Abstract
Purpose
Health care access and advanced cancer stage are associated with oncologic outcomes for numerous common cancers. However, the impact of patient travel distance to health care on stage at diagnosis has not been well characterized.
Methods
This study used a historical cohort of patients with colon cancer in the National Cancer Data Base from 2003 through 2010. The primary outcome, stage at diagnosis, was evaluated using hierarchical regression modeling. A secondary outcome was time to receipt of initial therapy that was evaluated using Cox shared frailty modeling.
Results
Among 296,474 patients with colon cancer (mean age, 68 ± 13.6 years; 47.6% male; 78.5% white), 3.9% traveled ≥ 50 miles to the diagnosing facility. Fewer black patients, patients with higher income, and patients with lower education traveled longer distances (trend test P < .001 for all). Patients traveling ≥ 50 miles were more likely to present with metastatic disease compared with those traveling less than 12.5 miles (odds ratio [OR], 1.18; 95% CI, 1.12 to 1.24) or 12.5 to 49.9 miles (OR, 1.18; 95% CI, 1.12 to 1.24). In sensitivity analyses, the association was robust to alternate methods of modeling travel distance (quintile stratification or continuous). Travel distance ≥ 50 miles was also associated with a higher likelihood of earlier initiation of therapy compared with travel distance of less than 12.5 miles (hazard ratio [HR], 1.10; 95% CI, 1.08 to 1.13) or 12.5 to 49.9 miles (HR, 1.11; 95% CI, 1.08 to 1.13).
Conclusion
Advanced colon cancer stage at diagnosis is associated with patient travel distance to health care, which may be a barrier to early cancer screening. Health care reform efforts designed to address only insurance coverage may not mitigate disparities based on difficulties accessing cancer care.
INTRODUCTION
Disparities in cancer treatment and outcomes are well-documented for various cancer sites.1–3 Numerous studies describe patient and provider factors associated with a patient's decision or ability to present for recommended cancer screening or therapy and the likelihood that screening or treatment are recommended by providers.1,4,5 However, the reasons and underlying mechanism for disparities in cancer care remain unclear.
Travel burden affects patient access to and use of health care.6,7 Numerous studies document that travel burden (variably measured as travel distance or travel time) can result in delays in diagnosis and can have an impact on the care that patients with a variety of common cancers ultimately receive.8–14 However, most of this work has used a variety of definitions for short and/or long travel distance, without clear consensus, and state-level data or smaller geographic units were used to define study cohorts. In the context of highly variable national geography (ie, travel distance in large states such as Texas or California may not be readily comparable to travel distance in New England), the generalizability of these findings may be limited. Alternatively, linked Surveillance, Epidemiology, and End Results-Medicare data could be used to evaluate travel burden among a national cohort. But studying only elderly patients who might already face difficulties with travel and/or transportation at baseline could potentially accentuate the effect of travel burden. To date, the association between travel distance and cancer stage at presentation has not been adequately evaluated in a national cohort.
The impact of travel distance on cancer stage is pertinent for two reasons. First, patients presenting with late-stage cancer have worse cancer-related outcomes, often with fewer therapeutic options after diagnosis and, as a result, might require additional systemic therapies compared with patients diagnosed and treated at an earlier stage.15 Second, given the variable regional geography of the United States, it important to understand whether any association between travel distance and advanced cancer stage at presentation is a national phenomenon or is restricted to more remote regions of the country. We therefore evaluated the impact of patient travel distance on cancer stage at presentation among a national cohort of patients with colon cancer in the United States. Our hypothesis was that increased travel distance is associated with more advanced cancer stage at presentation. As a secondary evaluation, we explored the relationship between travel distance and time to receipt of initial standard medical and/or surgical therapeutic interventions.
METHODS
Data
The National Cancer Data Base (NCDB) was used to conduct a historical cohort study of patients with colon cancer diagnosed from 2003 through 2010. A joint project of the American College of Surgeons Commission on Cancer (ACS CoC) and the American Cancer Society, the NCDB is a prospectively collected, hospital-based registry representing roughly 70% of all cancers diagnosed in the United States and has accumulated data on approximately 25 million cancer cases. This study was considered exempt by The University of Texas MD Anderson Cancer Center institutional review board, and the manuscript was approved by the ACS CoC.
Study Patients
The flow diagram in Figure 1 shows study exclusion criteria used to define the cohort. The NCDB provides patient travel distance to the reporting facility. Therefore, patients diagnosed at institutions other than the reporting facility were excluded. Patients without a reported travel distance were also excluded. In addition, patients whose travel distance exceeded 250 miles were excluded to minimize bias from those who may have electively sought care a long distance from their primary residence.16
Fig 1.
