” (0.7 ± 1.1, P = 0.008, 95 % CI 0.2–1.2, d = 0.67) and “social recognition” (0.5 ± 0.8, P = 0.010, 95 % CI 0.1–0.9, d = 0.63), and large increases in all remaining seven constructs from Phase A to Phase C (all P < 0.01, d > 1.0). Both “stress management” (0.4 ± 0.6, P = 0.013, 95 % CI 0.1–0.6, d = 0.61) and “social recognition” (0.6 ± 1.1, P = 0.033, 95 % CI 0.1–1.1, d = 0.51) constructs increased to a medium extent in the LEX group from Phase A to Phase C with no change in the other constructs. The ANCOVA revealed that differences in all 14 constructs of the EMI between groups at Phase A had a significant effect (P < 0.05) on the change in these constructs following training. When correcting for differences in baseline scores, there were greater increases in “stress” (P < 0.001, 95 % CI 0.56–1.8, ή 2 = 0.29), “revitalisation” (P = 0.043, 95 % CI 0.2–0.9, ή 2 = 0.11), “ill-health” (P = 0.049, 95 % CI 0.01–0.96, ή 2 = 0.10), “weight management” (P < 0.001, 95 % CI 0.9–0.1.7, ή 2 = 0.50), “appearance” (P < 0.001, 95 % CI 0.9–1.9, ή 2 = 0.46), “strength & endurance” (P = 0.048, 95 % CI 0.02–0.63, ή 2 = 0.10) and “nimbleness” (P = 0.013, 95 % CI 0.1–1.0, ή 2 = 0.16) in the SED group compared to the LEX group. On the other hand, the increase in “affiliation” was greater in the LEX group than in the SED group (P = 0.02, 95 % CI 0.1–1.6, ή 2 = 0.14). There were no differences in the change in the remaining constructs between groups when corrected for the scores at Phase A.
Discussion
In support of the primary hypothesis, findings suggest that low-volume HIIT increases perceptions of HRQL, exercise motives and aerobic fitness in older adults, to varying degrees, in both SED males and LEX. To the authors’ knowledge, this is the first study to demonstrate both positive psychological and physiological effects of HIIT in healthy ageing men. Previous research has shown that various types of exercise training (e.g. aerobic, strength, functional strength and flexibility) increase perceptions of HRQL in older adults (Reid et al. 2010; Rejeski and Mihalko 2001). King and colleagues (King et al. 2000) compared the effects of two 12-month exercise programmes (moderate-intensity endurance and strengthening exercises vs. stretching and flexibility exercises) on measured and perceived physical functioning and HRQL in 103 older adults. Both exercise programmes improved perceptions of HRQL yet the participants in the stretching and flexibility exercise group reported greater improvements in bodily pain. Rejeski and Mihalko (2001) advocate that HRQL is a critical component of public health for older adults; therefore, targeting specific components of HRQL that are valued by the participants should be central to the development of their exercise training programmes. Although there is limited research examining the effects of HIIT on HRQL in an older adult population, our findings support previous research highlighting the positive psychological effects of HIIT on quality of life in other population samples, particularly clinical populations (Nilsson et al. 2008; Wisløff et al. 2007).
Clarity in the conceptual understanding of HRQL is central to furthering our understanding of the effects of types of exercise training on this multi-dimensional construct. The most widely used definition and measure of HRQL is the MOS SF-36, which encompasses both physical and mental health. Across the eight domains of the MOS SF-36, HIIT had the greatest effect on physical functioning over time from Phase A to Phase C in the SED group compared to the LEX group. Similar findings were reported in a study of cancer survivors aged 24–73 years who followed an 18-week high-intensity strength training programme (De Backer et al. 2008). Perceptions of the physical functioning domain of HRQL increased by 17 % in cancer survivors as a result of the training programme. Our findings demonstrate that the HIIT improved perceptions of all domains of HRQL in the SED group except social functioning and role limitations due to emotional problems. These findings suggest that certain aspects of HRQL may be improved to a greater extent by certain types of exercise training, and in this study, it appears that HIIT is more effective at targeting increases in physical health as opposed to mental health in sedentary ageing men. The LEX group had higher perceptions of physical functioning and general health than the SED group which would be expected as they regularly participate in exercise. Over time, the HIIT had no effect on perceptions of HRQL in the LEX group with the exception of an increase in their perceptions of pain. This could be due to the new physical challenge of a novel type of exercise training programme. Overall, the findings further echo Rejeski and Mihalko’s (2001) observations that targeting those aspects of HRQL that are valued by the participants, either trained or untrained, is critical in developing an appropriate and effective exercise training programme.
