Abstract
Aims
Supernatants of serum-free cultured mononuclear cells (MNC) contain a mix of immunomodulating factors (secretome), which have been shown to attenuate detrimental inflammatory responses following myocardial ischaemia. Inflammatory dilated cardiomyopathy (iDCM) is a common cause of heart failure in young patients. Experimental autoimmune myocarditis (EAM) is a CD4+ T cell-dependent model, which mirrors important pathogenic aspects of iDCM. The aim of this study was to determine the influence of MNC secretome on myocardial inflammation in the EAM model.
Methods and results
BALB/c mice were immunized twice with an alpha myosin heavy chain peptide together with Complete Freund adjuvant. Supernatants from mouse mononuclear cells were collected, dialysed, and injected i.p. at Day 0, Day 7, or Day 14, respectively. Myocarditis severity, T cell responses, and autoantibody formation were assessed at Day 21. The impact of MNC secretome on CD4+ T cell function and viability was evaluated using in vitro proliferation and cell viability assays. A single high-dose application of MNC secretome, injected at Day 14 after the first immunization, effectively attenuated myocardial inflammation. Mechanistically, MNC secretome induced caspase-8-dependent apoptosis in autoreactive CD4+ T cells.
Conclusion
MNC secretome abrogated myocardial inflammation in a CD4+ T cell-dependent animal model of autoimmune myocarditis. This anti-inflammatory effect of MNC secretome suggests a novel and simple potential treatment concept for inflammatory heart diseases.
Keywords: Myocarditis, Conditioned medium, Secretome, Mononuclear cells
See page 650 for the editorial comment on this article (doi:10.1093/eurheartj/eht050)
Introduction
Myocarditis denotes inflammation of the heart muscle. Clinical presentations include subclinical disease to fatal courses with progressive heart failure, arrhythmia, and sudden death.1,2 The cause of myocarditis often remains unknown in the individual patient, but virus-triggered autoimmunity is thought to play an important role in disease development. Immunosuppressive regimens have failed to improve functional outcomes in large clinical trials of acute myocarditis,3–5 but are beneficial during chronic phases of disease in patients without evidence of viral genomes in heart muscle biopsies.6
The idea of using conditioned medium as a therapeutic agent evolved in the field of stem cell research. Many of the regenerative effects seen after administration of stem cells were rather mediated via paracrine signalling than by direct cellular interactions.7 Conditioned culture medium containing the secretome of mesenchymal stem cells is rich in angiogenic and chemotactic factors.8 Besides, there is growing evidence that stem cell conditioned medium has immunomodulating features as well.9,10
We have recently shown that a high-dose application of the secretome of peripheral blood mononuclear cells (PBMC) directly influences the endogenous inflammatory response after acute myocardial infarction (AMI). In a porcine closed-chest reperfusion infarction model, an i.v. injection of PBMC secretome effectively suppressed inflammatory responses and tissue damage.11–13 Moreover, we were able to show that PBMC secretome also attenuates microvascular obstruction, inhibits platelet aggregation, and causes vasodilation in a NOS-dependent manner.14 On the basis of these observations, we specifically addressed immunomodulatory features of MNC secretome and tested its anti-inflammatory effects in a model of autoimmune myocarditis.
Experimental autoimmune myocarditis (EAM) can be induced in susceptible mouse strains by immunization with a heart muscle myosin-specific peptide (MyHC-α614–629) together with a strong adjuvant. The majority of immunized mice develops myocarditis peaking 21 days after the first immunization.15 Experimental autoimmune myocarditis represents a CD4+ cell-mediated disease,16,17 accordingly, depletion of CD4+ cells effectively prevents disease development.18–20
Here, we provide for the first time evidence that high-dose application of MNC secretome attenuates EAM. Mechanistically, the secretome induces apoptosis of autoreactive CD4+ T cells.
Methods
Generation of murine and human mononuclear cell secretome
Spleens from donor Balb/c mice were removed and homogenized under sterile conditions. Splenocytes were resuspended in UltraCulture serum-free medium (Cambrex Corp., North Brunswick, NJ, USA; 1 × 106 cells/mL). After incubation for 24 h supernatants were dialysed against ammonium acetate (at a concentration of 50 mM, cut-off 3.5kD), sterile filtered, frozen, lyophilized, and kept frozen at −80°C until further used. Mononuclear cell secretome pooled from 10 different donor mice were used for further experiments. For some experiments, PBMC obtained from young healthy volunteers (ethics committee vote: 2010/034) were used for the production of MNC secretome. The mononuclear cell fraction was separated from venous whole-blood samples by Ficoll density-gradient centrifugation. Mononuclear cell secretome was produced according to the protocol described above. The content of mouse and human MNC secretome (obtained from 25 × 106 cells) was analysed using commercially available cytokine arrays (Proteome Profiler Arrays obtained from R&D, MN, USA) following the manufacturer's instructions.
Experimental autoimmune myocarditis induction
Animal experiments were approved by the University of Vienna, Austria (GZ66.009/0055-II/10b/2010). Experimental autoimmune myocarditis was induced in 6–8-week-old Balb/c mice by subcutaneous injection of 150 µg of the MyHC-α (MyHC-α614–629: Ac-SLKLMATLFSTYASAD) or ovalbumin emulsified 1:1 in PBS/CFA (1 mg/mL, H37Ra) with a 7-day interval between injections (on Day 0 and Day 7, respectively).21 Supernatant of 4 × 106 syngeneic, murine MNC cultures was i.p. injected at different time points (Day 0, Day 7, and Day 14). Injections of lyophilized culture medium served as a negative control. Mice were sacrificed on Day 21 (climax of inflammation) and hearts were evaluated for myocardial infiltrates.