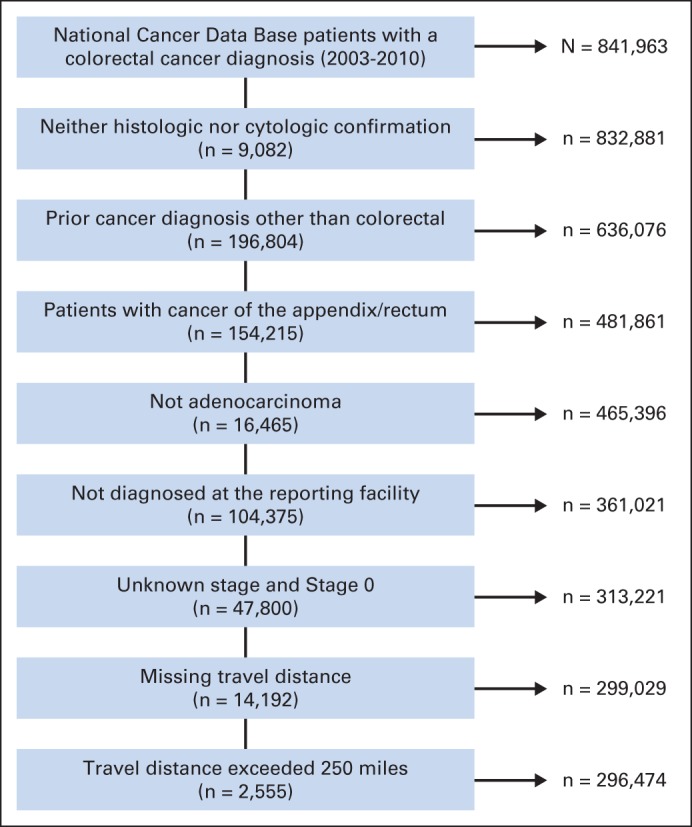
Flow chart of cohort inclusion/exclusion criteria. Values on the left side of the diagram indicate No. of patients excluded. Values on right side indicate No. of patients included.
Variables
Demographic and clinical data included age, sex, race/ethnicity (white, black, Hispanic, Asian, or other), indicators of income and education based on area of residence derived from Census 2000 data (used as proxies for patient socioeconomic status), insurance status (categorized as private, Medicare, Medicaid, other, or uninsured), rurality of residence, Charlson-Deyo comorbidity index17 (categorized 0, 1, and ≥ 2), and tumor characteristics (American Joint Committee on Cancer TNM classification). Hospital-level variables included type of facility (academic/research, comprehensive community cancer center, community cancer center, or other) and nine predefined geographic regions. Precalculated patient travel distance to the reporting facility is provided in the NCDB and represents an estimate of the “great circle” distance (in miles) between a patient's residential ZIP Code centroid and the reporting hospital's ZIP Code centroid (as calculated by using the Haversine formula). Three patient groups were created on the basis of travel distance: short (< 12.5 miles); intermediate (12.5 to 49.9 miles); long (≥ 50 miles). We selected 50 miles as our long-distance benchmark on the basis of work by Onega et al.9
Analysis
Categorical and continuous variable distributions were assessed by using standard descriptive statistics. A Cochran-Armitage test of trend was used to evaluate changes across travel distance categories. Cancer stage at presentation (distant metastatic v locoregional) was the primary outcome. Multivariable, two-level (patient and geographic region) hierarchical regression modeling was used to evaluate the association between travel distance and cancer stage at presentation. We chose multilevel modeling because we considered the data correlated—patients from the same geographic region are likely surrounded by a similar density of health care providers and facilities and thus may face similar travel difficulties when trying to access medical care.
