This editorial refers to ‘Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency’†, by P. Ponikowski et al., on page 657.
Iron is vital for our health as it plays a crucial role in many processes in the body. Due to its ability to accept and donate electrons, iron is a central part of numerous catalytic enzymes and proteins that are crucial for DNA synthesis, transport of oxygen, cell respiration, oxidative phosphorylation, and many other biochemical pathways. Iron is stored within ferritin and, although found in all cells of the body, the liver and the spleen serve as the main sites of ferritin storage. Although ferritin in the serum comprises only a minor part of total ferritin, it is a good indicator of total body iron. In the absence of a physiological excretion mechanism, iron homeostasis is regulated by absorption and recycling. As absorption capacity is limited, loss of iron—often caused by blood loss—may result quite rapidly in iron deficiency.
In chronic diseases, iron metabolism may be significantly disturbed, resulting in iron deficiency, either absolute or relative, even in the absence of blood loss.1 In chronic inflammatory diseases such as chronic kidney disease, SIRS (systemic inflammatory response syndrome), and advanced rheumatic disease, hepcidin, which plays an important regulatory role by inhibiting iron absorption, may also play an important role in relative iron deficiency. Augmented gastrointestinal loss and reduced availability of utilizable iron from the reticuloendothelial system may additionally contribute to iron deficiency.2 Recent observational data suggest that the pathophysiological mechanism in heart failure (HF) might be different.3 Still, the precise mechanism of iron deficiency in chronic diseases and potential differences between them remain to some extent unknown.
In HF, iron deficiency is related to the severity of the disease, is only partly related to anaemia, and is a poor prognostic sign, independently of other prognostic factors in both acute and chronic HF.4 In fact, the prognostic value of iron deficiency may significantly exceed that of anaemia. This might be explained by the fact that iron deficiency affects not only haemoglobin, but probably, already earlier, myoglobin. In addition, iron depletion may also take place in the myocardium of the failing heart.5 Although not tested properly yet, it is, therefore, tempting to speculate that iron depletion not only is a marker of advanced HF, but also plays an important pathophysiological role in its progression, in addition to contributing to symptoms due to reduced oxygen transport and utilization in the peripheral musculature. A logical step to improve symptoms positively and possibly also reverse remodelling, therefore, is to supplement iron in those patients with iron deficiency and HF. Ponikowski et al. now provide additional evidence that this may indeed result in beneficial effects for such patients.6
In CONFIRM-HF, the positive results of i.v. iron supplementation using ferric carboxymaltose (FCM) in HF seen in the FAIR-HF trial7 could indeed be confirmed. In fact, the results were rather consistent in the two trials regarding basically all outcomes measured, which is very reassuring. There was an increase in the 6 min walking distance by 33 m after 6 months compared with placebo,6 which is at least the extent found for most established therapies in HF.8 This improvement was seen irrespective of the presence of anaemia and the level of ferritin. Of note, the positive effects were not only seen in severely symptomatic patients as in FAIR-HF,7 but were seen irrespective of New York Heart Association (NYHA) class. Interestingly, the effects seemed to be larger in patients with diabetes or decreased kidney function, i.e. conditions in which repletion of iron deficiency may be particularly beneficial. The study was properly conducted, including proper blinding, which is not easy for iron application, and the investigators are to be commended.
CONFIRM-HF is more than just the confirmation of FAIR-HF.7 It shows that positive effects are not limited to a period of 6 months, but are sustained at least up to 1 year. More patients with NYHA class II were included, but patients still did not represent a real-world population, and were almost exclusively recruited in Eastern Europe. Importantly, iron was given as a bolus using a simplified dosing regimen without significant safety issues. This allowed rapid i.v. application with longer intervals between applications than in FAIR-HF. This is very important as it makes the treatment more easily applicable in daily practice in the outpatient setting, and will increase acceptance of i.v. iron therapy significantly.