Histopathological evaluation
Haematoxylin-eosin stained heart sections were scored according to a semi-quantitative scale (0, indicated no inflammatory infiltrates; 1, small foci of inflammatory cells between myocytes; 2, larger foci of >100 inflammatory cells; 3, <10% of a cross-section involved; 4, >30% of a cross-section involved), as previously described.22
Enzyme-linked immunosorbent assays
To characterize the impact of MNC secretome on the systemic inflammatory state, enzyme-linked immunosorbent assays (ELISA) were performed. IL-1β, IL-6, TNF-α, IFN-γ, IL-10, IL-17 and TGF-β1 were analysed in plasma samples obtained on Day 21 using commercially available kits (R&D, MN, USA). Formation of MyHC-α specific antibodies was determined by a solid phase ELISA, coating plates with 5 μg/mL MyHC-α. Since the original peptide sequence is hydrophobic, four lysine residues were added to the N-terminus to make the peptide water soluble (KKKKRSLKLMATLFSTYASADR). Plasma was diluted 1:10 for IgM, 1:50 for IgG1, 1:10 for IgG2a and IgG2b, and 1:50 for IgG3 and bound antibodies were detected with monoclonal rat anti-mouse IgM, IgG1, IgG2a, IgG2b, and IgG3 antibodies (Pharmingen, CA, USA) diluted 1:1000 and a HRP-coupled goat anti-rat antiserum (Amersham, Biosciences, UK) diluted 1:2000. The substrate for HRP was ABTS [60 mM/L citric acid, 77 mM/L Na2HPO4 × 2H2O, 1.7 mM/L ABTS (Sigma, MO, USA), 3 mM/L H2O2]. The content of sFAS, sFASL, sCD40, and sCD40L in MNC preparations was measured by commercially available ELISA kits (R&D Systems, Minneapolis, MN, USA).
Flow cytometry
Isolated mouse splenocytes and human PBMC were analysed for amounts of CD4+ T cells, CD8+ T cells, B cells, and monocytes. Mouse spleens were dissected and passed through a 40 mm cell strainer (BD Biosciences). Cells were washed with PBS and remaining erythrocytes were lysed with a commercially available haemolysis buffer (Morphisto, Frankfurt am Main, Germany). Isolated splenocytes and human PBMC were washed and analysed using the following fluorescence-labelled monoclonal antibodies: fluorescein isothiocyanate (FITC)-anti-CD4, phycoerythrin (PE)-anti-CD8 and PE-anti-CD19. All antibodies were obtained from Biozyme (Oldendorf, Germany). Appropriate isotype controls were included and gates were set according to isotype-matched controls. The content of monocytes was determined by placing a gate in the forward/side scatter dot blot. Analysis was performed on a FACSCalibur flow cytometer (BD Biosciences), and data were evaluated using the FlowJo software (Tree Star, Ashland, OR, USA). To test the CD4+/CD8+ cell ration in vivo, whole-blood samples were obtained from mice sacrificed 12 and 36 h after MNC secretome or medium control treatment on Day 14. Erythrocytes were lysed and cell pellets were stained with anti-CD4, anti-CD8 (both Acris, Herford, Germany) and 7-Aminoactinomycin D (7-AAD; Beckman Coulter, CA, USA). Numbers of CD4+ and CD8+ cells, CD4+/CD8+ ratio, and amount of 7-AAD positive CD4+ cells were determined by flow cytometry.
Proliferation assays
Spleens were homogenized and splenocytes (1 × 105) were cultured for 5 days with different concentrations of water-soluble MyHC-α. CD4+ cells were purified from spleens or human peripheral mononuclear cells obtained from healthy volunteers using the MACS bead system (Miltenyi Biotec, Bergisch Gladbach, Germany). 1×105 cells per well were either stimulated with phytohaemagglutinin (PHA, 7 µg/mL, Sigma, MO, USA) or a monoclonal antibody to CD3 (10µg/mL, Becton Dickinson, NJ, USA) in 96-well round-bottom plates. Human MNC secretome was added in different concentrations. Plates were incubated for 5 days and then pulsed for 18 h with 3[H]-thymidine. Proliferation of splenocytes and CD4+ cells was measured in a liquid scintillation counter.
Detection of apoptosis
Purified human CD4+ T cells, JURKAT cells (ATCC, VA, USA), or murine T cell lymphoma cells (CLS, Eppelheim, Germany) were incubated in a humidified atmosphere with or without human MNC secretome of 1.1 × 106 cells. Cell viability was monitored by Annexin V-fluorescein/propidium iodide (FITC/PI) co-staining (Becton Dickinson, Franklin Lakes, NJ, USA) at different time points (0, 6, 12, 24 h) or by determination of released histones (18 h) using a commercially available kit (Roche Molecular Biochemicals, Penzberg, Germany). Alternatively, purified CD4+ cells were pre-incubated for 30 min with 20 µM of different caspase inhibitors (Z-VAD, Z-DEDV, Z-IETD, Z-LEHD; purchased from R&D, MN, USA) before adding MNC secretome or lyophilized medium control. For antibody-blocking experiments, CD4+ cells were pre-incubated with antibodies directed against CD40L, FASL, VEGF, IL8, ENA78, MMP9, isotype control (all R&D, MN, USA), TRAIL1 or TRAIL2 (both Adipogen, Liestal, Switzerland) for 30 min. Mononuclear cell secretome of 1.1 × 106 cells was added and after 18 h of incubation, histone release was monitored.