In a secondary and exploratory analysis, we evaluated the association between travel distance and time to receipt of initial therapy after diagnosis in a subgroup who received therapy within 90 days of diagnosis and who were diagnosed and treated at the same facility (n = 254,797). Patients treated at a different facility were excluded for two reasons. First, those treated at a different hospital from the diagnosing facility have longer times to initiation of therapy after diagnosis.18 Second, NCDB data provides only travel distance to the reporting facility. To evaluate for potentially important differences among patients who were diagnosed and treated at the same versus different institutions, available demographic and clinical variables were compared. The two groups were generally similar with minimal differences in the distributions of categorical covariates. Multivariable Cox shared-frailty modeling (assuming a log-normal distribution) was chosen for the same reason that hierarchical modeling was selected in the primary analysis.19,20
Models were constructed using a nonparsimonious approach with age (categorized into < 60 years, 60 to 69, 70 to 79, ≥ 80 years), sex, race, insurance, income, education, rurality, and patient comorbidity selected as covariates. Missing data were uncommon, with less than 3% of patients missing any covariate data used in the regression analyses. Possible interactions between travel distance and insurance status and also between race and rurality were examined, but neither interaction term contributed to model performance. Sensitivity analyses were also conducted to ensure that study findings were not dependent on the defined categories for travel distance. In separate multivariable models, travel distance was categorized on the basis of quintiles and also as a continuous variable. When modeling distance continuously, two separate models were created, one with and another without a quadratic term (distance2), to account for a potential nonlinear relationship between travel distance and the outcome. All analyses were performed by using SAS 9.3 (SAS Institute, Cary, NC). Statistical comparisons were two-sided and were considered significant at the P < .05 level.
RESULTS
The cohort comprised 296,474 patients with colon cancer. Mean age was 68 ± 13.6 years, 47.6% were male, and 78.5% were white. Many had some burden of comorbid illness (30% had a Charlson score ≥ 1). The majority (53.9%) were diagnosed at a comprehensive community cancer center, and more than 50% of the patients sought care in the Southeast, Great Lakes, and Atlantic region. Nearly all patients received treatment at the hospital where they were diagnosed (97.7%).
Table 1 summarizes patient demographic and clinical characteristics as well as diagnosing facility characteristics stratified by patient travel distance to the diagnosing facility. The proportion of black patients (trend test P < .001) and patients with higher income (trend test P < .001) and lower education (trend test P < .001) significantly decreased with increasing travel distance. Patients traveling longer distances were significantly more likely to be diagnosed at an academic/research facility (trend test P < .001), although the opposite was true of patients diagnosed at community cancer centers (trend test P < .001).
Table 1.
Patient Demographic, Clinical, and Diagnosing Facility Characteristics Stratified by Travel Distance
| Characteristic | Travel Distance |
|||||
|---|---|---|---|---|---|---|
| Short (< 12.5 miles) |
Intermediate (12.5-49.9 miles) |
Long (≥ 50 miles) |
||||
| No. | % | No. | % | No. | % | |
| No. of patients | 215,561 | 69,296 | 11,617 | |||
| Demographic characteristics | ||||||
| Age, years | ||||||
| Median | 69 | 67 | 67 | |||