The reduction in HF-related hospitalization was substantial6 and is very promising, particularly as such a reduction is seen not only in CONFIRM-HF, but also in other trials. When pooling the four studies using i.v. iron in HF patients with iron deficiency (i.e. ferritin <100 ng/mL or 100–300 ng/mL and transferrin saturation <20%),6,7,9,10 a large and significant reduction of HF hospitalization (Figure 1) is seen. Of note, all studies reached their primary endpoint and achieved improved symptoms and quality of life.6,7,9,10 Notwithstanding the above, it must be noted that compared with CONFIRM-HF, FAIR-HF—being similarly large and with similar prevalence of ischaemic cardiomyopathy, diabetes, and renal dysfunction—did not show a significant reduction in HF hospitalization by itself. The latter may relate to shorter follow-up, but also to inclusion of sicker patients, with their HF represented by a lower ejection fraction and higher prevalence of NYHA class III, respectively. A pooled analysis might shed more light on this issue.
Figure 1.
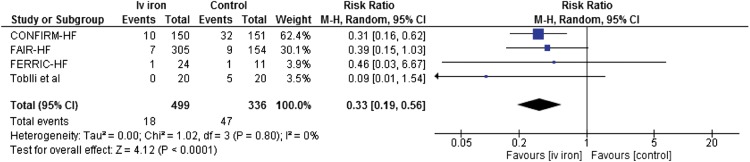
Forest plot of effects (risk ratio) of iron i.v. (using ferric carboxymaltose in the first CONFIRM-HF and FAIR-HF trials; iron sucrose complex in the remaining two trials) on heart failure hospitalization. CI, confidence interval.
Is parenteral iron treatment ready to be used for standard therapy in HF? The answer is: not yet. The increase in the 6 min walking distance by 33 m can be considered as clinically relevant, particularly as it is not the first study with such a finding and is additionally supported by positive results of most other endpoints. Improvement in 6 min walking distance has been accepted as sufficient to give therapies a class I indication in rare diseases such as pulmonary hypertension. However, HF is obviously not a rare disease. All treatments that are recommended with a class I indication for the treatment of chronic HF with reduced ejection fraction have been proven effective in large morbidity and mortality trials.11 Importantly, improvement in the 6 min walking distance has not been proven sufficiently robust for identification of effective pharmacological interventions.8 Long-term data particularly on mortality are not yet known and the effects seen so far on mortality are much less optimistic [pooled risk ratio of 0.82 (95% confidence interval 0.44–1.54)]6,7,9 than the effects on HF hospitalization. Moreover, the latter effect seems too good to be representative for a broad HF population.
An additional question that arises is why should a relatively expensive parenteral application be used if a cheap oral form is also available. One reason is that all studies so far used i.v. iron in HF. In addition, oral iron is neither suitable nor effective in all patients; oral iron is poorly absorbed, particularly in patients with chronic diseases such as HF, and is not well tolerated because of adverse gastrointestinal effects.12 Moreover, even if effective, it may take several months until iron stores are restored, whereas very rapid effects are seen with i.v. iron regarding not only iron stores but also clinical effects.13 Thus, i.v. iron supplementation may be superior. However, direct comparisons are scarce. Limited evidence in kidney disease suggests that i.v. iron is indeed more effective than oral iron.12,14 The evidence in HF is extremely limited. A very small study found improvement in VO2max by i.v. iron only, but not by oral iron despite a similar increase in haemoglobin.15
What recommendation for the use of iron in HF patients with iron deficiency can then be made at present? Obviously, standard therapy needs to be established first, based on current guidelines,11 also considering the underlying causes of HF. In addition, co-morbidities requiring class I interventions need to be addressed appropriately. If all treatments positively influencing prognosis are fully established, but patients still remain symptomatic, additional therapy should be considered. Given the very positive effects of iron supplementation on symptoms and quality of life in iron-deficient HF patients, such therapy is undoubtedly one of the first to be considered. Based on current knowledge, there is no rationale to try oral iron first, but rather i.v. FCM should be used in the doses and application used in CONFIRM-HF. Importantly, levels of ferritin and transferrin saturation must be controlled when following patients, as relapse of iron deficiency is likely. In less symptomatic HF, there is no reason at present to employ i.v. iron, and ferritin levels may even be increased in these patients.3
How should we proceed from this point? The authors state that ‘the study was not designed primarily to address the morbidity/mortality aspect of iron deficiency therapy with FCM, but our results constitute a strong background for such a study to be performed in the near future’.6 We fully agree with this statement; the sooner the better! When constructing such a trial, ample consideration should be given to long-term effects as well as co-morbid conditions prone to respond to iron, such as diabetes and kidney failure. If such a trial confirms the positive effects not only on functional capacity but also, much more importantly, on morbidity and mortality, i.v. iron supplementation may become the standard of care in HF patients with iron deficiency.