Endocytosis and dendritic cell activation assays
Blood was obtained from young healthy volunteers and monocytes were purified by CD14 positive selection using MACS beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were incubated at concentration of 1 × 106 cells/mL for 5 days with IL-4 (1000 U/mL) and GM-CSF (50 ng/mL; both Peprotech, NJ, USA). The phenotype of naive dendritic cells (DCs) was determined by flow cytometry using CD14, CD1a, CD11c, CD80, CD83, CD86, and human leucocyte antigen (HLA)-DR specific antibodies (all Beckman Coulter, CA, USA). Endocytic activity was assessed by flow cytometry after incubating cells for 1 h with either MNC secretome (obtained from 1.25 × 106 cells) or medium control together with 1 mg/mL FITC-Dextran (Sigma, MO, USA). In an additional set of experiments, naive DCs were incubated for 24 h with MNC secretome (obtained from 1.25 × 106 cells) or control medium. Then, 1 µg/mL lipopolysaccharide (LPS; Sigma, MO, USA) was added and the expression of maturation markers (CD80, CD83, CD86, HLA-DR) was determined by flow cytometry.
Statistical analysis
Results are depicted as means ± standard error of the mean and levels of significances were determined by the two-sided student's t-test, two-sided Mann–Whitney U test, or ANOVA adjusted by a Bonferroni correction for multiple testings. Data analysis was performed with SPSS 18.0 (SPSS, Inc., USA) and GraphPad Prism 5 (GraphPad Software, Inc., CA, USA). A P-value <0.05 was regarded as statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
Mononuclear cell secretome attenuates experimental autoimmune myocarditis
Mononuclear cell secretome has recently been shown to reduce the inflammatory response during AMI. We, therefore, tested its effects in the EAM model, which mirrors important aspects of human inflammatory dilated cardiomyopathy (iDCM). Myosin peptide immunized mice were treated i.p. with MNC secretome at different time points. Secretome treatment during the phase of immunization (Day 0 or Day 7) had no impact on the extent of myocardial inflammation as expressed by the myocarditis score at Day 21 (Day 0 injection: MNC secretome 2.8 ± 0.6; control medium: 2.3 ± 0.6; P = 0.606 / Day 7 injection: MNC secretome 3.1 ± 0.3; control medium: 3.4 ± 0.5; P = 0.639). In contrast, injection of MNC secretome on Day 14 almost completely abrogated myocarditis at Day 21 (MNC secretome: 0.1 ± 0.1; control medium: 2.4 ± 0.4; P = 0.0089; Figure 1A–D). Hearts from MNC secretome-treated animals had only sparse lymphocytic infiltrations and no areas of cardiomyocyte apoptosis and/or necrosis (Figure 1E), whereas hearts from mice treated with control medium consistently showed dense inflammatory infiltrates (Supplementary material online, Figure S1).
Figure 1.
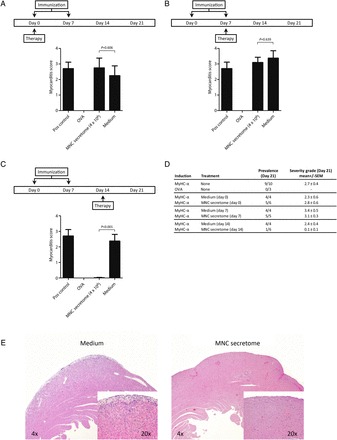
Pathological evaluations of explanted hearts are depicted in (A–D). Mononuclear cell secretome applied during the sensitization process (Day 0 and Day 7) had no impact on the development of myocarditis. Treating mice with mononuclear cell secretome on Day 14, significantly reduced the lymphocytic infiltrate (mononuclear cell secretome: 0.1 ± 0.1; control medium: 2.4 ± 0.4; Mann–Whitney U test: P = 0.0089). A representative sample of a heart section is shown in (E). In the heart of a control mouse, treated with ‘unconditioned’ medium a dense inflammatory infiltrate was seen, whereas only scattered, infiltrating lymphocytes can be found in the animal treated with mononuclear cell secretome.
Circulating levels of autoantibodies are only marginally affected by mononuclear cell secretome
Next, we evaluated the effect of MNC secretome on the formation of MyHC-α specific antibodies. Lower levels of circulating IgM and IgG1 were measured in MNC-treated animals, however, no differences in IgG2a, IgG2b, and IgG3 levels were found between the groups (Figure 2A).
Figure 2.

Evaluation of the autoantibody formation against MyHC-α is shown in (A). IgM and IgG1 levels were lower in mononuclear cell-treated animals, however, other immunoglobulin subtypes remained unchanged. Cytokine analysis of plasma samples obtained during scarification showed that the systemic inflammatory response was dampened in mice treated with mononuclear cell secretome when compared with control animals (B). Splenocytes obtained from mice after scarification evidenced a decreased proliferatory capacity against MyHC-α in a dose-dependent manner if animals were treated with mononuclear cell secretome (C).
Levels of inflammatory cytokines are reduced in mononuclear cell secretome-treated animals
To further characterize the anti-inflammatory effect of MNC secretome, we analysed plasma for levels of IL-1β, IL-6, TNF-α, IFN-γ, IL-10, IL-17, and TGF-β1. There were no detectable amounts of TNF-α, IFN-γ, IL-10, IL-17 in the circulation. There was a trend of lower IL-1, IL-6, and TGF-β1 levels in the treated group when compared with control animals, however, these observations did not reach significance (107.9 ± 35.5 vs. 43.7 ± 19.5 pg/mL; P = 0.115 / 45.9 ± 33.7 vs. 9.6 ± 3.1 pg/mL; P = 0.241 / 181.4 ± 103.7 vs. 12.5 ± 0.3 pg/mL; P = 0.083, respectively; Figure 2B).