| ± SD | 13.7 | 13.4 | 13.2 | |||
| ≤ 40 | 2.2 | 2.7 | 2.7 | |||
| 40-49 | 7.0 | 8.4 | 7.4 | |||
| 50-59 | 16.8 | 19.2 | 18.0 | |||
| 60-69 | 21.7 | 25.4 | 26.2 | |||
| 70-79 | 26.4 | 25.3 | 26.2 | |||
| ≥ 80 | 25.9 | 19.0 | 19.6 | |||
| Male sex | 46.7 | 50.0 | 50.7 | |||
| Race/ethnicity | ||||||
| White | 76.1 | 85.0 | 84.7 | |||
| Black | 14.5 | 8.9 | 7.5 | |||
| Hispanic | 5.2 | 3.2 | 4.2 | |||
| Other | 3.0 | 1.7 | 2.1 | |||
| Missing | 1.2 | 1.2 | 1.5 | |||
| Insurance status | ||||||
| Private | 33.1 | 36.2 | 30.6 | |||
| Medicare | 56.9 | 53.6 | 56.9 | |||
| Medicaid | 4.4 | 3.7 | 4.3 | |||
| Other | 0.5 | 0.8 | 1.1 | |||
| Uninsured | 3.4 | 3.8 | 4.4 | |||
| Missing | 1.7 | 1.9 | 2.8 | |||
| Income, $* | ||||||
| ≥ 46,000 | 41.6 | 31.4 | 9.1 | |||
| Missing | 1.1 | 1.3 | 1.7 | |||
| Education* | ||||||
| ≥ 29% without HS education | 38.2 | 23.8 | 14.0 | |||
| Missing | 1.1 | 1.3 | 1.7 | |||
| Rurality | ||||||
| Metropolitan | 91.8 | 65.7 | 25.5 | |||
| Suburban | 6.2 | 28.3 | 57.3 | |||
| Rural | 0.1 | 4.6 | 15.9 | |||
| Missing | 1.9 | 1.3 | 1.3 | |||
| Clinical characteristics | ||||||
| Comorbidity index | ||||||
| 0 | 69.7 | 70.7 | 70.0 | |||
| 1 | 22.1 | 21.4 | 21.5 | |||
| ≥ 2 | 8.2 | 7.9 | 8.5 | |||
| AJCC stage | ||||||
| I | 23.2 | 23.5 | 21.5 | |||
| II | 28.4 | 27.7 | 27.6 | |||
| III | 27.6 | 27.8 | 27.2 | |||
| IV | 20.9 | 21.1 | 23.7 | |||
| Facility characteristics | ||||||
| Hospital type | ||||||
| Academic/research | 22.8 | 21.2 | 33.1 | |||
| Comprehensive community cancer center | 53.4 | 55.4 | 53.2 | |||
| Community cancer center | 22.1 | 22.0 | 12.9 | |||
| Other | 1.7 | 1.4 | 0.8 | |||
| Region | ||||||
| Northeast | 7.7 | 4.7 | 2.1 | |||
| Atlantic | 17.2 | 9.5 | 4.5 | |||
| Southeast | 20.2 | 23.8 | 14.9 | |||
| Great Lakes | 21.0 | 18.4 | 10.5 | |||
| South | 5.4 | 11.4 | 12.9 | |||
| Midwest | 6.5 | 9.0 | 21.6 | |||
| West | 6.8 | 11.0 | 17.4 | |||
| Mountain | 3.4 | 3.4 | 8.2 | |||
| Pacific | 11.7 | 8.8 | 8.1 | |||
| Treating/reporting status† | 97.7 | 97.6 | 97.2 | |||
NOTE. Column percentages may not add up to 100% due to rounding.
Abbreviations: AJCC, American Joint Committee on Cancer; HS, high school; SD, standard deviation.
Based on Census 2000 data. For income, data represent the percentage of participants whose area of residence had a median household income ≥ $46,000. For education, data represent the percentage of participants whose area of residence had ≥ 29% adults who did not attain a high school education.
Hospital where the patient was diagnosed (ie, reporting facility) is the same as the treating hospital.
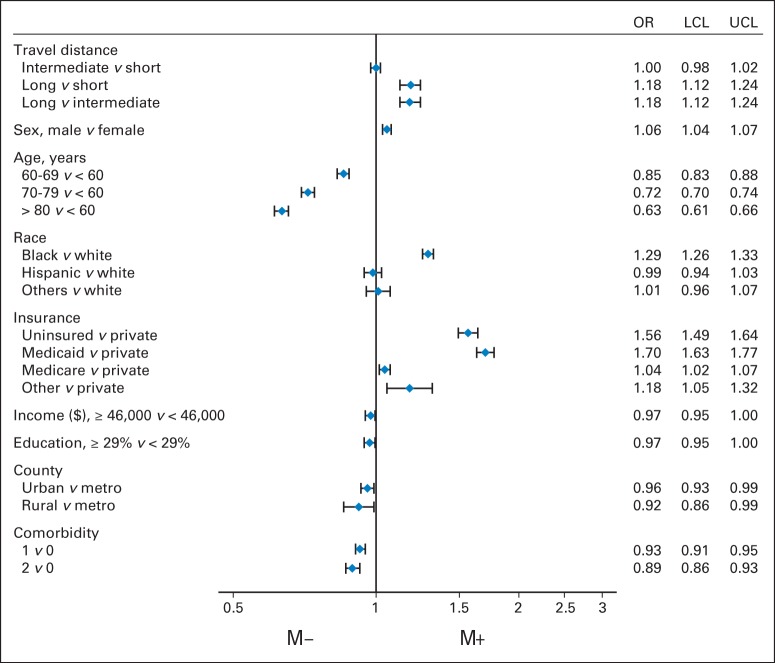
Multilevel model results are summarized in Figure 2. Patients traveling a long distance were more likely than those traveling a short distance (odds ratio [OR], 1.18; 95% CI, 1.12 to 1.24) to be diagnosed with metastatic colon cancer at presentation. Patients who traveled a long distance compared with an intermediate distance were also more likely (OR, 1.18; 95% CI, 1.12 to 1.24) to present with metastatic disease. There was no difference between those who traveled short and intermediate distances. Compared with patients age younger than 60 years, increasing age was associated with decreasing odds of metastatic disease at diagnosis (60 to 69 years: OR, 0.85; 95% CI, 0.83 to 0.88; 70 to 79 years: OR, 0.72; 95% CI, 0.70 to 0.74; ≥ 80 years: OR, 0.63; 95% CI, 0.61 to 0.66). Compared with white patients, black patients were more likely to have metastatic disease at diagnosis (OR, 1.29; 95% CI, 1.26 to 1.33). Compared with patients with private insurance, those who were uninsured (OR, 1.56; 95% CI, 1.49 to 1.64) and those with Medicaid (OR, 1.70; 95% CI, 1.63 to 1.77) were also at higher odds of presenting with metastatic disease.