In relation to the exercise motives of the participants, HIIT had the greatest effect on exercise motives over time from Phase A to Phase C, in the SED group compared to the LEX group, even when baseline values were controlled for in the analysis. Overall, the LEX group had higher motives related to social recognition, affiliation and competition than the SED group yet weight management motives were significantly higher in the SED group. To date, there is no known research examining the effect of HIIT on motives for exercise in older adults yet there is a wealth of research supporting a positive relationship between exercise and motivation (Duncan et al. 2010; Wilson et al. 2003). Individuals are motivated to initiate and maintain exercise for a variety of reasons including weight management, social integration and enjoyment. In the present study, weight management, stress, revitalisation, challenge, enjoyment, strength and nimbleness and appearance motives appeared to demonstrate the greatest increases over time in the SED group with large effect sizes reported. This finding suggests that as a result of the HIIT, the SED group were motivated to exercise for both intrinsic and extrinsic reasons. Weight management and appearance-related motives are common extrinsic motives in those individuals at the early stages of integrating physical activity and exercise into their daily lifestyle, which could be the case in this study. Yet, it was encouraging to note that other motives, including the intrinsic motives of enjoyment and challenge, also increased greatly over time in the SED group as a result of the HIIT. Overall, the majority of the motives for exercise did increase, suggesting that the HIIT had a positive effect on the motivational levels of previously sedentary ageing men.
Similar to other studies (Freyssin et al. 2012; Roditis et al. 2007) employing 30 s HIIT intervals, we report no adverse events relating to the exercise intervention. As such, HIIT subsequent to 6 weeks of cardiovascular conditioning appears to be well-tolerated across fitness levels in ageing men. Training block 1, which consisted primarily of moderate-intensity exercise, produced a medium (5 %) (d = 0.6) improvement in the SED group. This preconditioning exercise proved effective in that all SED participants who completed training block 1 also completed the HIIT intervention. The magnitude of improvement in aerobic fitness following preconditioning is in line with previous similar exercise interventions involving previously sedentary participants (Gormley et al. 2008; Huang et al. 2005). As expected, in the LEX group both prior to and following training block 1 was within the standard error of measurement.
Small increases in significantly improve mortality risk. Given that increased in both the SED and LEX groups [3.3 ml kg min−1 (10.5 %, d = 1.06) and 3.0 ml kg min−1 (6.9 %, d = 0.9), our findings have clinical relevance. There is absence of comparative data regarding the efficacy of reduced frequency of HIIT on aerobic capacity in healthy older men. Data in younger men indicate LfHIIT to be effective at improving cardiorespiratory fitness. Recently, Nakahara et al. (2014), using young healthy males, demonstrated improvements in aerobic capacity that are greater than that demonstrated in the present study. Using a low-frequency protocol comprising one session per week with bouts at 80 % maximum work rate to volitional exhaustion, they demonstrated a 13 % increase in over 3 months. Similarly, there is evidence that reducing the volume of work within HIIT sessions, but maintaining their frequency at three sessions per week does not adversely impact on the improvements in aerobic capacity in young men (Matsuo et al. 2014; Zelt et al. 2014).