Splenocyte proliferation to MyHC-α614–629 is strongly impaired in mononuclear cell secretome-treated animals
Previous studies have shown that proliferative responses to the myosin peptide in vitro are strongly linked to the development of EAM.22,23 We, therefore, isolated splenocytes from immunized mice, treated with either MNC secretome or control medium on Day 21. Splenocytes were stimulated with different concentrations of MyHC-α and proliferation was assessed by measuring 3[H]-thymidine uptake. As shown in Figure 2C, proliferation of splenocytes obtained from MNC secretome-treated animals was significantly impaired as calculated by ANOVA (Figure 2C).
Mononuclear cell secretome obtained from mouse splenocytes is comparable with mononuclear cell secretome obtained from human peripheral blood mononuclear cells
As a proof of principle that MNC secretome produced from mouse splenocyte cultures is comparable with MNC secretome from human PBMC cultures, we evaluated the distribution of CD4+ T cells, CD8+ T cells, B cells, and monocytes and performed cytokine arrays with both secretomes. Although splenocytes contained markedly more B cells, levels of secreted proteins were comparable in both preparation (Supplementary material online, Figure S2A and B, Figure 3A). Both secretomes contained considerable amounts of IL-1Ra, IL-16, MCP-1, RANTES, and sICAM-1. All other tested cytokines and chemokines were only present in low concentrations.
Figure 3.
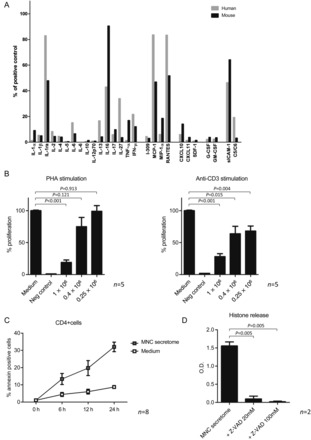
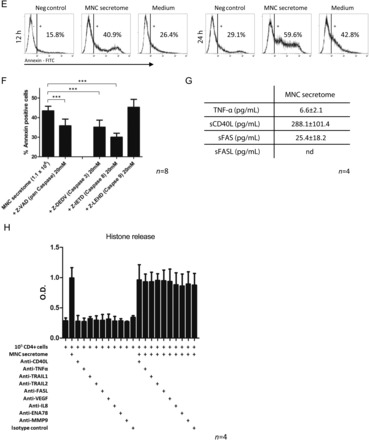
Results of cytokine arrays of mononuclear cell secretome obtained from cultured mouse splenocytes and mononuclear cell secretome obtained from cultured human peripheral blood mononuclear cell are depicted in (A). Both preparations were comparable regarding their secreted products. (B) The proliferative response of purified human CD4+ cells in the presence or absence of mononuclear cell secretome. CD4+ cells stimulated with phytohaemagglutinin or anti-CD3 showed lower proliferation rates when treated with mononuclear cell secretome. Unstimulated, purified CD4+ cells or a commercially available T cell line (JURKAT) undergo apoptosis in the presence of mononuclear cell secretome as shown by flow cytometry (C and E) and by histone release assay (D). This effect was partially reversible by pre-incubation with a caspase-3 and a caspase-8 inhibitor, indicating an external pathway-mediated effect (F). Interestingly, known pro-apoptotic cytokines were only found in marginal concentrations in the mononuclear cell secretome (G). Blocking pathways associated with apoptosis by neutralizing antibodies had no effect on mononuclear cell secretome-induced histone release (H).
Mononuclear cell secretome suppresses proliferation of CD4+ T cells in vitro but has no impact on dendritic cell function
Experimental autoimmune myocarditis is a CD4+ T cell-mediated disease. We, therefore, investigated the effect of MNC secretome on CD4+ cell proliferation in vitro. First, purified human CD4+ cells were stimulated either with PHA or with a monoclonal antibody to the T cell receptor. The addition of MNC secretome to the stimulation assays significantly reduced the proliferative response in a dose-dependent manner (Figure 3B). Since MNC secretome treatment on Day 0 and Day 7 had no impact on the development of myocarditis and dendritic cells (DCs) are considered pivotal during this sensitization process, we sought to further highlight the role of MNC secretome on DC function. Although the maturation of monocyte-derived dendritic cells seemed to be slightly impaired by MNC secretome treatment, endocytosis activity was not influenced (Supplementary material online, Figure S3A and B).
Mononuclear cell secretome induces apoptosis in CD4+ T cells, JURKAT, and murine T cell lymphoma cells
Next, we evaluated the effect of MNC secretome on cell viability. Co-incubation of unstimulated CD4+ T cells, a JURKAT cell line or a mouse lymphoma cell line with MNC secretome resulted in an apoptosis induction as determined by Annexin V/PI co-staining and by histone release assays (Figure 3C and E, Supplementary material online, Figure S2C). To exclude direct cytotoxic effects of MNC secretome, we pre-incubated cells with a pan-caspase inhibitor. Induction of apoptosis was inhibited by adding Z-VAD to the experimental setting (MNC secretome: 1.56 ± 0.11 O.D.; MNC secretome + Z-VAD 20 mM: 0.09 ± 0.07 O.D.; P = 0.008; MNC secretome + Z-VAD 100 mM: 0.01 ± 0.01 O.D.; P = 0.005; Figure 3D).
Caspase blocking experiments
To define whether apoptosis is mediated through external or mitochondrial pathways, we selectively blocked caspase-9, caspase-8, and caspase-3. Pre-incubation of purified CD4+ T cells with caspase-8 and caspase-3 but not caspase-9 inhibitors resulted in a significantly reduced Annexin staining (Figure 3F). These observations indicate that the external pathway is involved in MNC secretome-mediated apoptosis. Consequently, we evaluated known apoptosis-inducing factors in the secretome. As illustrated in Figure 3G, TNF-α, sCD40L, sFASL, and sFAS were only present in low concentrations in the MNC secretome. These findings suggest that the pro-apoptotic capacity is largely mediated by still unknown factor(s) or by a not yet understood interplay between several specific factors within the secretome.