Fig 2.
Forest plot of factors associated with stage IV disease at presentation. Income represents that a patient's area of residence (on basis of Census 2000 data) had a median household income ≥ $46,000 or < $46,000. Education represents that a patient's area of residence (on basis of Census 2000 data) had ≥ 29% or < 29% of adults who did not attain a high school education. M− represents a lower likelihood of metastatic disease. M+ represents a greater likelihood of metastatic disease. LCL, lower confidence limit; OR, odds ratio; UCL, upper confidence limit.
In an unadjusted time-to-event analysis, there appeared to be an association between increasing travel distance and receipt of therapy (Fig 3A-B). After adjustment for relevant covariates, long travel distance was associated with time to receipt of initial therapy. For patients with cancer at any stage and among the stage IV subgroup, those who traveled a long distance were 10% and 9%, respectively, more likely to initiate therapy earlier compared with patients traveling shorter distances (all stages: hazard ratio [HR], 1.10; 95% CI, 1.08 to 1.13; stage IV subgroup: HR, 1.09; 95% CI, 1.03 to 1.15); the effect was similar with short or intermediate (HR, 1.11; 95% CI, 1.08 to 1.13) distance used as the reference category. There was no difference in time to receipt of initial therapy among patients traveling an intermediate compared with a short distance.
Fig 3.
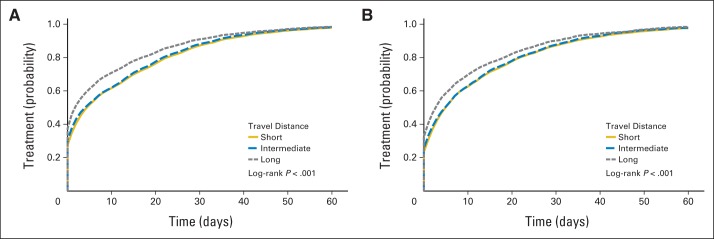
Unadjusted time to receipt of initial therapy among patients with (A) any stage disease and (B) stage IV disease.
Several sensitivity analyses were performed. Travel distance was modeled using both quintiles and as a continuous variable. The association between longer travel distance and metastatic disease at presentation was robust in all cases. The analyses were also repeated, stratified by race/ethnicity (white, black, Hispanic, and Asian). Although point estimates were generally similar across racial groups compared with the overall cohort, only the findings among white patients (approximately 80% of our cohort) were statistically significant.
DISCUSSION
In this national cohort study of patients in the United States who had colon cancer, we identified an association between patient travel distance and stage of disease at presentation. Patients traveling ≥ 50 miles to the diagnosing facility were more likely to present with metastatic disease than patients traveling shorter distances. Cancer stage at presentation significantly influences treatment planning as well as short- and long-term prognoses. Diagnosis at an earlier stage may allow for not only less invasive treatments but also for less treatment all together and therefore lower incurred health care costs.21,22 In the context of ongoing efforts to improve care and identify cost-saving strategies within the US health care system, and given the high and increasing costs associated with cancer care in the initial phase of treatment after diagnosis,23,24 finding ways to increase the proportion of patients with common diseases, such as colon cancer, who are initially diagnosed at an earlier stage could simultaneously markedly reduce costs and improve patient outcomes.
A variety of colon cancer screening methods represent cost-effective strategies for early cancer detection.25,26 For example, Gross et al27 recently demonstrated parallel increases in the use of colonoscopy and the detection of early-stage disease. Other recent work has demonstrated that colonoscopy is associated with a 70% to 80% decrease in the likelihood of patients presenting with advanced colon cancer.28,29 Yet national survey data suggest that rates of colon cancer screening in the United States are only 45% to 58%.30,31 Challenges obtaining transportation among certain subgroups of the general population may contribute to these low rates. Over the last decade, nonmedical financial obstacles (eg, transportation or lack of child care) and high travel burdens have been acknowledged by the Presidential Cancer Panel as key barriers to health care access.32,33 Issues with transportation are particularly problematic for underserved populations and are likely accentuated by increasing distance to medical care.34 Therefore, travel distance to medical care may act as a barrier for patients trying to access health care and could play a role in the low national rate of colon cancer screening.