The present data are also comparable to previous studies examining standard frequency HIIT in healthy young adults. A recent meta-analysis of 37 HIIT studies reported an average increase in of 0.51 L min−1 (95 % CI: 0.43 to 0.60 L min−1) (Bacon et al. 2013). These data are comparable, though higher than that achieved in the present study. The meta-analysis of Bacon et al. (2013) considered only studies of young sedentary/moderately active participants (n = 334) under the age of 45 years, engaging in HIIT training a minimum of three times per week for durations of between 6 and 13 weeks and encompassing a minimum of 10 min of high-intensity work per session, interspersed with a minimum of 1:1 recovery intervals. The comparatively lower effect size in the present study may be due to the shorter duration, methodological differences or may be a reflection of the greater scope for increased in younger cohorts. Aside from variations in training protocol, the meta-analysis of Bacon et al. (2013) required a minimum “total amount of time” engaged “high intensity work” per week to be ≥30 min. This weekly volume is less than the total time (27 min−1) participants in the present study engaged in high-intensity work across the duration of the 6 week LfHIIT intervention. Furthermore, there is supporting data from another recent meta-analysis (Weston et al. 2014b) which demonstrated that reducing the volume of HIIT sessions but maintaining frequency at ~3 week−1 (low-volume HIIT) resulted in a similar magnitude for improvement to the present study.
Another recent meta-analytic review (Weston et al. 2014a) identified HIIT to be more effective than moderate-intensity exercise at improving in patients with lifestyle-associated cardiometabolic disease. Weston et al. (2014a) reported an average HIIT-induced improvement of 5.4 ml kg min−1 (19.4 %) compared with 2.6 ml kg min−1 (10.3 %) increase following moderate-intensity training across 10 randomised controlled trials (Weston et al. 2014a). However, the majority of these randomised controlled trials included patients who had poor baseline aerobic fitness and symptoms of vascular disease or heart failure. The present large improvements in in the SED group are in line with the 11.5 % reported following 6 weeks treadmill HIIT in young overweight men (Macpherson et al. 2011) and the 9.5 % following 2 weeks of cycle exercise HIIT in young obese men (Whyte et al. 2010).
Taken together, it seems that the overall quantity of time engaged in high-intensity work may be less important than the frequency of high-intensity excursions within each session and the concomitant duration of recovery interval. Furthermore, the present study employed a work to recovery interval ratio of 1:6 compared with a ≥1:1 ratio in the meta-analytical study of Bacon et al. (2013). This has important implications for exercise prescription amongst previously sedentary ageing persons who are likely to better tolerate shorter work to rest ratios. This is further supported by the complete adherence to the LfHIIT protocol in the present study, which may further inform exercise prescription amongst older adults who are known to perceive exercise participation to be overly time-consuming (Chao et al. 2000).
The present study has some important limitations that should be noted. One concerns the proximity of the conditioning programme (training block 1) to the HIIT intervention (training block 2), which makes it impossible to rule out the contribution of training block 1 to the overall effect of the SED group at Phase C. At the time of writing this manuscript, the effects of HIIT on SED are currently unknown and the authors deemed it prudent to gradually prepare the SED cohort by introducing them to supervised progressive exercise training. This is further justified when one considers the complete adherence to the HIIT training subsequent to cardiovascular conditioning. Further, improvements in LEX in response to HIIT would indicate that similar improvements in SED are as a consequence of the HIIT stimulus, rather than a residual effect of the conditioning exercise in training block 1. Another potential limitation relates to the absence of questionnaire data for the LEX group at Phase B, which prevented a comparison between groups following conditioning exercise in SED. However, given that LEX simply maintained their usual exercise training (and supported by an absence of physiological change) from Phase A to B, repetition of questionnaire data collection was deemed unnecessary.
Conclusion
HIIT appears to enhance HRQL and exercise motives (especially appearance/weight management) in otherwise healthy sedentary ageing males. Although HIIT has a minimal effect on HRQL and motives in lifelong exercisers, It does show significant promise as an alternative and time efficient training modality. These effects are concurrent with an increase in as a result of the HIIT. Low-volume HIIT is emerging as a credible, well-tolerated and effective approach to improving aerobic fitness in ageing men, irrespective of initial fitness levels.