Blocking antibodies against known pro-apoptotic are ineffective in reversing CD4+ T cell apoptosis
To characterize the role of known pro-apoptotic factors in the apoptosis-inducing capacity of MNC secretome we co-incubated purified CD4+ T cells with blocking antibodies directed against CD40L, TRAIL1, TRAIL2, and FASL. Antibodies directed against different chemokines without an apoptosis-inducing capacity (VEGF, IL8, ENA78, and MMP9) served as control. Histone release of MNC secretome-treated CD4+ cells was not reduced by blocking these factors, indicating that MNC secretome display its cell death inducing feature aside commonly accepted pathways (Figure 3H).
CD4/CD8 cell ratio is reduced in mononuclear cell secretome-treated animals
On the basis of our in vitro findings, we measured CD4+ and CD8+ cells 12 and 36 h after treating EAM animals with MNC secretome or control medium. The CD4/CD8 ratio was reduced in mice receiving the treatment when compared with control animals although this trend reached significance only at the 36 h timepoint (12 h: 2.1 ± 0.3 vs. 1.7 ± 0.4; P = 0.441; 36 h: 2.9 ± 0.2 vs. 2.0 ± 0.1; P = 0.007; Table 1). In parallel, the number of 7-AAD positive circulating CD4+ cells was increased in MNC secretome-treated animals when compared with the control group.
Table 1.
The CD4+ and CD8+ cell counts in whole-blood samples obtained 12 and 36 h after mononuclear cell secretome treatment (n = 4–5)
| CD4+ (%) | CD8+ (%) | CD4/CD8 ratio | CD4+/7-AAD pos | |
|---|---|---|---|---|
| 12 h | ||||
| Medium | 19.9 ± 1.3 | 10.8 ± 1.9 | 2.1 ± 0.3 | 8.1 ± 0.7 |
| MNC secretome | 13.3 ± 1.0 | 9.3 ± 2.0 | 1.7 ± 0.4 | 8.8 ± 2.8 |
| 36 h | ||||
| Medium | 23.9 ± 1.6 | 7.4 ± 1.1 | 2.9 ± 0.2 | 5.4 ± 0.5 |
| MNC secretome | 19.1 ± 2.4 | 9.3 ± 0.5 | 2.0 ± 0.1 | 10.6 ± 1.1 |
The CD4+/CD8+ cell ratio was reduced in treated animals when compared with controls. Additionally, CD4+/7-AAD positive cells were found more frequently.
Discussion
In this study, we showed for the first time that a systemic, high-dose application of MNC secretome attenuates EAM. In vitro analysis revealed an apoptosis-inducing effect of MNC secretome on CD4+ T cells. This observation was reversible by blocking the external apoptosis pathway.
Myocarditis is one of the leading causes for iDCM. The pathophysiology underlying the disease is still not completely understood. Nevertheless, autoimmunity is considered a key factor promoting ongoing inflammation, fibrosis, and pathological remodelling. Accordingly, specific subgroups of affected patients may take advantage of immunosuppressive treatment. However, first clinical trials testing immunosuppression for acute myocarditis failed. In a study by Parrillo et al.24 no advantage of immunosuppressive treatment was found. The Myocarditis Treatment Trial, comparing a placebo group to two immunosuppressive regimens (prednisolone and azathioprine or prednisolone and cyclosporine) came up with similar results a few years later.4 Both trails suggest that immunosuppression is not an option for the treatment of acute, viral myocarditis and this view was followed in the guidelines.25,26 The question of immunosuppression for myocarditis was readdressed when knowledge on pathophysiological aspects of myocarditis increased. Whereas in earlier studies patients were recruited without excluding cases of an acute viral myocarditis, Wojnicz et al.27 treated patients with chronic inflammatory heart disease and increased HLA expression on heart biopsies, with either prednisolone and azathioprine or placebo for 3 months. LVEF improved significantly in the immunosuppression group, even 2 years after treatment. These encouraging results were confirmed by Frustaci et al.6,28 on patients which fulfilled criteria for inflammatory heart disease but had no evidence of viral genome in biopsy samples. Future clinical trials testing immunomodulatory or immunosuppressive drug regimens should carefully distinguish between patients with acute viral myocarditis and chronic inflammatory heart disease where autoimmunity is the prevailing cause for ongoing disease after clearance of the virus. Tests measuring autoantibody load might help to better define forms of autoimmune myocarditis and could be valuable to monitor disease severity in the future.29
The EAM model was first described by Neu et al.30 The experimental basis of the EAM model is an immunization with a cardiac specific peptide—MyHC-α. Susceptible mouse strains such as Balb/c are immunized by a subcutaneously injection of an homologous α-myosin fragment together with a strong adjuvant.15 The EAM model is currently considered the best available model mimicking autoimmune mechanisms of inflammatory heart disease. It offers the great advantage to study disease pathogenesis and treatment effects in vivo in the absence of an infective agent.31 However, despite the advantage in testing new and promising therapeutic targets, data from animal models should be estimated with caution and must not uncritically be extrapolated to the human system.