We further explored this hypothesis by evaluating the association between travel distance and the time to receipt of initial therapy after diagnosis. Surprisingly, we found greater travel distance was positively associated with receipt of therapy. One possible explanation for this seeming paradox is that travel distance may cause patients to delay recommended colon cancer screening (possibly because of the inconvenience of traveling to a provider or center offering colonoscopy), but patients will promptly seek medical care, regardless of the distance they need to travel, once diagnosed. Alternatively, because patients traveling longer distances were more likely to present with advanced disease, they may have been either more aggressive about pursuing therapy because they had metastatic disease or they were symptomatic and therefore more proactive in pursuing therapy. To this point, Bilimoria et al18 have shown that earlier-stage disease for several cancers, including colon cancer, is associated with increased time from diagnosis to initiation of treatment.
Several studies have described the influence of race as well as place of residence across the spectrum of cancer care.2,35–38 The Affordable Care Act (ACA) has been proposed as an important step in addressing national inequities in health care. Key features of the ACA are designed to improve health care coverage, in particular among those with low income, underserved minorities, and the uninsured.39 However, in the context of our findings, it is unclear whether this approach will entirely address the problem at hand. If travel distance is a barrier to recommended cancer screening services, simply increasing health care coverage may not necessarily translate into the desired outcome—increased access to health care. This raises the question of how to increase patient access to and use of effective cancer screening modalities. Increasing the number of and access to primary care providers (PCPs) has also been proposed as a central aspect of ongoing plans for health care reform, largely because of the value placed on PCPs as portals to requisite preventative and screening services.40,41 However, that alone would not address potential travel difficulties that patients, particularly underserved patients, encounter when trying to access health care, especially if travel distance to specialists who perform endoscopic procedures is unchanged.
Our study results could be interpreted to suggest that detection of cancer prompts patients to initiate cancer care. If, however, access to specialists who provide screening colonoscopy is limited by travel distance, a relatively simple solution could be increasing the use of noninvasive screening tests that can be ordered and/or performed by PCPs (ie, fecal occult blood testing). Current examples of this approach are the Veterans Affairs Health System (the largest integrated health care system in the United States), in which fecal occult blood testing is the dominant colorectal screening modality, and Kaiser Permanente, a large integrated health care system in which fecal-based screening is the recommended system-wide approach and has resulted in screening rates far exceeding nationally reported rates.42,43 Although noninvasive screening modalities cannot provide a definitive diagnosis of colon cancer, abnormal findings might prompt patients to act earlier and actively seek care from providers or centers offering more definitive diagnostic methods. If the underlying issue is that patients with abnormal screening tests have limited access to providers who offer colonoscopy for diagnostic purposes, simply increasing use of noninvasive screening modalities would not adequately address the overall problem. For these patients, focusing additional diagnostic resources in more remote communities would be more likely to achieve the desired effect. As Accountable Care Organizations develop, expand, and become a more integral part of the US health care system, development of satellite clinics closer to patients who have longer travel distances or bringing mobile cancer screening personnel and resources to patients in remote communities could make cancer screening more accessible.
Our study has several notable limitations. Only patients with colon cancer were considered; therefore, generalizability of our findings to other disease sites for which effective screening modalities exist remains unclear. NCDB does not include data regarding patients' ability to travel and patients' resources. It also lacks provider information, such as availability and location of physicians offering colonoscopy. Travel distance was estimated by using ZIP Code centroids for both patients and diagnosing facilities, which raises possible misclassification bias. An inherent assumption in our analysis was that patients traveled by using personal modes of transportation rather than public transit. For patients who use public transit, travel distance may actually have been longer than reported. In addition, because ZIP Code regions can range in size from a city block to many square miles, these estimates may under- or over-represent the true distance traveled for care.
Ideally, patient and hospital ZIP Code data would have been provided rather than calculated travel distance. Calculation of patient travel distance to all the surrounding hospitals and/or providers would have allowed ascertainment of whether patients traveled to the closest facility or voluntarily bypassed closer hospitals en route to the diagnosing facility. To at least partially address this issue, patients who traveled more than 250 miles were excluded to mitigate bias introduced by patients electively traveling a greater distance (eg, tertiary referral center). In our time-to-event analysis, we could not entirely account for intentional nonreceipt of therapy (ie, patients consciously electing not to pursue care after diagnosis). Although such patients likely constitute a small proportion of patients in the general community, we attempted to account for this possibility by limiting this analysis to patients who actually initiated therapy within 90 days of diagnosis. Finally, we recognize that travel distance may be only a proxy and likely does not entirely reflect all the factors contributing to a patient's access to health care providers, institutions, and medical resources.