Conflict of interest: none declared.
References
- 1.Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Mori C, von Eisenhart RB, Pocock S, Poole-Wilson PA, Ponikowski P. Rationale and design of Ferinject assessment in patients with IRon deficiency and chronic Heart Failure (FAIR-HF) study: a randomized, placebo-controlled study of intravenous iron supplementation in patients with and without anaemia. Eur J Heart Fail. 2009;11:1084–1091. doi: 10.1093/eurjhf/hfp140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Veldhuisen DJ, Anker SD, Ponikowski P, Macdougall IC. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol. 2011;8:485–493. doi: 10.1038/nrcardio.2011.77. [DOI] [PubMed] [Google Scholar]
- 3.Jankowska EA, Malyszko J, Ardehali H, Koc-Zorawska E, Banasiak W, von Haehling S, Macdougall IC, Weiss G, McMurray JJ, Anker SD, Gheorghiade M, Ponikowski P. Iron status in patients with chronic heart failure. Eur Heart J. 2013;34:827–834. doi: 10.1093/eurheartj/ehs377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jankowska EA, Kasztura M, Sokolski M, Bronisz M, Nawrocka S, Oleskowska-Florek W, Zymlinski R, Biegus J, Siwolowski P, Banasiak W, Anker SD, Filippatos G, Cleland JG, Ponikowski P. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J. 2014;35:2468–2476. doi: 10.1093/eurheartj/ehu235. [DOI] [PubMed] [Google Scholar]
- 5.Maeder MT, Khammy O, dos RC, Kaye DM. Myocardial and systemic iron depletion in heart failure: implications for anemia accompanying heart failure. J Am Coll Cardiol. 2011;58:474–480. doi: 10.1016/j.jacc.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36:657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anker SD, Comin CJ, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart RB, Pocock SJ, Poole-Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 8.Olsson LG, Swedberg K, Clark AL, Witte KK, Cleland JG. Six minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: a systematic review. Eur Heart J. 2005;26:778–793. doi: 10.1093/eurheartj/ehi162. [DOI] [PubMed] [Google Scholar]
- 9.Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole-Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 10.Toblli JE, Lombrana A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50:1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 11.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 12.Rozen-Zvi B, Gafter-Gvili A, Paul M, Leibovici L, Shpilberg O, Gafter U. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: systematic review and meta-analysis. Am J Kidney Dis. 2008;52:897–906. doi: 10.1053/j.ajkd.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Comin-Colet J, Lainscak M, Dickstein K, Filippatos GS, Johnson P, Luscher TF, Mori C, Willenheimer R, Ponikowski P, Anker SD. The effect of intravenous ferric carboxymaltose on health-related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR-HF study. Eur Heart J. 2013;34:30–38. doi: 10.1093/eurheartj/ehr504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albaramki J, Hodson EM, Craig JC, Webster AC. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. 2012;1 doi: 10.1002/14651858.CD007857.pub2. CD007857. [DOI] [PubMed] [Google Scholar]
- 15.Beck-da-Silva L, Piardi D, Soder S, Rohde LE, Pereira-Barretto AC, de Albuquerque D, Bocchi E, Vilas-Boas F, Moura LZ, Montera MW, Rassi S, Clausell N. IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol. 2013;168:3439–3442. doi: 10.1016/j.ijcard.2013.04.181. [DOI] [PubMed] [Google Scholar]