We have found that treating mice with MNC secretome inhibited the development of an autoimmune myocarditis. This finding, however, was restricted to an application of the compound on Day 14, because treatment on Day 0 and Day 7 had no impact on disease severity. The reason for this might be a time-limited effect of MNC secretome on CD4+ cell suppression in vivo. In the EAM model, the injected myosin fragment persists at the deposition site and the injected MyHC-α/PBS/CFA suspension can be still found at the time of scarification when opening the inguinal region. On the other hand the half-life of the MNC secretome is currently unknown, however, as the effective components are most likely peptides/proteins a rapid decline in function can be considered within 24 h. Another explanation for the time-dependent efficacy of MNC secretome could be distinct immunological processes at different stages of EAM. During the sensitization phase dendritic cell function is crucial. Dendritic cells take up the injected myosin homologue, process it, and present it to naïve CD4+ T cells. Interestingly, MNC secretome had only a marginal impact on DC function. Maturation to LPS stimulation was only minimally impaired and endocytosis was unaltered in the presence of MNC secretome.
A major limitation of this study is that mice were treated by a single-dose protocol and the effect was only monitored on Day 21. Data on long-term effects of MNC secretome treatment are still missing. In addition, myocarditis is a chronic disease in the clinical scenario, therefore, repeated treatment for a longer time period is necessary. We plan to address these two questions in a future study.
Stem cells have been shown to possess—besides their regenerative capacity—considerable immunomodulatory features, e.g. they can effectively reduce lymphocyte proliferation in vitro.32 It has been suggested that these anti-proliferative effects are mediated, at least partly, via paracrine mechanisms.10,33 The idea of using ‘conditioned’ medium from stem cell cultures instead of stem cells has recently developed mainly supported by research in the field of regenerative medicine. Several groups have so far reported encouraging results of using the secretome of mesenchymal or bone marrow-derived stem cells in treating myocardial infarction.7,8,34,35 We have recently expanded the concept of regeneratory, stem cell-derived paracrine factors, by showing that the secretome of PBMC also mediates myocyte protection following myocardial ischaemia.11–13 Our findings were corroborated by the work of Wollert and colleagues36 showing in a detailed analysis that the secretome derived from stem cells only slightly differs from the secretome from peripheral blood leucocytes. A major advantage of using paracrine factors from PBMC instead of stem cells is that they are easily accessible. Our protocol of dialysis and lyophilization was developed for an off-the shelf scenario for future clinical applications. For the dialysis step, a cut-off of 3.5 kD was used to avoid a loss of proteins. Neither dialysis nor lyophilization had an impact on observed effects (unpublished data). In the clinical setting, MNC secretome could be produced in analogy to other ‘biologicals’ (e.g. i.v. immunoglobulins) from blood donations of healthy volunteers. However, strict regulatory prerequisites (e.g. virus inactivation, potency assays, and mandated GMP facilities) have to be met in order to reach human clinical trials.
The main mechanistic finding of this work is the capacity of MNC secretome to induce apoptosis of CD4+ T cells in vitro and in vivo. We have thoroughly evaluated this observation in primary CD4+ T cell cultures, a human JURKAT, and a mouse CD4+ T cell lymphoma cell line. Although the observed effect might not be limited to T-helper cells, suppressing the CD4+ cell function is substantial in regard to treating myocarditis. Previous work has shown that treatment with anti-CD4 monoclonal antibody significantly improved cardiac functional parameters in a rat myocarditis model. In addition, lymphocytes obtained from treated animals showed no proliferative response after in vitro stimulation with a myosin fragment.18,20 In a tedious work by Valaperti et al.,16 the importance of CD4+ cell function in the EAM model was addressed by showing that treatment with CD11b+ monocytes, suppressed the CD4+ dependent, MyHC-α-specific autoimmune response. These findings are supported by the clinical observation that T cell depletion is a possible rescue therapy for fulminant autoimmune myocarditis.37,38
Autoantibody formation is a well-described feature in the pathogenesis of myocarditis. Antibodies against a wide range of receptors, mitochondrial, and contractile proteins have been found both in human and in animal models.39 Myosin-specific antibodies are detectable in 46% of sera from patients suffering from dilated cardiomyopathy in western blot analysis.40 In contrast to this clinical observation, autoantibody formation is not directly involved in the development of myocardial infiltrates in the EAM model, since B-cell deficient mice still develop a myocarditis.22 However, levels of anti-myosin antibodies can be considered a surrogate marker to monitor disease severity in the EAM model. In our study, decreased circulating anti-myosin IgG1 and IgM together with reduced levels of IL-1β, IL-6, and TGF-β underline the therapeutic effect of MNC secretome.
One limitation of this study is that the MNC secretome by definition comprises of a myriad of proteins.36 Currently, although a detailed mapping of the protein content of MNC secretome has been performed, we have only limited knowledge regarding the factors mediating observed effects. Unfortunately, this is an unsolved problem for most of the work done in the field of secretome research. In some studies, potential target proteins were inactivated by blocking antibodies, however, effects were uniformly at most partially reversible.12,35,41 In this present study, we tried to correlate the apoptosis-inducing capacity of MNC secretome to known apoptosis-relevant factors. TNF-α, sCD40L, and sFAS were only marginally present in MNC secretome, sFASL was not detectable as determined by the ELISA technique. In addition, blocking different pathways associated with programmed cell death by neutralizing antibodies did not affect histone release in CD4+ cell cultures. Interestingly, non-protein mediated mechanisms of conditioned medium were put up for discussion recently. Timmers and collegues42 could show that exosomes consisting of cholesterol, sphingomyelin, and phosphatidylcholine-mediated cardioprotective effect.
To the best of our knowledge, this is the first study evaluating immunosuppressive features of a high-dose application of MNC secretome in the murine EAM model. Further studies are warranted to perpetuate the concept of using MNC secretome for the treatment of myocarditis.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was funded by the Christian Doppler Research Association and the Medical University of Vienna. The Medical University of Vienna has claimed financial interest (patent number: EP2201954, WO2010070105-A1, filed 18 December 2008). Funding to pay the Open Access publication charges for this article was provided by the Christian Doppler Laboratory for Cardiac and Thoracic Diagnosis and Regeneration.