In 1999, the Institute of Medicine released its report Ensuring Quality Cancer Care. Among its recommendations for improving cancer care in the United States was a call to “…ensure entry to, and equitable treatment within, the cancer care system.”44 Our work suggests that perhaps this chasm has not yet been crossed. With growing attention to the quality of medical and cancer care and ongoing changes to the US health care system, finding effective ways to mitigate disparities in cancer care by understanding relevant modifiable and nonmodifiable patient factors affecting an individual's ability to access health care services may aid in the development of more effective cancer screening programs.
Acknowledgment
The authors thank Stephanie Deming and Sarah Bronson for editorial assistance.
Footnotes
Presented in part at the Annual Research Meeting of Academy Health, Seattle, WA, June 12-14, 2011.
The data used in this study are derived from a deidentified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Nader N. Massarweh, Yan Xing, George J. Chang, Alex B. Haynes, Y. Nancy You, Barry W. Feig, Janice N. Cormier
Financial support: Janice N. Cormier
Administrative support: Janice N. Cormier
Provision of study materials or patients: Janice N. Cormier
Collection and assembly of data: Nader N. Massarweh, Yi-Ju Chiang, Y. Nancy You, Janice N. Cormier
Data analysis and interpretation: Nader N. Massarweh, Yi-Ju Chiang, Yan Xing, George J. Chang, Alex B. Haynes, Y. Nancy You, Barry W. Feig, Janice N. Cormier
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 2.Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 3.Debarros M, Steele SR. Colorectal cancer screening in an equal access healthcare system. J Cancer. 2013;4:270–280. doi: 10.7150/jca.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson A. Unequal treatment: Confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94:666–668. [PMC free article] [PubMed] [Google Scholar]
- 5.Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 6.Bosanac EM, Parkinson RC, Hall DS. Geographic access to hospital care: A 30-minute travel time standard. Med Care. 1976;14:616–624. doi: 10.1097/00005650-197607000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Goodman DC, Fisher E, Stukel TA, et al. The distance to community medical care and the likelihood of hospitalization: Is closer always better? Am J Public Health. 1997;87:1144–1150. doi: 10.2105/ajph.87.7.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin I, Dalton H, Qiu Y, et al. Endometrial cancer surgery in Arizona: A statewide analysis of access to care. Gynecol Oncol. 2011;121:83–86. doi: 10.1016/j.ygyno.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Onega T, Duell EJ, Shi X, et al. Geographic access to cancer care in the U.S. Cancer. 2008;112:909–918. doi: 10.1002/cncr.23229. [DOI] [PubMed] [Google Scholar]
- 10.Scoggins JF, Fedorenko CR, Donahue SM, et al. Is distance to provider a barrier to care for Medicaid patients with breast, colorectal, or lung cancer? J Rural Health. 2012;28:54–62. doi: 10.1111/j.1748-0361.2011.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stitzenberg KB, Thomas NE, Dalton K, et al. Distance to diagnosing provider as a measure of access for patients with melanoma. Arch Dermatol. 2007;143:991–998. doi: 10.1001/archderm.143.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldwin LM, Cai Y, Larson EH, et al. Access to cancer services for rural colorectal cancer patients. J Rural Health. 2008;24:390–399. doi: 10.1111/j.1748-0361.2008.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroen AT, Brenin DR, Kelly MD, et al. Impact of patient distance to radiation therapy on mastectomy use in early-stage breast cancer patients. J Clin Oncol. 2005;23:7074–7080. doi: 10.1200/JCO.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 14.Celaya MO, Berke EM, Onega TL, et al. Breast cancer stage at diagnosis and geographic access to mammography screening (New Hampshire, 1998-2004) Rural Remote Health. 2010;10:1361. [PMC free article] [PubMed] [Google Scholar]
- 15.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975-2007. http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 16.Birkmeyer JD, Siewers AE, Marth NJ, et al. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003;290:2703–2708. doi: 10.1001/jama.290.20.2703. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: Trends and predictors of delays. Ann Surg. 2011;253:779–785. doi: 10.1097/SLA.0b013e318211cc0f. [DOI] [PubMed] [Google Scholar]
- 19.Callegaro A, Iacobelli S. The Cox shared frailty model with log-skew-normal frailties. Stat Model. 2012;12:399–418. [Google Scholar]