References
- Acree LS, et al. Physical activity is related to quality of life in older adults. Health Qual Life Outcomes. 2006;4(1):37. doi: 10.1186/1477-7525-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon AP, Carter RE, Ogle EA, Joyner MJ. VO2max trainability and high intensity interval training in humans: a meta-analysis. PLoS One. 2013;8:e73182. doi: 10.1371/journal.pone.0073182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A (1997) Self-efficacy: The exercise of control. WH Freeman
- Borg GA. Perceived exertion: a note on history and methods. Med Sci Sports Exerc. 1973;5(2):90–93. doi: 10.1249/00005768-197300520-00017. [DOI] [PubMed] [Google Scholar]
- Chao D, Foy CG, Farmer D. Exercise adherence among older adults: challenges and strategies. Control Clin Trials. 2000;21(5):S212–S217. doi: 10.1016/S0197-2456(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Chrysohoou C, et al. High intensity, interval exercise improves quality of life of patients with chronic heart failure: a randomized controlled trial. QJM. 2014;107(1):25–32. doi: 10.1093/qjmed/hct194. [DOI] [PubMed] [Google Scholar]
- Craig R, Mindell J (2012) Health survey for England. London: The Information Centre
- Dacey M, Baltzell A, Zaichkowsky L. Older adults’ intrinsic and extrinsic motivation toward physical activity. Am J Health Behav. 2008;32(6):570–582. doi: 10.5993/AJHB.32.6.2. [DOI] [PubMed] [Google Scholar]
- De Backer IC, Vreugdenhil G, Nijziel MR, Kester AD, Van Breda E, Schep G. Long-term follow-up after cancer rehabilitation using high-intensity resistance training: persistent improvement of physical performance and quality of life. Br J Cancer. 2008;99(1):30–36. doi: 10.1038/sj.bjc.6604433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health (2011) Start active, stay active. A report on physical activity for health from the four home countries’. Chief Medical Officers. The Department of Health
- Duncan LR, Hall CR, Wilson PM, Jenny O. Exercise motivation: a cross-sectional analysis examining its relationships with frequency, intensity, and duration of exercise. Int J Behav Nutr Phys Act. 2010;7(7):1–9. doi: 10.1186/1479-5868-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyssin C, Verkindt C, Prieur F, Benaich P, Maunier S, Blanc P. Cardiac rehabilitation in chronic heart failure: effect of an 8-week, high-intensity interval training versus continuous training. Arch Phys Med Rehabil. 2012;93(8):1359–1364. doi: 10.1016/j.apmr.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, Little JP, MacDonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590:1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley SE, et al. Effect of intensity of aerobic training on VO2max. Med Sci Sports Exerc. 2008;40(7):1336. doi: 10.1249/MSS.0b013e31816c4839. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Mackinnon SL, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on VO2peak and the capillary supply to skeletal muscle. J Appl Physiol. 1997;82(4):1305–1310. doi: 10.1152/jappl.1997.82.4.1305. [DOI] [PubMed] [Google Scholar]
- Herbert P, Sculthorpe N, Baker JS, Grace FM (2015a) Validation of a 6 second power test for the determination of peak power output. Res Sports Med. Feb 27: 1–11. doi:10.1080/15438627.2015.1005294 [DOI] [PubMed]
- Herbert P, Grace FM, Sculthorpe N (2015b) Exercising caution: prolonged recovery to a single session of high intensity interval training in older men. J Am Gerontol Soc [DOI] [PubMed]
- Hogan M. Physical and cognitive activity and exercise for older adults: a review. Int J Aging Hum Dev. 2005;60(2):95–126. doi: 10.2190/PTG9-XDVM-YETA-MKXA. [DOI] [PubMed] [Google Scholar]
- Huang G, Gibson CA, Tran ZV, Osness WH. Controlled endurance exercise training and VO2max changes in older adults: a meta‐analysis. Prev Cardiol. 2005;8(4):217–225. doi: 10.1111/j.0197-3118.2005.04324.x. [DOI] [PubMed] [Google Scholar]
- King AC, Pruitt LA, Phillips W, Oka R, Rodenburg A, Haskell WL. Comparative effects of two physical activity programs on measured and perceived physical functioning and other health-related quality of life outcomes in older adults. J Gerontol A Biol Sci Med Sci. 2000;55(2):74–83. doi: 10.1093/gerona/55.2.M74. [DOI] [PubMed] [Google Scholar]