Conflict of interest: H.J.A. is a shareholder of APOSIENCE AG, which owns the rights to commercialize MNC secretome for therapeutic use.
Supplementary Material
Acknowledgements
We are thankful to Barbara Bohle, Veronika Sexl, Barbara Neudert and Christoph Inci for intellectual and technical assistance. U.E. and P.B acknowledge support from the Swiss National Foundation.
References
- 1.Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 2.Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maisch B, Hufnagel G, Schonian U, Hengstenberg C. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Disease (ESETCID) Eur Heart J. 1995;16(Suppl O):173–175. doi: 10.1093/eurheartj/16.suppl_O.173. [DOI] [PubMed] [Google Scholar]
- 4.Mason JW, O'Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME, Moon TE. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 5.Hia CP, Yip WC, Tai BC, Quek SC. Immunosuppressive therapy in acute myocarditis: an 18 year systematic review. Arch Dis Child. 2004;89:580–584. doi: 10.1136/adc.2003.034686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J. 2009;30:1995–2002. doi: 10.1093/eurheartj/ehp249. [DOI] [PubMed] [Google Scholar]
- 7.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 8.Angoulvant D, Ivanes F, Ferrera R, Matthews PG, Nataf S, Ovize M. Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J Heart Lung Transplant. 2011;30:95–102. doi: 10.1016/j.healun.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Horn AP, Frozza RL, Grudzinski PB, Gerhardt D, Hoppe JB, Bruno AN, Chagastelles P, Nardi NB, Lenz G, Salbego C. Conditioned medium from mesenchymal stem cells induces cell death in organotypic cultures of rat hippocampus and aggravates lesion in a model of oxygen and glucose deprivation. Neurosci Res. 2009;63:35–41. doi: 10.1016/j.neures.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Kim SY, Cho HS, Yang SH, Shin JY, Kim JS, Lee ST, Chu K, Roh JK, Kim SU, Park CG. Soluble mediators from human neural stem cells play a critical role in suppression of T-cell activation and proliferation. J Neurosci Res. 2009;87:2264–2272. doi: 10.1002/jnr.22050. [DOI] [PubMed] [Google Scholar]
- 11.Ankersmit HJ, Hoetzenecker K, Dietl W, Soleiman A, Horvat R, Wolfsberger M, Gerner C, Hacker S, Mildner M, Moser B, Lichtenauer M, Podesser BK. Irradiated cultured apoptotic peripheral blood mononuclear cells regenerate infarcted myocardium. Eur J Clin Invest. 2009;39:445–456. doi: 10.1111/j.1365-2362.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenauer M, Mildner M, Hoetzenecker K, Zimmermann M, Podesser BK, Sipos W, Berenyi E, Dworschak M, Tschachler E, Gyongyosi M, Ankersmit HJ. Secretome of apoptotic peripheral blood cells (APOSEC) confers cytoprotection to cardiomyocytes and inhibits tissue remodelling after acute myocardial infarction: a preclinical study. Basic Res Cardiol. 2011;106:1283–1297. doi: 10.1007/s00395-011-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenauer M, Mildner M, Baumgartner A, Hasun M, Werba G, Beer L, Altmann P, Roth G, Gyongyosi M, Podesser BK, Ankersmit HJ. Intravenous and intramyocardial injection of apoptotic white blood cell suspensions prevents ventricular remodelling by increasing elastin expression in cardiac scar tissue after myocardial infarction. Basic Res Cardiol. 2011;106:645–655. doi: 10.1007/s00395-011-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoetzenecker K, Assinger A, Lichtenauer M, Mildner M, Schweiger T, Starlinger P, Jakab A, Berenyi E, Pavo N, Zimmermann M, Gabriel C, Plass C, Gyongyosi M, Volf I, Ankersmit HJ. Secretome of apoptotic peripheral blood cells (APOSEC) attenuates microvascular obstruction in a porcine closed chest reperfused acute myocardial infarction model: role of platelet aggregation and vasodilation. Basic Res Cardiol. 2012;107:292. doi: 10.1007/s00395-012-0292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pummerer CL, Luze K, Grassl G, Bachmaier K, Offner F, Burrell SK, Lenz DM, Zamborelli TJ, Penninger JM, Neu N. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest. 1996;97:2057–2062. doi: 10.1172/JCI118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valaperti A, Marty RR, Kania G, Germano D, Mauermann N, Dirnhofer S, Leimenstoll B, Blyszczuk P, Dong C, Mueller C, Hunziker L, Eriksson U. CD11b+ monocytes abrogate Th17 CD4+ T cell-mediated experimental autoimmune myocarditis. J Immunol. 2008;180:2686–2695. doi: 10.4049/jimmunol.180.4.2686. [DOI] [PubMed] [Google Scholar]
- 17.Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991;147:2141–2147. [PubMed] [Google Scholar]
- 18.Yuan HT, Liao YH, Wang Z, Dong JH, Cao LS, Wang ZH, Wang JP, Fu ML. Prevention of myosin-induced autoimmune myocarditis in mice by anti-L3T4 monoclonal antibody. Can J Physiol Pharmacol. 2003;81:84–88. doi: 10.1139/y02-159. [DOI] [PubMed] [Google Scholar]
- 19.Inomata T, Watanabe T, Haga M, Hirahara H, Abo T, Okura Y, Hanawa H, Kodama M, Izumi T. Anti-CD2 monoclonal antibodies prevent the induction of experimental autoimmune myocarditis. Jpn Heart J. 2000;41:507–517. doi: 10.1536/jhj.41.507. [DOI] [PubMed] [Google Scholar]