- 20.Hougaard P. Frailty models for survival data. Lifetime Data Anal. 1995;1:255–273. doi: 10.1007/BF00985760. [DOI] [PubMed] [Google Scholar]
- 21.Taplin SH, Barlow W, Urban N, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87:417–426. doi: 10.1093/jnci/87.6.417. [DOI] [PubMed] [Google Scholar]
- 22.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 23.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frazier AL, Colditz GA, Fuchs CS, et al. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954–1961. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 26.Sonnenberg A, Delcò F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med. 2000;133:573–584. doi: 10.7326/0003-4819-133-8-200010170-00007. [DOI] [PubMed] [Google Scholar]
- 27.Gross CP, Andersen MS, Krumholz HM, et al. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA. 2006;296:2815–2822. doi: 10.1001/jama.296.23.2815. [DOI] [PubMed] [Google Scholar]
- 28.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 29.Doubeni CA, Weinmann S, Adams K, et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: A nested case-control study. Ann Intern Med. 2013;158:312–320. doi: 10.7326/0003-4819-158-5-201303050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole AM, Jackson JE, Doescher M. Urban-rural disparities in colorectal cancer screening: Cross-sectional analysis of 1998-2005 data from the Centers for Disease Control's Behavioral Risk Factor Surveillance Study. Cancer Med. 2012;1:350–356. doi: 10.1002/cam4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapiro JA, Klabunde CN, Thompson TD, et al. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21:895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman HP, Reuben SH. President's Cancer Panel, Report of the Chairman, 2000-2001. Bethesda, MD: National Cancer Institute; 2001. Voices of a broken system: Real people, real problems. [Google Scholar]
- 33.Reuben SH, Milliken EL, Paradis LJ. President's Cancer Panel, 2009-2010 Annual Report. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2011. America's demographic and cultural transformation: Implications for cancer. [Google Scholar]
- 34.Guidry JJ, Aday LA, Zhang D, et al. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5:361–366. [PubMed] [Google Scholar]
- 35.Cooper GS, Koroukian SM. Racial disparities in the use of and indications for colorectal procedures in Medicare beneficiaries. Cancer. 2004;100:418–424. doi: 10.1002/cncr.20014. [DOI] [PubMed] [Google Scholar]
- 36.Onega T, Duell EJ, Shi X, et al. Influence of place of residence in access to specialized cancer care for African Americans. J Rural Health. 2010;26:12–19. doi: 10.1111/j.1748-0361.2009.00260.x. [DOI] [PubMed] [Google Scholar]
- 37.Morris AM, Billingsley KG, Baxter NN, et al. Racial disparities in rectal cancer treatment: A population-based analysis. Arch Surg. 2004;139:151–155. doi: 10.1001/archsurg.139.2.151. [DOI] [PubMed] [Google Scholar]
- 38.Calsoyas I, Stratton MS. Prostate cancer screening: A racial dichotomy. Arch Intern Med. 2004;164:1830–1832. doi: 10.1001/archinte.164.17.1830. [DOI] [PubMed] [Google Scholar]
- 39.Sommers BD, Bindman AB. New physicians, the Affordable Care Act, and the changing practice of medicine. JAMA. 2012;307:1697–1698. doi: 10.1001/jama.2012.523. [DOI] [PubMed] [Google Scholar]
- 40.Barnes KA, Kroening-Roche JC, Comfort BW. The developing vision of primary care. N Engl J Med. 2012;367:891–893. doi: 10.1056/NEJMp1204487. [DOI] [PubMed] [Google Scholar]
- 41.Bindman AB, Blum JD, Kronick R. Medicare's transitional care payment: A step toward the medical home. N Engl J Med. 2013;368:692–694. doi: 10.1056/NEJMp1214122. [DOI] [PubMed] [Google Scholar]
- 42.El-Serag HB, Petersen L, Hampel H, et al. The use of screening colonoscopy for patients cared for by the Department of Veterans Affairs. Arch Intern Med. 2006;166:2202–2208. doi: 10.1001/archinte.166.20.2202. [DOI] [PubMed] [Google Scholar]
- 43.Moiel D, Thompson J. Early detection of colon cancer: The Kaiser Permanente Northwest 30-year history—How do we measure success? Is it the test, the number of tests, the stage, or the percentage of screen-detected patients? Perm J. 2011;15:30–38. doi: 10.7812/tpp/11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spinks T, Albright HW, Feeley TW, et al. Ensuring quality cancer care: A follow-up review of the Institute of Medicine's 10 recommendations for improving the quality of cancer care in America. Cancer. 2012;118:2571–2582. doi: 10.1002/cncr.26536. [DOI] [PMC free article] [PubMed] [Google Scholar]