- Macpherson RE, Hazell TJ, Olver TD, Paterson DH, Lemon PW. Run sprint interval training improves aerobic performance but not maximal cardiac output. Med Sci Sports Exerc. 2011;43(1):115–122. doi: 10.1249/MSS.0b013e3181e5eacd. [DOI] [PubMed] [Google Scholar]
- Markland D, Ingledew DK. The measurement of exercise motives: factorial validity and invariance across gender of a revised. Exercise motivations inventory. Br J Health Psychol. 1997;2(4):361–376. doi: 10.1111/j.2044-8287.1997.tb00549.x. [DOI] [Google Scholar]
- Marsh AP, Miller ME, Rejeski WJ, Hutton SL, Kritchevsky SB. Lower extremity muscle function after strength or power training in older adults. J Aging Phys Act. 2009;17:416–443. doi: 10.1123/japa.17.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Saotome K, Seino S, Shimojo N, Matsushita A, Iemitsu M, Ohshima H, Tanaka K, Mukai C. Effects of a low-volume aerobic-type interval exercise on VO2max and cardiac mass. Med Sci Sports Exerc. 2014;46:42–50. doi: 10.1249/MSS.0b013e3182a38da8. [DOI] [PubMed] [Google Scholar]
- McAuley E, Konopack JF, Motl RW, Morris KS, Doerksen SE, Rosengren KR. Physical activity and quality of life in older adults: influence of health status and self-efficacy. Ann Behav Med. 2006;31(1):99–103. doi: 10.1207/s15324796abm3101_14. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Ueda SY, Miyamoto T (2014). Low-frequency severe-intensity interval training improves cardiorespiratory functions. Med Sci Sports Exerc [DOI] [PubMed]
- Nilsson BB, Westheim A, Risberg MA. Long-term effects of a group-based high-intensity aerobic interval-training program in patients with chronic heart failure. Am J Cardiol. 2008;2(9):1220–1224. doi: 10.1016/j.amjcard.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18(2):189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Pollock ML. Submaximal and maximal working capacity of elite distance runners. Part I: cardiorespiratory aspects. Ann N Y Acad Sci. 1977;301:310–322. doi: 10.1111/j.1749-6632.1977.tb38209.x. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, Zee PC. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010;11(9):934–940. doi: 10.1016/j.sleep.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeski WJ, Mihalko SL. Physical activity and quality of life in older adults. J Gerontol A Biol Sci Med Sci. 2001;56(suppl 2):23–35. doi: 10.1093/gerona/56.suppl_2.23. [DOI] [PubMed] [Google Scholar]
- Roditis P, et al. The effects of exercise training on the kinetics of oxygen uptake in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2007;14(2):304–311. doi: 10.1097/hjr.0b013e32808621a3. [DOI] [PubMed] [Google Scholar]
- Snowden M, et al. Effect of exercise on cognitive performance in community‐dwelling older adults: review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc. 2011;59(4):704–716. doi: 10.1111/j.1532-5415.2011.03323.x. [DOI] [PubMed] [Google Scholar]
- Storer TW, Davis JA, Caiozzo VJ. Accurate prediction of VO2max in cycle ergometry. Med Sci Sports Exerc. 1990;22(5):704–712. doi: 10.1249/00005768-199010000-00024. [DOI] [PubMed] [Google Scholar]
- Vickers AJ, Altman DG. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- Weston KS, Wisløff U, Coombes JS (2014a) High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 48(16):1227–1234 [DOI] [PubMed]
- Weston M, Taylor KL, Batterham AM, Hopkins WG. Effects of low-volume high-intensity interval training (HIT) on fitness in adults: a meta-analysis of controlled and non-controlled trials. Sports Med. 2014;44:1005–1017. doi: 10.1007/s40279-014-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte LJ, Gill JM, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010;59(10):1421–1428. doi: 10.1016/j.metabol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Wilson PM, Rodgers WM, Blanchard CM, Gessell J. The relationship between psychological needs, self‐determined motivation, exercise attitudes, and physical fitness. J Appl Soc Psychol. 2003;33(11):2373–2392. doi: 10.1111/j.1559-1816.2003.tb01890.x. [DOI] [Google Scholar]
- Wisløff U, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- Zelt JG, et al. Reducing the volume of sprint interval training does not diminish maximal and submaximal performance gains in healthy men. Eur J Appl Phys. 2014;114(11):2427–2436. doi: 10.1007/s00421-014-2960-4. [DOI] [PubMed] [Google Scholar]