- 20.Wang QQ, Wang YL, Yuan HT, Liu FQ, Jin YP, Han B. Immune tolerance to cardiac myosin induced by anti-CD4 monoclonal antibody in autoimmune myocarditis rats. J Clin Immunol. 2006;26:213–221. doi: 10.1007/s10875-006-9018-2. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson U, Ricci R, Hunziker L, Kurrer MO, Oudit GY, Watts TH, Sonderegger I, Bachmaier K, Kopf M, Penninger JM. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003;9:1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson U, Kurrer MO, Schmitz N, Marsch SC, Fontana A, Eugster HP, Kopf M. Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003;107:320–325. doi: 10.1161/01.CIR.0000043802.38699.66. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M. Dual role of the IL-12/IFN-gamma axis in the development of autoimmune myocarditis: induction by IL-12 and protection by IFN-gamma. J Immunol. 2001;167:5464–5469. doi: 10.4049/jimmunol.167.9.5464. [DOI] [PubMed] [Google Scholar]
- 24.Parrillo JE, Cunnion RE, Epstein SE, Parker MM, Suffredini AF, Brenner M, Schaer GL, Palmeri ST, Cannon RO, III, Alling D, Wittes J, Ferrans V, Rodriguez R, Fauci A. A prospective, randomized, controlled trial of prednisone for dilated cardiomyopathy. N Engl J Med. 1989;321:1061–1068. doi: 10.1056/NEJM198910193211601. [DOI] [PubMed] [Google Scholar]
- 25.Howlett JG, McKelvie RS, Arnold JM, Costigan J, Dorian P, Ducharme A, Estrella-Holder E, Ezekowitz JA, Giannetti N, Haddad H, Heckman GA, Herd AM, Isaac D, Jong P, Kouz S, Liu P, Mann E, Moe GW, Tsuyuki RT, Ross HJ, White M. Canadian Cardiovascular Society Consensus Conference guidelines on heart failure, update 2009: diagnosis and management of right-sided heart failure, myocarditis, device therapy and recent important clinical trials. Can J Cardiol. 2009;25:85–105. doi: 10.1016/S0828-282X(09)70477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guidelines for diagnosis and treatment of myocarditis (JCS 2009): digest version. Circ J. 2011;75:734–743. doi: 10.1253/circj.CJ-88-0008. [DOI] [PubMed] [Google Scholar]
- 27.Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, Glanowska G, Wilczewski P, Niklewski T, Zembala M, Polonski L, Rozek MM, Wodniecki J. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation. 2001;104:39–45. doi: 10.1161/01.CIR.104.1.39. [DOI] [PubMed] [Google Scholar]
- 28.Frustaci A, Chimenti C, Calabrese F, Pieroni M, Thiene G, Maseri A. Immunosuppressive therapy for active lymphocytic myocarditis: virological and immunologic profile of responders versus nonresponders. Circulation. 2003;107:857–863. doi: 10.1161/01.CIR.0000048147.15962.31. [DOI] [PubMed] [Google Scholar]
- 29.Caforio AL, Tona F, Bottaro S, Vinci A, Dequal G, Daliento L, Thiene G, Iliceto S. Clinical implications of anti-heart autoantibodies in myocarditis and dilated cardiomyopathy. Autoimmunity. 2008;41:35–45. doi: 10.1080/08916930701619235. [DOI] [PubMed] [Google Scholar]
- 30.Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 31.Kania G, Blyszczuk P, Eriksson U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc Med. 2009;19:247–252. doi: 10.1016/j.tcm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 33.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Santo S, Yang Z, Wyler von Ballmoos M, Voelzmann J, Diehm N, Baumgartner I, Kalka C. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One. 2009;4:e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korf-Klingebiel M, Kempf T, Sauer T, Brinkmann E, Fischer P, Meyer GP, Ganser A, Drexler H, Wollert KC. Bone marrow cells are a rich source of growth factors and cytokines: implications for cell therapy trials after myocardial infarction. Eur Heart J. 2008;29:2851–2858. doi: 10.1093/eurheartj/ehn456. [DOI] [PubMed] [Google Scholar]
- 37.Ankersmit HJ, Ullrich R, Moser B, Hoetzenecker K, Hacker S, German P, Krenn C, Horvat R, Grimm M, Wolner E, Zuckermann A. Recovery from giant cell myocarditis with ECMO support and utilisation of polyclonal antithymocyte globulin: a case report. Thorac Cardiovasc Surg. 2006;54:278–280. doi: 10.1055/s-2006-923803. [DOI] [PubMed] [Google Scholar]
- 38.Cooper LT, Jr, Hare JM, Tazelaar HD, Edwards WD, Starling RC, Deng MC, Menon S, Mullen GM, Jaski B, Bailey KR, Cunningham MW, Dec GW. Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol. 2008;102:1535–1539. doi: 10.1016/j.amjcard.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kallwellis-Opara A, Dorner A, Poller WC, Noutsias M, Kuhl U, Schultheiss HP, Pauschinger M. Autoimmunological features in inflammatory cardiomyopathy. Clin Res Cardiol. 2007;96:469–480. doi: 10.1007/s00392-007-0524-x. [DOI] [PubMed] [Google Scholar]
- 40.Caforio AL, Grazzini M, Mann JM, Keeling PJ, Bottazzo GF, McKenna WJ, Schiaffino S. Identification of alpha- and beta-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation. 1992;85:1734–1742. doi: 10.1161/01.CIR.85.5.1734. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, von Ballmoos MW, Faessler D, Voelzmann J, Ortmann J, Diehm N, Kalka-Moll W, Baumgartner I, Di Santo S, Kalka C. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis. 2010;211:103–109. doi: 10.1016/j.atherosclerosis.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


